Nitroxide—HMP—Protects Human Trophoblast HTR-8/SVneo Cells from H2O2-Induced Oxidative Stress by Reducing the HIF1A Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Antioxidant Assay
2.3. Cell Culture Studies
2.4. Cell Viability Assay
2.5. Biochemical Measurements
2.6. HIF1A Immunofluorescence
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. COX In Situ Enzyme Chemistry
2.9. Human Subjects
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. Radical Scavenging Activity of HMP
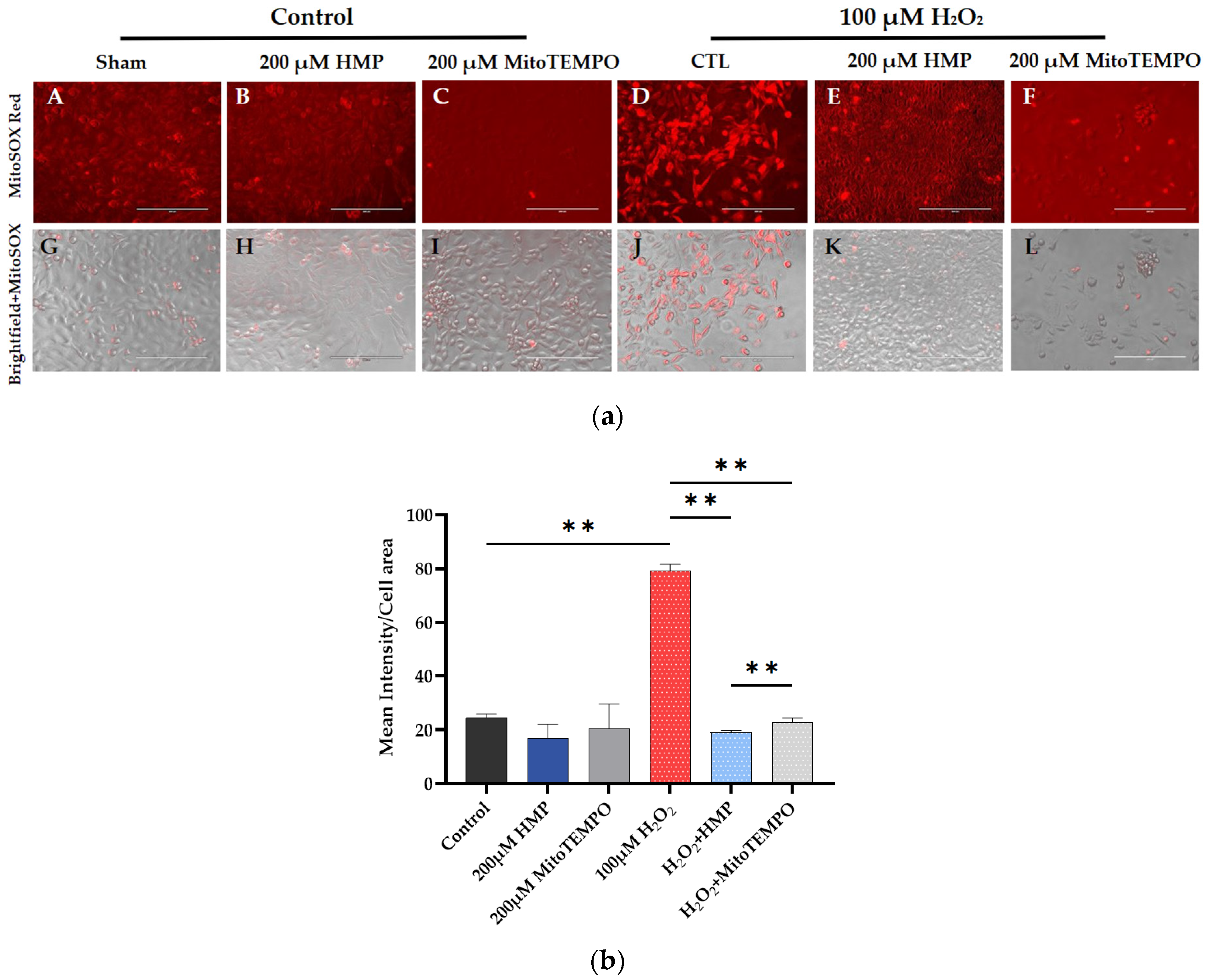
3.2. HMP Pre-Treatment Reduced Mitochondrial-Derived ROS Production in H2O2-Exposed Trophoblast HTR-8/SVneo Cells
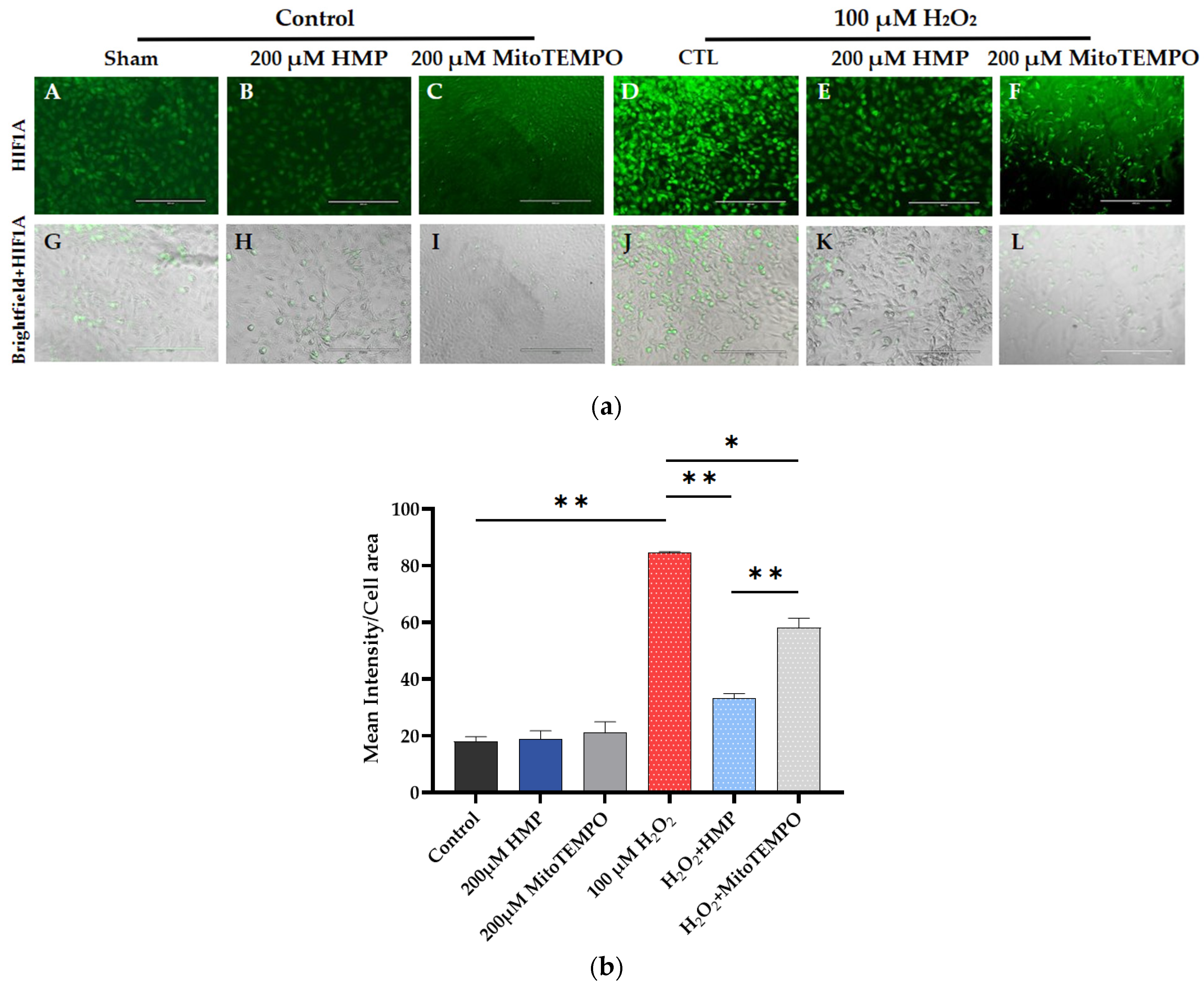
3.3. HMP Pre-Treatment Reduced HIF1A Expression in H2O2-Exposed Trophoblast HTR-8/SVneo Cells
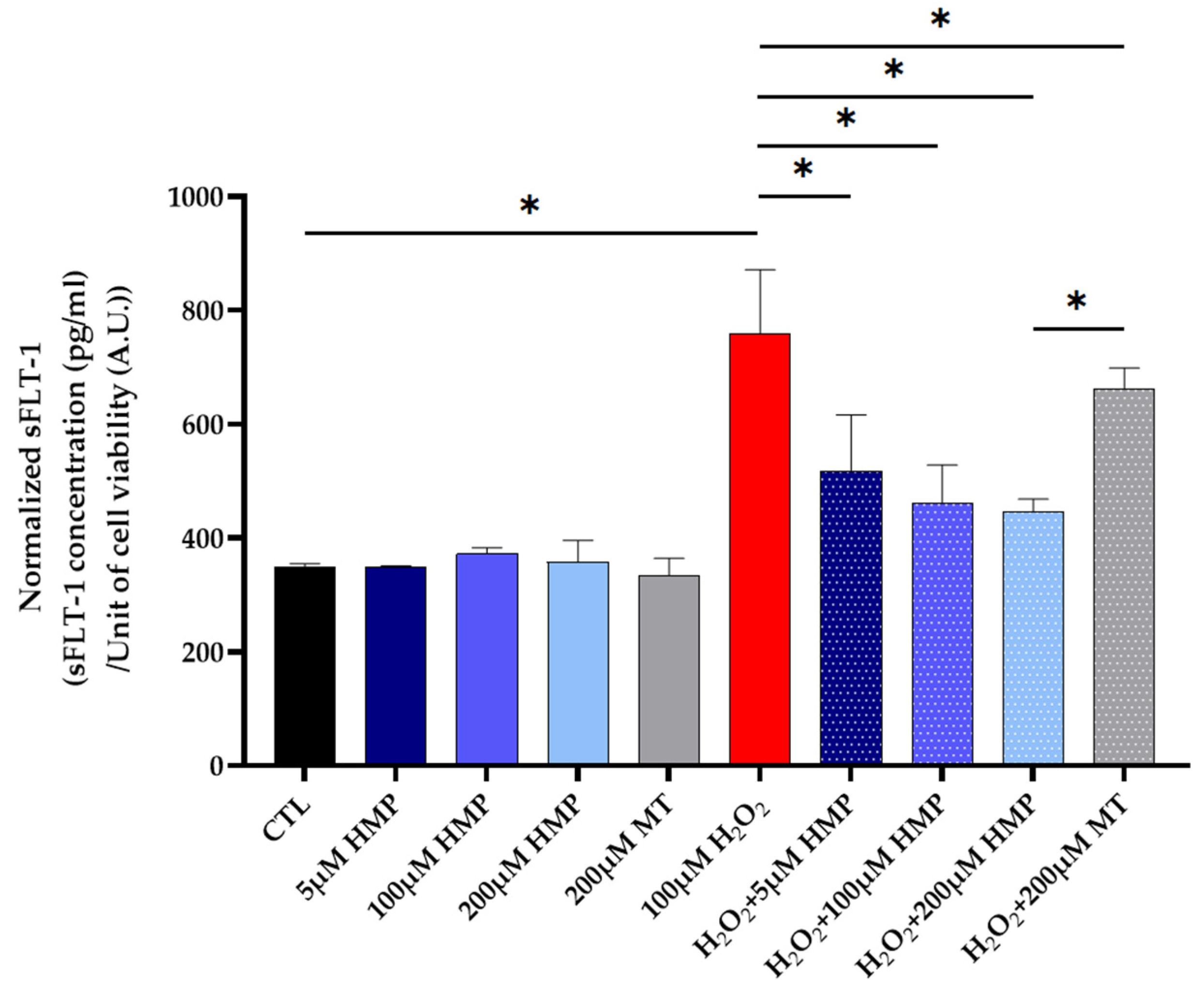
3.4. HMP Pre-Treatment Reduced sFLT1 Protein Expression in H2O2-Exposed Trophoblast HTR-8/SVneo Cells
3.5. HMP Pre-Treatment Improved the Mitochondrial Energetics in H2O2-Exposed Trophoblast HTR-8/SVneo Cells
3.6. HMP Pre-Treatment Improved the Mitochondrial COX Activity in H2O2-Exposed Trophoblast HTR-8/SVneo Cells
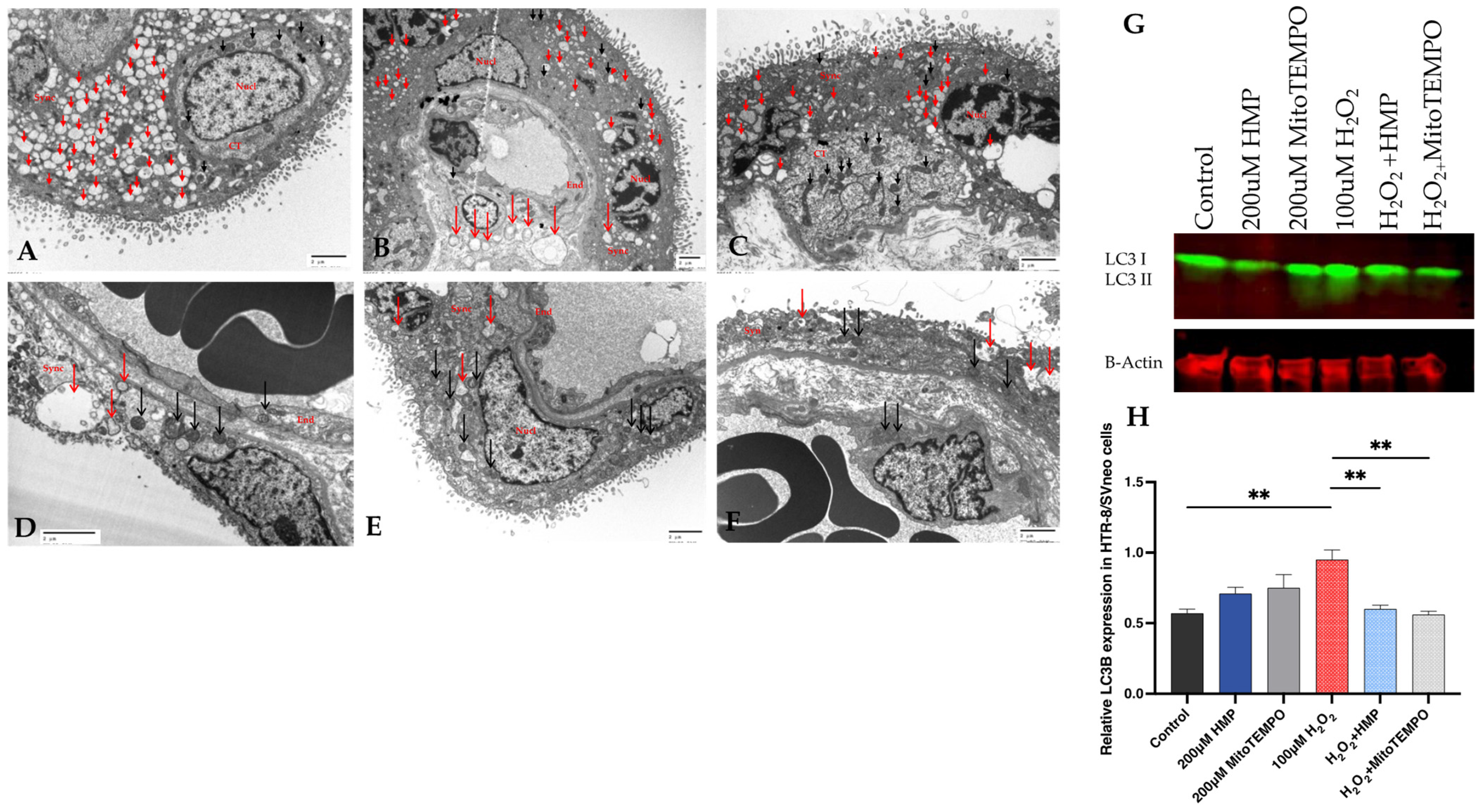
3.7. Autophagy and Mitochondrial Dysfunction in Human Pregnancy and in HTR-8/SVneo Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- MacKay, A.P.; Berg, C.J.; Atrash, H.K. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet. Gynecol. 2001, 97, 533–538. [Google Scholar]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [CrossRef]
- Nisell, H.; Palm, K.; Wolff, K. Prediction of maternal and fetal complications in preeclampsia. Acta Obstet. Gynecol. Scand. 2000, 79, 19–23. [Google Scholar]
- Odegard, R.A.; Vatten, L.J.; Nilsen, S.T.; Salvesen, K.A.; Austgulen, R. Preeclampsia and fetal growth. Obstet. Gynecol. 2000, 96, 950–955. [Google Scholar]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Nicolaides, K.H.; Poon, L.C. Prevention of preeclampsia with aspirin. Am. J. Obstet. Gynecol. 2022, 226, S1108–S1119. [Google Scholar] [CrossRef]
