Oxidative Stress and Performance after Training in Professional Soccer (European Football) Players
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Measures
2.3. Yo-Yo Test
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Performance, Hormones, and Vitamins
4.2. Performance and Oxidative Stress
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, R.; Goodpaster, B.H.; Koch, L.G.; Sarzynski, M.A.; Kohrt, W.M.; Johannsen, N.M.; Skinner, J.S.; Castro, A.; Irving, B.A.; Noland, R.C.; et al. Precision exercise medicine: Understanding exercise response variability. Br. J. Sports Med. 2019, 53, 1141–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, D.; Lombardi, G.; Strollo, M.; Pontillo, M.; Motta, A.; Locatelli, M. A Possible Antioxidant Role for Vitamin D in Soccer Players: A Retrospective Analysis of Psychophysical Stress Markers in a Professional Team. Int. J. Environ. Res. Public Health 2020, 17, 3484. [Google Scholar] [CrossRef]
- de la Puente Yagüe, M.; Collado Yurrita, L.; Ciudad Cabañas, M.J.; Cuadrado Cenzual, M.A. Role of Vitamin D in Athletes and Their Performance: Current Concepts and New Trends. Nutrients 2020, 12, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalczyk, M.M.; Gołaś, A.; Maszczyk, A.; Kaczka, P.; Zając, A. Influence of Sunlight and Oral D3 Supplementation on Serum 25(OH)D Concentration and Exercise Performance in Elite Soccer Players. Nutrients 2020, 12, 1311. [Google Scholar] [CrossRef] [PubMed]
- Rojano-Ortega, D.; Berral-de la Rosa, F.J. Effects of vitamin D supplementation on muscle function and recovery after exercise-induced muscle damage: A systematic review. J. Hum. Nutr. Diet. 2022, 36, 1068–1078. [Google Scholar] [CrossRef]
- Molina-López, J.; Molina, J.M.; Chirosa, L.J.; Florea, D.I.; Sáez, L.; Planells, E. Effect of folic acid supplementation on homocysteine concentration and association with training in handball players. J. Int. Soc. Sports Nutr. 2013, 10, 10. [Google Scholar] [CrossRef]
