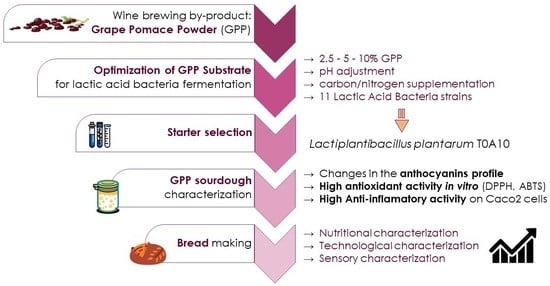
Up-Cycling Grape Pomace through Sourdough Fermentation: Characterization of Phenolic Compounds, Antioxidant Activity, and Anti-Inflammatory Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grape Pomace
2.2. Microrganisms
2.3. Starter Selection
2.3.1. Grape Pomace-Derived Substrates
2.3.2. Growth and Kinetics of Acidification
2.3.3. In Vitro Antioxidant Activity
2.4. Sourdough Fermentation and Characterization
2.5. Analysis of Anthocyanins by UHPLC-DAD-MS/MS
2.6. Antioxidant Activity on Caco2 Cells
2.6.1. Caco2 Cells Culture
2.6.2. Citotoxicity
2.6.3. RNA Extraction and Real-Time-PCR
2.7. Breadmaking
2.8. Bread Characterization
2.8.1. Biochemical and Nutritional Characterization
2.8.2. Technological Characterization
2.8.3. Sensory Analysis
2.9. Statistical Analysis
3. Results
3.1. LAB Strain Selection
3.2. Sourdough Fermentation
3.3. Anthocyanins Identification and Quantification
3.4. Cytotoxicity and Anti-Inflammatory Effects
3.5. Breads
3.5.1. Biochemical and Nutritional Characterization
3.5.2. Technological Characterization
3.6. Sensory Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machado, N.F.; Domínguez-Perles, R. Addressing facts and gaps in the phenolics chemistry of winery by-products. Molecules 2017, 22, 286. [Google Scholar] [CrossRef] [Green Version]
- Beres, C.; Costa, G.N.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.; Cruz, A.P.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Perra, M.; Bacchetta, G.; Muntoni, A.; De Gioannis, G.; Castangia, I.; Rajha, H.N.; Manca, M.L.; Manconi, M. An outlook on modern and sustainable approaches to the management of grape pomace by integrating green processes, biotechnologies and advanced biomedical approaches. J. Funct. Foods 2022, 98, 105276. [Google Scholar] [CrossRef]
- Yang, C.; Han, Y.; Tian, X.; Sajid, M.; Mehmood, S.; Wang, H.; Li, H. Phenolic composition of grape pomace and its metabolism. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape pomace valorization: A systematic review and meta-analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Shiferaw Terefe, N.; Augustin, M.A. Fermentation for tailoring the technological and health related functionality of food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2887–2913. [Google Scholar] [CrossRef]
- Campanella, D.; Rizzello, C.G.; Fasciano, C.; Gambacorta, G.; Pinto, D.; Marzani, B.; Scarano, N.; De Angelis, M.; Gobbetti, M. Exploitation of grape marc as functional substrate for lactic acid bacteria and bifidobacteria growth and enhanced antioxidant activity. Food Microbiol. 2017, 65, 25–35. [Google Scholar] [CrossRef]
- Mišković Špoljarić, K.; Šelo, G.; Pešut, E.; Martinović, J.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Antioxidant and antiproliferative potentials of phenolic-rich extracts from biotransformed grape pomace in colorectal Cancer. BMC Complement. Med. 2023, 23, 29. [Google Scholar] [CrossRef]