- Covarrubias, A.E.; Lecarpentier, E.; Lo, A.; Salahuddin, S.; Gray, K.J.; Karumanchi, S.A.; Zsengellér, Z.K. AP39, a Modulator of Mitochondrial Bioenergetics, Reduces Antiangiogenic Response and Oxidative Stress in Hypoxia-Exposed Trophoblasts: Relevance for Preeclampsia Pathogenesis. Am. J. Pathol. 2019, 189, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Zsengeller, Z.K.; Rajakumar, A.; Hunter, J.T.; Salahuddin, S.; Rana, S.; Stillman, I.E.; Ananth Karumanchi, S. Trophoblast mitochondrial function is impaired in preeclampsia and correlates negatively with the expression of soluble fms-like tyrosine kinase 1. Pregnancy Hypertens. 2016, 6, 313–319. [Google Scholar] [CrossRef]
- Nezu, M.; Souma, T.; Yu, L.; Sekine, H.; Takahashi, N.; Wei, A.Z.; Ito, S.; Fukamizu, A.; Zsengeller, Z.K.; Nakamura, T.; et al. Nrf2 inactivation enhances placental angiogenesis in a preeclampsia mouse model and improves maternal and fetal outcomes. Sci. Signal. 2017, 10, aam5711. [Google Scholar] [CrossRef]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Walsh, S.W. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin. Reprod. Endocrinol. 1998, 16, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Walsh, S.W. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 1998, 19, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Stanek, J.; Eis, A.L.; Myatt, L. Nitrotyrosine immunostaining correlates with increased extracellular matrix: Evidence of postplacental hypoxia. Placenta 2001, 22 (Suppl. SA), S56–S62. [Google Scholar] [CrossRef]
- Hubel, C.A. Oxidative stress in the pathogenesis of preeclampsia. Proc. Soc. Exp. Biol. Med. 1999, 222, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Placental oxidative stress: From miscarriage to preeclampsia. J. Soc. Gynecol. Investig. 2004, 11, 342–352. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.P.; Skepper, J.N.; Burton, G.J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Haram, K.; Mortensen, J.H.; Myking, O.; Magann, E.F.; Morrison, J.C. The role of oxidative stress, adhesion molecules and antioxidants in preeclampsia. Curr. Hypertens. Rev. 2019, 15, 105–112. [Google Scholar] [CrossRef]
