Modulation of TLR4/NFκB Pathways in Autoimmune Myocarditis
Abstract
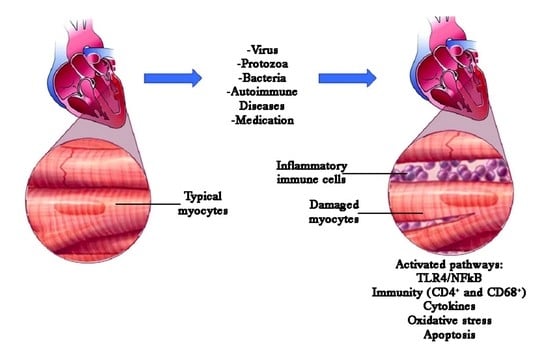
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Coriolus Versicolor Extract
2.3. Induction of Autoimmune Myocarditis
2.4. Experimental Groups
- -
- Control: The animals were orally administered with vehicle for 21 days;
- -
- Control + Coriolus versicolor: The animals were orally administered with Coriolus versicolor (200 mg/Kg) for 21 days;
- -
- EAM: The rats were subjected to EAM as previously described and treated
- -
- orally with vehicle every day for 21 days;
- -
- EAM + Coriolus versicolor: The rats were subjected to EAM as previously described and treated orally with Coriolus versicolor (200 mg/Kg) every day for 21 days.
2.5. Body and Heart Weights
2.6. Blood Pressure and Heart Rate Measurements
2.7. Oxidative Stress Evaluation
2.8. Cytokines Measurements
2.9. RNA Extraction and cDNA Synthesis
2.10. Real-Time PCR
2.11. Histological Analysis
2.12. Immunohistochemical Analysis
2.13. Western Blot Analysis
2.14. Tunel Assay
2.15. Statistical Evaluation
3. Results
3.1. Modulation of TLR4/NFκB Pathway
3.2. Modulation of Pro-Inflammatory Cell Recruitment
3.3. Modulation of Pro-Inflammatory Cytokines and Oxidative Stress
3.4. Modulation of Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kuhl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammirati, E.; Moslehi, J.J. Diagnosis and Treatment of Acute Myocarditis: A Review. JAMA 2023, 329, 1098–1113. [Google Scholar] [CrossRef] [PubMed]
- Blyszczuk, P. Myocarditis in Humans and in Experimental Animal Models. Front. Cardiovasc. Med. 2019, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Kawai, C. From myocarditis to cardiomyopathy: Mechanisms of inflammation and cell death: Learning from the past for the future. Circulation 1999, 99, 1091–1100. [Google Scholar] [CrossRef] [Green Version]
- Rose, N.R. Myocarditis: Infection versus autoimmunity. J. Clin. Immunol. 2009, 29, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Camelliti, P.; Green, C.R.; Kohl, P. Structural and functional coupling of cardiac myocytes and fibroblasts. Cardiovasc. Gap Junctions 2006, 42, 132–149. [Google Scholar]
- Amoah, B.P.; Yang, H.; Zhang, P.; Su, Z.; Xu, H. Immunopathogenesis of Myocarditis: The Interplay Between Cardiac Fibroblast Cells, Dendritic Cells, Macrophages and CD4+ T Cells. Scand. J. Immunol. 2015, 82, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, J.; He, L.; Ma, H.; Zhang, X.; Zhu, X.; Dolence, E.K.; Ren, J.; Li, J. Deficiency in TLR4 signal transduction ameliorates cardiac injury and cardiomyocyte contractile dysfunction during ischemia. J. Cell Mol. Med. 2009, 13, 1513–1525. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.A.; Tsen, M.F.; White, D.J.; Horton, J.W. TLR4 inactivation and rBPI(21) block burn-induced myocardial contractile dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1645–H1655. [Google Scholar] [CrossRef] [Green Version]
- Edfeldt, K.; Swedenborg, J.; Hansson, G.K.; Yan, Z.Q. Expression of toll-like receptors in human atherosclerotic lesions: A possible pathway for plaque activation. Circulation 2002, 105, 1158–1161. [Google Scholar] [CrossRef] [Green Version]
