Sulfated Polysaccharide from Caulerpa racemosa Attenuates the Obesity-Induced Cardiometabolic Syndrome via Regulating the PRMT1-DDAH-ADMA with mTOR-SIRT1-AMPK Pathways and Gut Microbiota Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Collecting Caulerpa racemosa and Preparing–Isolating Sulfated Polysaccharides from Caulerpa racemosa
2.2. Design of In Vivo Study
2.2.1. Animal Handling Procedures and Ethical Approval
2.2.2. Study Design of Treatments
2.2.3. Composition of Feed and Production of CFED
2.2.4. Biomedical Analysis of Blood Samples
2.3. The Sequencing and Analysis of the 16S rRNA Gene in Rat Feces for Gut Microbiota
2.4. Data Analysis and Management
3. Results
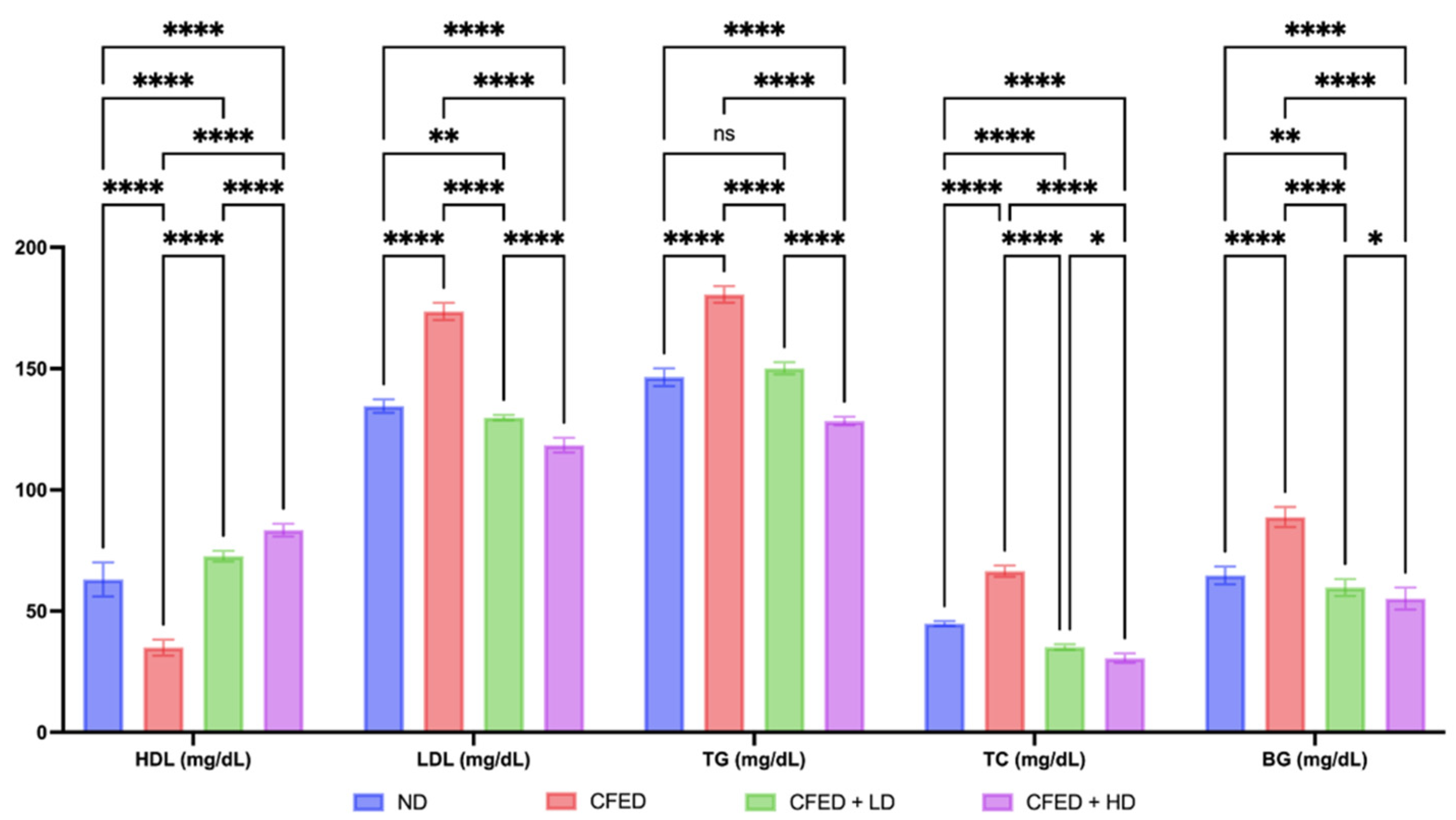
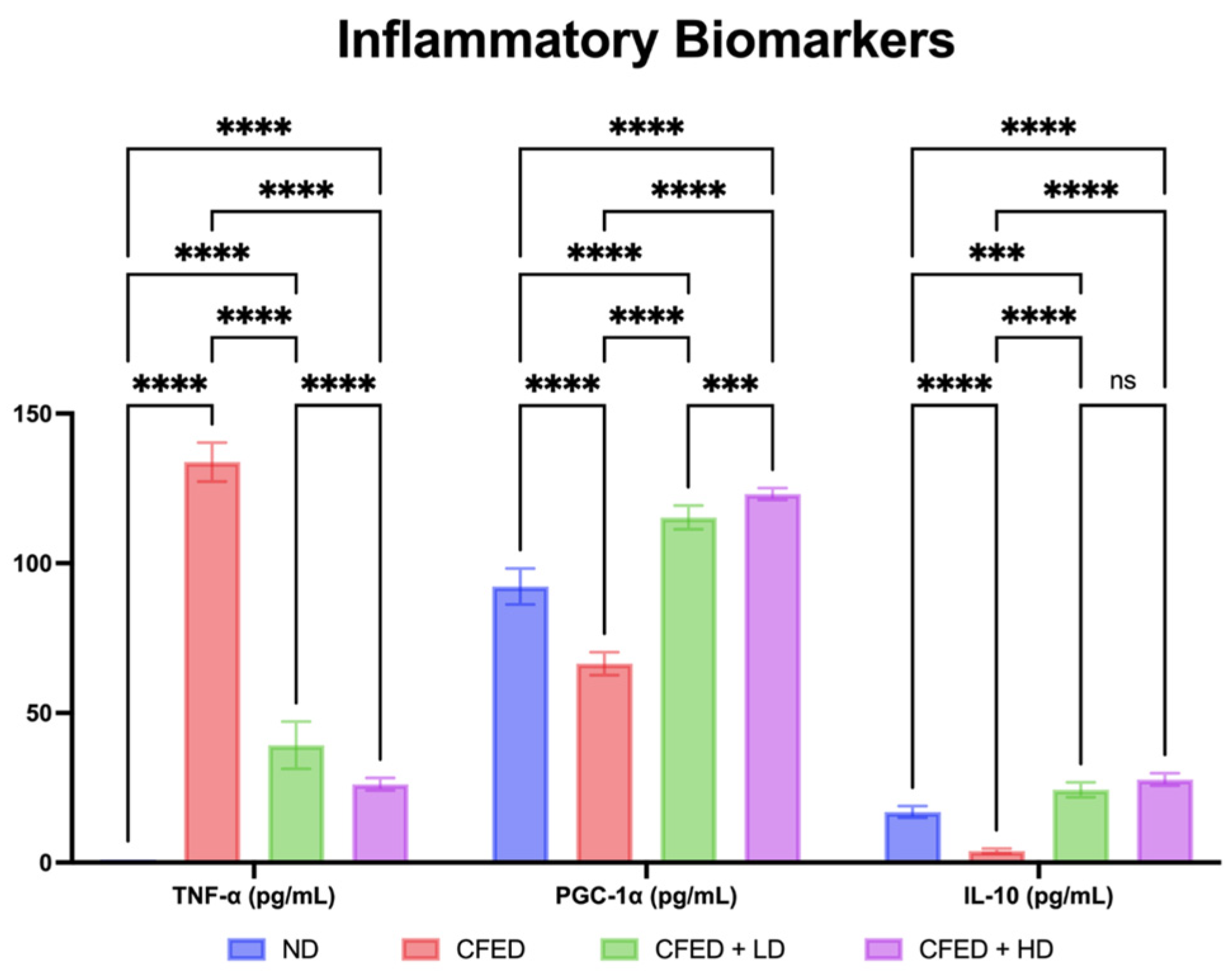
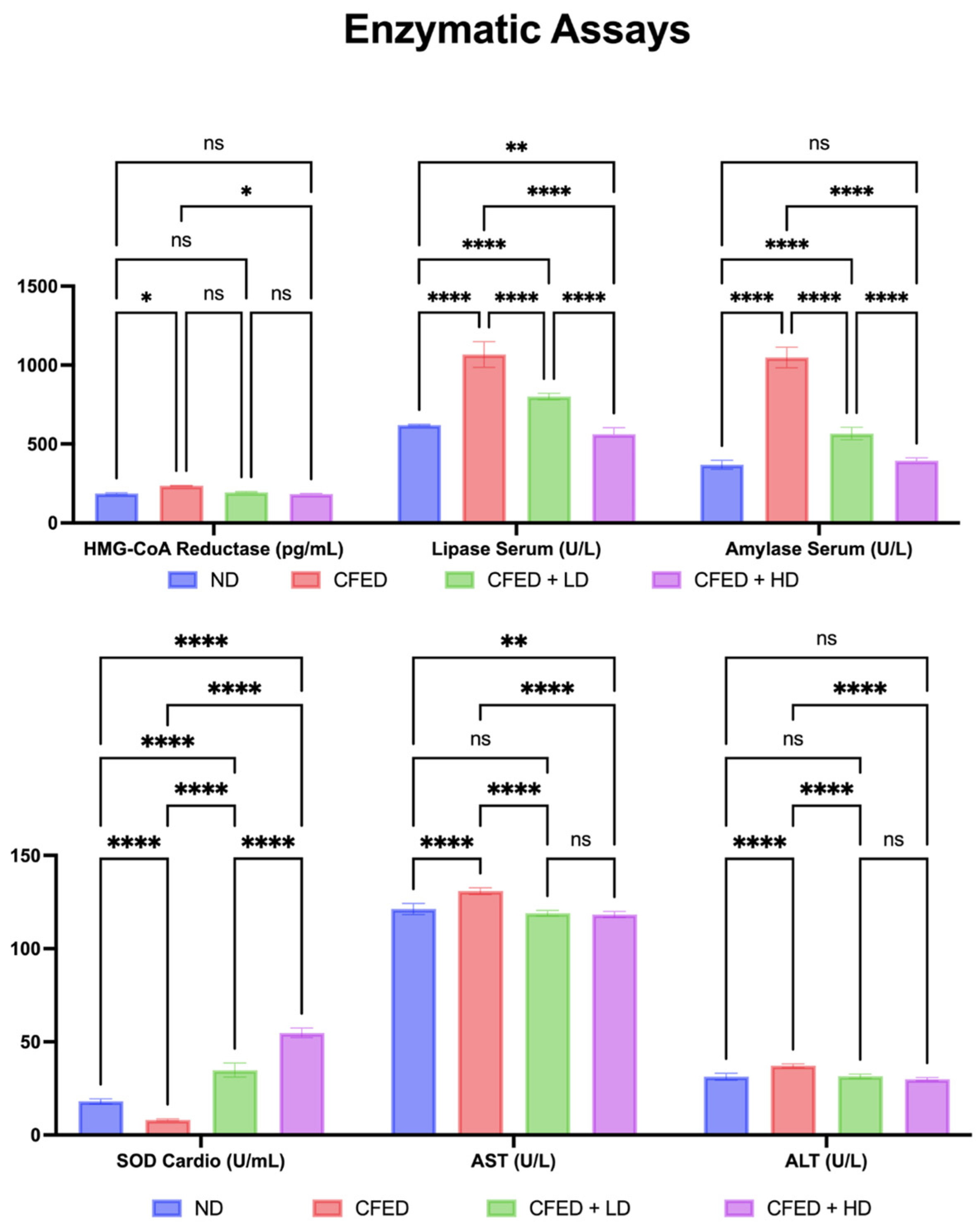
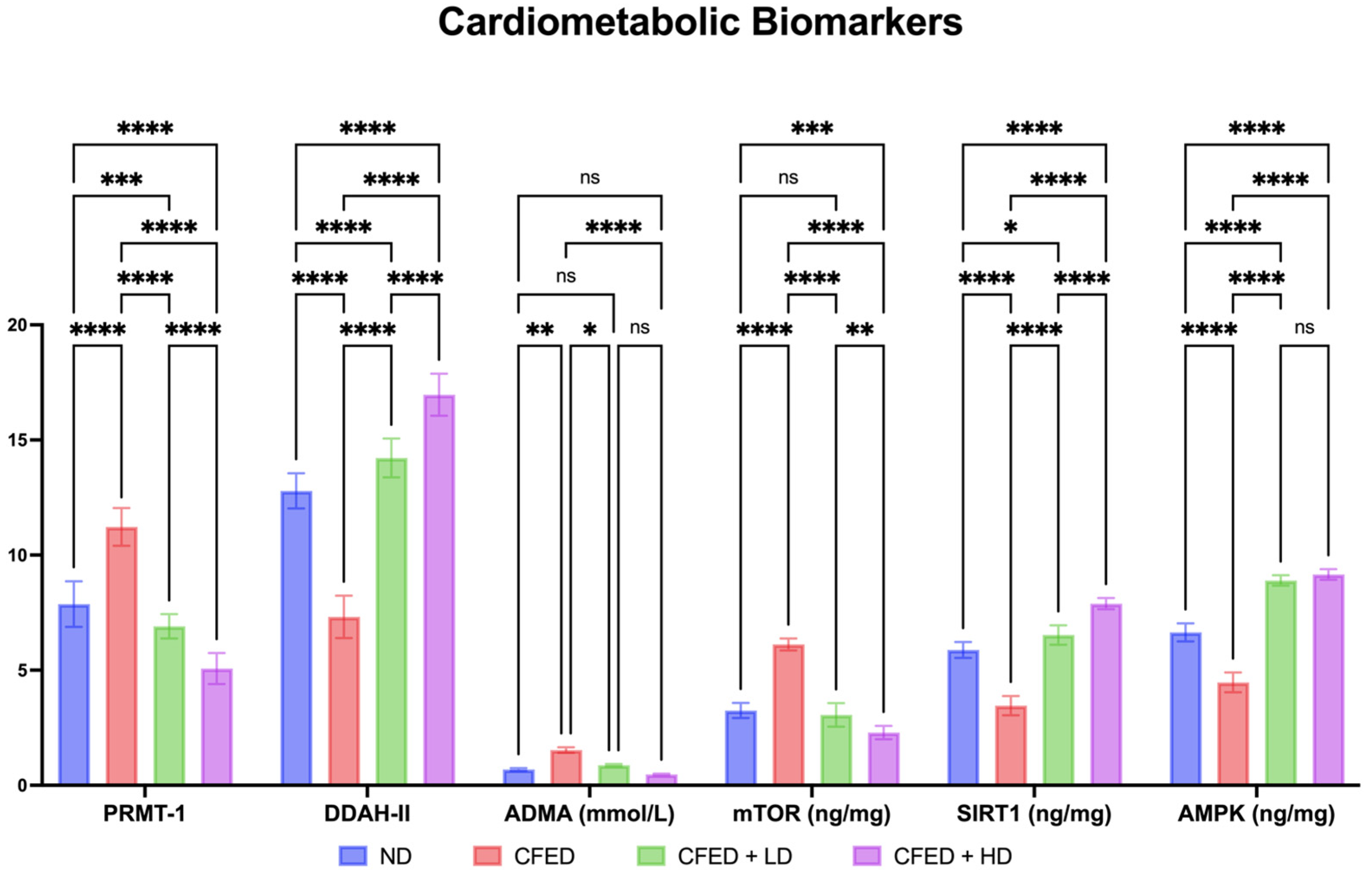
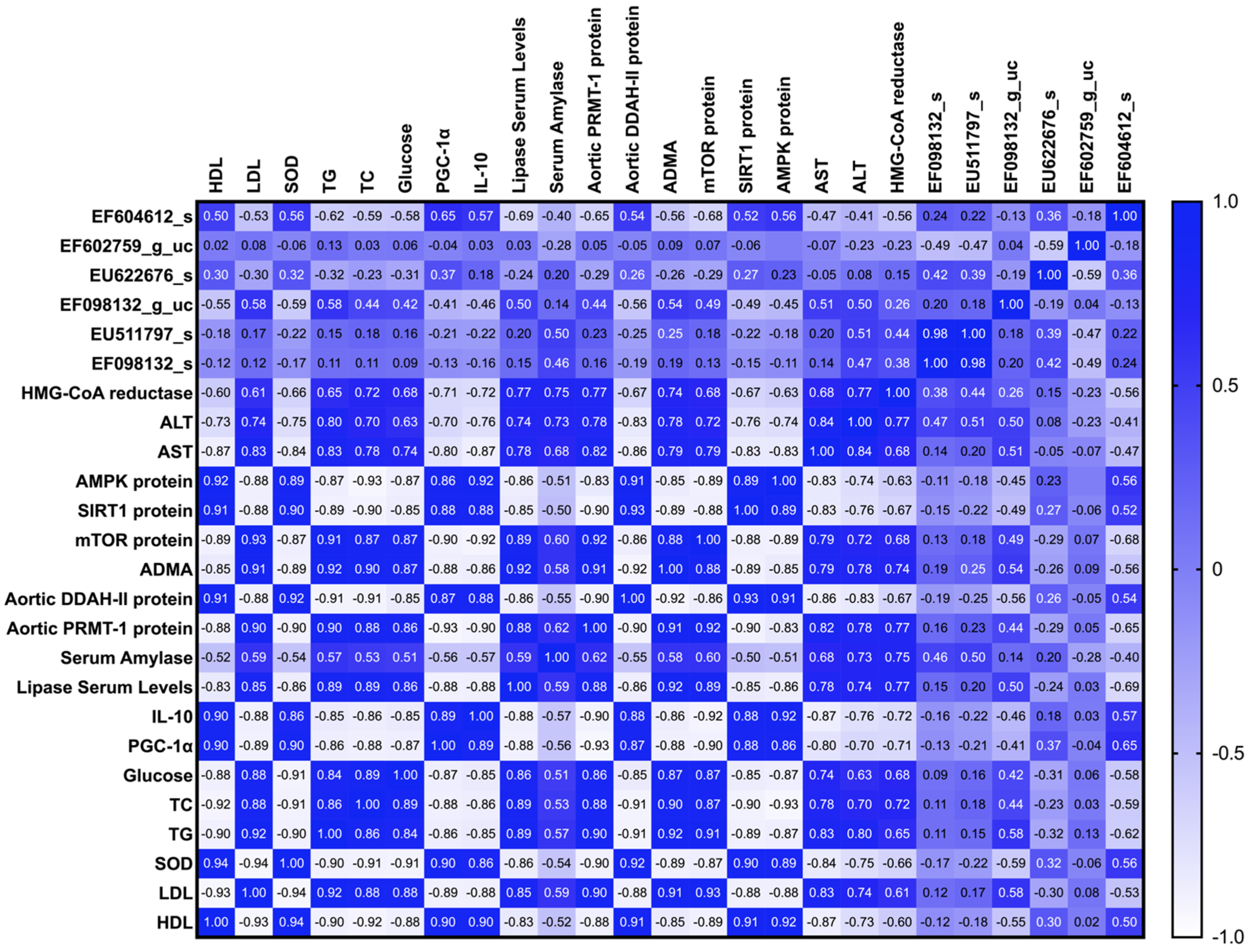
3.1. Effects of Sulfated Polysaccharides of Caulerpa racemosa on the Cardiometabolic Biomarkers in Rats
3.2. Modulation of Gut Microbiome in Rats Fed with Cholesterol- and Fat-Enriched Diet Supplemented with Sulfated Polysaccharides of Caulerpa racemosa
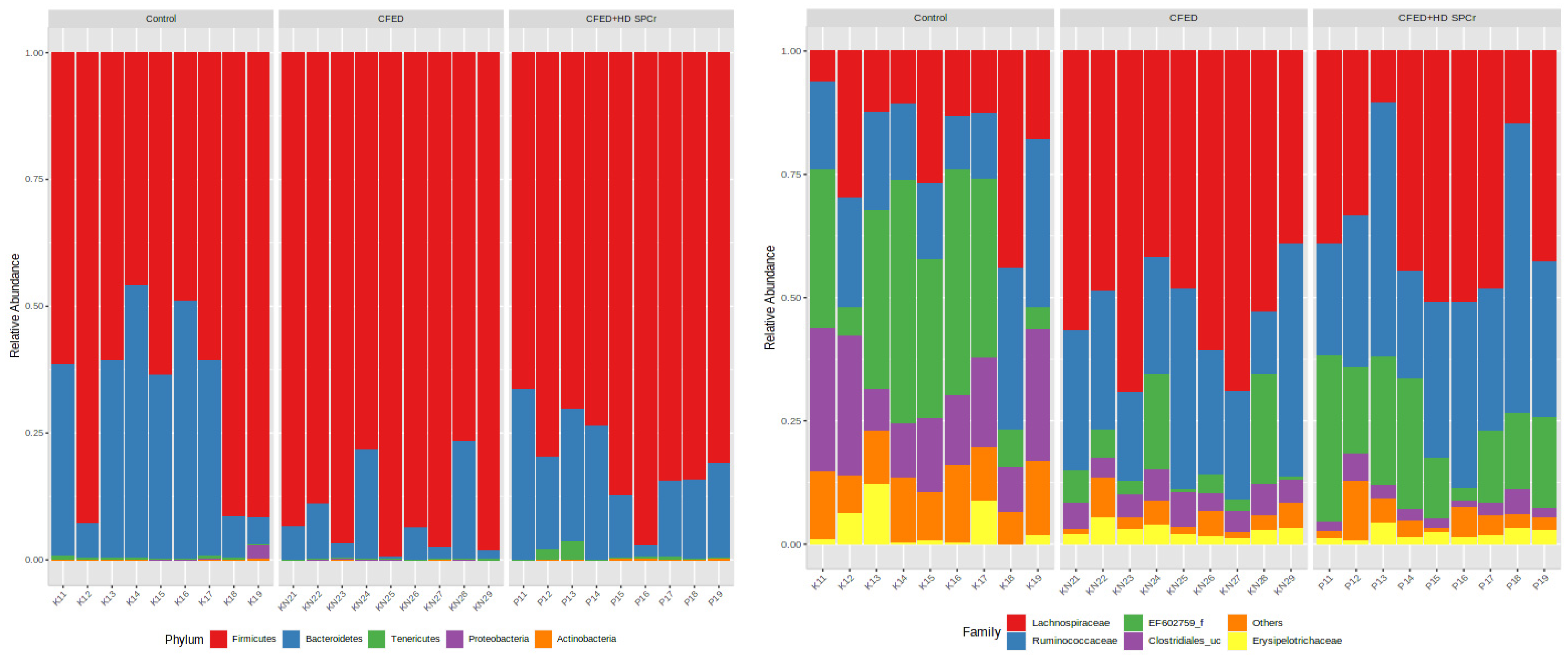
3.2.1. Effect of Sulfated Polysaccharides of Caulerpa racemosa on Gut Microbiota Composition
3.2.2. Effect of Sulfated Polysaccharides of Caulerpa racemosa on Gut Microbiota Diversity
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Putra, I.C.S.; Kamarullah, W.; Prameswari, H.S.; Pramudyo, M.; Iqbal, M.; Achmad, C.; Akbar, M.R.; Tiksnadi, B.B. Metabolically Unhealthy Phenotype in Normal Weight Population and Risk of Mortality and Major Adverse Cardiac Events: A Meta-Analysis of 41 Prospective Cohort Studies. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102635. [Google Scholar] [CrossRef]
