Response Surface Optimization for the Enhancement of the Extraction of Bioactive Compounds from Citrus limon Peel
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample and Extract Preparation
2.3. Design of the Experiment and the Response Surface Methodology (RSM) Optimization
2.4. Determinations
2.4.1. Total Phenolic Content (TPC)
2.4.2. Total Flavonoid Content (TFC)
2.4.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4.4. DPPH Radical Scavenging Activity
2.4.5. Hydrogen Peroxide (H2O2) Scavenging Assay
2.4.6. Ascorbic Acid (AA) Content
2.4.7. Total Carotenoid Content (TCC)
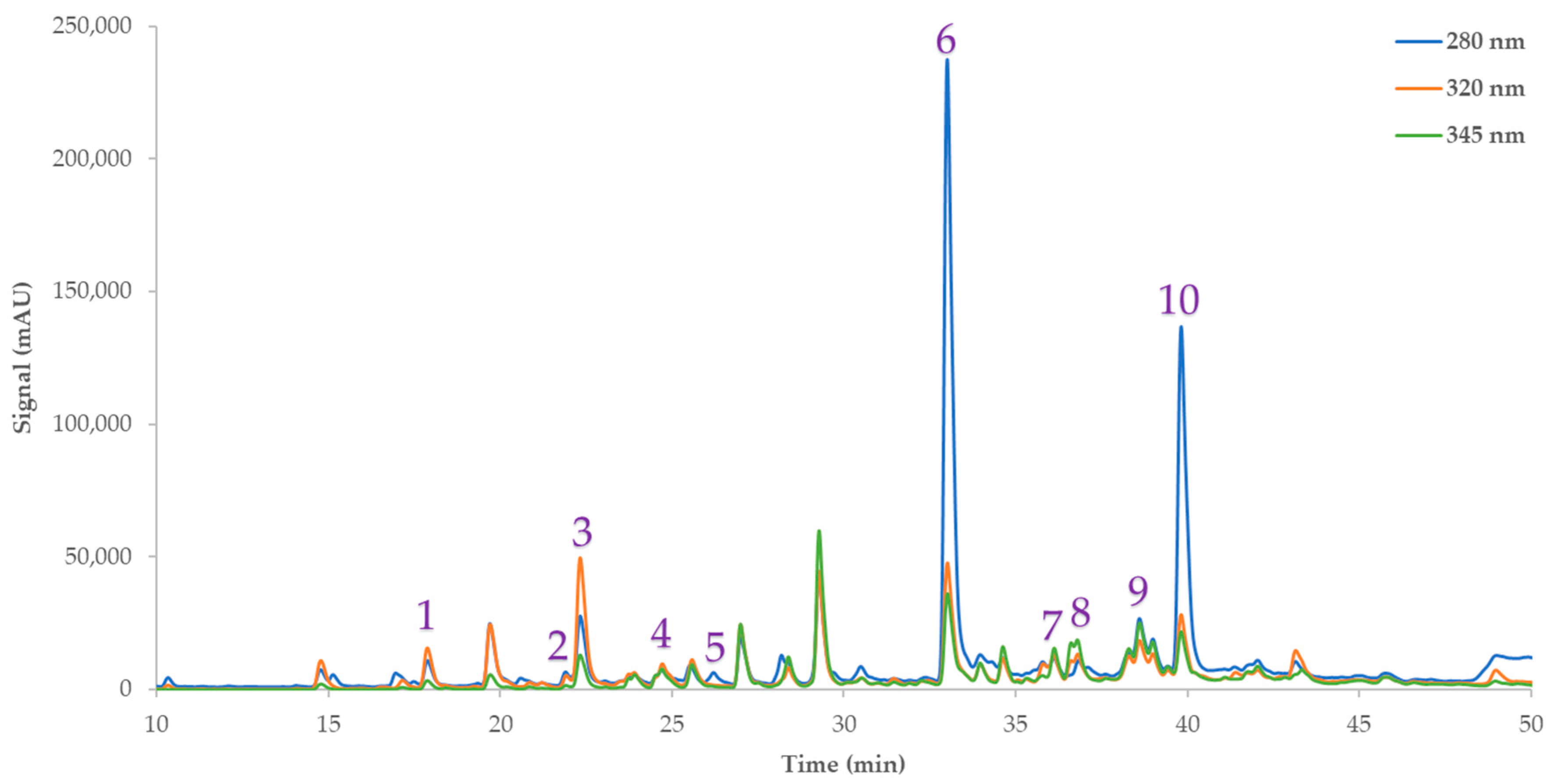
2.4.8. HPLC-Based Determination of the Eriocitrin Content and Other Phenolic Compounds
2.5. Statistical Analysis
3. Results and Discussion
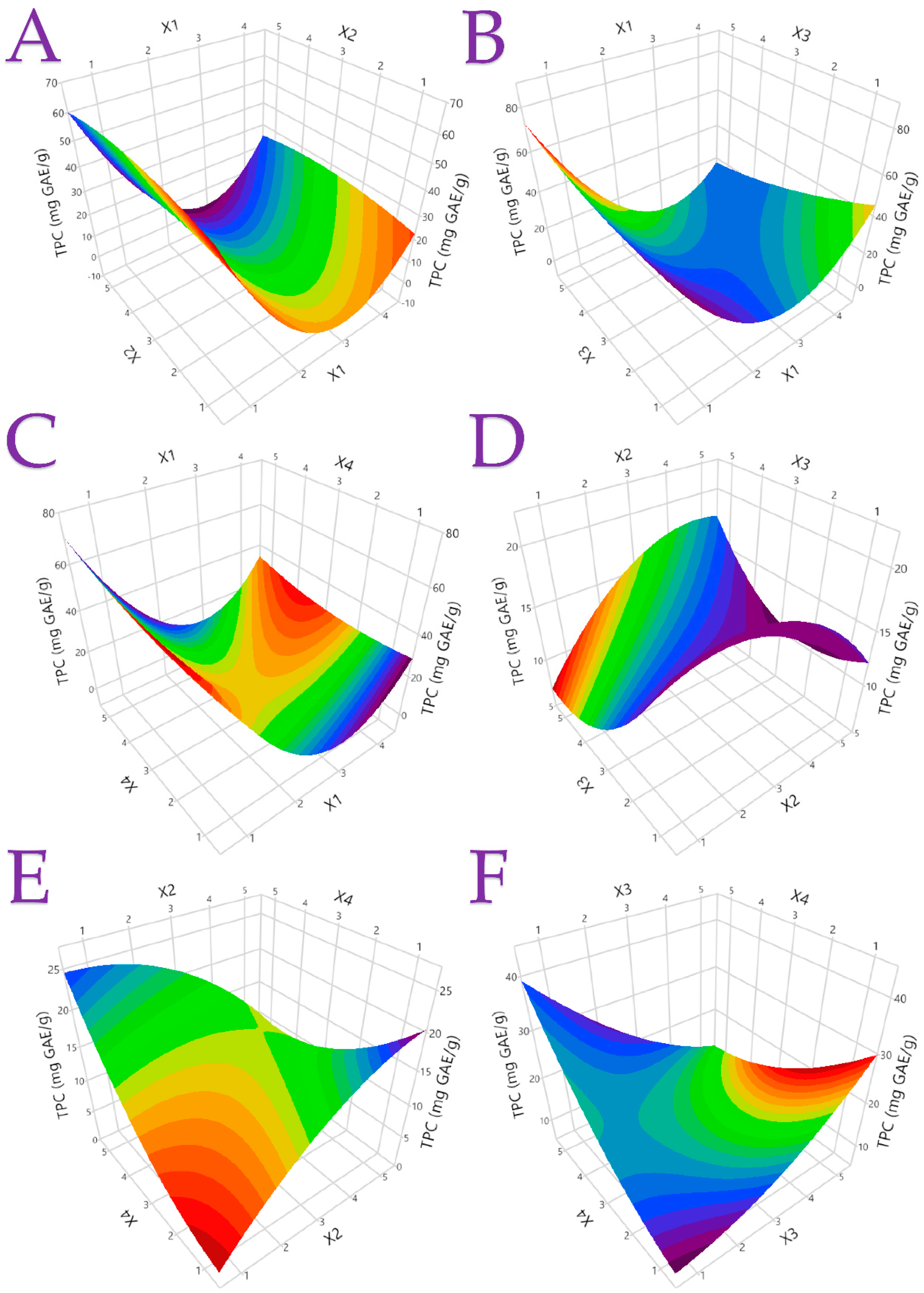
3.1. Extraction Optimization
3.2. Analysis of the Extracts
3.2.1. Total Phenolic Content (TPC) and Flavonoid Content (TFC) of the Extracts
3.2.2. Eriocitrin Content and Other Phenolics of the Extracts
3.2.3. Antioxidant Properties of the Extracts
3.2.4. Ascorbic Acid Content of the Extracts
3.2.5. Total Carotenoid Content (TCC) of the Extracts
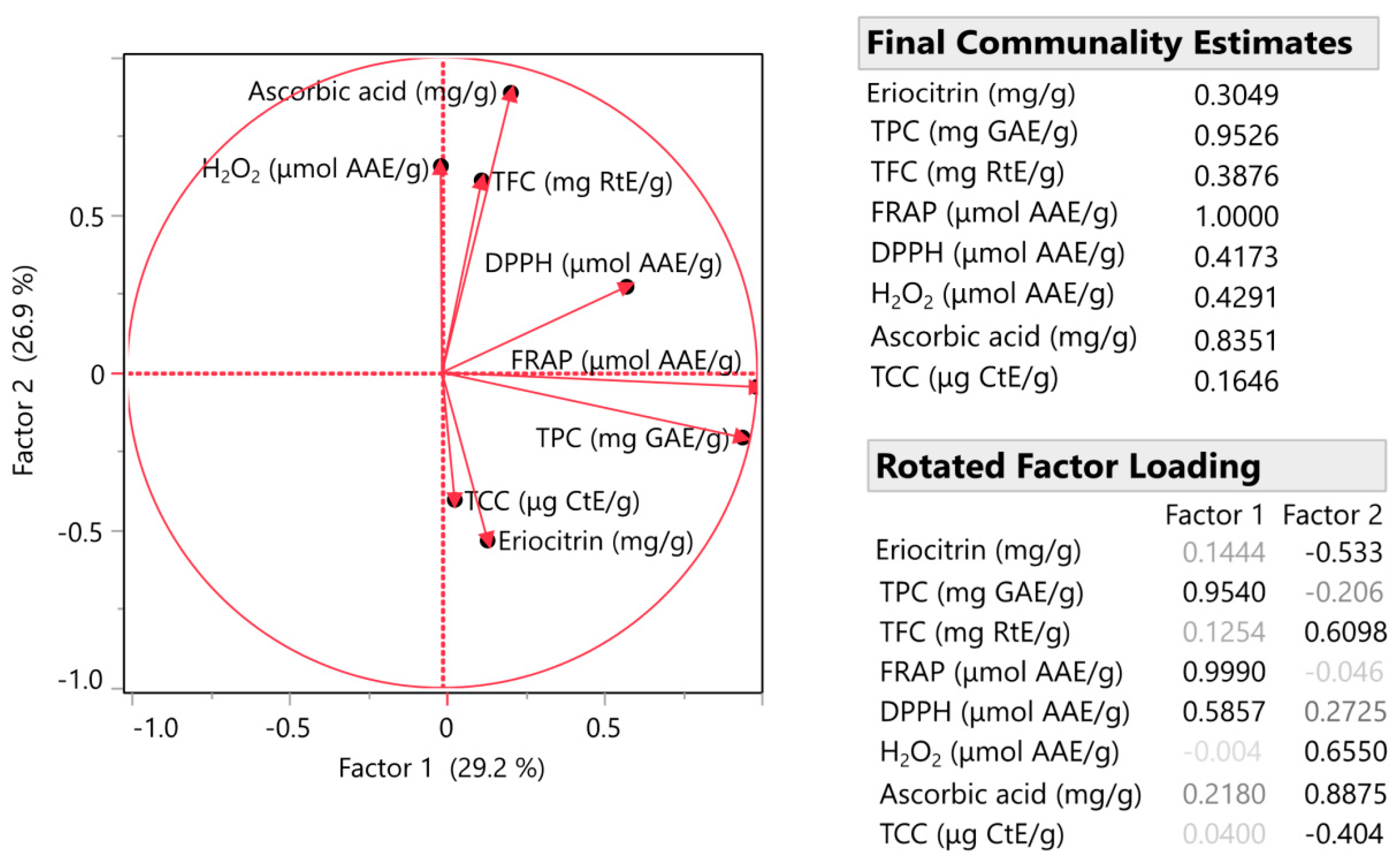
3.3. Factor Analysis (FA) and Multivariate Correlation Analysis (MCA)
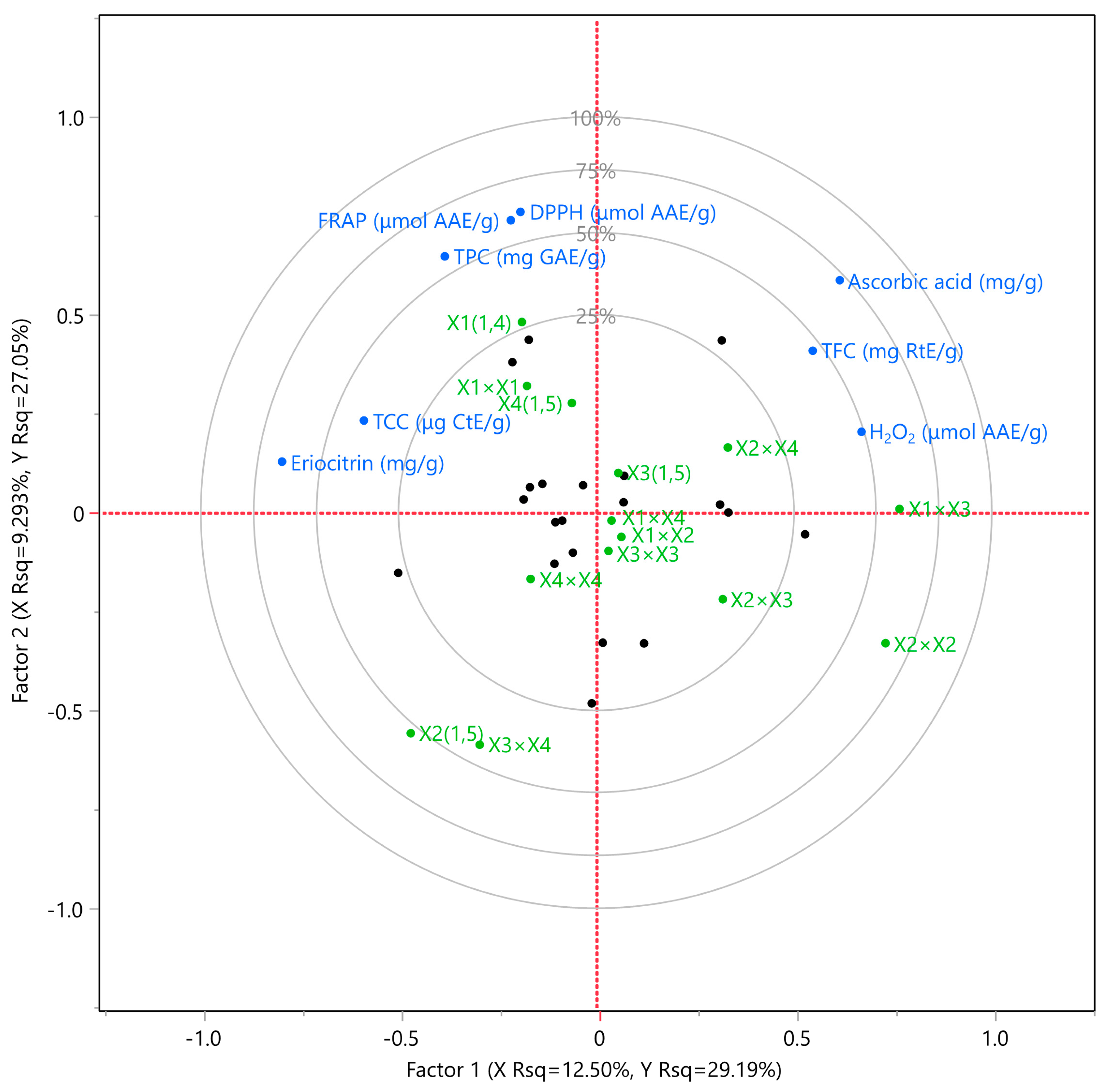
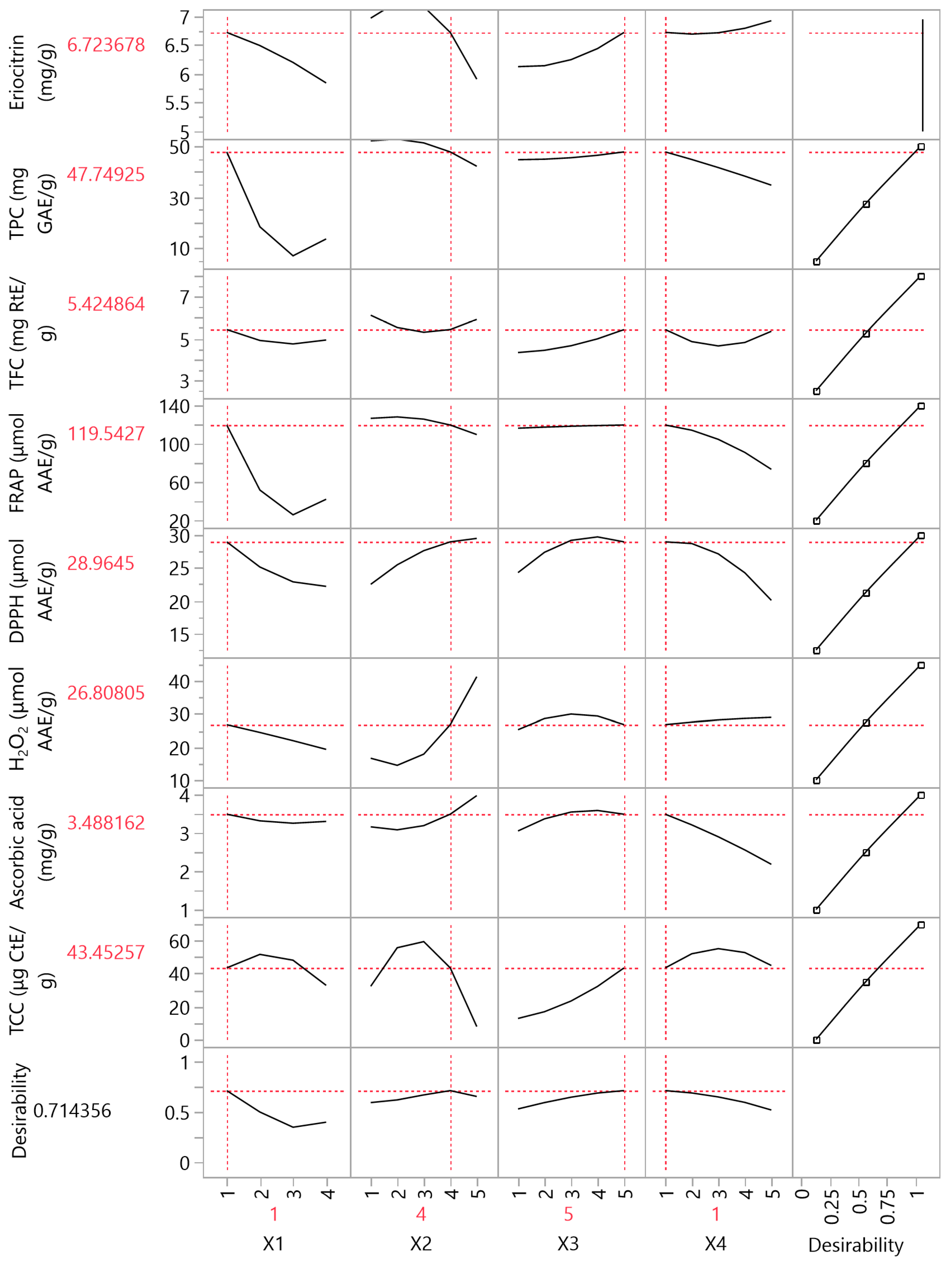
3.4. Partial Least Squares (PLS) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Appelhans, M.S.; Bayly, M.J.; Heslewood, M.M.; Groppo, M.; Verboom, G.A.; Forster, P.I.; Kallunki, J.A.; Duretto, M.F. A new subfamily classification of the Citrus family (Rutaceae) based on six nuclear and plastid markers. Taxon 2021, 70, 1035–1061. [Google Scholar] [CrossRef]
- Suri, S.; Singh, A.; Nema, P.K. Current applications of citrus fruit processing waste: A scientific outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, Global Distribution, and Nutritional Importance of Citrus Fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Sadaka, C.; Generalić Mekinić, I.; Garzoli, S.; Švarc-Gajić, J.; Rodrigues, F.; Morais, S.; Moreira, M.M.; Ferreira, E.; Spigno, G.; et al. The Chemical Variability, Nutraceutical Value, and Food-Industry and Cosmetic Applications of Citrus Plants: A Critical Review. Antioxidants 2023, 12, 481. [Google Scholar] [CrossRef] [PubMed]
- Lorente, J.; Vegara, S.; Martí, N.; Ibarz, A.; Coll, L.; Hernández, J.; Valero, M.; Saura, D. Chemical guide parameters for Spanish lemon (Citrus limon (L.) Burm.) juices. Food Chem. 2014, 162, 186–191. [Google Scholar] [CrossRef]
- Magalhães, D.; Vilas-Boas, A.A.; Teixeira, P.; Pintado, M. Functional Ingredients and Additives from Lemon by-Products and Their Applications in Food Preservation: A Review. Foods 2023, 12, 1095. [Google Scholar] [CrossRef]