- Kodama, M.; Matsumoto, Y.; Fujiwara, M.; Masani, F.; Izumi, T.; Shibata, A. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin. Immunol. Immunopathol. 1990, 57, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Kodama, M.; Hanawa, H.; Saeki, M.; Hosono, H.; Inomata, T.; Suzuki, K.; Shibata, A. Rat dilated cardiomyopathy after autoimmune giant cell myocarditis. Circ. Res. 1994, 75, 278–284. [Google Scholar]
- Caforio, A.L.; Mahon, N.J.; Tona, F.; McKenna, W.J. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: Pathogenetic and clinical significance. Eur. J. Heart Fail. 2002, 4, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Lauer, B.; Schannwell, M.; Kuhl, U.; Strauer, B.E.; Schultheiss, H.P. Antimyosin autoantibodies are associated with deterioration of systolic and diastolic left ventricular function in patients with chronic myocarditis. J. Am. Coll. Cardiol. 2000, 35, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herskowitz, A.; Ahmed-Ansari, A.; Neumann, D.A.; Beschorner, W.E.; Rose, N.R.; Soule, L.M.; Burek, C.L.; Sell, K.W.; Baughman, K.L. Induction of major histocompatibility complex antigens within the myocardium of patients with active myocarditis: A nonhistologic marker of myocarditis. J. Am. Coll. Cardiol. 1990, 15, 624–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seko, Y.; Takahashi, N.; Ishiyama, S.; Nishikawa, T.; Kasajima, T.; Hiroe, M.; Suzuki, S.; Ishiwata, S.; Kawai, S.; Azuma, M.; et al. Expression of costimulatory molecules B7-1, B7-2, and CD40 in the heart of patients with acute myocarditis and dilated cardiomyopathy. Circulation 1998, 97, 637–639. [Google Scholar] [CrossRef] [Green Version]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Helio, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediat. Inflamm. 2014, 2014, 805841. [Google Scholar] [CrossRef] [Green Version]
- Paterson, R.R.M.; Lima, N. Biomedical effects of mushrooms with emphasis on pure compounds. Biomed. J. 2014, 37, 357–368. [Google Scholar] [CrossRef]
- Wasser, S. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Komura, D.L.; Ruthes, A.C.; Carbonero, E.R.; Gorin, P.A.; Iacomini, M. Water-soluble polysaccharides from Pleurotus ostreatus var. florida mycelial biomass. Int. J. Biol. Macromol. 2014, 70, 354–359. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Trovato Salinaro, A.; Fusco, R.; Cordaro, M.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Lo Dico, G.; Cuzzocrea, S.; Di Paola, R. Hericium erinaceus and Coriolus versicolor modulate molecular and biochemical changes after traumatic brain injury. Antioxidants 2021, 10, 898. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Tomasello, M.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Interdonato, L.; Abdelhameed, A.S.; Fusco, R.; Calabrese, V.; Cuzzocrea, S.; et al. Mechanism of Action of Natural Compounds in Peripheral Multiorgan Dysfunction and Hippocampal Neuroinflammation Induced by Sepsis. Antioxidants 2023, 12, 635. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Fusco, R.; Genovese, T.; Cordaro, M.; D’Amico, R.; Trovato Salinaro, A.; Ontario, M.L.; Modafferi, S.; Cuzzocrea, S.; Di Paola, R.; et al. Coriolus Versicolor Downregulates TLR4/NF-kappaB Signaling Cascade in Dinitrobenzenesulfonic Acid-Treated Mice: A Possible Mechanism for the Anti-Colitis Effect. Antioxidants 2022, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Ontario, M.L.; Bua, O.; Di Mauro, P.; Toscano, M.A.; Petralia, C.C.T.; Maiolino, L.; et al. Redox modulation of cellular stress response and lipoxin A4 expression by Hericium erinaceus in rat brain: Relevance to Alzheimer’s disease pathogenesis. Immun. Ageing 2016, 13, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Fronte, V.; Koverech, G.; Luca, M.; Serra, A.; Toscano, M.A.; Petralia, A.; et al. Redox modulation of cellular stress response and lipoxin A4 expression by Coriolus versicolor in rat brain: Relevance to Alzheimer’s disease pathogenesis. Neurotoxicology 2016, 53, 350–358. [Google Scholar] [CrossRef]
- Cordaro, M.; Modafferi, S.; D’Amico, R.; Fusco, R.; Genovese, T.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Interdonato, L.; Di Paola, D.; et al. Natural Compounds Such as Hericium erinaceus and Coriolus versicolor Modulate Neuroinflammation, Oxidative Stress and Lipoxin A4 Expression in Rotenone-Induced Parkinson’s Disease in Mice. Biomedicines 2022, 10, 2505. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Fusco, R.; Cordaro, M.; Interdonato, L.; Crupi, R.; Gugliandolo, E.; Di Paola, D.; Peritore, A.F.; Siracusa, R.; Impellizzeri, D.; et al. Modulation of NRF-2 Pathway Contributes to the Therapeutic Effects of Boswellia serrata Gum Resin Extract in a Model of Experimental Autoimmune Myocarditis. Antioxidants 2022, 11, 2129. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jin, X.; Zhang, L.; Yang, L. A study on the antioxidant effect of Coriolus versicolor polysaccharide in rat brain tissues. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 481–484. [Google Scholar] [CrossRef] [Green Version]
- Di Paola, R.; Cordaro, M.; Crupi, R.; Siracusa, R.; Campolo, M.; Bruschetta, G.; Fusco, R.; Pugliatti, P.; Esposito, E.; Cuzzocrea, S. Protective effects of ultramicronized palmitoylethanolamide (PEA-um) in myocardial ischaemia and reperfusion injury in vivo. Shock 2016, 46, 202–213. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.A.; Metwally, M.E.; El-khawanki, M.M.; Hashim, A.M. Protective effect of captopril against clozapine-induced myocarditis in rats: Role of oxidative stress, proinflammatory cytokines and DNA damage. Chem.-Biol. Interact. 2014, 216, 43–52. [Google Scholar] [CrossRef]
- Draginic, N.D.; Jakovljevic, V.L.; Jeremic, J.N.; Srejovic, I.M.; Andjic, M.M.; Rankovic, M.R.; Sretenovic, J.Z.; Zivkovic, V.I.; Ljujic, B.T.; Mitrovic, S.L.; et al. Melissa officinalis L. Supplementation Provides Cardioprotection in a Rat Model of Experimental Autoimmune Myocarditis. Oxid. Med. Cell. Longev. 2022, 2022, 1344946. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Campolo, M.; Latteri, S.; Carughi, A.; Mandalari, G.; Cuzzocrea, S. The Antioxidant Activity of Pistachios Reduces Cardiac Tissue Injury of Acute Ischemia/Reperfusion (I/R) in Diabetic Streptozotocin (STZ)-Induced Hyperglycaemic Rats. Front. Pharmacol. 2018, 9, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crupi, R.; Palma, E.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; Cordaro, M.; Impellizzeri, D.; De Caro, C.; Calzetta, L.; Cuzzocrea, S.; et al. Protective Effect of Hydroxytyrosol Against Oxidative Stress Induced by the Ochratoxin in Kidney Cells: In vitro and in vivo Study. Front. Vet. Sci. 2020, 7, 136. [Google Scholar] [CrossRef] [Green Version]