- Bays, H.E.; Bindlish, S.; Clayton, T.L. Obesity, Diabetes Mellitus, and Cardiometabolic Risk: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2023. Obes. Pillars 2023, 5, 100056. [Google Scholar] [CrossRef]
- Mei, X.; Zeng, J.; Liu, D.-F.; Zhao, Y.; Yang, H.-L.; Li, Y.; Qiu, P.; Tang, M.-W. Abnormalities of the PRMT1-ADMA-DDAH1 Metabolism Axis and Probucol Treatment in Diabetic Patients and Diabetic Rats. Ann. Palliat. Med. 2021, 10, 3343–3353. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-B.; Sui, G.-G.; Lu, X.-Y.; Sun, Z.-L. Elevated Levels of ADMA Are Associated with Lower DDAH2 and Higher PRMT1 in LPS-Induced Endometritis Rats. Inflammation 2018, 41, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.; Cerrah, S.; Arabul, M.; Akalin, A. Relation of Asymmetric Dimethylarginine Levels to Macrovascular Disease and Inflammation Markers in Type 2 Diabetic Patients. J. Diabetes Res. 2014, 2014, 139215. [Google Scholar] [CrossRef]
- Sadria, M.; Layton, A.T. Interactions among MTORC, AMPK and SIRT: A Computational Model for Cell Energy Balance and Metabolism. Cell Commun. Signal. 2021, 19, 57. [Google Scholar] [CrossRef]
- Giovannini, L.; Bianchi, S. Role of Nutraceutical SIRT1 Modulators in AMPK and MTOR Pathway: Evidence of a Synergistic Effect. Nutrition 2017, 34, 82–96. [Google Scholar] [CrossRef]
- Nurkolis, F.; Taslim, N.A.; Subali, D.; Kurniawan, R.; Hardinsyah, H.; Gunawan, W.B.; Kusuma, R.J.; Yusuf, V.M.; Pramono, A.; Kang, S.; et al. Dietary Supplementation of Caulerpa Racemosa Ameliorates Cardiometabolic Syndrome via Regulation of PRMT-1/DDAH/ADMA Pathway and Gut Microbiome in Mice. Nutrients 2023, 15, 909. [Google Scholar]
