Natural Products in Renal-Associated Drug Discovery
Abstract
:1. Introduction
2. Plant-Based Extracts with Antioxidant and Anti-Inflammatory Properties
3. Plant Sources and Activity
4. Active Ingredients
5. Meta-Analysis of Transcriptome Datasets Related to Natural Products
5.1. Comparison and Functional Annotation of Differentially Regulated Genes
5.2. Construction of a Gene Regulatory Network
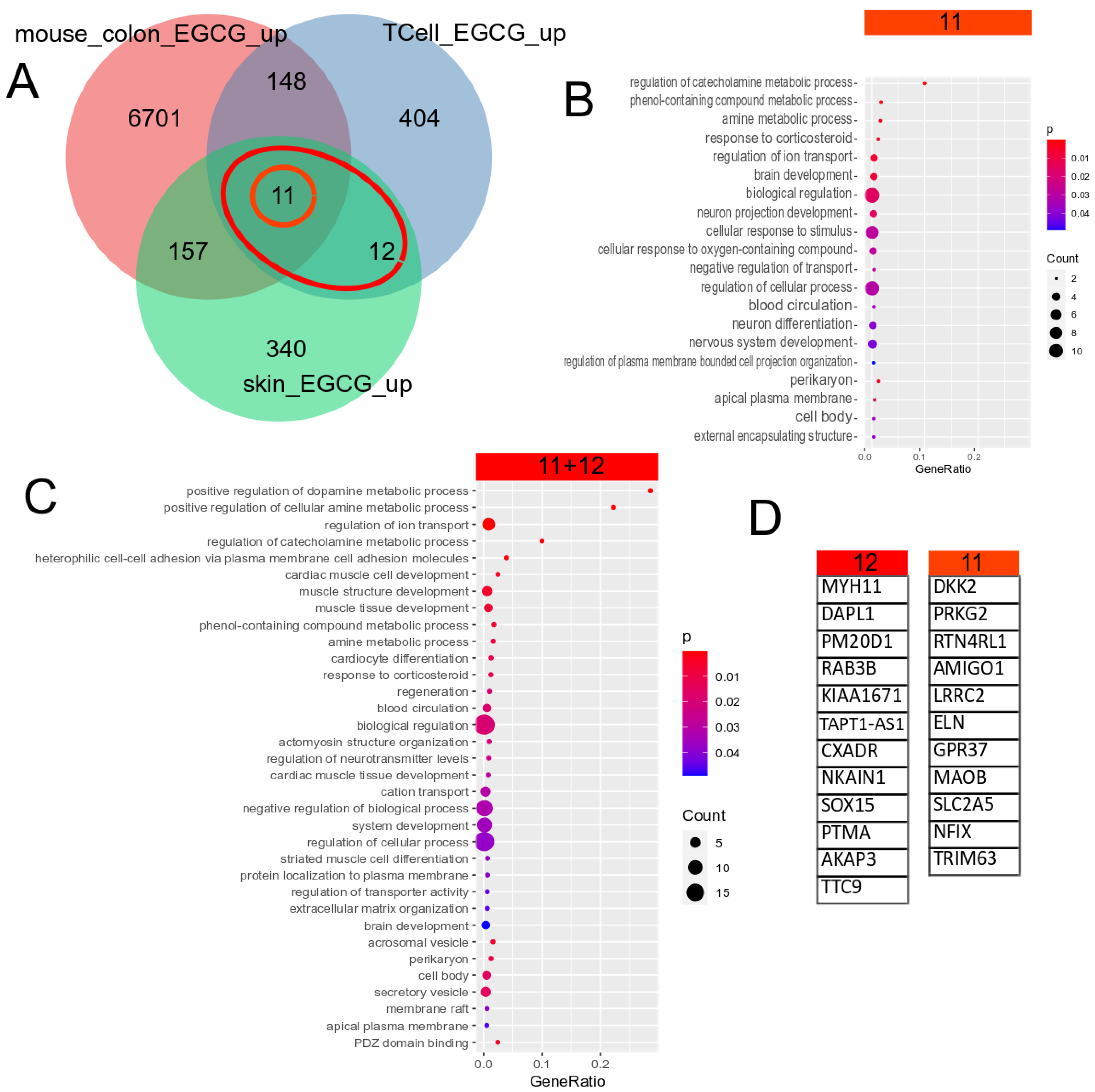
5.3. EGCG Upregulated Gene Signature
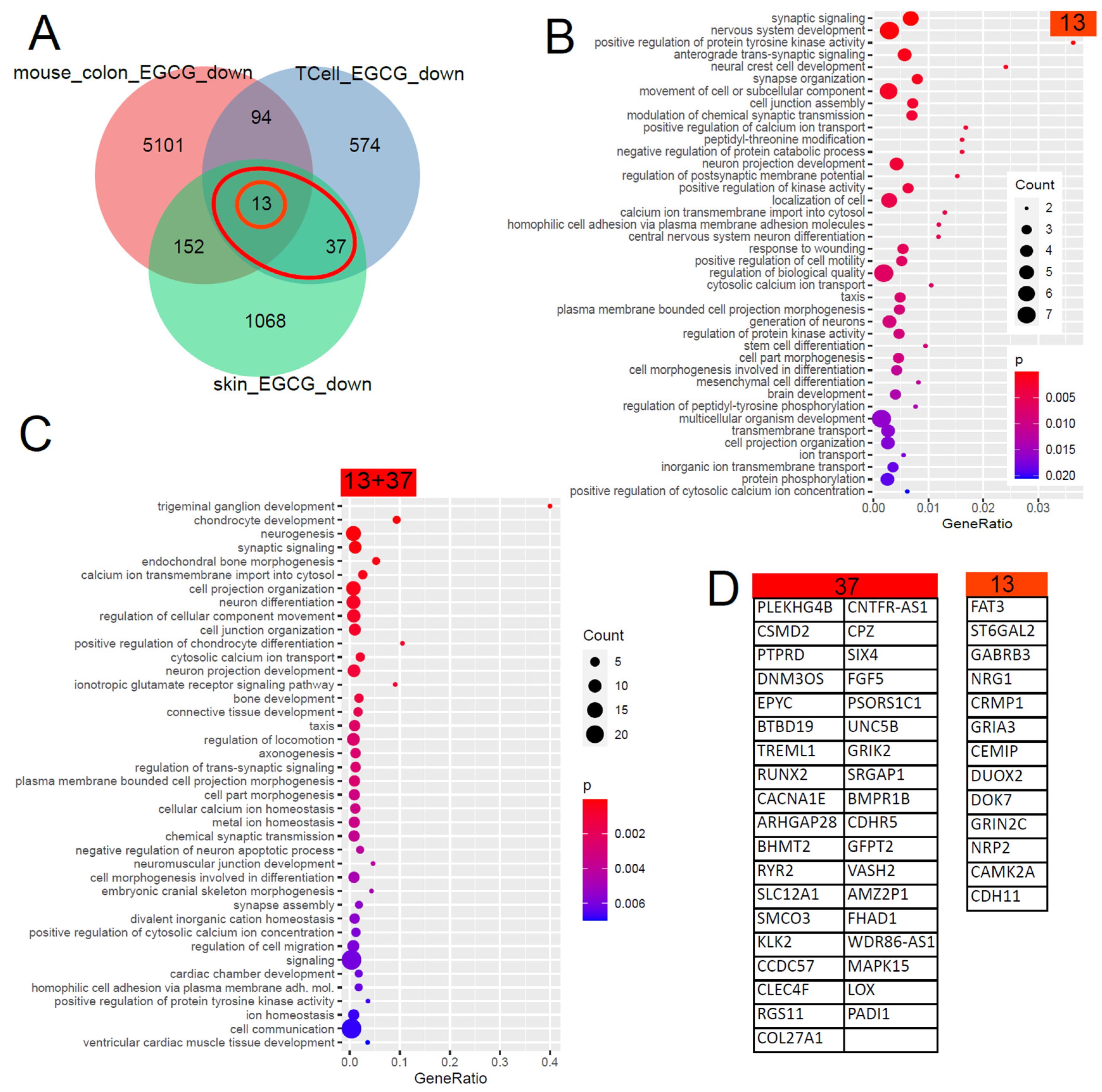
5.4. Gene Signature Downregulated by EGCG
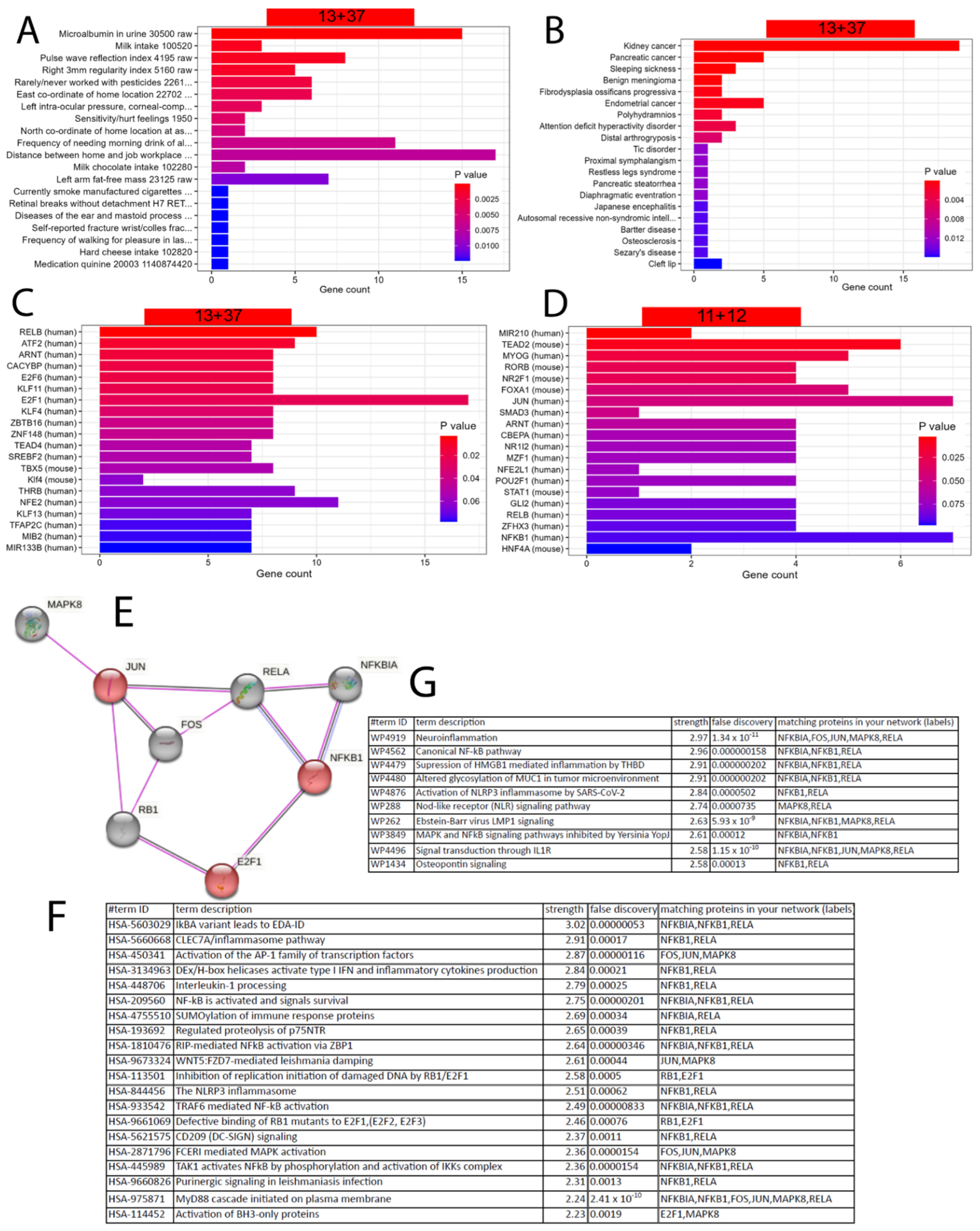
5.5. Refined Characterisation and Transcription Factor Analysis of the EGCG Gene Signatures
5.6. Transfer of EGCG-Associated Gene Signatures to Kidney Datasets
| GEO_Search | Accession | Cell Type | Treatment | PMID/DOI |
|---|---|---|---|---|
| (Camellia) AND “Homo sapiens” | GSE152781 | human skin | EGCG (green tea) | PMID: 33917842 [73] |
| green tea AND kidney | GSE41644 | Mouse colon | EGCG (green tea) | PMID: 23643524 [75] |
| human T cells EGCG | GSE53448 | human CD4+CD25− T cells | EGCG (green tea) | PMID: 24476360 [74] |
| kidney AND “natural products” | GSE198890 | HEK cells | VT01454 | https://doi.org/10.1038/s41589-022-01061-z (accessed on 1 June 2023) [84] |
| kidney AND “natural products” | GSE159656 | mouse kidney | Ganoderma lucidum | PMID: 33374283 [85] |
5.7. Comparison of Ganoderma Lucidum-Associated Gene Signatures to Datasets of Kidney Disease Models
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute Kidney Injury and Chronic Kidney Disease as Interconnected Syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J. Chronic Kidney Disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G.; et al. National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Wruck, W.; Boima, V.; Erichsen, L.; Thimm, C.; Koranteng, T.; Kwakyi, E.; Antwi, S.; Adu, D.; Adjaye, J. Urine-Based Detection of Biomarkers Indicative of Chronic Kidney Disease in a Patient Cohort from Ghana. J. Pers. Med. 2022, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Bazzi, C. Pathophysiology of Proteinuria. Kidney Int. 2003, 63, 809–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schena, F.P.; Gesualdo, L. Pathogenetic Mechanisms of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2005, 16, S30–S33. [Google Scholar] [CrossRef] [Green Version]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Al-Jaghbeer, M.; Dealmeida, D.; Bilderback, A.; Ambrosino, R.; Kellum, J.A. Clinical Decision Support for In-Hospital AKI. J. Am. Soc. Nephrol. JASN 2018, 29, 654–660. [Google Scholar] [CrossRef] [Green Version]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute Kidney Injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar]
- Erichsen, L.; Thimm, C.; Wruck, W.; Kaierle, D.; Schless, M.; Huthmann, L.; Dimski, T.; Kindgen-Milles, D.; Brandenburger, T.; Adjaye, J. Secreted Cytokines within the Urine of AKI Patients Modulate TP53 and SIRT1 Levels in a Human Podocyte Cell Model. Int. J. Mol. Sci. 2023, 24, 8228. [Google Scholar] [CrossRef]
