Oxidative Model of Retinal Neurodegeneration Induced by Sodium Iodate: Morphofunctional Assessment of the Visual Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Housing
2.2. Anesthesia
2.3. Implantation of a Chronic Electrode
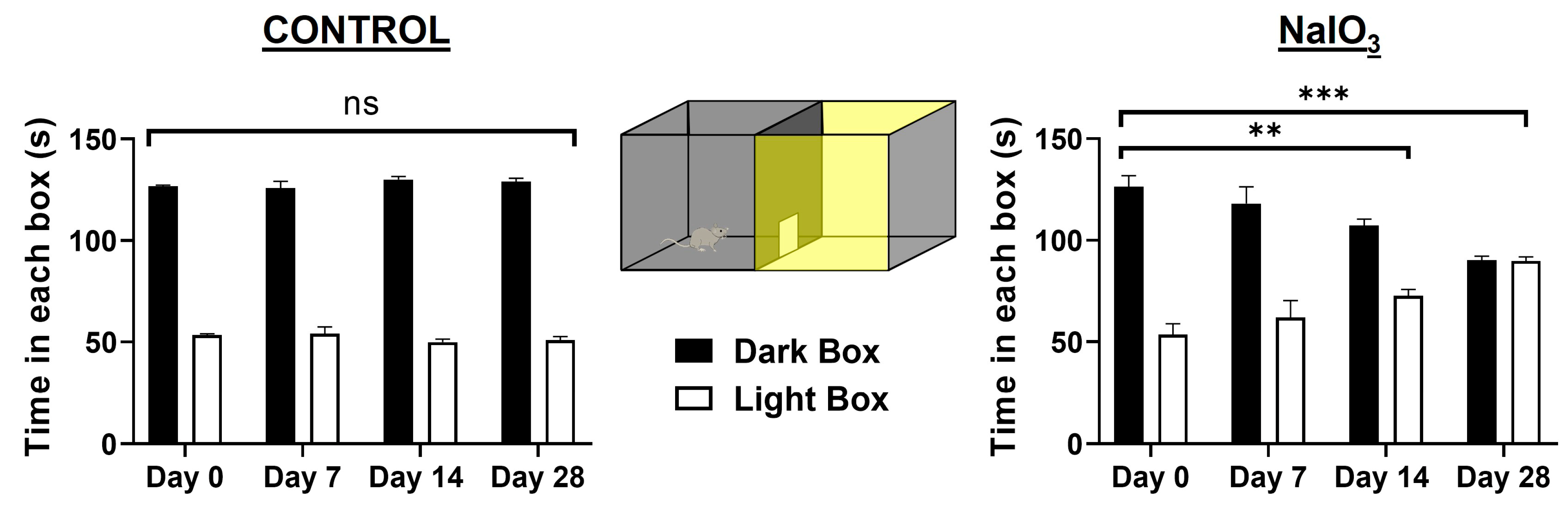
2.4. Light/Dark Transition Test
2.5. Optomotor Test
2.6. Pupillary Light Reflex (PLR)
2.7. Full-Field Electroretinogram (ERG)
2.8. Visual Evoked Potentials (VEP)
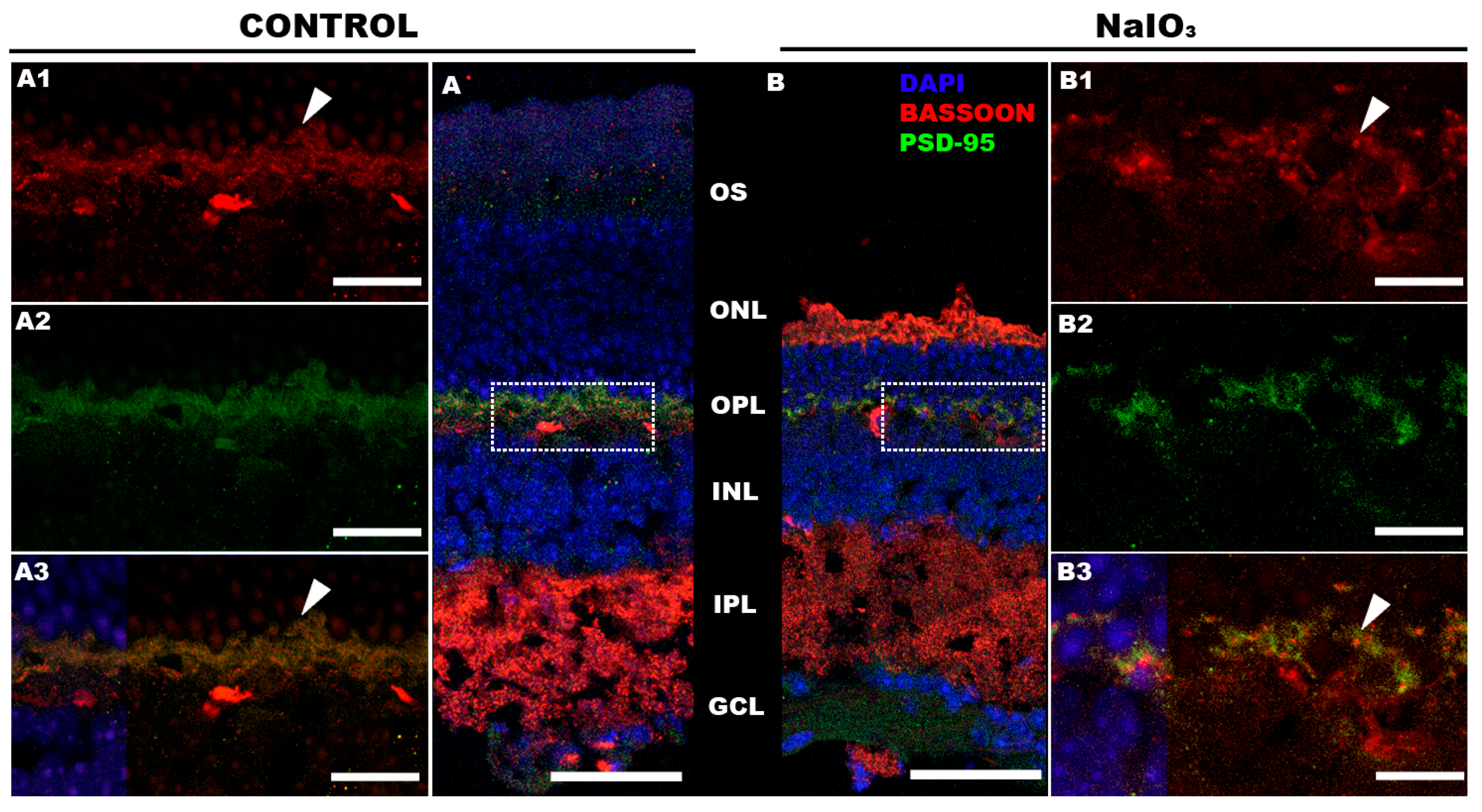
2.9. Inmunohistochemistry
2.10. Statistical Analysis
3. Results
3.1. Light/Dark Transition Test
3.2. Optomotor Test
3.3. Pupillary Light Reflex (PLR)
3.4. Full-Field Electroretinogram
3.5. Visual Evoked Potentials
3.6. Inmunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, B.; Hawes, N.L.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Heckenlively, J.R. Retinal degeneration mutants in the mouse. Vis. Res. 2002, 42, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Veleri, S.; Lazar, C.H.; Chang, B.; Sieving, P.A.; Banin, E.; Swaroop, A. Biology and therapy of inherited retinal degenerative disease: Insights from mouse models. Dis. Model. Mech. 2015, 8, 109–129. [Google Scholar] [CrossRef] [Green Version]
- Lucas, R.J.; Hattar, S.; Takao, M.; Berson, D.M.; Foster, R.G.; Yau, K.W. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 2003, 299, 245–247. [Google Scholar] [CrossRef] [Green Version]
- Barnard, A.R.; Hattar, S.; Hankins, M.W.; Lucas, R.J. Melanopsin regulates visual processing in the mouse retina. Curr. Biol. 2006, 16, 389–395. [Google Scholar] [CrossRef]
- Doyle, S.E.; Yoshikawa, T.; Hillson, H.; Menaker, M. Retinal pathways influence temporal niche. Proc. Natl. Acad. Sci. USA 2008, 105, 13133–13138. [Google Scholar] [CrossRef]
- Morin, L.P.; Studholme, K.M. Separation of function for classical and ganglion cell photoreceptors with respect to circadian rhythm entrainment and induction of photosomnolence. Neuroscience 2011, 199, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [Green Version]
- Ao, J.; Wood, J.P.; Chidlow, G.; Gillies, M.C.; Casson, R.J. Retinal pigment epithelium in the pathogenesis of age-related macular degeneration and photobiomodulation as a potential therapy? Clin. Exp. Ophthalmol. 2018, 46, 670–686. [Google Scholar] [CrossRef] [Green Version]
- Napoli, D.; Biagioni, M.; Billeri, F.; Di Marco, B.; Orsini, N.; Novelli, E.; Strettoi, E. Retinal Pigment Epithelium Remodeling in Mouse Models of Retinitis Pigmentosa. Int. J. Mol. Sci. 2021, 22, 5381. [Google Scholar] [CrossRef]
- Hu, M.L.; Edwards, T.L.; O’Hare, F.; Hickey, D.G.; Wang, J.H.; Liu, Z.; Ayton, L.N. Gene therapy for inherited retinal diseases: Progress and possibilities. Clin. Exp. Optom. 2021, 104, 444–454. [Google Scholar] [CrossRef]