- Penniston, K.L.; Nakada, S.Y.; Holmes, R.P.; Assimos, D.G. Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J. Endourol. 2008, 22, 567–570. [Google Scholar] [CrossRef]
- Arbona, V.; Iglesias, D.J.; Gómez-Cadenas, A. Non-targeted metabolite profiling of citrus juices as a tool for variety discrimination and metabolite flow analysis. BMC Plant Biol. 2015, 15, 38. [Google Scholar] [CrossRef]
- Sánchez-Bravo, P.; Noguera-Artiaga, L.; Martínez-Tomé, J.; Hernández, F.; Sendra, E. Effect of Organic and Conventional Production on the Quality of Lemon “Fino 49”. Agronomy 2022, 12, 980. [Google Scholar] [CrossRef]
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in global vitamin C recommendations: A review of RDA criteria and underlying health perspectives. Crit. Rev. Food Sci. Nutr. 2021, 61, 742–755. [Google Scholar] [CrossRef]
- Del Río, J.A.; Fuster, M.D.; Gómez, P.; Porras, I.; García-Lidón, A.; Ortuño, A. Citrus limon: A source of flavonoids of pharmaceutical interest. Food Chem. 2004, 84, 457–461. [Google Scholar] [CrossRef]
- Turner, T.; Burri, B.J. Potential nutritional benefits of current citrus consumption. Agriculture 2013, 3, 170–187. [Google Scholar] [CrossRef]
- Tounsi, M.S.; Wannes, W.A.; Ouerghemmi, I.; Jegham, S.; Njima, Y.B.; Hamdaoui, G.; Zemni, H.; Marzouk, B. Juice components and antioxidant capacity of four Tunisian Citrus varieties. J. Sci. Food Agric. 2011, 91, 142–151. [Google Scholar] [CrossRef]
- Sir Elkhatim, K.A.; Elagib, R.A.A.; Hassan, A.B. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Kauser, T.; Aslam, J.; Quddoos, M.Y.; Ali, A.; Kauser, S.; Kabir, K.; Rafique, A.; Noreen, S.; Iftikhar, K.; et al. Comparison of Different Techno-Functional Properties of Raw Lemon Pomace and Lemon Pomace Powder, and Development of Nutritional Biscuits by Incorporation of Lemon Pomace Powder. Caraka Tani J. Sustain. Agric. 2023, 38, 176–192. [Google Scholar] [CrossRef]
- Younus, N.K. Iraqi Lemon Peel Extract (Citrus limon) as Antibacterial and Antioxidant Agent. E3S Web Conf. 2023, 391, 01121. [Google Scholar] [CrossRef]
- Moosavy, M.H.; Hassanzadeh, P.; Mohammadzadeh, E.; Mahmoudi, R.; Khatibi, S.A.; Mardani, K. Antioxidant and antimicrobial activities of essential oil of lemon (Citrus limon) peel In Vitro and in a food model. J. Food Qual. Hazards Control 2017, 4, 42–48. [Google Scholar]