- Krzywański, J.; Mikulski, T.; Pokrywka, A.; Mlyńczak, M.; Krysztofiak, H.; Frączek, B.; Ziemba, A. Vitamin B12 Status and Optimal Range for Hemoglobin Formation in Elite Athletes. Nutrients 2020, 12, 1038. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Kück, M.; Radziwolek, L.; Kerling, A. Iron Deficiency in Adolescent and Young Adult German Athletes—A Retrospective Study. Nutrients 2022, 14, 4511. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.; Alvero-Cruz, J.R.; Solla, J.; García-Bastida, J.; García-Coll, V.; Rivilla, I.; Ruiz, E.; García-Romero, J.; Carnero, E.A.; Clemente-Suárez, V.J. Competition Seriousness and Competition Level Modulate Testosterone and Cortisol Responses in Soccer Players. Int. J. Environ. Res. Public Health 2020, 17, 350. [Google Scholar] [CrossRef] [Green Version]
- Perroni, F.; Migliaccio, S.; Borrione, P.; Vetrano, M.; Amatori, S.; Sisti, D.; Rocchi, M.B.L.; Salerno, G.; Del Vescovo, R.; Cavarretta, E.; et al. Can Haematological and Hormonal Biomarkers Predict Fitness Parameters in Youth Soccer Players? A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 6294. [Google Scholar] [CrossRef]
- Abate, M.; Dicarlo, L.; Cocco, G.; Cocco, A.; Salini, V. Testosterone, cortisol, vitamin D and oxidative stress and their relationships in professional soccer players. J. Sports Med. Phys. Fitness 2022, 62, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hou, L.; Cui, M.; Mourot, L.; Zhu, W. Acute effects of low-volume intermittent versus higher-volume continuous exercise on arterial stiffness in healthy young men. Sci. Rep. 2022, 12, 1749. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Carretero, A.; Millan-Domingo, F.; Garcia-Dominguez, E.; Correas, A.G.; Olaso-Gonzalez, G.; Viña, J. Redox-related biomarkers in physical exercise. Redox Biol. 2021, 42, 101956. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wiltshire, H.D.; Baker, J.S.; Wang, Q. Effects of High Intensity Exercise on Oxidative Stress and Antioxidant Status in Untrained Humans: A Systematic Review. Biology 2021, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [Green Version]
- Thirupathi, A.; Wang, M.; Lin, J.K.; Fekete, G.; István, B.; Baker, J.S.; Gu, Y. Effect of Different Exercise Modalities on Oxidative Stress: A Systematic Review. Biomed Res. Int. 2021, 2021, 1947928. [Google Scholar] [CrossRef]
- Luti, S.; Modesti, A.; Modesti, P.A. Inflammation, Peripheral Signals and Redox Homeostasis in Athletes Who Practice Different Sports. Antioxidants 2020, 9, 1065. [Google Scholar] [CrossRef]
