A Combination of Polymethoxyflavones from Citrus sinensis and Prenylflavonoids from Humulus lupulus Counteracts IL-1β-Induced Differentiated Caco-2 Cells Dysfunction via a Modulation of NF-κB/Nrf2 Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. PMFF and PFF Isolation
2.3. Cell Culturing and Treatments
2.4. Cell Viability
2.5. Nitrite Assay
2.6. Estimation of Combination Index
2.7. PGE2 Assay
2.8. Western Blot Analysis
2.9. Reactive Oxygen and Nitrogen Species (RONs)
2.10. GSH Measurements
2.11. Statistical Analysis
3. Results
3.1. Evaluation of PMFF and PFF Chemical Composition
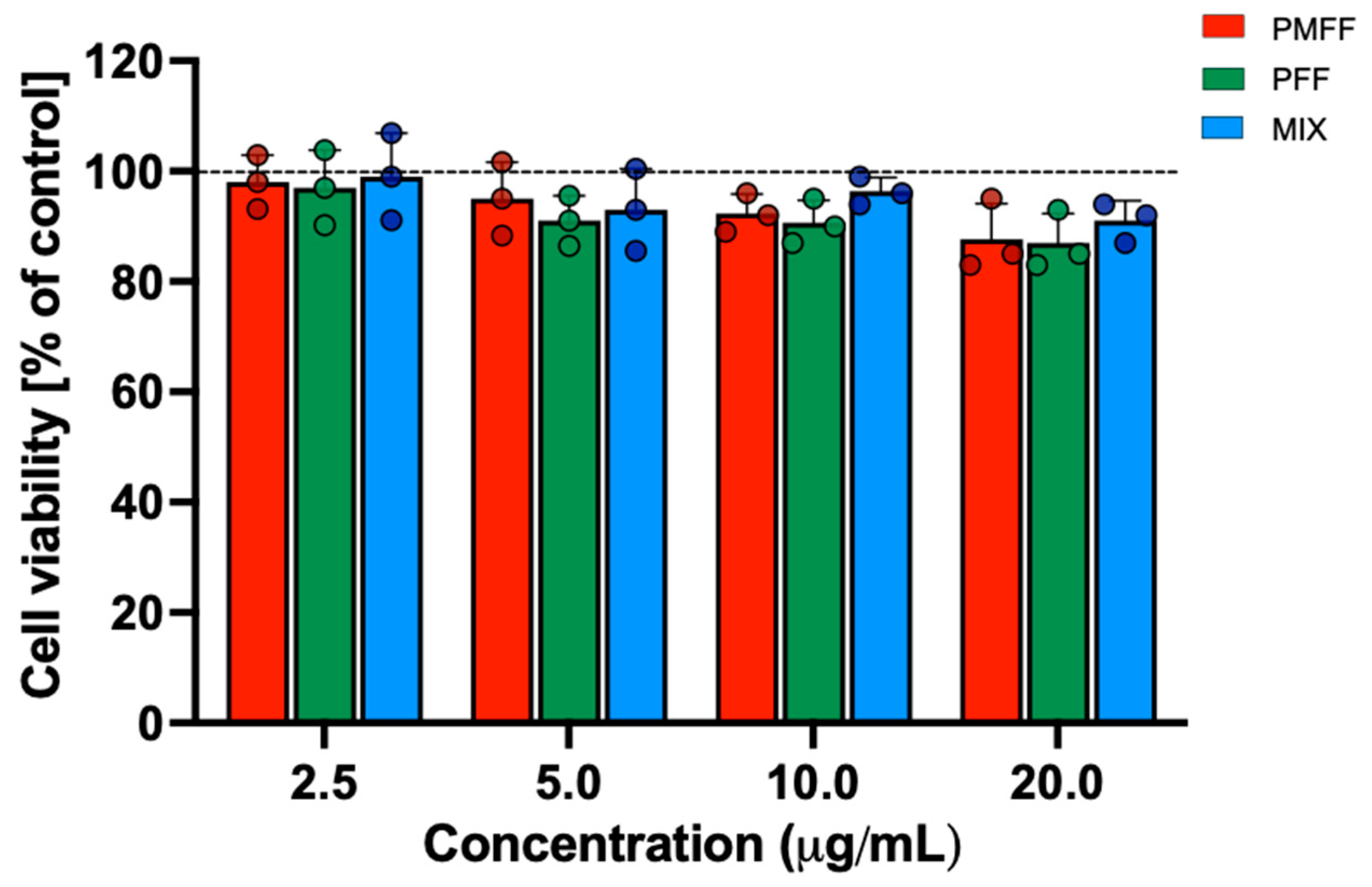