- Hamdan, D.; El-Readi, M.Z.; Nibret, E.; Sporer, F.; Farrag, N.; El-Shazly, A.; Wink, M. Chemical composition of the essential oils of two Citrus species and their biological activities. Pharmazie 2010, 65, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Heleno, S.; Carocho, M.; Pinela, J.; Prieto, M.; Ferreira, I.; Barros, L.; Fernandes, F.; Heleno, S.; Carocho, M.; et al. Optimization of citric acid extraction from citrus peels. In Proceedings of the 1st International Electronic Conference on Food Science and Functional Foods, Online, 10–25 November 2020. [Google Scholar]
- Haida, Z.; Ab Ghani, S.; Juju Nakasha, J.; Hakiman, M. Determination of experimental domain factors of polyphenols, phenolic acids and flavonoids of lemon (Citrus limon) peel using two-level factorial design: Determination of experimental domain factors. Saudi J. Biol. Sci. 2022, 29, 574–582. [Google Scholar] [CrossRef]
- Pappas, V.M.; Lakka, A.; Palaiogiannis, D.; Athanasiadis, V.; Bozinou, E.; Ntourtoglou, G.; Makris, D.P.; Dourtoglou, V.G.; Lalas, S.I. Optimization of Pulsed Electric Field as Standalone “Green” Extraction Procedure for the Recovery of High Value-Added Compounds from Fresh Olive Leaves. Antioxidants 2021, 10, 1554. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Pappas, V.M.; Palaiogiannis, D.; Chatzimitakos, T.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Pulsed Electric Field-Based Extraction of Total Polyphenols from Sideritis raiseri Using Hydroethanolic Mixtures. Oxygen 2022, 2, 91–98. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Kaltsa, O.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. The Effect of Ultrasonication Pretreatment on the Production of Polyphenol-Enriched Extracts from Moringa oleifera L. (Drumstick Tree) Using a Novel Bio-Based Deep Eutectic Solvent. Appl. Sci. 2019, 10, 220. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Palaiogiannis, D.; Poulianiti, K.; Bozinou, E.; Lalas, S.I.; Makris, D.P. Extraction of Polyphenolic Antioxidants from Red Grape Pomace and Olive Leaves: Process Optimization Using a Tailor-Made Tertiary Deep Eutectic Solvent. Sustainability 2022, 14, 6864. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimized Isolation Procedure for the Extraction of Bioactive Compounds from Spent Coffee Grounds. Appl. Sci. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimization of the Extraction Parameters for the Isolation of Bioactive Compounds from Orange Peel Waste. Sustainability 2022, 14, 13926. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Kotsou, K.; Palaiogiannis, D.; Lalas, S.I. Optimization of Extraction Parameters for Enhanced Recovery of Bioactive Compounds from Quince Peels Using Response Surface Methodology. Foods 2023, 12, 2099. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.H.; Mohamad, A.B. Hydrogen peroxide scavenging activity of novel coumarins synthesized using different approaches. PLoS ONE 2015, 10, 2–10. [Google Scholar] [CrossRef]