- Monés, J.; Leiva, M.; Peña, T.; Martínez, G.; Biarnés, M.; Garcia, M.; Serrano, A.; Fernandez, E. A Swine Model of Selective Geographic Atrophy of Outer Retinal Layers Mimicking Atrophic AMD: A Phase I Escalating Dose of Subretinal Sodium Iodate. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3974–3983. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.; Lubiński, W.; Kłos, P.; Kawa, M.; Baumert, B.; Penkala, K.; Grzegrzółka, R.; Karczewicz, D.; Wiszniewska, B.; Machaliński, B.; et al. Sodium iodate selectively injuries the posterior pole of the retina in a dose-dependent manner: Morphological and electrophysiological study. Neurochem. Res. 2010, 35, 1819–1827. [Google Scholar]
- Redfern, W.S.; Storey, S.; Tse, K.; Hussain, Q.; Maung, K.P.; Valentin, J.P.; Ahmed, G.; Bigley, A.; Heathcote, D.; McKay, J.S.; et al. Evaluation of a convenient method of assessing rodent visual function in safety pharmacology studies: Effects of sodium iodate on visual acuity and retinal morphology in albino and pigmented rats and mice. J. Pharmacol. Toxicol. Methods 2011, 63, 102–114. [Google Scholar] [CrossRef]
- Yang, Y.; Ng, T.K.; Ye, C.; Yip, Y.W.; Law, K.; Chan, S.O.; Pang, C.P. Assessing sodium iodate-induced outer retinal changes in rats using confocal scanning laser ophthalmoscopy and optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1696–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Iacovelli, J.; Spencer, C.; Saint-Geniez, M. Direct effect of sodium iodate on neurosensory retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Kadkhodaeian, H.A.; Tiraihi, T.; Daftarian, N.; Ahmadieh, H.; Ziaei, H.; Taheri, T. Histological and, Electrophysiological Changes in the Retinal Pigment, Epithelium after Injection of Sodium Iodate in the Orbital Venus Plexus of Pigmented Rats. J. Ophthalmic. Vis. Res. 2016, 11, 70–77. [Google Scholar] [PubMed] [Green Version]
- Chowers, G.; Cohen, M.; Marks-Ohana, D.; Stika, S.; Eijzenberg, A.; Banin, E.; Obolensky, A. Course of, Sodium Iodate-Induced Retinal Degeneration in Albino and Pigmented Mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- Hanus, J.; Anderson, C.; Sarraf, D.; Ma, J.; Wang, S. Retinal pigment epithelial cell necroptosis in response to sodium iodate. Cell Death. Discov. 2016, 2, 16054. [Google Scholar] [CrossRef] [Green Version]
- Sorsby, A. Experimental Pigmentary Degeneration Of The Retina By Sodium Iodate. Br. J. Ophthalmol. 1941, 25, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Machalińska, A.; Lejkowska, R.; Duchnik, M.; Kawa, M.; Rogińska, D.; Wiszniewska, B.; Machaliński, B. Dose-dependent retinal changes following sodium iodate administration: Application of spectral-domain optical coherence tomography for monitoring of retinal injury and endogenous regeneration. Curr. Eye Res. 2014, 39, 1033–1041. [Google Scholar] [CrossRef]