- Webb, R.; Hughes, M.G.; Thomas, A.W.; Morris, K. The Ability of Exercise-Associated Oxidative Stress to Trigger Redox-Sensitive Signalling Responses. Antioxidants 2017, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Jakovljević, V.; Zlatković, M.; Čubrilo, D.; Pantić, I.; Djurić, D. The effects of progressive exercise on cardiovascular function in elite athletes: Focus on oxidative stress. Acta Physiol. Hung. 2011, 98, 51–58. [Google Scholar] [CrossRef]
- Fatouros, I.G.; Chatzinikolaou, A.; Douroudos, I.I.; Nikolaidis, M.G.; Kyparos, A.; Margonis, K.; Michailidis, Y.; Vantarakis, A.; Taxildaris, K.; Katrabasas, I.; et al. Time-course of changes in oxidative stress and antioxidant status responses following a soccer game. J. Strength Cond. Res. 2010, 24, 3278–3286. [Google Scholar] [CrossRef]
- Ponce-Gonzalez, J.G.; Corral-Pérez, J.; De Villarreal, E.S.; Gutierrez-Manzanedo, J.V.; De Castro-Maqueda, G.; Casals, C. Antioxidants Markers of Professional Soccer Players during the Season and their Relationship with Competitive Performance. J. Hum. Kinet. 2021, 80, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Krępa, E.; Bańkowski, S.; Kargul, A.; Iskra, J. Changes in blood antioxidant status in American football players and soccer players over a training macrocycle. J. Exerc. Sci. Fit. 2021, 19, 229–233. [Google Scholar] [CrossRef]
- Powers, S.K.; Goldstein, E.; Schrager, M.; Ji, L.L. Exercise Training and Skeletal Muscle Antioxidant Enzymes: An Update. Antioxidants 2022, 12, 39. [Google Scholar] [CrossRef]
- Silva, J.R.; Rebelo, A.; Marques, F.; Pereira, L.; Seabra, A.; Ascensão, A.; Magalhães, J. Biochemical impact of soccer: An analysis of hormonal, muscle damage, and redox markers during the season. Appl. Physiol. Nutr. Metab. 2014, 39, 432–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassalle, C. An easy and reliable automated method to estimate oxidative stress in the clinical setting. Methods Mol. Biol. 2008, 477, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Maughan, R.J.; Gleeson, M.; Bilsborough, J.; Jeukendrup, A.; Morton, J.P.; Phillips, S.M.; Armstrong, L.; Burke, L.M.; Close, G.L.; et al. UEFA expert group statement on nutrition in elite football. Current evidence to inform practical recommendations and guide future research. Br. J. Sports Med. 2021, 55, 416. [Google Scholar] [CrossRef]