- Wardy, W.; Saalia, F.K.; Steiner-Asiedu, M.; Budu, A.S.; Sefa-Dedeh, S. A comparison of some physical, chemical and sensory attributes of three pineapple (Ananas comosus) varieties grown in Ghana. Afr. J. Food Sci. 2009, 3, 22–025. [Google Scholar]
- Chen, Y.; Pan, H.; Hao, S.; Pan, D.; Wang, G.; Yu, W. Evaluation of phenolic composition and antioxidant properties of different varieties of Chinese citrus. Food Chem. 2021, 364, 130413. [Google Scholar] [CrossRef]
- Al-Ashaal, H.A.; El-Sheltawy, S.T. Antioxidant capacity of hesperidin from Citrus peel using electron spin resonance and cytotoxic activity against human carcinoma cell lines. Pharm. Biol. 2011, 49, 276–282. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of phenolic profile and antioxidant capacity of different fruit part from lemon (Citrus limon Burm.) cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef]
- Rizaldy, D.; Insanu, M.; Sabila, N.; Haniffadli, A.; Zahra, A.A.; Pratiwi, S.N.E.; Mudrika, S.N.; Hartati, R.; Fidrianny, I. Lemon (Citrus limon L.): Antioxidative Activity and Its Marker Compound. Biointerface Res. Appl. Chem. 2023, 13, 21. [Google Scholar] [CrossRef]
- Kalompatsios, D.; Athanasiadis, V.; Palaiogiannis, D.; Lalas, S.I.; Makris, D.P. Valorization of Waste Orange Peels: Aqueous Antioxidant Polyphenol Extraction as Affected by Organic Acid Addition. Beverages 2022, 8, 71. [Google Scholar] [CrossRef]
- Dugo, P.; Lo Presti, M.; Öhman, M.; Fazio, A.; Dugo, G.; Mondello, L. Determination of flavonoids in citrus juices by micro-HPLC-ESI/MS. J. Sep. Sci. 2005, 28, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Chuang, Y.C.; Ku, Y.H. Quantitation of bioactive compounds in citrus fruits cultivated in Taiwan. Food Chem. 2007, 102, 1163–1171. [Google Scholar] [CrossRef]
- Makni, M.; Jemai, R.; Kriaa, W.; Chtourou, Y.; Fetoui, H. Citrus limon from Tunisia: Phytochemical and Physicochemical Properties and Biological Activities. Biomed Res. Int. 2018, 2018, 6251546. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, A.; Wang, X.; Sun, H. A kaempferol-3-O-β-D-glucoside, intervention effect of astragalin on estradiol metabolism. Steroids 2019, 149, 108413. [Google Scholar] [CrossRef]
- Tzima, K.; Kallithraka, S.; Kotseridis, Y.; Makris, D.P. A Comparative Evaluation of Aqueous Natural Organic Acid Media for the Efficient Recovery of Flavonoids from Red Grape (Vitis vinifera) Pomace. Waste Biomass Valorization 2015, 6, 391–400. [Google Scholar] [CrossRef]
- Alasalvar, H.; Kaya, M.; Berktas, S.; Basyigit, B.; Cam, M. Pressurised hot water extraction of phenolic compounds with a focus on eriocitrin and hesperidin from lemon peel. Int. J. Food Sci. Technol. 2023, 58, 2060–2066. [Google Scholar] [CrossRef]