- Yadav, A.; Sharma, R.; Mehrotra, R. Algal Bioactive Components: Sources, Health Benefits, and Sustainability. In Bioactive Components: A Sustainable System for Good Health and Well-Being; Thakur, M., Belwal, T., Eds.; Springer Nature: Singapore, 2023; pp. 85–101. ISBN 978-981-19-2366-1. [Google Scholar]
- Zhao, A.; Chen, Y.; Li, Y.; Lin, D.; Yang, Z.; Wang, Q.; Chen, H.; Xu, Q.; Chen, J.; Zhu, P.; et al. Sulfated Polysaccharides from Enteromorpha Prolifera Attenuate Lipid Metabolism Disorders in Mice with Obesity Induced by a High-Fat Diet via a Pathway Dependent on AMP-Activated Protein Kinase. J. Nutr. 2022, 152, 939–949. [Google Scholar] [CrossRef]
- Chaves Filho, G.P.; de Paula Oliveira, R.; de Medeiros, S.R.B.; Rocha, H.A.O.; Moreira, S.M.G. Sulfated Polysaccharides from Green Seaweed Caulerpa Prolifera Suppress Fat Accumulation. J. Appl. Phycol. 2020, 32, 4299–4307. [Google Scholar] [CrossRef]
- Nurkolis, F.; Kurniawan, R.; Kurniatanty, I.; Park, M.N.; Moon, M.; Fatimah, S.; Gunawan, W.B.; Surya, R.; Taslim, N.A.; Song, H.; et al. New Insight on In Vitro Biological Activities of Sulfated Polysaccharides from Ulvophyte Green Algae. Molecules 2023, 28, 4531. [Google Scholar]
- Permatasari, H.K.; Nurkolis, F.; Gunawan, W.B.; Yusuf, V.M.; Yusuf, M.; Kusuma, R.J.; Sabrina, N.; Muharram, F.R.; Taslim, N.A.; Mayulu, N.; et al. Modulation of Gut Microbiota and Markers of Metabolic Syndrome in Mice on Cholesterol and Fat Enriched Diet by Butterfly Pea Flower Kombucha. Curr. Res. Food Sci. 2022, 5, 1251–1265. [Google Scholar] [CrossRef]