- Wu, L.; Gokden, N.; Mayeux, P.R. Evidence for the Role of Reactive Nitrogen Species in Polymicrobial Sepsis-Induced Renal Peritubular Capillary Dysfunction and Tubular Injury. J. Am. Soc. Nephrol. 2007, 18, 1807–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonventre, J.V.; Zuk, A. Ischemic Acute Renal Failure: An Inflammatory Disease? Kidney Int. 2004, 66, 480–485. [Google Scholar] [CrossRef] [Green Version]
- Nath, K.A.; Norby, S.M. Reactive Oxygen Species and Acute Renal Failure. Am. J. Med. 2000, 109, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, J.; Chen, X.; Xie, J.; Yang, Q.; Wang, J.; Deng, X. Senkyunolide I Alleviates Renal Ischemia-Reperfusion Injury by Inhibiting Oxidative Stress, Endoplasmic Reticulum Stress and Apoptosis. Int. Immunopharmacol. 2022, 102, 108393. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Tanaka, T.; Yoshida, Y.; Inagi, R. Physiological and Pathophysiological Role of Reactive Oxygen Species and Reactive Nitrogen Species in the Kidney. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1097–1105. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Lei, C.-T.; Zhang, C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Magno, A.; Herat, L.; Carnagarin, R.; Schlaich, M.; Matthews, V. Current Knowledge of IL-6 Cytokine Family Members in Acute and Chronic Kidney Disease. Biomedicines 2019, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Bonventre, J.V.; Yang, L. Cellular Pathophysiology of Ischemic Acute Kidney Injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chang, A.; Hack, B.K.; Eadon, M.T.; Alper, S.L.; Cunningham, P.N. TNF-Mediated Damage to Glomerular Endothelium Is an Important Determinant of Acute Kidney Injury in Sepsis. Kidney Int. 2014, 85, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, M.A.; Vaidya, V.S.; Bonventre, J.V. Biomarkers of Nephrotoxic Acute Kidney Injury. Toxicology 2008, 245, 182–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinke, H.; Masuda, S.; Togashi, Y.; Ikemi, Y.; Ozawa, A.; Sato, T.; Kim, Y.H.; Mishima, M.; Ichimura, T.; Bonventre, J.V.; et al. Urinary Kidney Injury Molecule-1 and Monocyte Chemotactic Protein-1 Are Noninvasive Biomarkers of Cisplatin-Induced Nephrotoxicity in Lung Cancer Patients. Cancer Chemother. Pharmacol. 2015, 76, 989–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Vaidya, V.S.; Brown, R.P.; Zhang, J.; Rosenzweig, B.A.; Thompson, K.L.; Miller, T.J.; Bonventre, J.V.; Goering, P.L. Comparison of Kidney Injury Molecule-1 and Other Nephrotoxicity Biomarkers in Urine and Kidney Following Acute Exposure to Gentamicin, Mercury, and Chromium. Toxicol. Sci. 2008, 101, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Choucry, M.A.; Khalil, M.N.A.; El Awdan, S.A. Protective Action of Crateva Nurvala Buch. Ham Extracts against Renal Ischaemia Reperfusion Injury in Rats via Antioxidant and Anti-Inflammatory Activities. J. Ethnopharmacol. 2018, 214, 47–57. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, B.; Li, Y.; Zhang, H. Protective Effect of Luteolin Against Renal Ischemia/Reperfusion Injury via Modulation of Pro-Inflammatory Cytokines, Oxidative Stress and Apoptosis for Possible Benefit in Kidney Transplant. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 5720–5727. [Google Scholar] [CrossRef] [Green Version]