- Cao, X.; He, Y.; Kong, Y.; Mei, X.; Huo, Y.; He, Y.; Liu, J. Elucidating the interaction mechanism of eriocitrin with β-casein by multi-spectroscopic and molecular simulation methods. Food Hydrocoll. 2019, 94, 63–70. [Google Scholar] [CrossRef]
- Yao, L.; Liu, W.; Bashir, M.; Nisar, M.F.; Wan, C. (Craig) Eriocitrin: A review of pharmacological effects. Biomed. Pharmacother. 2022, 154, 113563. [Google Scholar] [CrossRef] [PubMed]
- Nezamodini, Z.S.; Rezvani, Z.; Kian, K. Electronic Physician (ISSN: 2008-5842). Electron. Physician 2017, 9, 3592–3597. [Google Scholar]
- Dong, X.; Hu, Y.; Li, Y.; Zhou, Z. The maturity degree, phenolic compounds and antioxidant activity of Eureka lemon [Citrus limon (L.) Burm. f.]: A negative correlation between total phenolic content, antioxidant capacity and soluble solid content. Sci. Hortic. 2019, 243, 281–289. [Google Scholar] [CrossRef]
- Kim, D.B.; Shin, G.H.; Kim, J.M.; Kim, Y.H.; Lee, J.H.; Lee, J.S.; Song, H.J.; Choe, S.Y.; Park, I.J.; Cho, J.H.; et al. Antioxidant and anti-ageing activities of citrus-based juice mixture. Food Chem. 2016, 194, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Bartosz, G.; Stefaniuk, I.; Pichla, M.; Namieśnik, J.; Sadowska-Bartosz, I. Dietary antioxidants as a source of hydrogen peroxide. Food Chem. 2019, 278, 692–699. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin c—Sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Jacob, R.A.; Sotoudeh, G. Vitamin C function and status in chronic disease. Nutr. Clin. Care 2002, 5, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Di Matteo, A.; Di Rauso Simeone, G.; Cirillo, A.; Rao, M.A.; Di Vaio, C. Morphological characteristics, ascorbic acid and antioxidant activity during fruit ripening of four lemon (Citrus limon (L.) Burm. F.) cultivars. Sci. Hortic. (Amsterdam). 2021, 276, 109741. [Google Scholar] [CrossRef]
- Fatin Najwa, R.; Azrina, A. Comparison of vitamin C content in citrus fruits by titration and high performance liquid chromatography (HPLC) methods. Int. Food Res. J. 2017, 24, 726–733. [Google Scholar]
- Dutta, D.; Chaudhuri, U.R.; Chakraborty, R. Structure, health benefits, antioxidant property and processing and storage of carotenoids. African J. Biotechnol. 2005, 4, 1510–1520. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chuang, Y.C.; Hsu, H.W. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 2008, 106, 277–284. [Google Scholar] [CrossRef]


| Independent Variables | Code Units | Coded Variable Level | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Technique | X1 | ST | PEF + ST | US + ST | PEF + US + ST | – |
| C (%, v/v) | X2 | 0 | 25 | 50 | 75 | 100 |