- De Oliveira, J.C.; Scomparin, D.X.; Andreazzi, A.E.; Branco, R.C.S.; Martins, A.G.; Gravena, C.; Grassiolli, S.; Rinaldi, W.; Barbosa, F.B.; Mathias, P.C.F. Metabolic Imprinting by Maternal Protein Malnourishment Impairs Vagal Activity in Adult Rats. J. Neuroendocrinol. 2011, 23, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin Resistance, Cardiovascular Stiffening and Cardiovascular Disease. Metab. Clin. Exp. 2021, 119, 154766. [Google Scholar] [CrossRef]
- Kalita, P.; Ahmed, A.B.; Sen, S.; Chakraborty, R. A Comprehensive Review on Polysaccharides with Hypolipidemic Activity: Occurrence, Chemistry and Molecular Mechanism. Int. J. Biol. Macromol. 2022, 206, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides. Molecules 2019, 24, 2556. [Google Scholar]
- Cindana Mo’o, F.R.; Wilar, G.; Devkota, H.P.; Wathoni, N. Ulvan, a Polysaccharide from Macroalga Ulva Sp.: A Review of Chemistry, Biological Activities and Potential for Food and Biomedical Applications. Appl. Sci. 2020, 10, 5488. [Google Scholar]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S.; Sandhu, M.A. PGC-1 α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]
- Eddie Ip, W.K.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-Inflammatory Effect of IL-10 Mediated by Metabolic Reprogramming of Macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar]
- Jayawardena, T.U.; Sanjeewa, K.K.A.; Nagahawatta, D.P.; Lee, H.-G.; Lu, Y.-A.; Vaas, A.P.J.P.; Abeytunga, D.T.U.; Nanayakkara, C.M.; Lee, D.-S.; Jeon, Y.-J. Anti-Inflammatory Effects of Sulfated Polysaccharide from Sargassum Swartzii in Macrophages via Blocking TLR/NF-Κb Signal Transduction. Mar. Drugs 2020, 18, 601. [Google Scholar] [PubMed]