- Punuru, P.; Sujatha, D.; Kumari, B.P.; Charisma, V.V.L. Evaluation of Aqueous Extract of Murraya Koenigii in Unilateral Renal Ischemia Reperfusion Injury in Rats. Indian J. Pharmacol. 2014, 46, 171–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, C.; Yang, J.; Yang, H.; Li, X.; Wang, G. Attenuation of Renal Ischemia/Reperfusion Injury by Oleanolic Acid Preconditioning via Its Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities. Mol. Med. Rep. 2016, 13, 4697–4704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Q.; Liu, H.; Wang, J. Polydatin Ameliorates Renal Ischemia/Reperfusion Injury by Decreasing Apoptosis and Oxidative Stress through Activating Sonic Hedgehog Signaling Pathway. Food Chem. Toxicol. 2016, 96, 215–225. [Google Scholar] [CrossRef]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Mol. Basel Switz. 2022, 27, 7823. [Google Scholar] [CrossRef]
- Kang, H.G.; Lee, H.K.; Cho, K.B.; Park, S.I. A Review of Natural Products for Prevention of Acute Kidney Injury. Medicina (Mex.) 2021, 57, 1266. [Google Scholar] [CrossRef]
- Mestry, S.N.; Gawali, N.B.; Pai, S.A.; Gursahani, M.S.; Dhodi, J.B.; Munshi, R.; Juvekar, A.R. Punica Granatum Improves Renal Function in Gentamicin-Induced Nephropathy in Rats via Attenuation of Oxidative Stress. J. Ayurveda Integr. Med. 2020, 11, 16–23. [Google Scholar] [CrossRef]
- El Bohi, K.M.; Abdel-Motal, S.M.; Khalil, S.R.; Abd-Elaal, M.M.; Metwally, M.M.M.; ELhady, W.M. The Efficiency of Pomegranate (Punica granatum) Peel Ethanolic Extract in Attenuating the Vancomycin-Triggered Liver and Kidney Tissues Injury in Rats. Environ. Sci. Pollut. Res. 2021, 28, 7134–7150. [Google Scholar] [CrossRef] [PubMed]
- Nerdy, N.; Ritarwan, K. Hepatoprotective Activity and Nephroprotective Activity of Peel Extract from Three Varieties of the Passion Fruit (Passiflora sp.) in the Albino Rat. Open Access Maced. J. Med. Sci. 2019, 7, 536–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Yousef, H.M.; Alqahtani, A.S.; Ghani, A.S.A.; El-Toumy, S.A.; El-Dougdoug, W.I.A.; Hassan, W.H.B.; Hassan, H.M. Nephroprotective, Cytotoxic and Antioxidant Activities of Euphorbia Paralias. Saudi J. Biol. Sci. 2021, 28, 785–792. [Google Scholar] [CrossRef]
- Heidarian, E.; Jafari-Dehkordi, E.; Valipour, P.; Ghatreh-Samani, K.; Ashrafi-Eshkaftaki, L. Nephroprotective and Anti-Inflammatory Effects of Pistacia atlantica Leaf Hydroethanolic Extract Against Gentamicin-Induced Nephrotoxicity in Rats. J. Diet. Suppl. 2017, 14, 489–502. [Google Scholar] [CrossRef]