- Chan, C.; Huang, D.; Sekar, P.; Hsu, S.; Lin, W. Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate-induced cell death. J. Biomed. Sci. 2019, 26, 40. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, M.; Nakamura, S.; Inoue, Y.; Nishinaka, A.; Nakamura, M.; Shimazawa, M.; Hara, H. Irreversible, Photoreceptors and RPE Cells Damage by Intravenous Sodium Iodate in Mice Is Related to Macrophage Accumulation. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3476–3487. [Google Scholar] [CrossRef] [Green Version]
- Kannan, R.; Hinton, D.R. Sodium iodate induced retinal degeneration: New insights from an old model. Neural Regen. Res. 2014, 9, 2044–2045. [Google Scholar] [PubMed]
- Enzmann, V.; Row, B.W.; Yamauchi, Y.; Kheirandish, L.; Gozal, D.; Kaplan, H.J.; McCall, M.A. Behavioral and anatomical abnormalities in a sodium iodate-induced model of retinal pigment epithelium degeneration. Exp. Eye Res. 2006, 82, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Reisenhofer, M.H.; Balmer, J.M.; Enzmann, V. What Can Pharmacological Models of Retinal Degeneration Tell Us? Curr. Mol. Med. 2017, 17, 100–107. [Google Scholar] [CrossRef]
- Enzbrenner, A.; Zulliger, R.; Biber, J.; Pousa, A.M.Q.; Schäfer, N.; Stucki, C.; Giroud, N.; Berrera, M.; Kortvely, E.; Schmucki, R.; et al. Sodium Iodate-Induced Degeneration Results in Local Complement Changes and Inflammatory Processes in Murine Retina. Int. J. Mol. Sci. 2021, 22, 9218. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W.; Shah, A.; Yu, M.; Liu, Y.; He, L.; Dang, J.; Yang, L.; Yan, M.; Ying, Y.; et al. Sodium iodate induces ferroptosis in human retinal pigment epithelium, ARPE-19 cells. Cell Death Dis. 2021, 12, 230. [Google Scholar] [CrossRef]
- Koh, A.E.; Alsaeedi, H.A.; Rashid, M.B.A.; Lam, C.; Harun, M.H.N.; Saleh, M.F.B.M.; Luu, C.D.; Kumar, S.S.; Ng, M.H.; Isa, H.M.; et al. Retinal degeneration rat model: A study on the structural and functional changes in the retina following injection of sodium iodate. J. Photochem. Photobiol. B 2019, 196, 111514. [Google Scholar] [CrossRef]
- Polosukhina, A.; Litt, J.; Tochitsky, I.; Nemargut, J.; Sychev, Y.; De Kouchkovsky, I.; Huang, T.; Borges, K.; Trauner, D.; Van Gelder, R.N.; et al. Photochemical restoration of visual responses in blind mice. Neuron 2012, 75, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Rovira, X.; Trapero, A.; Pittolo, S.; Zussy, C.; Faucherre, A.; Jopling, C.; Giraldo, J.; Pin, J.P.; Gorostiza, P.; Goudet, C.; et al. OptoGluNAM4.1, a Photoswitchable Allosteric Antagonist for Real-Time Control of mGlu4 Receptor Activity. Cell Chem. Biol. 2016, 23, 929–934. [Google Scholar] [CrossRef] [Green Version]