- Bangsbo, J.; Iaia, F.M.; Krustrup, P. The Yo-Yo intermittent recovery test: A useful tool for evaluation of physical performance in intermittent sports. Sports Med. 2008, 38, 37–51. [Google Scholar] [CrossRef]
- Krustrup, P.; Mohr, M.; Amstrup, T.; Rysgaard, T.; Johansen, J.; Steensberg, A.; Pedersen, P.K.; Bangsbo, J. The yo-yo intermittent recovery test: Physiological response, reliability, and validity. Med. Sci. Sports Exerc. 2003, 35, 697–705. [Google Scholar] [CrossRef]
- Svensson, M.; Drust, B. Testing soccer players. J. Sports Sci. 2005, 23, 601–618. [Google Scholar] [CrossRef]
- Singer, J.D.; Willett, J.B. Applied Longitudinal Data Analysis; Oxford University Press: New York, NY, USA, 2003; ISBN 0195152964. [Google Scholar]
- Singer, J.D. Using SAS PROC MIXED to Fit Multilevel Models, Hierarchical Models, and Individual Growth Models. J. Educ. Behav. Stat. 1998, 23, 323–355. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, R.; Sparvieri, E.; Di Blasio, A.; Barassi, G.; Murgia, M.; Ripari, P.; Di Iorio, A. Ankle-Brachial Index and Arterial Stiffness, Modulate the Exertional Capacity of High-Frequency Training Athletes. J. Cardiovasc. Dev. Dis. 2022, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Paganelli, R.; Abate, M.; Ireland, A.; Molino-Lova, R.; Sorbi, S.; Macchi, C.; Pellegrino, R.; Di Iorio, A.; Cecchi, F. Leukocyte-derived ratios are associated with late-life any type dementia: A cross-sectional analysis of the Mugello study. GeroScience 2021, 43, 2785–2793. [Google Scholar] [CrossRef]
- Zivkovic, V.; Lazarevic, P.; Djuric, D.; Cubrilo, D.; MacUra, M.; Vuletic, M.; Barudzic, N.; Nesic, M.; Jakovljevic, V. Alteration in basal redox state of young male soccer players after a six-month training programme. Acta Physiol. Hung. 2013, 100, 64–76. [Google Scholar] [CrossRef]
- Le Moal, E.; Groussard, C.; Paillard, T.; Chaory, K.; Le Bris, R.; Plantet, K.; Pincemail, J.; Zouhal, H. Redox Status of Professional Soccer Players is Influenced by Training Load Throughout a Season. Int. J. Sports Med. 2016, 37, 680–686. [Google Scholar] [CrossRef]