- Gil-Sánchez, I.; Bartolomé Suáldea, B.; Victoria Moreno-Arribas, M. Malolactic Fermentation. In Red Wine Technology; Morata, A., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 85–98. ISBN 9780128144008. [Google Scholar]
- Sabel, A.; Bredefeld, S.; Schlander, M.; Claus, H. Wine phenolic compounds: Antimicrobial properties against yeasts, lactic acid and acetic acid bacteria. Beverages 2017, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Nionelli, L.; Montemurro, M.; Pontonio, E.; Verni, M.; Gobbetti, M.; Rizzello, C.G. Pro-technological and functional characterization of lactic acid bacteria to be used as starters for hemp (Cannabis sativa L.) sourdough fermentation and wheat bread fortification. Int. J. Food Microbiol. 2018, 279, 14–25. [Google Scholar] [CrossRef]
- Nionelli, L.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Use of hop extract as antifungal ingredient for bread making and selection of autochthonous resistant starters for sourdough fermentation. Int. J. Food Microbiol. 2018, 266, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Pontonio, E.; Verni, M.; Dingeo, C.; Diaz-de-Cerio, E.; Pinto, D.; Rizzello, C.G. Impact of enzymatic and microbial bioprocessing on antioxidant properties of hemp (Cannabis sativa L.). Antioxidants 2020, 9, 1258. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; De Angelis, M.; Gobbetti, M. Effect of sourdough fermentation on stabilisation, and chemical and nutritional characteristics of wheat germ. Food Chem. 2010, 119, 1079–1089. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Russo, V.; Pinto, D.; Marzani, B.; Gobbetti, M. Improving the antioxidant properties of quinoa flour through fermentation with selected autochthonous lactic acid bacteria. Int. J. Food Microbiol. 2017, 241, 252–261. [Google Scholar] [CrossRef]
- AACC. Approved Methods of Analysis, 11th ed.; AOAC: St. Paul, MN, USA, 2010. [Google Scholar]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K.J.A.E.M. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Weiss, W.; Vogelmeier, C.; Görg, A. Electrophoretic characterization of wheat grain allergens from different cultivars involved in bakers’ asthma. Electrophoresis 1993, 14, 805–816. [Google Scholar] [CrossRef]
- Verni, M.; Dingeo, C.; Rizzello, C.G.; Pontonio, E. Lactic acid bacteria fermentation and endopeptidase treatment improve the functional and nutritional features of Arthrospira platensis. Front. Microbiol. 2021, 12, 744437. [Google Scholar] [CrossRef]
- Trani, A.; Verrastro, V.; Punzi, R.; Faccia, M.; Gambacorta, G. Phenols, volatiles and sensory properties of Primitivo wines from the” Gioia Del Colle” PDO Area. S. Afr. J. Enol. Vitic. 2016, 37, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.; Alhinho da Silva, M.; Teixeira, N.; De Freitas, V.; Salas, E. Screening of anthocyanins and anthocyanin-derived pigments in red wine grape pomace using LC-DAD/MS and MALDI-TOF techniques. J. Agric. Food Chem. 2015, 63, 7636–7644. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Mackey, K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide-and proteoglycan-rich sources. Biotechniques 1995, 19, 942–945. [Google Scholar]