- Wen, Z.-S.; Xiang, X.-W.; Jin, H.-X.; Guo, X.-Y.; Liu, L.-J.; Huang, Y.-N.; OuYang, X.-K.; Qu, Y.-L. Composition and Anti-Inflammatory Effect of Polysaccharides from Sargassum Horneri in RAW264.7 Macrophages. Int. J. Biol. Macromol. 2016, 88, 403–413. [Google Scholar] [CrossRef]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Gunawan, W.B.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids Composition of Green Algae Caulerpa racemosa and Their Antidiabetic, Anti-Obesity, Antioxidant, and Anti-Inflammatory Properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef]
- Patil, P.; Mandal, S.; Tomar, S.K.; Anand, S. Food Protein-Derived Bioactive Peptides in Management of Type 2 Diabetes. Eur. J. Nutr. 2015, 54, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-T.; Liu, X.-T.; Chen, Q.-X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef]
- Barteková, M.; Adameová, A.; Görbe, A.; Ferenczyová, K.; Pecháňová, O.; Lazou, A.; Dhalla, N.S.; Ferdinandy, P.; Giricz, Z. Natural and Synthetic Antioxidants Targeting Cardiac Oxidative Stress and Redox Signaling in Cardiometabolic Diseases. Free Radic. Biol. Med. 2021, 169, 446–477. [Google Scholar] [CrossRef]
- Iqbal, D.; Khan, M.S.; Khan, M.S.; Ahmad, S.; Hussain, M.S.; Ali, M. Bioactivity Guided Fractionation and Hypolipidemic Property of a Novel HMG-CoA Reductase Inhibitor from Ficus Virens Ait. Lipids Health Dis. 2015, 14, 15. [Google Scholar] [CrossRef]