| t (min) | X3 | 30 | 60 | 90 | 120 | 150 |
| T (°C) | X4 | 20 | 35 | 50 | 65 | 80 |
| Design Point | Independent Variables | Responses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Eriocitrin (mg/g) | TPC (mg GAE/g) | TFC (mg RtE/g) | FRAP (μmol AAE/g) | DPPH (μmol AAE/g) | H2O2 (μmol AAE/g) | Ascorbic Acid (mg/g) | TCC (μg CtE/g) | |
| 1 | 3 | 1 | 3 | 4 | 6.09 | 5.90 | 2.47 | 20.81 | 18.97 | 22.48 | 2.55 | 9.47 |
| 2 | 3 | 2 | 1 | 3 | 6.47 | 13.13 | 2.66 | 40.04 | 23.89 | 15.70 | 2.85 | 59.21 |
| 3 | 2 | 3 | 4 | 3 | 6.74 | 13.56 | 3.66 | 41.25 | 23.44 | 21.86 | 2.62 | 64.90 |
| 4 | 2 | 4 | 5 | 4 | 6.84 | 12.48 | 4.45 | 34.91 | 21.16 | 27.77 | 2.50 | 54.39 |
| 5 | 3 | 5 | 4 | 2 | 5.10 | 6.61 | 5.65 | 22.63 | 23.52 | 42.46 | 3.71 | 14.99 |
| 6 | 4 | 1 | 4 | 5 | 5.58 | 12.20 | 2.81 | 25.66 | 14.31 | 24.19 | 1.55 | 6.25 |
| 7 | 4 | 2 | 3 | 1 | 6.20 | 11.80 | 3.08 | 38.69 | 26.87 | 13.96 | 3.01 | 39.40 |
| 8 | 1 | 3 | 3 | 2 | 6.52 | 41.52 | 4.14 | 113.19 | 28.70 | 21.94 | 3.04 | 57.83 |
| 9 | 1 | 4 | 4 | 1 | 6.45 | 51.24 | 4.86 | 128.89 | 30.31 | 29.37 | 3.70 | 25.55 |
| 10 | 1 | 5 | 1 | 4 | 5.69 | 40.57 | 5.93 | 120.67 | 23.08 | 34.48 | 3.66 | 18.83 |
| 11 | 1 | 1 | 2 | 3 | 6.10 | 40.15 | 3.75 | 113.30 | 23.59 | 18.29 | 3.17 | 11.63 |
| 12 | 1 | 2 | 5 | 5 | 6.95 | 44.50 | 4.10 | 85.51 | 17.82 | 17.29 | 1.20 | 50.39 |
| 13 | 4 | 3 | 2 | 4 | 6.86 | 23.92 | 4.40 | 62.06 | 24.27 | 22.06 | 3.01 | 36.59 |
| 14 | 3 | 4 | 2 | 5 | 6.42 | 13.41 | 6.61 | 32.21 | 18.51 | 29.59 | 3.07 | 45.29 |
| 15 | 2 | 5 | 3 | 5 | 6.09 | 11.77 | 7.09 | 38.77 | 20.33 | 41.80 | 3.58 | 10.47 |
| 16 | 2 | 1 | 1 | 1 | 6.19 | 5.13 | 4.02 | 21.21 | 18.27 | 20.62 | 3.17 | 5.38 |
| 17 | 2 | 2 | 2 | 2 | 6.20 | 11.18 | 3.48 | 37.81 | 23.26 | 19.28 | 2.79 | 60.25 |
| 18 | 3 | 3 | 5 | 1 | 6.54 | 10.31 | 4.23 | 29.25 | 20.89 | 14.15 | 3.01 | 55.99 |
| 19 | 4 | 4 | 1 | 2 | 6.49 | 22.15 | 5.25 | 40.28 | 21.79 | 31.01 | 3.12 | 9.91 |
| 20 | 4 | 5 | 5 | 3 | 5.42 | 9.60 | 6.45 | 36.88 | 19.84 | 38.38 | 3.50 | 3.34 |
| Design Point | Independent Variables | Responses (mg/g) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | NCA | CA | CGA | CFA | SA | LG | KR | KG | HES | |
| 1 | 3 | 1 | 3 | 4 | 0.17 | 0.22 | 0.66 | 0.07 | 0.07 | 0.14 | 0.27 | 0.41 | 2.66 |
| 2 | 3 | 2 | 1 | 3 | 0.16 | 0.20 | 0.76 | 0.09 | 0.07 | 0.16 | 0.26 | 0.44 | 2.91 |
| 3 | 2 | 3 | 4 | 3 | 0.13 | 0.09 | 0.73 | 0.08 | 0.06 | 0.16 | 0.30 | 0.44 | 3.74 |
| 4 | 2 | 4 | 5 | 4 | 0.15 | 0.04 | 0.68 | 0.07 | 0.03 | 0.16 | 0.29 | 0.44 | 4.63 |
| 5 | 3 | 5 | 4 | 2 | 0.04 | 0.31 | 0.40 | 0.04 | 0.04 | 0.13 | 0.21 | 0.38 | 2.75 |
| 6 | 4 | 1 | 4 | 5 | 0.14 | 0.19 | 0.51 | 0.07 | 0.05 | 0.13 | 0.23 | 0.39 | 2.26 |