- Ying, J.; Cen, X.; Yu, P. Effects of Eccentric Exercise on Skeletal Muscle Injury: From An Ultrastructure Aspect: A Review. Phys. Act. Health 2021, 6, 15–20. [Google Scholar] [CrossRef]
- Cheng, A.J.; Yamada, T.; Rassier, D.E.; Andersson, D.C.; Westerblad, H.; Lanner, J.T. Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery. J. Physiol. 2016, 594, 5149–5160. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wang, X.; Liu, Y.; Zhang, Z. Effects of Exercise-Induced ROS on the Pathophysiological Functions of Skeletal Muscle. Oxid. Med. Cell. Longev. 2021, 2021, 3846122. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.N.; Lamberts, R.P.; Lambert, M.I. High responders and low responders: Factors associated with individual variation in response to standardized training. Sports Med. 2014, 44, 1113–1124. [Google Scholar] [CrossRef]
- Bonafiglia, J.T.; Preobrazenski, N.; Gurd, B.J. A Systematic Review Examining the Approaches Used to Estimate Interindividual Differences in Trainability and Classify Individual Responses to Exercise Training. Front. Physiol. 2021, 12, 1881. [Google Scholar] [CrossRef] [PubMed]
- Peternelj, T.T.; Coombes, J.S. Antioxidant supplementation during exercise training: Beneficial or detrimental? Sports Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Chen, C.; Teo, E.C.; Zhang, Y.; Huang, J.; Xu, Y.; Gu, Y. Intracellular Oxidative Stress Induced by Physical Exercise in Adults: Systematic Review and Meta-Analysis. Antioxidants 2022, 11, 1751. [Google Scholar] [CrossRef] [PubMed]
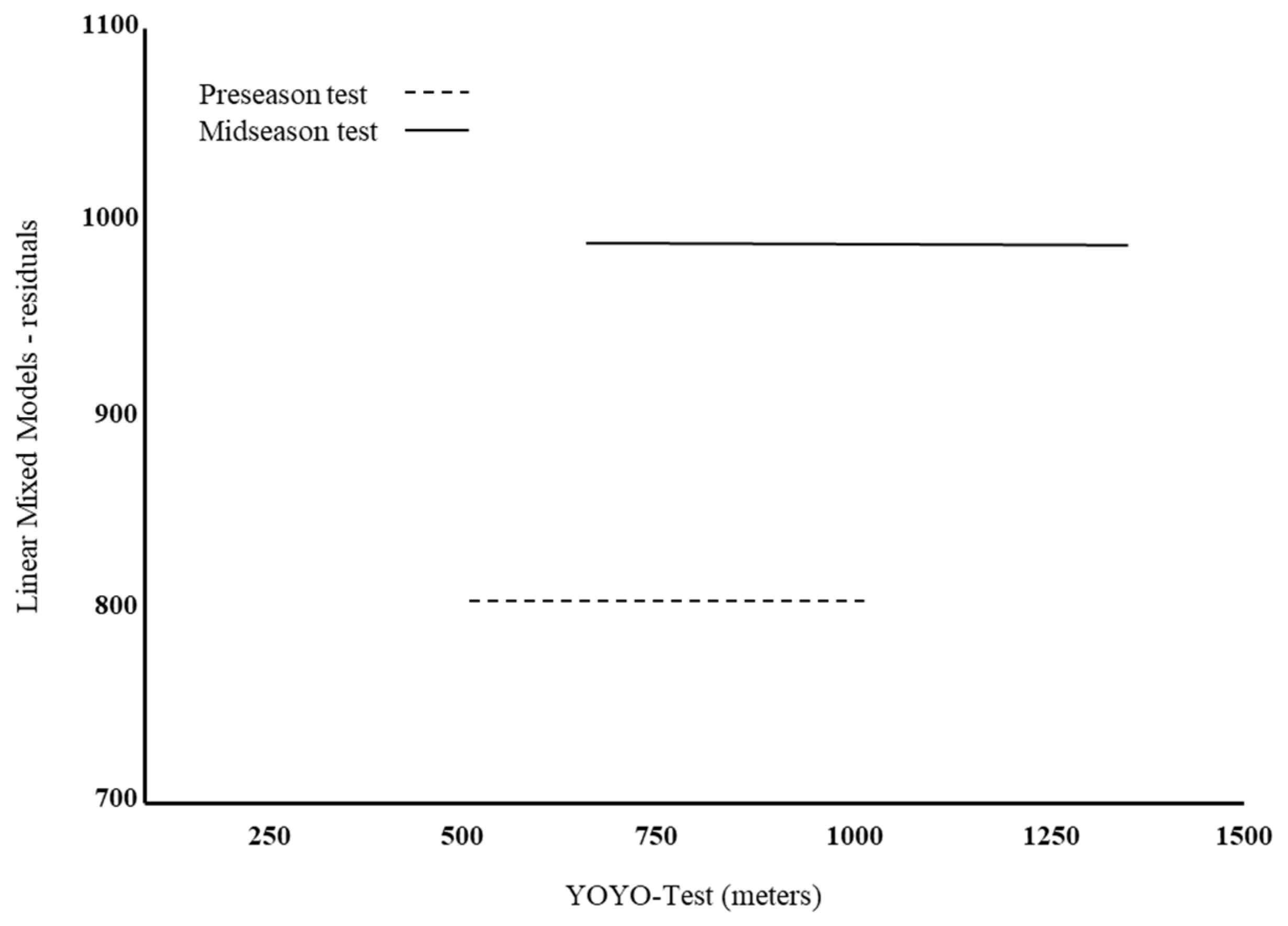
| Pre-Season | Mid-Season | p-Value | |
|---|---|---|---|
| Number | 82 | 82 | |
| Age (year) | 25.3 ± 4.2 | 25.3 ± 4.2 | |
| Weight (kg) | 75.1 ± 6.2 | 73.7 ± 4.8 | 0.42 |
| Height (cm) | 176.8 ± 5.9 | 176.8 ± 6 | 0.99 |
| BMI (kg/m2) | 23.9 ± 1 | 23.5 ± 0.8 | 0.36 |
| Yo-Yo test (m) | 799.8 ± 116.4 | 984.1 ± 153.6 | <0.001 * |
| Hemoglobin (g/dL) | 15 ± 0.6 | 14.9 ± 0.5 | 0.07 |
| Iron (µg/dL) | 97.6 ± 25.3 | 96 ± 21.9 | 0.76 |
| Vitamin B12 (pg/mL) | 667.6 ± 174.8 | 655.1 ± 166.1 | 0.98 |
| Folic Acid (ng/mL) | 82.2 ± 24.3 | 79.4 ± 20.6 | 0.83 |
| Vitamin D (ng/dL) | 29.1 ± 6.6 | 26 ± 6.8 | 0.16 |
| Testosterone (ng/mL) | 86.9 ± 24 | 77.6 ± 24 | 0.17 |
| Cortisol (µg/dL) | 117.7 ± 44.4 | 177.4 ± 77.7 | 0.001 * |
| Creatin kinase (µg/L) | 231.1 ± 106.5 | 233.8 ± 116.4 | 0.70 |