- Chinnappan, S.M.; George, A.; Thaggikuppe, P.; Choudhary, Y.; Choudhary, V.K.; Ramani, Y.; Dewangan, R. Nephroprotective Effect of Herbal Extract Eurycoma longifolia on Paracetamol-Induced Nephrotoxicity in Rats. Evid. Based Complement. Alternat. Med. 2019, 2019, 4916519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anyasor, G.N.; Onajobi, F.D.; Osilesi, O.; Adebawo, O. Proximate Composition, Mineral Content and in Vitro Antioxidant Activity of Leaf and Stem of Costus afer (Ginger Lily). J. Intercult. Ethnopharmacol. 2014, 3, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ezejiofor, A.N.; Udowelle, N.A.; Orisakwe, O.E. Nephroprotective and Antioxidant Effect of Aqueous Leaf Extract of Costus Afer Ker Gawl on Cyclosporin-a (Csa) Induced Nephrotoxicity. Clin. Phytoscience 2017, 2, 11. [Google Scholar] [CrossRef] [Green Version]
- Anyanwu, B.O.; Orish, C.N.; Ezejiofor, A.N.; Nwaogazie, I.L.; Orisakwe, O.E. Neuroprotective Effect of Costus Afer on Low Dose Heavy Metal Mixture (Lead, Cadmium and Mercury) Induced Neurotoxicity via Antioxidant, Anti-Inflammatory Activities. Toxicol. Rep. 2020, 7, 1032–1038. [Google Scholar] [CrossRef]
- Genfi, A.K.A.; Larbie, C.; Emikpe, B.O.; Oyagbemi, A.A.; Firempong, C.K.; Adjei, C.O. Modulation of Oxidative Stress and Inflammatory Cytokines as Therapeutic Mechanisms of Ocimum Americanum L Extract in Carbon Tetrachloride and Acetaminophen-Induced Toxicity in Rats. J. Evid.-Based Integr. Med. 2020, 25, 2515690X2093800. [Google Scholar] [CrossRef]
- Nyarko, A.K.; Asare-Anane, H.; Ofosuhene, M.; Addy, M.E. Extract of Ocimum Canum Lowers Blood Glucose and Facilitates Insulin Release by Isolated Pancreatic β-Islet Cells. Phytomedicine 2002, 9, 346–351. [Google Scholar] [CrossRef]
- Ranfaing, J.; Dunyach-Remy, C.; Louis, L.; Lavigne, J.-P.; Sotto, A. Propolis Potentiates the Effect of Cranberry (Vaccinium macrocarpon) against the Virulence of Uropathogenic Escherichia Coli. Sci. Rep. 2018, 8, 10706. [Google Scholar] [CrossRef] [Green Version]
- Amin, R.; Thalluri, C.; Docea, A.O.; Sharifi-Rad, J.; Calina, D. Therapeutic Potential of Cranberry for Kidney Health and Diseases. eFood 2022, 3, e33. [Google Scholar] [CrossRef]
- Csikós, E.; Horváth, A.; Ács, K.; Papp, N.; Balázs, V.L.; Dolenc, M.S.; Kenda, M.; Kočevar Glavač, N.; Nagy, M.; Protti, M.; et al. Treatment of Benign Prostatic Hyperplasia by Natural Drugs. Molecules 2021, 26, 7141. [Google Scholar] [CrossRef]
- Carneiro, D.M.; Freire, R.C.; Honório, T.C.D.D.; Zoghaib, I.; Cardoso, F.F.D.S.E.S.; Tresvenzol, L.M.F.; De Paula, J.R.; Sousa, A.L.L.; Jardim, P.C.B.V.; Cunha, L.C.D. Randomized, Double-Blind Clinical Trial to Assess the Acute Diuretic Effect of Equisetum arvense (Field Horsetail) in Healthy Volunteers. Evid. Based Complement. Alternat. Med. 2014, 2014, 760683. [Google Scholar] [CrossRef] [Green Version]
- Pallag, A.; Filip, G.A.; Olteanu, D.; Clichici, S.; Baldea, I.; Jurca, T.; Micle, O.; Vicaş, L.; Marian, E.; Soriţău, O.; et al. Equisetum arvense L. Extract Induces Antibacterial Activity and Modulates Oxidative Stress, Inflammation, and Apoptosis in Endothelial Vascular Cells Exposed to Hyperosmotic Stress. Oxid. Med. Cell. Longev. 2018, 2018, 3060525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alabi, T.D.; Brooks, N.L.; Oguntibeju, O.O. Leaf Extracts of Anchomanes Difformis Ameliorated Kidney and Pancreatic Damage in Type 2 Diabetes. Plants 2021, 10, 300. [Google Scholar] [CrossRef]