- Di Paola, D.; Capparucci, F.; Lanteri, G.; Crupi, R.; Marino, Y.; Franco, G.A.; Cuzzocrea, S.; Spano, N.; Gugliandolo, E.; Peritore, A.F. Environmental Toxicity Assessment of Sodium Fluoride and Platinum-Derived Drugs Co-Exposure on Aquatic Organisms. Toxics 2022, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, D.; Natale, S.; Iaria, C.; Crupi, R.; Cuzzocrea, S.; Spano, N.; Gugliandolo, E.; Peritore, A.F. Environmental Co-Exposure to Potassium Perchlorate and Cd Caused Toxicity and Thyroid Endocrine Disruption in Zebrafish Embryos and Larvae (Danio rerio). Toxics 2022, 10, 198. [Google Scholar] [CrossRef]
- D’Amico, R.; Gugliandolo, E.; Siracusa, R.; Cordaro, M.; Genovese, T.; Peritore, A.F.; Crupi, R.; Interdonato, L.; Di Paola, D.; Cuzzocrea, S.; et al. Toxic Exposure to Endocrine Disruptors Worsens Parkinson’s Disease Progression through NRF2/HO-1 Alteration. Biomedicines 2022, 10, 1073. [Google Scholar] [CrossRef]
- Hirakawa, H.; Zempo, H.; Ogawa, M.; Watanabe, R.; Suzuki, J.; Akazawa, H.; Komuro, I.; Isobe, M. A DPP-4 inhibitor suppresses fibrosis and inflammation on experimental autoimmune myocarditis in mice. PLoS ONE 2015, 10, e0119360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, L.Q.; Li, H.Q.; Wu, J.; Bian, N.N.; Yan, G. Beneficial effects of andrographolide in a rat model of autoimmune myocarditis and its effects on PI3K/Akt pathway. Korean J. Physiol. Pharmacol. 2019, 23, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peritore, A.F.; D’Amico, R.; Siracusa, R.; Cordaro, M.; Fusco, R.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Di Paola, R.; Cuzzocrea, S.; et al. Management of Acute Lung Injury: Palmitoylethanolamide as a New Approach. Int. J. Mol. Sci. 2021, 22, 5533. [Google Scholar] [CrossRef]
- Fusco, R.; Salinaro, A.T.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cordaro, M.; Cuzzocrea, S.; et al. Hidrox((R)) Counteracts Cyclophosphamide-Induced Male Infertility through NRF2 Pathways in a Mouse Model. Antioxidants 2021, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Fusco, R.; Crupi, R.; Rizzarelli, E.; Cuzzocrea, S.; et al. Protective effect of a new hyaluronic acid-carnosine conjugate on the modulation of the inflammatory response in mice subjected to collagen-induced arthritis. Biomed. Pharmacother. 2020, 125, 110023. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; D’Amico, R.; Fusco, R.; Genovese, T.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Interdonato, L.; Di Paola, D.; Di Paola, R.; et al. Acai Berry Mitigates Vascular Dementia-Induced Neuropathological Alterations Modulating Nrf-2/Beclin1 Pathways. Cells 2022, 11, 2616. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; Impellizzeri, D.; D’Amico, R.; Fusco, R.; Peritore, A.F.; Di Paola, D.; Interdonato, L.; Gugliandolo, E.; Crupi, R.; Di Paola, R.; et al. Role of Bevacizumab on Vascular Endothelial Growth Factor in Apolipoprotein E Deficient Mice after Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 4162. [Google Scholar] [CrossRef]
- Cordaro, M.; Fusco, R.; D’Amico, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Mandalari, G.; Cuzzocrea, S.; et al. Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein. Antioxidants 2020, 9, 992. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. Consumption of Anacardium occidentale L. (Cashew Nuts) Inhibits Oxidative Stress through Modulation of the Nrf2/HO-1 and NF-κB Pathways. Molecules 2020, 25, 4426. [Google Scholar] [CrossRef]