- Taravati, A.; Tohidi, F. Comprehensive analysis of oxidative stress markers and antioxidants status in preeclampsia. Taiwan J. Obstet. Gynecol. 2018, 57, 779–790. [Google Scholar] [CrossRef]
- Rajakumar, A.; Brandon, H.M.; Daftary, A.; Ness, R.; Conrad, K.P. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta 2004, 25, 763–769. [Google Scholar] [CrossRef]
- Rajakumar, A.; Jeyabalan, A.; Markovic, N.; Ness, R.; Gilmour, C.; Conrad, K.P. Placental HIF-1 alpha, HIF-2 alpha, membrane and soluble VEGF receptor-1 proteins are not increased in normotensive pregnancies complicated by late-onset intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R766–R774. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Michael, H.M.; Daftary, A.; Jeyabalan, A.; Gilmour, C.; Conrad, K.P. Proteasomal activity in placentas from women with preeclampsia and intrauterine growth restriction: Implications for expression of HIF-alpha proteins. Placenta 2008, 29, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Furuya, M.; Kurasawa, K.; Nagahama, K.; Kawachi, K.; Nozawa, A.; Takahashi, T.; Aoki, I. Disrupted balance of angiogenic and antiangiogenic signalings in preeclampsia. J. Pregnancy 2011, 2011, 123717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagamatsu, T.; Fujii, T.; Kusumi, M.; Zou, L.; Yamashita, T.; Osuga, Y.; Momoeda, M.; Kozuma, S.; Taketani, Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: An implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 2004, 145, 4838–4845. [Google Scholar] [CrossRef] [Green Version]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.H.; Yuan, H.T.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, R.J.; Lam, C.; Qian, C.; Yu, K.F.; Maynard, S.E.; Sachs, B.P.; Sibai, B.M.; Epstein, F.H.; Romero, R.; Thadhani, R.; et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 2006, 355, 992–1005. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Chambers, J.C.; Fusi, L.; Malik, I.S.; Haskard, D.O.; De Swiet, M.; Kooner, J.S. Association of maternal endothelial dysfunction with preeclampsia. JAMA J. Am. Med. Assoc. 2001, 285, 1607–1612. [Google Scholar] [CrossRef] [Green Version]