- Rodríguez-Fierros, F.L.; Guarner-Lans, V.; Soto, M.E.; Manzano-Pech, L.; Díaz-Díaz, E.; Soria-Castro, E.; Rubio-Ruiz, M.E.; Jiménez-Trejo, F.; Pérez-Torres, I. Modulation of Renal Function in a Metabolic Syndrome Rat Model by Antioxidants in Hibiscus sabdariffa L. Molecules 2021, 26, 2074. [Google Scholar] [CrossRef]
- Benzer, F.; Kandemir, F.M.; Kucukler, S.; Comaklı, S.; Caglayan, C. Chemoprotective Effects of Curcumin on Doxorubicin-Induced Nephrotoxicity in Wistar Rats: By Modulating Inflammatory Cytokines, Apoptosis, Oxidative Stress and Oxidative DNA Damage. Arch. Physiol. Biochem. 2018, 124, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S. Melissa Officinalis Extract in the Treatment of Patients with Mild to Moderate Alzheimer’s Disease: A Double Blind, Randomised, Placebo Controlled Trial. J. Neurol. Neurosurg. Psychiatry 2003, 74, 863–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarei, A.; Changizi-Ashtiyani, S.; Taheri, S.; Hosseini, N. A Brief Overview of the Effects of Melissa Officinalis L. Extract on the Function of Various Body Organs. Zahedan J. Res. Med. Sci. 2015, 17. [Google Scholar] [CrossRef] [Green Version]
- Haji, S.A.; Movahed, A. Update on Digoxin Therapy in Congestive Heart Failure. Am. Fam. Physician 2000, 62, 409–416. [Google Scholar]
- Sesso, H.D.; Liu, S.; Gaziano, J.M.; Buring, J.E. Dietary Lycopene, Tomato-Based Food Products and Cardiovascular Disease in Women. J. Nutr. 2003, 133, 2336–2341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viuda-Martos, M.; Sanchez-Zapata, E.; Sayas-Barberá, E.; Sendra, E.; Pérez-Álvarez, J.A.; Fernández-López, J. Tomato and Tomato Byproducts. Human Health Benefits of Lycopene and Its Application to Meat Products: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1032–1049. [Google Scholar] [CrossRef]
- Chaudhuri, K.N.; Ghosh, B.; Tepfer, D.; Jha, S. Genetic Transformation of Tylophora Indica with Agrobacterium rhizogenes A4: Growth and Tylophorine Productivity in Different Transformed Root Clones. Plant Cell Rep. 2005, 24, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and Their Effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Bhathena, S.J. Dietary Phytoestrogens: A Possible Role in Renal Disease Protection. Am. J. Kidney Dis. 2001, 37, 1056–1068. [Google Scholar] [CrossRef]