- Prischich, D.; Gomila, A.M.J.; Milla-Navarro, S.; Sangüesa, G.; Diez-Alarcia, R.; Preda, B.; Matera, C.; Batlle, M.; Ramírez, L.; Giralt, E.; et al. Adrenergic Modulation With Photochromic Ligands. Angew. Chem. Int. Ed. Engl. 2021, 60, 3625–3631. [Google Scholar] [CrossRef] [PubMed]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, S.; Nayak, S.K.; Campo, B.; Walker, J.R.; Hogenesch, J.B.; Jegla, T. Illumination of the melanopsin signaling pathway. Science 2005, 307, 600–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detwiler, P.B. Phototransduction in Retinal Ganglion Cells. Yale J. Biol. Med. 2018, 91, 49–52. [Google Scholar]
- Domenici, L.; Origlia, N.; Falsini, B.; Cerri, E.; Barloscio, D.; Fabiani, C.; Sansò, M.; Giovannini, L. Rescue of retinal function by BDNF in a mouse model of glaucoma. PLoS ONE 2014, 9, e115579. [Google Scholar] [CrossRef] [Green Version]
- Makowiecki, K.; Garrett, A.; Clark, V.; Graham, S.L.; Rodger, J. Reliability of, VEP Recordings Using Chronically Implanted Screw Electrodes in Mice. Transl. Vis. Sci. Technol. 2015, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Kunimi, H.; Miwa, Y.; Inoue, H.; Tsubota, K.; Kurihara, T. A Novel HIF Inhibitor Halofuginone, Prevents Neurodegeneration in a Murine Model of Retinal Ischemia-Reperfusion. Int. J. Mol. Sci. 2019, 20, 10. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Atorf, J.; Ramos, D.; Thiele, F.; Weber, S.; Dalke, C.; Sun, M.; Puk, O.; Michel, D.; Fuchs, H.; et al. German Mouse Clinic Consortium. Mutation in Bmpr1b Leads to Optic Disc Coloboma and Ventral Retinal Gliosis in Mice. Investig. Ophthalmol. Vis. Sci. 2020, 61, 44. [Google Scholar] [CrossRef] [Green Version]
- Bourin, M.; Hascoët, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Milla-Navarro, S.; Pazo-González, M.; Germain, F.; de la Villa, P. Phenotype Characterization of a Mice Genetic Model of Absolute Blindness. Int. J. Mol. Sci. 2022, 23, 8152. [Google Scholar] [CrossRef]
- Kiuchi, K.; Yoshizawa, K.; Shikata, N.; Moriguchi, K.; Tsubura, A. Morphologic characteristics of retinal degeneration induced by sodium iodate in mice. Curr. Eye Res. 2002, 25, 373–379. [Google Scholar] [CrossRef]
- Tanaka, M.; Machida, S.; Ohtaka, K.; Tazawa, Y.; Nitta, J. Third-order neuronal responses contribute to shaping the negative electroretinogram in sodium iodate-treated rats. Curr. Eye Res. 2005, 30, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.J.; Seo, J.M.; Yu, H.G.; Chung, H. Monocular retinal degeneration induced by intravitreal injection of sodium iodate in rabbit eyes. Jpn. J. Ophthalmol. 2016, 60, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Shen, H.; Yuan, S.; Lu, G.; Zhang, Y.; Wang, H.; Zhao, Y.; Sun, X.; Liu, Q. Combined Transplantation With Human Mesenchymal Stem Cells Improves Retinal Rescue Effect of Human Fetal RPE Cells in Retinal Degeneration Mouse Model. Investig. Ophthalmol. Vis. Sci. 2020, 61, 9. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.; Girardot, P.E.; Chrenek, M.A.; Sellers, J.T.; Li, Y.; Kim, Y.K.; Summers, V.R.; Ferdous, S.; Shelton, D.A.; et al. Electrophysiologic and Morphologic, Strain Differences in a Low-Dose NaIO3-Induced Retinal Pigment Epithelium Damage Model. Transl. Vis. Sci. Technol. 2021, 10, 10. [Google Scholar] [CrossRef]