- D’Amico, R.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Interdonato, L.; Crupi, R.; Gugliandolo, E.; Macri, F.; Di Paola, D.; Peritore, A.F.; et al. Regulation of Apoptosis and Oxidative Stress by Oral Boswellia Serrata Gum Resin Extract in a Rat Model of Endometriosis. Int. J. Mol. Sci. 2022, 23, 15348. [Google Scholar] [CrossRef]
- Engel, A.L.; Sun, G.C.; Gad, E.; Rastetter, L.R.; Strobe, K.; Yang, Y.; Dang, Y.; Disis, M.L.; Lu, H. Protein-bound polysaccharide activates dendritic cells and enhances OVA-specific T cell response as vaccine adjuvant. Immunobiology 2013, 218, 1468–1476. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.F.; Zhuang, T.F.; Si, Y.M.; Qi, K.Y.; Zhao, J. Coriolus versicolor mushroom polysaccharides exert immunoregulatory effects on mouse B cells via membrane Ig and TLR-4 to activate the MAPK and NF-kappaB signaling pathways. Mol. Immunol. 2015, 64, 144–151. [Google Scholar] [CrossRef]
- Price, L.A.; Wenner, C.A.; Sloper, D.T.; Slaton, J.W.; Novack, J.P. Role for toll-like receptor 4 in TNF-alpha secretion by murine macrophages in response to polysaccharide Krestin, a Trametes versicolor mushroom extract. Fitoterapia 2010, 81, 914–919. [Google Scholar] [CrossRef]
- Jedrzejewski, T.; Sobocinska, J.; Pawlikowska, M.; Dzialuk, A.; Wrotek, S. Extract from the Coriolus versicolor Fungus as an Anti-Inflammatory Agent with Cytotoxic Properties against Endothelial Cells and Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9063. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, B.; Feng, Z.; Yu, S.; Bao, Y. A study on immunomodulatory mechanism of Polysaccharopeptide mediated by TLR4 signaling pathway. BMC Immunol. 2015, 16, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Barcena, M.L.; Jeuthe, S.; Niehues, M.H.; Pozdniakova, S.; Haritonow, N.; Kuhl, A.A.; Messroghli, D.R.; Regitz-Zagrosek, V. Sex-Specific Differences of the Inflammatory State in Experimental Autoimmune Myocarditis. Front. Immunol. 2021, 12, 686384. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, M.; Arsenovic-Ranin, N.; Stojic-Vukanic, Z.; Bufan, B.; Vucicevic, D.; Jancic, I. Quercetin ameliorates experimental autoimmune myocarditis in rats. J. Pharm. Pharm. Sci. 2010, 13, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Vdovenko, D.; Eriksson, U. Regulatory Role of CD4(+) T Cells in Myocarditis. J. Immunol. Res. 2018, 2018, 4396351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmerler, P.; Jeuthe, S.; O h-Ici, D.; Wassilew, K.; Lauer, D.; Kaschina, E.; Kintscher, U.; Muller, S.; Muench, F.; Kuehne, T.; et al. Mortality and morbidity in different immunization protocols for experimental autoimmune myocarditis in rats. Acta Physiol. 2014, 210, 889–898. [Google Scholar] [CrossRef]
- Watanabe, K.; Nakazawa, M.; Fuse, K.; Hanawa, H.; Kodama, M.; Aizawa, Y.; Ohnuki, T.; Gejyo, F.; Maruyama, H.; Miyazaki, J. Protection against autoimmune myocarditis by gene transfer of interleukin-10 by electroporation. Circulation 2001, 104, 1098–1100. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Hanawa, H.; Toba, K.; Watanabe, H.; Watanabe, R.; Yoshida, K.; Abe, S.; Kato, K.; Kodama, M.; Aizawa, Y. Expression of immunological molecules by cardiomyocytes and inflammatory and interstitial cells in rat autoimmune myocarditis. Cardiovasc. Res. 2005, 68, 278–288. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Hanawa, H.; Liu, H.; Yoshida, T.; Hayashi, M.; Watanabe, R.; Abe, S.; Toba, K.; Yoshida, K.; Elnaggar, R.; et al. Hydrodynamic-based delivery of an interleukin-22-Ig fusion gene ameliorates experimental autoimmune myocarditis in rats. J. Immunol. 2006, 177, 3635–3643. [Google Scholar] [CrossRef] [Green Version]