- Ríos-Silva, M.; Santos-Álvarez, R.; Trujillo, X.; Cárdenas-María, R.; López-Zamudio, M.; Bricio-Barrios, J.; Leal, C.; Saavedra-Molina, A.; Huerta-Trujillo, M.; Espinoza-Mejía, K.; et al. Effects of Chronic Administration of Capsaicin on Biomarkers of Kidney Injury in Male Wistar Rats with Experimental Diabetes. Molecules 2018, 24, 36. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.K.; Vij, A.S.; Sharma, M. Mechanisms and Clinical Uses of Capsaicin. Eur. J. Pharmacol. 2013, 720, 55–62. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkara, P.J.; Sabina, E.P. Pre-Treatment with Beta Carotene Gives Protection Against Nephrotoxicity Induced by Bromobenzene via Modulation of Antioxidant System, Pro-Inflammatory Cytokines and Pro-Apoptotic Factors. Appl. Biochem. Biotechnol. 2020, 190, 616–633. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Stahl, W.; Rock, C.L. Beta Carotene: From Biochemistry to Clinical Trials. Nutr. Rev. 2009, 58, 39–53. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Tea Catechins and Polyphenols: Health Effects, Metabolism, and Antioxidant Functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Wongmekiat, O.; Peerapanyasut, W.; Kobroob, A. Catechin Supplementation Prevents Kidney Damage in Rats Repeatedly Exposed to Cadmium through Mitochondrial Protection. Naunyn. Schmiedebergs Arch. Pharmacol. 2018, 391, 385–394. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic Potential of Resveratrol: The In Vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Schempp, C.M.; Pelz, K.; Wittmer, A.; Schöpf, E.; Simon, J.C. Antibacterial Activity of Hyperforin from St John’s Wort, against Multiresistant Staphylococcus Aureus and Gram-Positive Bacteria. Lancet 1999, 353, 2129. [Google Scholar] [CrossRef]
- Prassas, I.; Diamandis, E.P. Novel Therapeutic Applications of Cardiac Glycosides. Nat. Rev. Drug Discov. 2008, 7, 926–935. [Google Scholar] [CrossRef]
- Harvey, A. Natural Products in Drug Discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Thomford, N.; Senthebane, D.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [Green Version]
- Alice, C.B.; Vargas, V.M.F.; Silva, G.A.A.B.; De Siqueira, N.C.S.; Schapoval, E.E.S.; Gleye, J.; Henriques, J.A.P.; Henriques, A.T. Screening of Plants Used in South Brazilian Folk Medicine. J. Ethnopharmacol. 1991, 35, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, J.O.; Krettli, A.U. Potential Antimalarials from Nigerian Plants: A Review. J. Ethnopharmacol. 2011, 133, 289–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ud-Din, S.; Wilgus, T.A.; McGeorge, D.D.; Bayat, A. Pre-Emptive Priming of Human Skin Improves Cutaneous Scarring and Is Superior to Immediate and Delayed Topical Anti-Scarring Treatment Post-Wounding: A Double-Blind Randomised Placebo-Controlled Clinical Trial. Pharmaceutics 2021, 13, 510. [Google Scholar] [CrossRef] [PubMed]
- Kehrmann, J.; Tatura, R.; Zeschnigk, M.; Probst-Kepper, M.; Geffers, R.; Steinmann, J.; Buer, J. Impact of 5-Aza-2′-Deoxycytidine and Epigallocatechin-3-Gallate for Induction of Human Regulatory T Cells. Immunology 2014, 142, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.P.G.; Cooney, J.M.; Dommels, Y.E.M.; Nones, K.; Brewster, D.T.; Park, Z.; Butts, C.A.; McNabb, W.C.; Laing, W.A.; Roy, N.C. Modulation of Colonic Inflammation in Mdr1a(−/−) Mice by Green Tea Polyphenols and Their Effects on the Colon Transcriptome and Proteome. J. Nutr. Biochem. 2013, 24, 1678–1690. [Google Scholar] [CrossRef]