- Germain, A.M.; Romanik, M.C.; Guerra, I.; Solari, S.; Reyes, M.S.; Johnson, R.J.; Price, K.; Karumanchi, S.A.; Valdes, G. Endothelial dysfunction: A link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension 2007, 49, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Yinon, Y.; Kingdom, J.C.; Odutayo, A.; Moineddin, R.; Drewlo, S.; Lai, V.; Cherney, D.Z.; Hladunewich, M.A. Vascular dysfunction in women with a history of preeclampsia and intrauterine growth restriction: Insights into future vascular risk. Circulation 2010, 122, 1846–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.M.; Myatt, L.; Spong, C.Y.; Thom, E.A.; Hauth, J.C.; Leveno, K.J.; Pearson, G.D.; Wapner, R.J.; Varner, M.W.; Thorp, J.M., Jr.; et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N. Engl. J. Med. 2010, 362, 1282–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, Y.; Nakamura, K.; Abe, J.; Hyodo, M.; Haga, S.; Ozaki, M.; Harashima, H. Mitochondrial delivery of Coenzyme Q10 via systemic administration using a MITO-Porter prevents ischemia/reperfusion injury in the mouse liver. J. Control. Release 2015, 213, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dare, A.J.; Logan, A.; Prime, T.A.; Rogatti, S.; Goddard, M.; Bolton, E.M.; Bradley, J.A.; Pettigrew, G.J.; Murphy, M.P.; Saeb-Parsy, K. The mitochondria-targeted anti-oxidant MitoQ decreases ischemia-reperfusion injury in a murine syngeneic heart transplant model. J. Heart Lung Transplant. 2015, 34, 1471–1480. [Google Scholar] [CrossRef] [Green Version]