- Mitrousis, N.; Hacibekiroglu, S.; Ho, M.T.; Sauvé, Y.; Nagy, A.; van der Kooy, D.; Shoichet, M.S. Hydrogel-mediated co-transplantation of retinal pigmented epithelium and photoreceptors restores vision in an animal model of advanced retinal degeneration. Biomaterials 2020, 257, 120233. [Google Scholar] [CrossRef]
- Franco, L.M.; Zulliger, R.; Wolf-Schnurrbusch, U.E.; Katagiri, Y.; Kaplan, H.J.; Wolf, S.; Enzmann, V. Decreased visual function after patchy loss of retinal pigment epithelium induced by low-dose sodium iodate. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4004–4010. [Google Scholar] [CrossRef] [Green Version]
- Carido, M.; Zhu, Y.; Postel, K.; Benkner, B.; Cimalla, P.; Karl, M.O.; Kurth, T.; Paquet-Durand, F.; Koch, E.; Münch, T.A.; et al. Characterization of a mouse model with complete, RPE loss and its use for, RPE cell transplantation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5431–5444. [Google Scholar] [CrossRef] [Green Version]
- Güler, A.D.; Ecker, J.L.; Lall, G.S.; Haq, S.; Altimus, C.M.; Liao, H.W.; Barnard, A.R.; Cahill, H.; Badea, T.C.; Zhao, H.; et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 2008, 453, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Brozou, C.; Fotiou, D.; Androudi, S.; Theodoridou, E.; Giantselidis, C.; Alexandridis, A.; Brazitikos, P. Pupillometric characteristics in patients with choroidal neovascularization due to age-related macular degeneration. Eur. J. Ophthalmol. 2009, 19, 254–262. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Aleman, T.S.; Cideciyan, A.V.; Sumaroka, A.; Schwartz, S.B.; Windsor, E.A.; Swider, M.; Herrera, W.; Stone, E.M. Leber congenital amaurosis caused by, Lebercilin (LCA5) mutation: Retained photoreceptors adjacent to retinal disorganization. Mol. Vis. 2009, 15, 1098–1106. [Google Scholar] [PubMed]
- Richter, P.; Wilhelm, H.; Peters, T.; Luedtke, H.; Kurtenbach, A.; Jaegle, H.; Wilhelm, B. The diagnostic accuracy of chromatic pupillary light responses in diseases of the outer and inner retina. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 519–527. [Google Scholar] [CrossRef]
- He, Y.; Tang, H.; Wang, G.; Ren, B.; Wang, Y.; Liu, Y. Correlation between Transient Pupillary Light Reflex and Retinal Function Impairment in Patients with Retinitis Pigmentosa. J. Ophthalmol. 2018, 2018, 2519375. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, L.; Adachi-Usami, E.; Mizota, A.; Hanawa, T.; Kimura, T. Early effects of sodium iodate injection on ERG in mice. Acta. Ophthalmol. 1993, 71, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Adachi-Usami, E.; Saeki, M.; Takeda, N.; Kimura, T. Electrophysiological studies on light damage in the mouse retina after sodium iodate injection. Ophthalmologica 1994, 208, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, R.; Xie, J.; Hu, J.; Huang, X.; Ren, F.; Li, L. Protective Effect of Hydrogen on Sodium Iodate-Induced Age-Related Macular Degeneration in Mice. Front Aging Neurosci. 2018, 10, 389. [Google Scholar] [CrossRef]