- Mohammadi, F.; Qorbani, M.; Kelishadi, R.; Baygi, F.; Ardalan, G.; Taslimi, M.; Mahmoudarabi, M.; Motlagh, M.-E.; Asayesh, H.; Larijani, B.; et al. Association of Cardiometabolic Risk Factors and Hepatic Enzymes in a National Sample of Iranian Children and Adolescents: The CASPIAN-III Study. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 463–468. [Google Scholar] [CrossRef]
- Song, X.; Cui, W.; Gao, Z.; Zhang, J.; Jia, L. Structural Characterization and Amelioration of Sulfated Polysaccharides from Ganoderma Applanatum Residue against CCl4-Induced Hepatotoxicity. Int. Immunopharmacol. 2021, 96, 107554. [Google Scholar] [CrossRef]
- Couto e Silva, A.; Wu, C.Y.-C.; Citadin, C.T.; Clemons, G.A.; Possoit, H.E.; Grames, M.S.; Lien, C.-F.; Minagar, A.; Lee, R.H.-C.; Frankel, A.; et al. Protein Arginine Methyltransferases in Cardiovascular and Neuronal Function. Mol. Neurobiol. 2020, 57, 1716–1732. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, X.; Yang, D.; Lu, Y.; Wei, G.; Yu, W.; Liu, X.; Zheng, Q.; Ying, J.; Hua, F. Relieving Cellular Energy Stress in Aging, Neurodegenerative, and Metabolic Diseases, SIRT1 as a Therapeutic and Promising Node. Front. Aging Neurosci. 2021, 13, 738686. [Google Scholar] [CrossRef]
- Fiedler, L. The DDAH/ADMA Pathway Is a Critical Regulator of NO Signalling in Vascular Homeostasis. Cell Adh. Migr. 2008, 2, 149–150. [Google Scholar] [CrossRef][Green Version]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Vesa, C.M.; Bungau, S.G. Novel Molecules in Diabetes Mellitus, Dyslipidemia and Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 4029. [Google Scholar] [CrossRef]
- Vesa, C.M.; Popa, L.; Popa, A.R.; Rus, M.; Zaha, A.A.; Bungau, S.; Tit, D.M.; Corb Aron, R.A.; Zaha, D.C. Current Data Regarding the Relationship between Type 2 Diabetes Mellitus and Cardiovascular Risk Factors. Diagnostics 2020, 10, 314. [Google Scholar] [CrossRef]