| Oxidative stress (U CARR) | 242.5 ± 74.2 | 287.7 ± 56.6 | 0.02 * |
| BAP (μmol/L) | 2233.8 ± 772.7 | 2443.2 ± 467 | <0.001 * |
| BAP/U CARR ratio | 9.6 ± 2.9 | 8.7 ± 2.2 | 0.58 |
| Model A | Model B | Model C | Model D | Model E | ||
|---|---|---|---|---|---|---|
| Initial status | ||||||
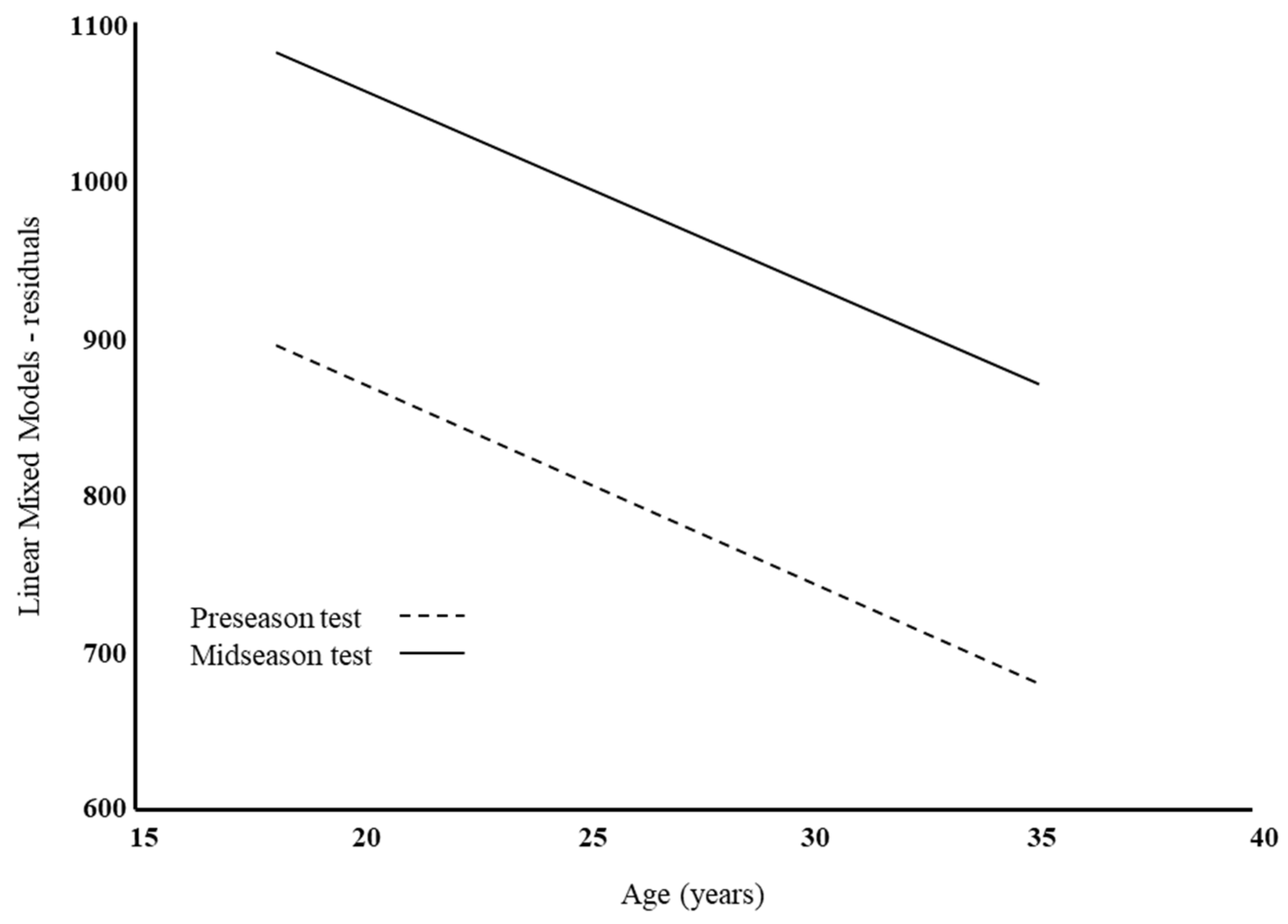
| Intercept | γ00 | 893.4 ± 13.9 *** | 985.6 ± 14.8 *** | 1105 ± 94 *** | 1303 ± 154 *** | 1131 ± 128 *** |
| Age | γ01 | −12.02 ± 2.8 *** | −0.2 ± 5.7 | −18.7 ± 4.5 *** | ||
| BAP | γ02 | 0.09 ± 0.02 *** | ||||
| UCARR | γ03 | 0.1 ± 0.5 | ||||
| Ratio | γ04 | 15.4 ± 11.05 | ||||
| Age*U CARR | γ01*03 | −0.05 ± 0.02 * | ||||
| Age*Ratio | γ01*04 | 0.8 ± 0.4 | ||||
| Rate of change | ||||||
| Intercept | γ10 | −184.3 ± 10.4 *** | −28.6 ± 51.7 | 502.2 ± 8.2 *** | 123.3 ± 37.2 ** | |
| Time*BAP | γ12 | −0.07 ± 0.02 *** | ||||
| Time*U CARR | γ13 | −1.09 ± 0.1 *** | ||||
| Time*Ratio | γ14 | −34.7 ± 3.7 *** | ||||
| Within person | δ2e | 21,495 ± 3336 *** | 4505 ± 699 *** | 3559 ± 553 *** | 2640 ± 412 *** | 1563 ± 244 *** |
| In initial status | δ20 | 5313 ± 3000 * | 13,808 ± 2517 *** | 10,259 ± 1890 *** | 10,155 ± 1805 *** | 10,089 ± 1704 *** |
| AIC | 2166 | 2039 | 2003 | 1974 | 1927 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abate, M.; Pellegrino, R.; Di Iorio, A.; Salini, V. Oxidative Stress and Performance after Training in Professional Soccer (European Football) Players. Antioxidants 2023, 12, 1470. https://doi.org/10.3390/antiox12071470
Abate M, Pellegrino R, Di Iorio A, Salini V. Oxidative Stress and Performance after Training in Professional Soccer (European Football) Players. Antioxidants. 2023; 12(7):1470. https://doi.org/10.3390/antiox12071470
Chicago/Turabian StyleAbate, Michele, Raffaello Pellegrino, Angelo Di Iorio, and Vincenzo Salini. 2023. "Oxidative Stress and Performance after Training in Professional Soccer (European Football) Players" Antioxidants 12, no. 7: 1470. https://doi.org/10.3390/antiox12071470
APA StyleAbate, M., Pellegrino, R., Di Iorio, A., & Salini, V. (2023). Oxidative Stress and Performance after Training in Professional Soccer (European Football) Players. Antioxidants, 12(7), 1470. https://doi.org/10.3390/antiox12071470