- Szczepanek, K.; Xu, A.; Hu, Y.; Thompson, J.; He, J.; Larner, A.C.; Salloum, F.N.; Chen, Q.; Lesnefsky, E.J. Cardioprotective function of mitochondrial-targeted and transcriptionally inactive STAT3 against ischemia and reperfusion injury. Basic Res. Cardiol. 2015, 110, 53. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Horváth, B.; Zsengellėr, Z.; Bátkai, S.; Cao, Z.; Kechrid, M.; Holovac, E.; Erdėlyi, K.; Tanchian, G.; Liaudet, L.; et al. Mitochondrial reactive oxygen species generation triggers inflammatory response and tissue injury associated with hepatic ischemia-reperfusion: Therapeutic potential of mitochondrially targeted antioxidants. Free Radic. Biol. Med. 2012, 53, 1123–1138. [Google Scholar] [CrossRef] [Green Version]
- Nuzzo, A.M.; Camm, E.J.; Sferruzzi-Perri, A.N.; Ashmore, T.J.; Yung, H.W.; Cindrova-Davies, T.; Spiroski, A.M.; Sutherland, M.R.; Logan, A.; Austin-Williams, S.; et al. Placental Adaptation to Early-Onset Hypoxic Pregnancy and Mitochondria-Targeted Antioxidant Therapy in a Rodent Model. Am. J. Pathol. 2018, 188, 2704–2716. [Google Scholar] [CrossRef] [Green Version]
- Vaka, V.R.; McMaster, K.M.; Cunningham, M.W.; Ibrahim, T.; Hazlewood, R.; Usry, N.; Cornelius, D.C.; Amaral, L.M.; LaMarca, B. Role of Mitochondrial Dysfunction and Reactive Oxygen Species in Mediating Hypertension in the Reduced Uterine Perfusion Pressure Rat Model of Preeclampsia. Hypertension 2018, 72, 703–711. [Google Scholar] [CrossRef]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef] [Green Version]
- Griesser, M.; Shah, R.; Van Kessel, A.T.; Zilka, O.; Haidasz, E.A.; Pratt, D.A. The Catalytic Reaction of Nitroxides with Peroxyl Radicals and Its Relevance to Their Cytoprotective Properties. J. Am. Chem. Soc. 2018, 140, 3798–3808. [Google Scholar] [CrossRef]
- Lewandowski, M.; Gwozdzinski, K. Nitroxides as Antioxidants and Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; Zielonka, M.; Dranka, B.; Kumar, S.N.; Myers, C.R.; Bennett, B.; Garces, A.M.; Dias Duarte Machado, L.G.; Thiebaut, D.; Ouari, O.; et al. Detection of mitochondria-generated reactive oxygen species in cells using multiple probes and methods: Potentials, pitfalls, and the future. J. Biol. Chem. 2018, 293, 10363–10380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S.I.; Nazarewicz, R.R.; Bikineyeva, A.; Hilenski, L.; Lassegue, B.; Griendling, K.K.; Harrison, D.G.; Dikalova, A.E. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid. Redox Signal. 2014, 20, 281–294. [Google Scholar] [CrossRef] [Green Version]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef] [PubMed]