- Vigetti, D.; Viola, M.; Karousou, E.; Rizzi, M.; Moretto, P.; Genasetti, A.; Clerici, M.; Hascall, V.C.; De Luca, G.; Passi, A. Hyaluronan-CD44-ERK1/2 regulate human aortic smooth muscle cell motility during aging. J. Biol. Chem. 2008, 283, 4448–4458. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, J.I.; Motilva, M.J.; Tomas, M.; et al. Functional implications of bound phenolic compounds and phenolics–food interaction: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 811–842. [Google Scholar] [CrossRef]
- Verni, M.; Verardo, V.; Rizzello, C.G. How fermentation affects the antioxidant properties of cereals and legumes. Foods 2019, 8, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; de Souza, T.S.P.; Wu, H.; Holland, B.; Dunshea, F.R.; Barrow, C.J.; Suleria, H.A.R. Development of Phenolic-Rich Functional Foods by Lactic Fermentation of Grape Marc: A Review. Food Rev. Int. 2023, 1–20. [Google Scholar] [CrossRef]
- Singh, V.; Haque, S.; Niwas, R.; Srivastava, A.; Pasupuleti, M.; Tripathi, C. Strategies for fermentation medium optimization: An in-depth review. Front. Microbiol. 2017, 7, 2087. [Google Scholar] [CrossRef] [Green Version]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Anghel, L.; Milea, A.S.; Constantin, O.E.; Barbu, V.; Chitescu, C.; Enachi, E.; Râpeanu, G.; Mocanu, G.D.; Stănciuc, N. Dried grape pomace with lactic acid bacteria as a potential source for probiotic and antidiabetic value-added powders. Food Chem. 2023, X, 100777. [Google Scholar] [CrossRef]
- Kungsuwan, K.; Singh, K.; Phetkao, S.; Utama-ang, N. Effects of pH and anthocyanin concentration on color and antioxidant activity of Clitoria ternatea extract. Food Appl. Biosc. J. 2014, 2, 31–46. [Google Scholar] [CrossRef]
- Han, F.; Ju, Y.; Ruan, X.; Zhao, X.; Yue, X.; Zhuang, X.; Quin, M.; Fang, Y. Color, anthocyanin, and antioxidant characteristics of young wines produced from spine grapes (Vitis davidii Foex) in China. Food Nutr. Res. 2017, 61, 1339552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocsan, I.C.; Măgureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, Ș.O.; Pătrașca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and anti-inflammatory actions of polyphenols from red and white grape pomace in ischemic heart diseases. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef] [PubMed]
- Klepacka, J.; Fornal, Ł. Ferulic acid and its position among the phenolic compounds of wheat. Crit. Rev. Food Sci. Nutr. 2006, 46, 639–647. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch–a review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 639–647. [Google Scholar] [CrossRef]
- Yogeswara, I.B.A.; Maneerat, S.; Haltrich, D. Glutamate decarboxylase from lactic acid bacteria—A key enzyme in GABA synthesis. Microorganisms 2020, 8, 1923. [Google Scholar] [CrossRef]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty years of knowledge on sourdough fermentation: A systematic review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]