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.F.; Vesa, C.M.; Behl, T.; Bungau, A.F.; et al. Oxidative stress–Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed. Pharmacother. 2022, 152, 113238. [Google Scholar] [CrossRef]
- Behl, T.; Gupta, A.; Sehgal, A.; Sharma, S.; Singh, S.; Sharma, N.; Diaconu, C.C.; Rahdar, A.; Hafeez, A.; Bhatia, S.; et al. A spotlight on underlying the mechanism of AMPK in diabetes complications. Inflamm. Res. 2021, 70, 939–957. [Google Scholar] [CrossRef]
- Sabir, F.; Barani, M.; Mukhtar, M.; Rahdar, A.; Cucchiarini, M.; Zafar, M.N.; Behl, T.; Bungau, S. Nanodiagnosis and Nanotreatment of Cardiovascular Diseases: An Overview. Chemosensors 2021, 9, 67. [Google Scholar] [CrossRef]


| Group | Normal | CFED | CFED + Low Dose SPCr | CFED + High Dose SPCr | p ** |
|---|---|---|---|---|---|
| Initial BW (g) | 224.62 ± 10.75 | 227.11 ± 15.46 | 227.19 ± 12.94 | 226.20 ± 10.39 | 0.7691 |
| Final BW (g) | 256.93 ± 4.97 | 277.37 ± 7.22 | 244.78 ± 4.03 | 242.68 ± 3.49 | <0.0001 |
| p * | <0.000001 | 0.000002 | 0.000438 | 0.001952 | |
| Weight gain (g/day) | 0.7 ± 0.20 | 1.09 ± 0.29 | 0.38 ± 0.28 | 0.36 ± 0.21 | 0.9989 |
| Food intake (g) | 5.43 ± 0.32 | 4.93 ± 0.72 | 5.13 ± 1.12 | 5.10 ± 0.48 | 0.9976 |
| Water intake (mL) | 5.81 ± 0.56 | 5.74 ± 0.87 | 5.35 ± 1.00 | 5.21 ± 0.53 | 0.9971 |