- Nishio, R.; Matsumori, A.; Shioi, T.; Ishida, H.; Sasayama, S. Treatment of experimental viral myocarditis with interleukin-10. Circulation 1999, 100, 1102–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Li, W.; Liu, W.; Gao, C.; Zhou, B.; Li, S.; Li, Y.; Kong, Y. IL-10 gene modified dendritic cells induced antigen-specific tolerance in experimental autoimmune myocarditis. Clin. Immunol. 2006, 121, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Sonderegger, I.; Rohn, T.A.; Kurrer, M.O.; Iezzi, G.; Zou, Y.; Kastelein, R.A.; Bachmann, M.F.; Kopf, M. Neutralization of IL-17 by active vaccination inhibits IL-23-dependent autoimmune myocarditis. Eur. J. Immunol. 2006, 36, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Rangachari, M.; Mauermann, N.; Marty, R.R.; Dirnhofer, S.; Kurrer, M.O.; Komnenovic, V.; Penninger, J.M.; Eriksson, U. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J. Exp. Med. 2006, 203, 2009–2019. [Google Scholar] [CrossRef]
- Afanasyeva, M.; Wang, Y.; Kaya, Z.; Stafford, E.A.; Dohmen, K.M.; Sadighi Akha, A.A.; Rose, N.R. Interleukin-12 receptor/STAT4 signaling is required for the development of autoimmune myocarditis in mice by an interferon-gamma-independent pathway. Circulation 2001, 104, 3145–3151. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Hanawa, H.; Yoshida, T.; Hayashi, M.; Liu, H.; Ding, L.; Otaki, K.; Hao, K.; Yoshida, K.; Kato, K.; et al. Alteration of IL-17 related protein expressions in experimental autoimmune myocarditis and inhibition of IL-17 by IL-10-Ig fusion gene transfer. Circ. J. 2008, 72, 813–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, J.R.; Neumann, D.A.; Lafond-Walker, A.; Herskowitz, A.; Rose, N.R. Interleukin 1 or tumor necrosis factor can promote Coxsackie B3-induced myocarditis in resistant B10.A mice. J. Exp. Med. 1992, 175, 1123–1129. [Google Scholar] [CrossRef]
- Smith, S.C.; Allen, P.M. Neutralization of endogenous tumor necrosis factor ameliorates the severity of myosin-induced myocarditis. Circ. Res. 1992, 70, 856–863. [Google Scholar] [CrossRef]
- Bachmaier, K.; Pummerer, C.; Kozieradzki, I.; Pfeffer, K.; Mak, T.W.; Neu, N.; Penninger, J.M. Low-molecular-weight tumor necrosis factor receptor p55 controls induction of autoimmune heart disease. Circulation 1997, 95, 655–661. [Google Scholar] [CrossRef]
- Varga, Z.V.; Giricz, Z.; Liaudet, L.; Haskó, G.; Ferdinandy, P.; Pacher, P. Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2015, 1852, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Stockklauser-Farber, K.; Ballhausen, T.; Laufer, A.; Rosen, P. Influence of diabetes on cardiac nitric oxide synthase expression and activity. Biochim. Biophys. Acta 2000, 1535, 10–20. [Google Scholar] [CrossRef]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiordelisi, A.; Iaccarino, G.; Morisco, C.; Coscioni, E.; Sorriento, D. NFkappaB is a Key Player in the Crosstalk between Inflammation and Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, O.; Picatoste, B.; Ares-Carrasco, S.; Ramírez, E.; Egido, J.; Tuñón, J. Potential role of nuclear factor B in diabetic cardiomyopathy. Mediat. Inflamm. 2011, 2011, 652097. [Google Scholar] [CrossRef] [Green Version]