| Strain | A | Vmax | λ |
|---|---|---|---|
| L. plantarum T0A10 | 2.83 ± 0.08 a | 0.41 ± 0.02 b | 3.39 ± 0.06 d |
| Ln. mesenteroides 12MM1 | 2.47 ± 0.05 b | 0.41 ± 0.03 b | 3.46 ± 0.05 d |
| L. plantarum LB1 | 2.41 ± 0.06 c | 0.39 ± 0.01 b | 3.31 ± 0.02 d |
| L. plantarum T6B10 | 2.40 ± 0.09 c | 0.40 ± 0.03 b | 4.39 ± 0.05 c |
| L. plantarum 18S9 | 2.36 ± 0.08 d | 0.31 ± 0.02 c | 3.31 ± 0.02 d |
| L. plantarum H18 | 2.46 ± 0.05 b | 0.44 ± 0.02 a | 5.19 ± 0.07 a |
| P. pentosaceus H22 | 2.44 ± 0.02 b | 0.47 ± 0.03 a | 5.44 ± 0.07 a |
| F. rossiae T0A16 | 2.88 ± 0.02 a | 0.46 ± 0.03 a | 4.94 ± 0.02 b |
| L. plantarum H64 | 2.87 ± 0.04 a | 0.47 ± 0.02 a | 4.92 ± 0.03 b |
| SD0 | SD2.5 | SD5 | ||||
|---|---|---|---|---|---|---|
| t0 | tf | t0 | tf | t0 | tf | |
| pH | 5.69 ± 0.04 a | 3.61 ± 0.03 d | 5.12 ± 0.02 b | 3.63 ± 0.01 d | 4.73 ± 0.01 c | 3.59 ± 0.02 d |
| TTA | 1.82 ± 0.01 e | 10.2 ± 0.18 b | 4.21 ± 0.03 d | 12.8 ± 0.26 a | 6.22 ± 0.05 c | 14.03 ± 0.32 a |
| Lactic acid bacteria (Log ufc/g) | 2.03 ± 0.10 c | 9.96 ± 0.09 a | 2.74 ± 0.30 c | 9.24 ± 0.07 b | 2.80 ± 0.25 c | 9.05 ± 0.13 b |
| Lactic acid (mmol/kg) | nd | 90.41 ± 2.31 a | nd | 85.23 ± 4.01 b | nd | 80.21 ± 3.87 c |
| Acetic acid (mmol/kg) | nd | 24.64 ± 1.05 a | 1.22 ± 0.07 b | 19.21 ± 2.27 b | 1.98 ± 0.10 b | 16.12 ± 1.54 c |
| QF | - | 3.67 ± 0.16 c | - | 4.43 ± 0.29 b | - | 4.97 ± 0.17 a |
| Total free amino acids (mg/kg) | 314 ± 12 d | 576 ± 32 a | 309 ± 16 d | 485 ± 27 b | 290 ± 10 e | 375 ± 21 c |
| DPPH Radical Scavenging Activity (%) | 7.0 ± 0.3 f | 13.1 ± 0.7 e | 34.3 ± 0.9 d | 58.4 ± 0.8 c | 74.1 ± 1.3 b | 95.2 ± 1.6 a |
| ABTS Radical scavenging (mM Trolox eq) | 0.11 ± 0.03 d | 0.18 ± 0.05 d | 0.30 ± 0.08 c | 0.48 ± 0.15 b | 0.62 ± 0.10 b | 1.02 ± 0.03 a |
| Kinetics of acidification parameters | ||||||
| A | 2.04 ± 0.12 a | 1.48 ± 0.07 b | 1.17 ± 0.04 c | |||
| Vmax (ΔpH/Δh) | 0.42 ± 0.02 a | 0.25 ± 0.01 b | 0.22 ± 0.01 b | |||
| λ (h) | 3.72 ± 0.18 b | 3.89 ± 0.13 b | 4.28 ± 0.10 a | |||
| Peak | RT (min) | Concentration (mg/kg) | MH+ (m/z) | Fragments | λmax | Anthocyanins | |
|---|---|---|---|---|---|---|---|
| SD5-T0 | SD5-T24 | ||||||
| 1. | 6.68 | 41.7 ± 1.6 a | 28.4 ± 0.9 b | 479 | 317 | 278,524 | Petunidin 3-O-glucoside |
| 2. | 7.16 | - | 23.3 ± 0.1 | 655 | 331,493 | 276,530 | Malvidin 3,5-O-diglucoside |
| 3. | 7.37 | 40.3 ± 3.7 a | 11.8 ± 0.8 b | 463 | 301 | 282,522 | Peonidin 3-O-glucoside |
| 4. | 7.66 | 847.5 ± 13.5 a | 755.3 ± 22.0 b | 493 | 331 | 258,528 | Malvidin 3-O-glucoside |
| 5. | 8.12 | 12.6 ± 0.9 b | 21.9 ± 0.6 a | 561 | 399 | 280,512 | Carboxypyranomalvidin-3-O-glucoside |
| 6. | 9.49 | 31.4 ± 2.3 a | 21.0 ± 1.5 b | 535 | 331 | 280,522 | Malvidin-3-O-acetylglucoside |
| 7. | 9.99 | 30.2 ± 1.5 a | 25.6 ± 0.3 b | 655 | 331 | 280,530 | Malvidin-3-O-caffeoylglucoside |
| 8. | 10.13 | 34.9 ± 0.01 a | 32.3 ± 1.4 a | 625 | 317 | 282,530 | Petunidin-3-O-coumaroylglucoside |
| 9. | 10.68 | 41.5 ± 0.2 a | 34.7 ± 1.3 b | 609 | 301 | 282,522 | Peonidin 3-O-coumaroylglucoside |