| 7 | 4 | 2 | 3 | 1 | 0.15 | 0.17 | 0.72 | 0.09 | 0.06 | 0.15 | 0.28 | 0.42 | 2.62 |
| 8 | 1 | 3 | 3 | 2 | 0.13 | 0.11 | 0.72 | 0.08 | 0.05 | 0.16 | 0.31 | 0.43 | 3.64 |
| 9 | 1 | 4 | 4 | 1 | 0.12 | 0.16 | 0.64 | 0.06 | 0.03 | 0.15 | 0.28 | 0.42 | 3.82 |
| 10 | 1 | 5 | 1 | 4 | 0.06 | 0.39 | 0.47 | 0.06 | 0.04 | 0.13 | 0.22 | 0.40 | 3.55 |
| 11 | 1 | 1 | 2 | 3 | 0.17 | 0.21 | 0.72 | 0.08 | 0.06 | 0.14 | 0.26 | 0.40 | 3.04 |
| 12 | 1 | 2 | 5 | 5 | 0.14 | 0.18 | 0.50 | 0.08 | 0.05 | 0.17 | 0.30 | 0.43 | 4.40 |
| 13 | 4 | 3 | 2 | 4 | 0.12 | 0.11 | 0.71 | 0.08 | 0.06 | 0.16 | 0.31 | 0.43 | 4.37 |
| 14 | 3 | 4 | 2 | 5 | 0.14 | 0.32 | 0.65 | 0.07 | 0.03 | 0.16 | 0.33 | 0.42 | 6.44 |
| 15 | 2 | 5 | 3 | 5 | 0.02 | 0.37 | 0.46 | 0.04 | 0.01 | 0.14 | 0.27 | 0.41 | 3.64 |
| 16 | 2 | 1 | 1 | 1 | 0.18 | 0.21 | 0.76 | 0.09 | 0.06 | 0.14 | 0.26 | 0.44 | 3.09 |
| 17 | 2 | 2 | 2 | 2 | 0.16 | 0.17 | 0.74 | 0.09 | 0.05 | 0.15 | 0.27 | 0.43 | 2.58 |
| 18 | 3 | 3 | 5 | 1 | 0.13 | 0.09 | 0.72 | 0.08 | 0.05 | 0.16 | 0.30 | 0.42 | 3.43 |
| 19 | 4 | 4 | 1 | 2 | 0.11 | 0.06 | 0.63 | 0.07 | 0.03 | 0.15 | 0.30 | 0.43 | 3.53 |
| 20 | 4 | 5 | 5 | 3 | 0.05 | 0.36 | 0.43 | 0.06 | 0.03 | 0.13 | 0.27 | 0.41 | 2.89 |
| Responses | Second-order Polynomial Equations (Models) | R2 | p | Equation |
|---|---|---|---|---|
| Eriocitrin | Y = 5.78 + 0.05X1 − 0.65X2 + 1X3 − 0.08X4 + 0.08X12 − 0.01X22 + 0.1X32 − 0.13X42 + 0.01X1X2 − 0.31X1X3 + 0.13X1X4 − 0.11X2X3 + 0.25X2X4 − 0.09X3X4 | 0.9934 | 0.0002 | (8) |
| TPC | Y = 60 − 51.54X1 + 5.29X2 + 3.07X3 + 6.89X4 + 9.64X12 − 0.58X22 + 0.65X32 + 0.69X42 + 0.09X1X2 − 1.97X1X3 − 0.03X1X4 + 0.73X2X3 − 1.3X2X4 − 1.99X3X4 | 0.9564 | 0.0164 | (9) |
| TFC | Y = 8.82 − 1.84X1 − 1.23X2 + 0.5X3 − 2.02X4 + 0.05X12 + 0.16X22 + 0.002X32 + 0.21X42 + 0.38X1X2 − 0.007X1X3 + 0.17X1X4 − 0.14X2X3 + 0.17X2X4 − 0.03X3X4 | 0.9523 | 0.0202 | (10) |
| FRAP | Y = 160.55 − 132.65X1 + 5.35X2 + 6.77X3 + 29.87X4 + 24.68X12 − 1.08X22 + 0.57X32 − 0.8X42 − 2.01X1X2 − 2.45X1X3 − 0.25X1X4 + 3.42X2X3 − 1.21X2X4 − 6.22X3X4 | 0.9623 | 0.0117 | (11) |
| DPPH | Y = 9.9 − 3.04X1 + 5.88X2 + 2.23X3 + 5.75X4 + 1.56X12 − 0.88X22 − 0.83X32 − 0.36X42 − 0.99X1X2 − 0.28X1X3 − 0.74X1X4 + 1.28X2X3 − 0.4X2X4 − 0.44X3X4 | 0.9614 | 0.0124 | (12) |
| H2O2 | Y = 26.38 + 1.6X1 − 10.44X2 + 5.48X3 − 4.83X4 − 0.85X12 + 2.85X22 − 0.76X32 − 0.17X42 + 0.49X1X2 − 0.66X1X3 + 1.21X1X4 − 0.64X2X3 − 0.08X2X4 + 0.92X3X4 | 0.9563 | 0.0165 | (13) |
| Ascorbic acid | Y = 3.96 − 0.26X1 − 1.51X2 + 0.8X3 + 0.12X4 + 0.08X12 + 0.2X22 − 0.02X32 − 0.07X42 + 0.01X1X2 − 0.13X1X3 + 0.06X1X4 + 0.03X2X3 + 0.13X2X4 − 0.16X3X4 | 0.9725 | 0.0056 | (14) |
| TCC | Y = −108.57 + 25.91X1 + 135.53X2 − 50.15X3 + 20.91X4 − 7X12 − 20.5X22 − 2.16X32 + 3.14X42 − 2.89X1X2 + 11.09X1X3 − 7.5X1X4 + 6.6X2X3 − 7.44X2X4 + 2.76X3X4 | 0.9805 | 0.0024 | (15) |