- Yamashita, H.; Yamasaki, K.; Sugihara, K.; Miyata, H.; Tsutsumi, S.; Iwaki, Y. Full-field electroretinography obtained using a contact lens electrode with built-in high-intensity white-light-emitting diodes can be utilized in toxicological assessments in rats. Ophthalmic. Res. 2009, 42, 15–20. [Google Scholar] [CrossRef]
- Strain, G.M.; Tedford, B.L. Flash and pattern reversal visual evoked potentials in C57BL/6J and B6CBAF1/J mice. Brain Res. Bull. 1993, 32, 57–63. [Google Scholar] [CrossRef]
- Ridder, W.H.; 3rd Nusinowitz, S. The visual evoked potential in the mouse--origins and response characteristics. Vis. Res. 2006, 46, 902–913. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Sugimoto, S.; Chiba, S. Effects of sodium iodate, iodoacetic acid and ethambutol on electroretinogram and visual evoked potential in rats. J. Toxicol. Sci. 1984, 9, 389–399. [Google Scholar] [CrossRef]
- Sabel, B.A.; Flammer, J.; Merabet, L.B. Residual vision activation and the brain-eye-vascular triad: Dysregulation, plasticity and restoration in low vision and blindness—A review. Restor. Neurol. Neurosci. 2018, 36, 767–791. [Google Scholar]
- Salinas-Navarro, M.; Jiménez-López, M.; Valiente-Soriano, F.J.; Alarcón-Martínez, L.; Avilés-Trigueros, M.; Mayor, S.; Holmes, T.; Lund, R.D.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; et al. Retinal ganglion cell population in adult albino and pigmented mice: A computerized analysis of the entire population and its spatial distribution. Vis. Res. 2009, 49, 637–647. [Google Scholar] [CrossRef] [Green Version]
- Salinas-Navarro, M.; Mayor-Torroglosa, S.; Jiménez-López, M.; Avilés-Trigueros, M.; Holmes, T.M.; Lund, R.D.; Villegas-Pérez, M.P.; Vidal-Sanz, M. A computerized analysis of the entire retinal ganglion cell population and its spatial distribution in adult rats. Vis. Res. 2009, 49, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Balmer, J.; Zulliger, R.; Roberti, S.; Enzmann, V. Retinal Cell Death Caused by Sodium Iodate Involves Multiple Caspase-Dependent and Caspase-Independent Cell-Death Pathways. Int. J. Mol. Sci. 2015, 16, 15086–15103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Yao, J.; Jia, L.; Lin, C.; Zacks, D.N. Protective Effect of Met12, a Small Peptide Inhibitor of Fas, on the Retinal Pigment Epithelium and Photoreceptor After Sodium Iodate Injury. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1801–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Yu, M.; Wang, Y.; Peng, Y.; Li, X.; Lam, D.M.; Chen, X.; Liu, X. Non-mitogenic human acidic fibroblast growth factor reduces retinal degeneration induced by sodium iodate. J. Ocul. Pharmacol. Ther. 2009, 25, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Nam, S.M.; Chang, B.J.; Nahm, S.S.; Lee, J.H. Ultrastructural Changes and Expression of PCNA and RPE65 in Sodium Iodate-Induced Acute, Retinal Pigment Epithelium Degeneration Model. Neurochem. Res. 2018, 43, 1010–1019. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Wang, C.; Zhang, Y.; Su, G. Morphologic and histopathologic change of sodium iodate-induced retinal degeneration in adult rats. Int. J. Clin. Exp. Pathol. 2019, 12, 443–454. [Google Scholar] [PubMed]
- Hariri, S.; Tam, M.C.; Lee, D.; Hileeto, D.; Moayed, A.A.; Bizheva, K. Noninvasive imaging of the early effect of sodium iodate toxicity in a rat model of outer retina degeneration with spectral domain optical coherence tomography. J. Biomed. Opt. 2013, 18, 26017. [Google Scholar] [CrossRef]
- Henkind, P.; Gartner, S. The relationship between retinal pigment epithelium and the choriocapillaris. Trans. Ophthalmol. Soc. UK 1983, 103, 444–447. [Google Scholar]
- Korte, G.E.; Reppucci, V.; Henkind, P. RPE destruction causes choriocapillary atrophy. Investig. Ophthalmol. Vis. Sci. 1984, 25, 1135–1145. [Google Scholar]
- Kohno, T.; Miki, T.; Shiraki, K.; Moriwaki, M. Indocyanine green angiography in choriocapillary atrophy induced by sodium iodate. Graefes Arch. Clin. Exp. Ophthalmol. 1995, 233, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.M.; Cano, M.; Handa, J.T. Nrf2 signaling is impaired in the aging, RPE given an oxidative insult. Exp. Eye Res. 2014, 119, 111–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of Kaempferol on Oxidative Stress-Induced Retinal Pigment Epithelial Cell Damage. Oxid. Med. Cell Longev. 2018, 2018, 1610751. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Lin, T.B.; Peng, H.Y.; Liu, H.J.; Lee, A.S.; Lin, C.H.; Tseng, K.W. Cytoprotective, Potential of Fucoxanthin in Oxidative Stress-Induced Age-Related Macular Degeneration and Retinal Pigment Epithelial Cell Senescence In Vivo and In Vitro. Mar. Drugs 2021, 19, 114. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, Y.; Gao, C.; Fariss, R.N.; Tam, J.; Wong, W.T. Monocyte infiltration and proliferation reestablish myeloid cell homeostasis in the mouse retina following retinal pigment epithelial cell injury. Sci. Rep. 2017, 7, 8433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zieger, M.; Punzo, C. Improved cell metabolism prolongs photoreceptor survival upon retinal-pigmented epithelium loss in the sodium iodate induced model of geographic atrophy. Oncotarget 2016, 7, 9620–9633. [Google Scholar] [CrossRef] [Green Version]