| 10. | 10.84 | 351.3 ± 13.4 b | 410.8 ± 10.1 a | 639 | 331 | 282,534 | Malvidin-3-O-trans-coumaroylglucoside |
| cY-B | SD0-B | SD5-B | |
|---|---|---|---|
| Volume increase (mL/min) | 0.212 ± 0.013 a | 0.198 ± 0.009 a | 0.205 ± 0.008 a |
| pH | 5.61 ± 0.02 a | 4.59 ± 0.01 b | 4.48 ± 0.02 b |
| TTA (mL NaOH 0.1 M) | 2.5 ± 0.13 b | 4.82 ± 0.24 a | 5.10 ± 0.16 a |
| Lactic acid (mmol/kg) | 1.32 ± 0.11 b | 23.13 ± 0.49 a | 24.18 ± 0.62 a |
| Acetic acid (mmol/kg) | 1.40 ± 0.21 c | 6.32 ± 0.28 a | 4.64 ± 0.36 b |
| QF | - | 3.66 ± 0.19 b | 5.21 ± 0.14 a |
| Total Free Amino acid (mg/kg) | 111 ± 4 b | 279 ± 8 a | 267 ± 6 a |
| DPPH Radical Scavenging Activity (%) | 7.3 ± 0.2 c | 12.0 ± 1.3 b | 46.2 ± 2.3 a |
| cY-B | SD0-B | SD5-B | |
|---|---|---|---|
| Specific volume (cm3/g) | 3.12 ± 0.06 a | 3.06 ± 0.03 ab | 2.92 ± 0.04 b |
| Hardness (N) | 52.51 ± 1.20 a | 51.76 ± 2.05 a | 52.76 ± 0.14 a |
| Cohesiveness | 0.57 ± 0.00 a | 0.46 ± 0.05 b | 0.35 ± 0.02 c |
| Springiness | 0.84 ± 0.02 b | 0.84 ± 0.01 b | 1.15 ± 0.10 a |
| Chewiness (N) | 27.06 ± 3.37 b | 19.95 ± 0.95 c | 44.30 ± 0.07 a |
| Crust color | |||
| L | 62.86 ± 1.43 a | 57.51 ± 0.34 a | 46.15 ± 2.79 b |
| a | −1.61 ± 0.24 b | −1.90 ± 0.34 b | 1.20 ± 0.27 a |
| b | 18.93 ± 0.38 a | 16.54 ± 1.57 a | 12.18 ± 0.89 b |
| ΔE | 33.99 ± 1.34 b | 38.07 ± 3.56 b | 47.97 ± 2.61 a |
| Crumb color | |||
| L | 60.95 ± 3.63 a | 56.61 ± 0.20 b | 45.37 ± 2.05 c |
| a | −4.3 ± 0.52 a | −3.63 ±0.20 a | −0.15 ± 0.15 b |
| b | 13.54 ± 0.4 a | 12.59 ± 0.67 a | 8.75 ± 0.14 b |
| ΔE | 34.02 ± 3.47 c | 37.88 ± 0.11 b | 48.24 ± 2.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torreggiani, A.; Demarinis, C.; Pinto, D.; Papale, A.; Difonzo, G.; Caponio, F.; Pontonio, E.; Verni, M.; Rizzello, C.G. Up-Cycling Grape Pomace through Sourdough Fermentation: Characterization of Phenolic Compounds, Antioxidant Activity, and Anti-Inflammatory Potential. Antioxidants 2023, 12, 1521. https://doi.org/10.3390/antiox12081521
Torreggiani A, Demarinis C, Pinto D, Papale A, Difonzo G, Caponio F, Pontonio E, Verni M, Rizzello CG. Up-Cycling Grape Pomace through Sourdough Fermentation: Characterization of Phenolic Compounds, Antioxidant Activity, and Anti-Inflammatory Potential. Antioxidants. 2023; 12(8):1521. https://doi.org/10.3390/antiox12081521
Chicago/Turabian StyleTorreggiani, Andrea, Chiara Demarinis, Daniela Pinto, Angela Papale, Graziana Difonzo, Francesco Caponio, Erica Pontonio, Michela Verni, and Carlo Giuseppe Rizzello. 2023. "Up-Cycling Grape Pomace through Sourdough Fermentation: Characterization of Phenolic Compounds, Antioxidant Activity, and Anti-Inflammatory Potential" Antioxidants 12, no. 8: 1521. https://doi.org/10.3390/antiox12081521
APA StyleTorreggiani, A., Demarinis, C., Pinto, D., Papale, A., Difonzo, G., Caponio, F., Pontonio, E., Verni, M., & Rizzello, C. G. (2023). Up-Cycling Grape Pomace through Sourdough Fermentation: Characterization of Phenolic Compounds, Antioxidant Activity, and Anti-Inflammatory Potential. Antioxidants, 12(8), 1521. https://doi.org/10.3390/antiox12081521