- Baier, A.K.A.; Horton, W.; Gizińska, E.; Pandey, G.; Szyszka, R.; Török, B.; Török, M. Organofluorine Hydrazone Derivatives as Multifunctional Anti-Alzheimer’s Agents with CK2 Inhibitory and Antioxidant Features. ChemMedChem 2021, 16, 1927–1932. [Google Scholar] [CrossRef]
- Horton, W.P.S.; Török, B.; Török, M. Theoretical and experimental analysis of the antioxidant features of substituted phenol and aniline model compounds. Struct. Chem. 2019, 30, 23–35. [Google Scholar] [CrossRef]
- Seligman, A.M.; Karnovsky, M.J.; Wasserkrug, H.L.; Hanker, J.S. Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB). J. Cell Biol. 1968, 38, 1–14. [Google Scholar] [CrossRef]
- Anderson, W.A.; Bara, G.; Seligman, A.M. The ultrastructural localization of cytochrome oxidase via cytochrome. J. Histochem. Cytochem. 1975, 23, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Bertoni-Freddari, C.; Fattoretti, P.; Casoli, T.; Di Stefano, G.; Solazzi, M.; Gracciotti, N.; Pompei, P. Mapping of mitochondrial metabolic competence by cytochrome oxidase and succinic dehydrogenase cytochemistry. J. Histochem. Cytochem. 2001, 49, 1191–1192. [Google Scholar] [CrossRef] [Green Version]
- Zsengeller, Z.K.; Aljinovic, N.; Teot, L.A.; Korson, M.; Rodig, N.; Sloan, J.L.; Venditti, C.P.; Berry, G.T.; Rosen, S. Methylmalonic acidemia: A megamitochondrial disorder affecting the kidney. Pediatr. Nephrol. 2014, 29, 2139–2146. [Google Scholar] [CrossRef]
- Zsengeller, Z.K.; Ellezian, L.; Brown, D.; Horvath, B.; Mukhopadhyay, P.; Kalyanaraman, B.; Parikh, S.M.; Karumanchi, S.A.; Stillman, I.E.; Pacher, P. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J. Histochem. Cytochem. 