- Smyth, G.K. Linear Models and Empirical Bayes Methods for Assessing Differential Expression in Microarray Experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 3. [Google Scholar] [CrossRef]
- Chen, H.; Boutros, P.C. VennDiagram: A Package for the Generation of Highly-Customizable Venn and Euler Diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Falcon, S.; Gentleman, R. Using GOstats to Test Gene Lists for GO Term Association. Bioinforma. Oxf. Engl. 2007, 23, 257–258. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Use R!; Springer: New York, NY, USA, 2009; ISBN 978-0-387-98140-6. [Google Scholar]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING V10: Protein–Protein Interaction Networks, Integrated over the Tree of Life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.; Wruck, W.; Erichsen, L.; Graffmann, N.; Adjaye, J. The Nephrotoxin Puromycin Aminonucleoside Induces Injury in Kidney Organoids Differentiated from Induced Pluripotent Stem Cells. Cells 2022, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, L.; Thimm, C.; Bohndorf, M.; Rahman, M.S.; Wruck, W.; Adjaye, J. Activation of the Renin–Angiotensin System Disrupts the Cytoskeletal Architecture of Human Urine-Derived Podocytes. Cells 2022, 11, 1095. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-L.; Fu, V.; Liu, G.; Tang, T.; Konradi, A.W.; Peng, X.; Kemper, E.; Cravatt, B.F.; Franklin, J.M.; Wu, Z.; et al. Hippo Pathway Regulation by Phosphatidylinositol Transfer Protein and Phosphoinositides. Nat. Chem. Biol. 2022, 18, 1076–1086. [Google Scholar] [CrossRef]
- Romero-Córdoba, S.L.; Salido-Guadarrama, I.; Meneses, M.E.; Cosentino, G.; Iorio, M.V.; Tagliabue, E.; Torres, N.; Sánchez-Tapia, M.; Bonilla, M.; Castillo, I.; et al. Mexican Ganoderma Lucidum Extracts Decrease Lipogenesis Modulating Transcriptional Metabolic Networks and Gut Microbiota in C57BL/6 Mice Fed with a High-Cholesterol Diet. Nutrients 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Kürbitz, C.; Heise, D.; Redmer, T.; Goumas, F.; Arlt, A.; Lemke, J.; Rimbach, G.; Kalthoff, H.; Trauzold, A. Epicatechin Gallate and Catechin Gallate Are Superior to Epigallocatechin Gallate in Growth Suppression and Anti-Inflammatory Activities in Pancreatic Tumor Cells. Cancer Sci. 2011, 102, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Yang, C.S. Mechanisms of Cancer Prevention by Tea Constituents. J. Nutr. 2003, 133, 3262S–3267S. [Google Scholar] [CrossRef] [Green Version]
- Kanlaya, R.; Khamchun, S.; Kapincharanon, C.; Thongboonkerd, V. Protective Effect of Epigallocatechin-3-Gallate (EGCG) via Nrf2 Pathway against Oxalate-Induced Epithelial Mesenchymal Transition (EMT) of Renal Tubular Cells. Sci. Rep. 2016, 6, 30233. [Google Scholar] [CrossRef]
- Ermak, G.; Davies, K.J.A. Calcium and Oxidative Stress: From Cell Signaling to Cell Death. Mol. Immunol. 2002, 38, 713–721. [Google Scholar] [CrossRef]