| Responses | Optimal Conditions | ||||
|---|---|---|---|---|---|
| Maximum Predicted Response | Technique (X1) | C (%, v/v) (X2) | t (min) (X3) | T (°C) (X4) | |
| Eriocitrin (mg/g) | 7.2 ± 0.2 | ST (1) | 50 (3) | 120 (4) | 50 (3) |
| TPC (mg GAE/g) | 51 ± 14 | ST (1) | 75 (4) | 120 (4) | 20 (1) |
| TFC (mg RtE/g) | 7 ± 1 | PEF + ST (2) | 100 (5) | 60 (2) | 80 (5) |
| FRAP (μmol AAE/g) | 128 ± 33 | ST (1) | 75 (4) | 120 (4) | 20 (1) |
| DPPH (μmol AAE/g) | 30 ± 3 | ST (1) | 75 (4) | 120 (4) | 35 (2) |
| H2O2 (μmol AAE/g) | 45 ± 9 | US + ST (3) | 100 (5) | 120 (4) | 65 (4) |
| Ascorbic acid (mg/g) | 3.9 ± 0.5 | PEF + ST (2) | 100 (5) | 120 (4) | 35 (2) |
| TCC (μg CtE/g) | 81 ± 12 | PEF + ST (2) | 50 (3) | 120 (4) | 65 (4) |
| Variables | PLS Model Values | Experimental Values |
|---|---|---|
| Eriocitrin (mg/g) | 6.72 | 6.6 ± 0.4 |
| TPC (mg GAE/g) | 47.75 | 45 ± 1 |
| TFC (mg RtE/g) | 5.43 | 5.3 ± 0.5 |
| FRAP (μmol AAE/g) | 119.54 | 112 ± 4 |
| DPPH (μmol AAE/g) | 28.97 | 28.0 ± 0.8 |
| H2O2 (μmol AAE/g) | 26.81 | 26.0 ± 0.7 |
| Ascorbic acid (mg/g) | 3.49 | 3.4 ± 0.2 |
| TCC (μg CtE/g) | 43.45 | 41 ± 1 |
| Phenolic Compounds | Optimal Extract (mg/g) |
|---|---|
| Neochlorogenic acid | 0.17 ± 0.01 |
| Catechin | 0.35 ± 0.02 |
| Chlorogenic acid | 0.64 ± 0.03 |
| Caffeic acid | 0.07 ± 0.01 |
| Syringic acid | 0.06 ± 0.01 |
| Luteolin 7-glycoside | 0.16 ± 0.01 |
| Kaempferol 3-O-β-rutinoside | 0.32 ± 0.02 |
| Kaempferol 3-glycoside | 0.44 ± 0.03 |
| Hesperidin | 6.3 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Bozinou, E.; Lalas, S.I. Response Surface Optimization for the Enhancement of the Extraction of Bioactive Compounds from Citrus limon Peel. Antioxidants 2023, 12, 1605. https://doi.org/10.3390/antiox12081605
Chatzimitakos T, Athanasiadis V, Kotsou K, Bozinou E, Lalas SI. Response Surface Optimization for the Enhancement of the Extraction of Bioactive Compounds from Citrus limon Peel. Antioxidants. 2023; 12(8):1605. https://doi.org/10.3390/antiox12081605
Chicago/Turabian StyleChatzimitakos, Theodoros, Vassilis Athanasiadis, Konstantina Kotsou, Eleni Bozinou, and Stavros I. Lalas. 2023. "Response Surface Optimization for the Enhancement of the Extraction of Bioactive Compounds from Citrus limon Peel" Antioxidants 12, no. 8: 1605. https://doi.org/10.3390/antiox12081605
APA StyleChatzimitakos, T., Athanasiadis, V., Kotsou, K., Bozinou, E., & Lalas, S. I. (2023). Response Surface Optimization for the Enhancement of the Extraction of Bioactive Compounds from Citrus limon Peel. Antioxidants, 12(8), 1605. https://doi.org/10.3390/antiox12081605