2012, 60, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Rajakumar, A.; Cerdeira, A.S.; Rana, S.; Zsengeller, Z.; Edmunds, L.; Jeyabalan, A.; Hubel, C.A.; Stillman, I.E.; Parikh, S.M.; Karumanchi, S.A. Transcriptionally active syncytial aggregates in the maternal circulation may contribute to circulating soluble fms-like tyrosine kinase 1 in preeclampsia. Hypertension 2012, 59, 256–264. [Google Scholar] [CrossRef]
- Charlton, N.C.; Mastyugin, M.; Torok, B.; Torok, M. Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef] [PubMed]
- Sebaugh, J.L. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Pringle, K.G.; Kind, K.L.; Sferruzzi-Perri, A.N.; Thompson, J.G.; Roberts, C.T. Beyond oxygen: Complex regulation and activity of hypoxia inducible factors in pregnancy. Hum. Reprod. Update 2010, 16, 415–431. [Google Scholar] [CrossRef] [Green Version]
- Akaishi, R.; Yamada, T.; Nakabayashi, K.; Nishihara, H.; Furuta, I.; Kojima, T.; Morikawa, M.; Fujita, N.; Minakami, H. Autophagy in the placenta of women with hypertensive disorders in pregnancy. Placenta 2014, 35, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, A.; Yamanaka-Tatematsu, M.; Fujita, N.; Koizumi, K.; Shima, T.; Yoshida, T.; Nikaido, T.; Okamoto, A.; Yoshimori, T.; Saito, S. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy 2013, 9, 303–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koklanaris, N.; Nwachukwu, J.C.; Huang, S.J.; Guller, S.; Karpisheva, K.; Garabedian, M.; Lee, M.J. First-trimester trophoblast cell model gene response to hypoxia. Am. J. Obstet. Gynecol. 2006, 194, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, T.; Wu, X.; An, P.; Dang, H. Autophagy in the HTR-8/SVneo Cell Oxidative Stress Model Is Associated with the NLRP1 Inflammasome. Oxid. Med. Cell. Longev. 2021, 2021, 2353504. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Shaish, A.; Barshack, I.; Polak-Charcon, S.; Afek, A.; Volkov, A.; Feldman, B.; Avivi, C.; Harats, D. Effects of hypoxia-inducible factor-1alpha overexpression in pregnant mice: Possible implications for preeclampsia and intrauterine growth restriction. Am. J. Pathol. 