- Kytö, V.; Saraste, A.; Saukko, P.; éronique Henn, V.; Pulkki, K.; Vuorinen, T.; Voipio-Pulkki, L.-M. Apoptotic cardiomyocyte death in fatal myocarditis. Am. J. Cardiol. 2004, 94, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Moe, G.W.; Marín-García, J. Role of cell death in the progression of heart failure. Heart Fail. Rev. 2016, 21, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, H.; Watanabe, K.; Veeraveedu, P.T.; Harima, M.; Thandavarayan, R.A.; Arozal, W.; Tachikawa, H.; Kodama, M.; Aizawa, Y. The antioxidant edaravone attenuates ER-stress-mediated cardiac apoptosis and dysfunction in rats with autoimmune myocarditis. Free. Radic. Res. 2010, 44, 1082–1090. [Google Scholar] [CrossRef]
- Abukhalil, M.H.; Althunibat, O.Y.; Aladaileh, S.H.; Al-Amarat, W.; Obeidat, H.M.; Al-Khawalde, A.A.A.; Hussein, O.E.; Alfwuaires, M.A.; Algefare, A.I.; Alanazi, K.M.; et al. Galangin attenuates diabetic cardiomyopathy through modulating oxidative stress, inflammation and apoptosis in rats. Biomed. Pharmacother. 2021, 138, 111410. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 139–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guicciardi, M.E.; Gores, G.J. Life and death by death receptors. FASEB J. 2009, 23, 1625–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bredesen, D.E.; Rao, R.V.; Mehlen, P. Cell death in the nervous system. Nature 2006, 443, 796–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghavami, S.; Kerkhoff, C.; Los, M.; Hashemi, M.; Sorg, C.; Karami-Tehrani, F. Mechanism of apoptosis induced by S100A8/A9 in colon cancer cell lines: The role of ROS and the effect of metal ions. J. Leukoc. Biol. 2004, 76, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R. Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer 2005, 103, 1551–1560. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Interdonato, L.; Impellizzeri, D.; D’Amico, R.; Cordaro, M.; Siracusa, R.; D’Agostino, M.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Fusco, R.; et al. Modulation of TLR4/NFκB Pathways in Autoimmune Myocarditis. Antioxidants 2023, 12, 1507. https://doi.org/10.3390/antiox12081507
Interdonato L, Impellizzeri D, D’Amico R, Cordaro M, Siracusa R, D’Agostino M, Genovese T, Gugliandolo E, Crupi R, Fusco R, et al. Modulation of TLR4/NFκB Pathways in Autoimmune Myocarditis. Antioxidants. 2023; 12(8):1507. https://doi.org/10.3390/antiox12081507
Chicago/Turabian StyleInterdonato, Livia, Daniela Impellizzeri, Ramona D’Amico, Marika Cordaro, Rosalba Siracusa, Melissa D’Agostino, Tiziana Genovese, Enrico Gugliandolo, Rosalia Crupi, Roberta Fusco, and et al. 2023. "Modulation of TLR4/NFκB Pathways in Autoimmune Myocarditis" Antioxidants 12, no. 8: 1507. https://doi.org/10.3390/antiox12081507
APA StyleInterdonato, L., Impellizzeri, D., D’Amico, R., Cordaro, M., Siracusa, R., D’Agostino, M., Genovese, T., Gugliandolo, E., Crupi, R., Fusco, R., Cuzzocrea, S., & Di Paola, R. (2023). Modulation of TLR4/NFκB Pathways in Autoimmune Myocarditis. Antioxidants, 12(8), 1507. https://doi.org/10.3390/antiox12081507