| (A) Primary Antibodies Table | ||||||
| Primary Antibodies | Type | Host | Concentration | Provider | Labeling | RRID Number |
| Anti-Cone Arrestin | Polyclonal | Rabbit | 1:10,000 | Merk-Millipore | Cones | AB_1163387 |
| Anti Rhodopsin | Monoclonal | Mouse | 1:200 | Merk-Millipore | Rod Outer Segments | AB_2178961 |
| Anti-PKCα | Polyclonal | Rabbit | 1:1000 | SIGMA | Bipolar Cells | AB_477345 |
| Anti-Brn3 | Polyclonal | Goat | 1:200 | Quimigen (Santa Cruz) | Retinal Ganglion Cells | AB_2167511 |
| Anti-Bassoon | Monoclonal | Mouse | 1:400 | BIONOVA | Photoreceptors Presynaptic Zone | AB_2313990 |
| Anti-PSD-95 | Polyclonal | Rabbit | 1:200 | Abcam | PSD-95 | AB_444362 |
| (B) Secondary Antibodies Table | ||||||
| Secondary Antibodies | Fluorochrome | Host | Concentration | Provider | Color | RRID Number |
| Goat Anti-IgG | CyTM2 | Donkey | 1:200 | VITRO (Jackson) | Green | AB_2307341 |
| Mouse Anti-IgG | CyTM2 | Chicken | 1:700 | VITRO (Jackson) | Green | AB_2535786 |
| Rabbit Anti-IgG | CyTM3 | Donkey | 1:200 | VITRO (Jackson) | Red | AB_2307443 |
| Nucleus | Retinal Thickness | Thickness Ratio | |||||
|---|---|---|---|---|---|---|---|
| ONL | INL | Total Thickness | ONL Thickness | INL Thickness | ONL/Total | INL/Total | |
| Control (n = 3) | 10.00 (0.62) | 5.53 (0.30) | 170.42 (13.10) | 55.17 (5.02) | 41.88 (5.74) | 0.324 (0.018) | 0.245 (0.019) |
| NaIO3 (n = 6) | 4.67 (1.20) | 5.22 (0.38) | 136.77 (3.39) | 33.20 (4.93) | 37.18 (1.23) | 0.243 (0.042) | 0.272 (0.002) |
| p-value | <0.001 (***) | 0.224 (ns) | 0.005 (**) | <0.001 (***) | 0.224 (ns) | 0.008 (**) | 0.066 (ns) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espitia-Arias, M.D.; de la Villa, P.; Paleo-García, V.; Germain, F.; Milla-Navarro, S. Oxidative Model of Retinal Neurodegeneration Induced by Sodium Iodate: Morphofunctional Assessment of the Visual Pathway. Antioxidants 2023, 12, 1594. https://doi.org/10.3390/antiox12081594
Espitia-Arias MD, de la Villa P, Paleo-García V, Germain F, Milla-Navarro S. Oxidative Model of Retinal Neurodegeneration Induced by Sodium Iodate: Morphofunctional Assessment of the Visual Pathway. Antioxidants. 2023; 12(8):1594. https://doi.org/10.3390/antiox12081594
Chicago/Turabian StyleEspitia-Arias, Michael D., Pedro de la Villa, Victor Paleo-García, Francisco Germain, and Santiago Milla-Navarro. 2023. "Oxidative Model of Retinal Neurodegeneration Induced by Sodium Iodate: Morphofunctional Assessment of the Visual Pathway" Antioxidants 12, no. 8: 1594. https://doi.org/10.3390/antiox12081594
APA StyleEspitia-Arias, M. D., de la Villa, P., Paleo-García, V., Germain, F., & Milla-Navarro, S. (2023). Oxidative Model of Retinal Neurodegeneration Induced by Sodium Iodate: Morphofunctional Assessment of the Visual Pathway. Antioxidants, 12(8), 1594. https://doi.org/10.3390/antiox12081594