- Peng, T.-I.; Jou, M.-J. Oxidative Stress Caused by Mitochondrial Calcium Overload. Ann. N. Y. Acad. Sci. 2010, 1201, 183–188. [Google Scholar] [CrossRef]
- Vandekerckhove, L.; Vermeulen, Z.; Liu, Z.Z.; Boimvaser, S.; Patzak, A.; Segers, V.F.M.; De Keulenaer, G.W. Neuregulin-1 Attenuates Development of Nephropathy in a Type 1 Diabetes Mouse Model with High Cardiovascular Risk. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E495–E504. [Google Scholar] [CrossRef] [Green Version]
- Denechaud, P.-D.; Fajas, L.; Giralt, A. E2F1, a Novel Regulator of Metabolism. Front. Endocrinol. 2017, 8, 311. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, R.; Zhou, J. E2F1 and NF-ΚB: Key Mediators of Inflammation-Associated Cancers and Potential Therapeutic Targets. Curr. Cancer Drug Targets 2016, 16, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The Complexity of NF-ΚB Signaling in Inflammation and Cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze-Osthoff, K.; Ferrari, D.; Riehemann, K.; Wesselborg, S. Regulation of NF-ΚB Activation by MAP Kinase Cascades. Immunobiology 1997, 198, 35–49. [Google Scholar] [CrossRef]
- Liang, Y.-J.; Jian, J.-H.; Liu, Y.-C.; Juang, S.-J.; Shyu, K.-G.; Lai, L.-P.; Wang, B.-W.; Leu, J.-G. Advanced Glycation End Products-Induced Apoptosis Attenuated by PPARδ Activation and Epigallocatechin Gallate through NF-ΚB Pathway in Human Embryonic Kidney Cells and Human Mesangial Cells. Diabetes Metab. Res. Rev. 2010, 26, 406–416. [Google Scholar] [CrossRef]
- Boima, V.; Tannor, E.K.; Osafo, C.; Awuku, Y.A.; Mate-Kole, M.; Davids, M.R.; Adu, D. The Ghana Renal Registry—A First Annual Report. Afr. J. Nephrol. 2021, 24, 19–24. [Google Scholar] [CrossRef]
- Osafo, C.; Mate-Kole, M.; Affram, K.; Adu, D. Prevalence of Chronic Kidney Disease in Hypertensive Patients in Ghana. Ren. Fail. 2011, 33, 388–392. [Google Scholar] [CrossRef]

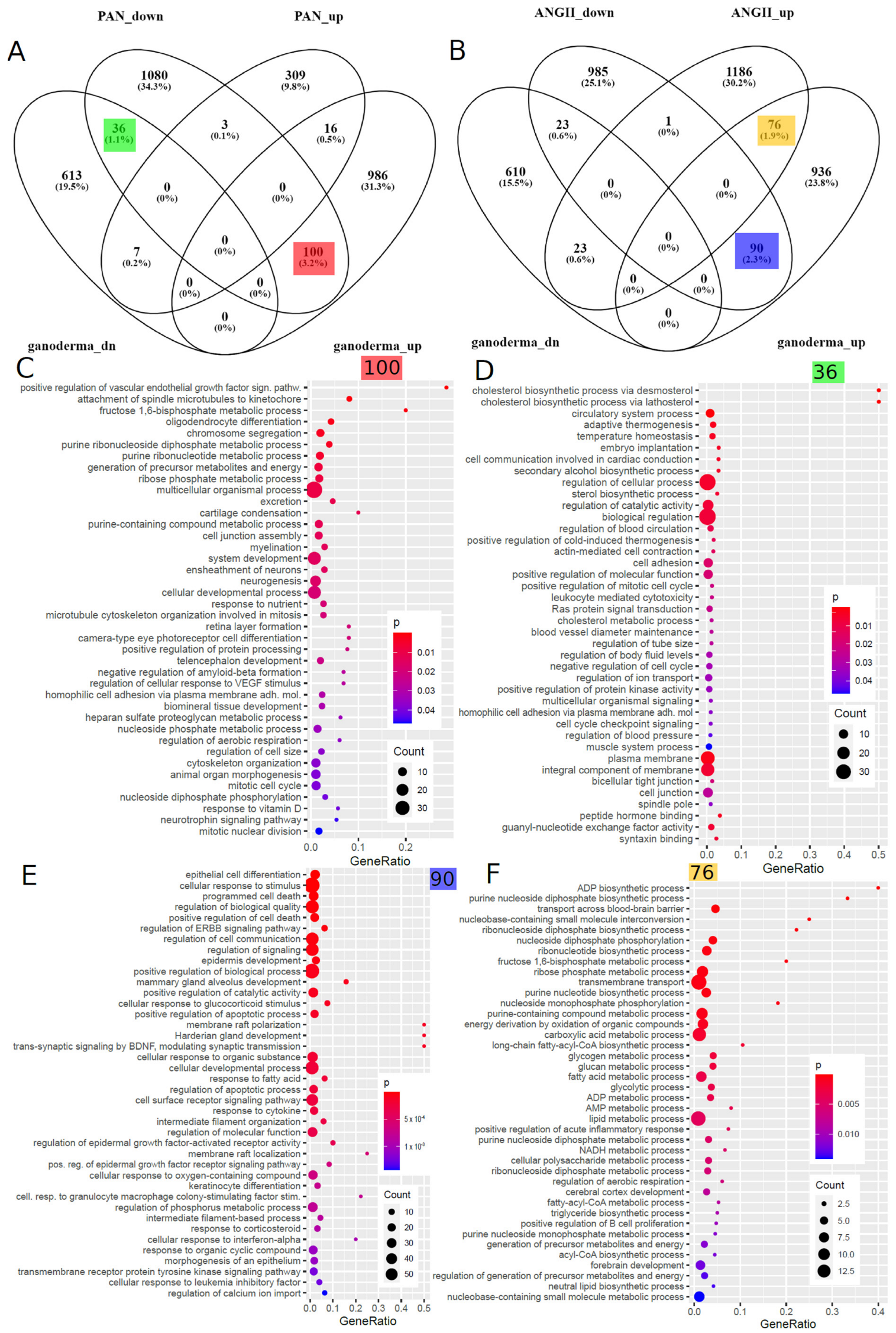
| Term | Ganoderma Lucidum | ANGII | p-Value | Odds Ratio | Genes |
|---|---|---|---|---|---|
| positive regulation of acute inflammatory response | up | up | 0.0043 | 22.34 | C3, OSMR |
| response to wounding | up | down | 0.0207 | 5.41 | DDR1, DSP, HMOX1, ID, PLAT, PLEC, USF1, ZFP36, ZFP36L2 |
| wound healing | up | down | 0.0473 | 2.65 | DDR1, DSP, HMOX1, PLAT, PLEC, USF1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wruck, W.; Genfi, A.K.A.; Adjaye, J. Natural Products in Renal-Associated Drug Discovery. Antioxidants 2023, 12, 1599. https://doi.org/10.3390/antiox12081599
Wruck W, Genfi AKA, Adjaye J. Natural Products in Renal-Associated Drug Discovery. Antioxidants. 2023; 12(8):1599. https://doi.org/10.3390/antiox12081599
Chicago/Turabian StyleWruck, Wasco, Afua Kobi Ampem Genfi, and James Adjaye. 2023. "Natural Products in Renal-Associated Drug Discovery" Antioxidants 12, no. 8: 1599. https://doi.org/10.3390/antiox12081599
APA StyleWruck, W., Genfi, A. K. A., & Adjaye, J. (2023). Natural Products in Renal-Associated Drug Discovery. Antioxidants, 12(8), 1599. https://doi.org/10.3390/antiox12081599