2010, 177, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Chuaiphichai, S.; Yu, G.Z.; Tan, C.M.J.; Whiteman, C.; Douglas, G.; Dickinson, Y.; Drydale, E.N.; Appari, M.; Zhang, W.; Crabtree, M.J.; et al. Endothelial GTPCH (GTP Cyclohydrolase 1) and Tetrahydrobiopterin Regulate Gestational Blood Pressure, Uteroplacental Remodeling, and Fetal Growth. Hypertension 2021, 78, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Chatre, L.; Ducat, A.; Spradley, F.T.; Palei, A.C.; Chereau, C.; Couderc, B.; Thomas, K.C.; Wilson, A.R.; Amaral, L.M.; Gaillard, I.; et al. Increased NOS coupling by the metabolite tetrahydrobiopterin (BH4) reduces preeclampsia/IUGR consequences. Redox. Biol. 2022, 55, 102406. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintye, D.; Sziva, R.E.; Mastyugin, M.; Török, M.; Jacas, S.; Lo, A.; Salahuddin, S.; Zsengellér, Z.K. Nitroxide—HMP—Protects Human Trophoblast HTR-8/SVneo Cells from H2O2-Induced Oxidative Stress by Reducing the HIF1A Signaling Pathway. Antioxidants 2023, 12, 1578. https://doi.org/10.3390/antiox12081578
Pintye D, Sziva RE, Mastyugin M, Török M, Jacas S, Lo A, Salahuddin S, Zsengellér ZK. Nitroxide—HMP—Protects Human Trophoblast HTR-8/SVneo Cells from H2O2-Induced Oxidative Stress by Reducing the HIF1A Signaling Pathway. Antioxidants. 2023; 12(8):1578. https://doi.org/10.3390/antiox12081578
Chicago/Turabian StylePintye, Diana, Réka Eszter Sziva, Maxim Mastyugin, Marianna Török, Sonako Jacas, Agnes Lo, Saira Salahuddin, and Zsuzsanna K. Zsengellér. 2023. "Nitroxide—HMP—Protects Human Trophoblast HTR-8/SVneo Cells from H2O2-Induced Oxidative Stress by Reducing the HIF1A Signaling Pathway" Antioxidants 12, no. 8: 1578. https://doi.org/10.3390/antiox12081578
APA StylePintye, D., Sziva, R. E., Mastyugin, M., Török, M., Jacas, S., Lo, A., Salahuddin, S., & Zsengellér, Z. K. (2023). Nitroxide—HMP—Protects Human Trophoblast HTR-8/SVneo Cells from H2O2-Induced Oxidative Stress by Reducing the HIF1A Signaling Pathway. Antioxidants, 12(8), 1578. https://doi.org/10.3390/antiox12081578