| FER (%) | 13.13 ± 4.25 | 22.37 ± 6.46 | 7.39 ± 5.61 | 7.17 ± 4.35 | 0.0035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayulu, N.; Gunawan, W.B.; Park, M.N.; Chung, S.; Suh, J.Y.; Song, H.; Kusuma, R.J.; Taslim, N.A.; Kurniawan, R.; Kartawidjajaputra, F.; et al. Sulfated Polysaccharide from Caulerpa racemosa Attenuates the Obesity-Induced Cardiometabolic Syndrome via Regulating the PRMT1-DDAH-ADMA with mTOR-SIRT1-AMPK Pathways and Gut Microbiota Modulation. Antioxidants 2023, 12, 1555. https://doi.org/10.3390/antiox12081555
Mayulu N, Gunawan WB, Park MN, Chung S, Suh JY, Song H, Kusuma RJ, Taslim NA, Kurniawan R, Kartawidjajaputra F, et al. Sulfated Polysaccharide from Caulerpa racemosa Attenuates the Obesity-Induced Cardiometabolic Syndrome via Regulating the PRMT1-DDAH-ADMA with mTOR-SIRT1-AMPK Pathways and Gut Microbiota Modulation. Antioxidants. 2023; 12(8):1555. https://doi.org/10.3390/antiox12081555
Chicago/Turabian StyleMayulu, Nelly, William Ben Gunawan, Moon Nyeo Park, Sanghyun Chung, Jin Young Suh, Hangyul Song, Rio Jati Kusuma, Nurpudji Astuti Taslim, Rudy Kurniawan, Felicia Kartawidjajaputra, and et al. 2023. "Sulfated Polysaccharide from Caulerpa racemosa Attenuates the Obesity-Induced Cardiometabolic Syndrome via Regulating the PRMT1-DDAH-ADMA with mTOR-SIRT1-AMPK Pathways and Gut Microbiota Modulation" Antioxidants 12, no. 8: 1555. https://doi.org/10.3390/antiox12081555
APA StyleMayulu, N., Gunawan, W. B., Park, M. N., Chung, S., Suh, J. Y., Song, H., Kusuma, R. J., Taslim, N. A., Kurniawan, R., Kartawidjajaputra, F., Nurkolis, F., & Kim, B. (2023). Sulfated Polysaccharide from Caulerpa racemosa Attenuates the Obesity-Induced Cardiometabolic Syndrome via Regulating the PRMT1-DDAH-ADMA with mTOR-SIRT1-AMPK Pathways and Gut Microbiota Modulation. Antioxidants, 12(8), 1555. https://doi.org/10.3390/antiox12081555