3.2. Evaluation of PMFF and PFF Cytotoxicity, Alone or in Combination, on Differentiated Caco-2 Cells
3.3. PMFF and PFF in Combination Inhibit NO and PGE2 Release in Differentiated, IL-1β-Stimulated Caco-2 Cells
3.4. PMFF and PFF in Combination Reduce iNOS and COX-2 Expression Levels in Differentiated, IL-1β-Activated Caco-2 Cells
3.5. PMFF and PFF in Combination Potentiate the Inhibition of IL-1β-Dependent Activation of NF-κB in Differentiated Caco-2 Cells
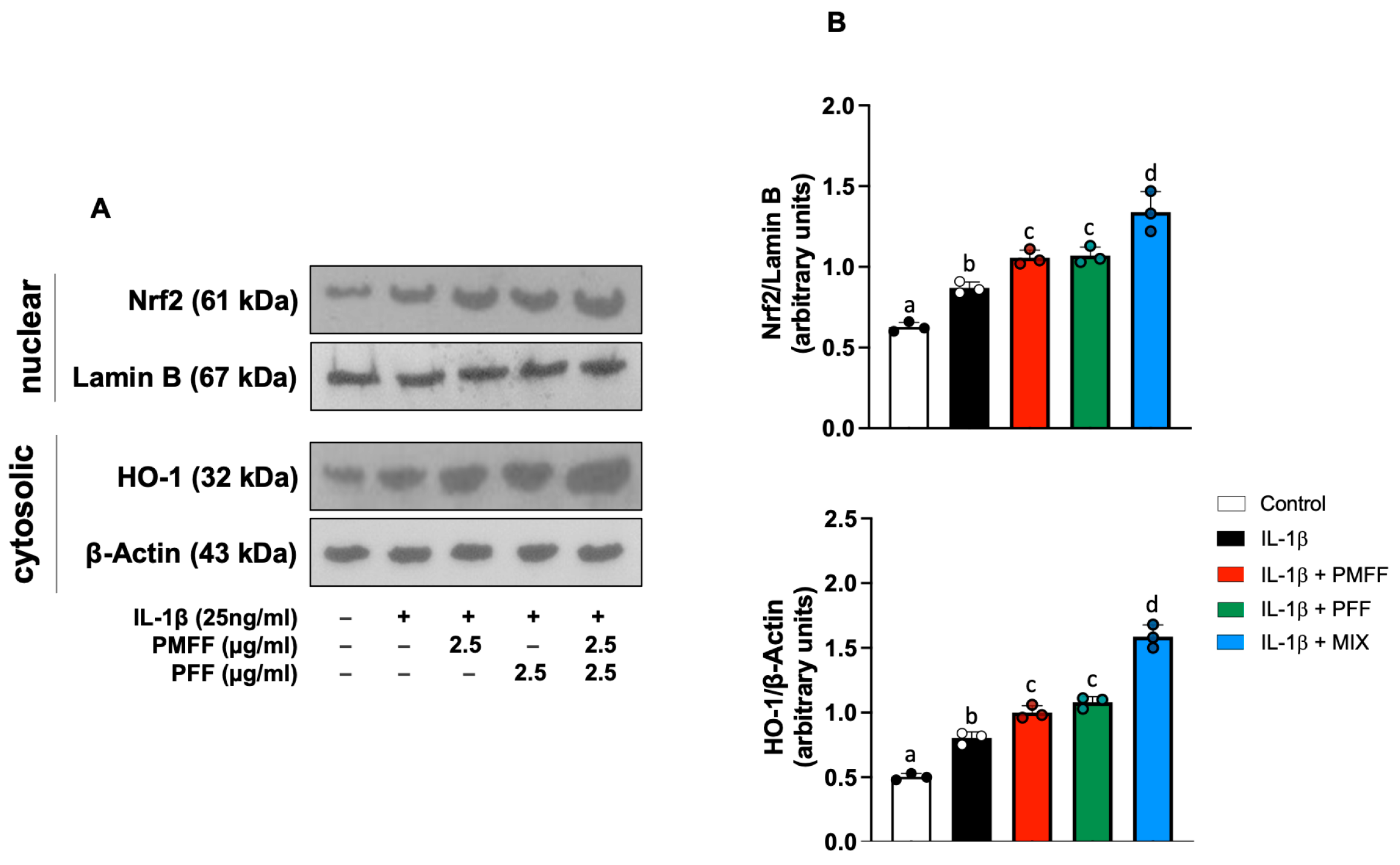
3.6. PMFF and PFF in Combination Enhances the Nuclear Translocation of Nrf2 and the Expression of HO-1 in Differentiated Caco-2 Cells
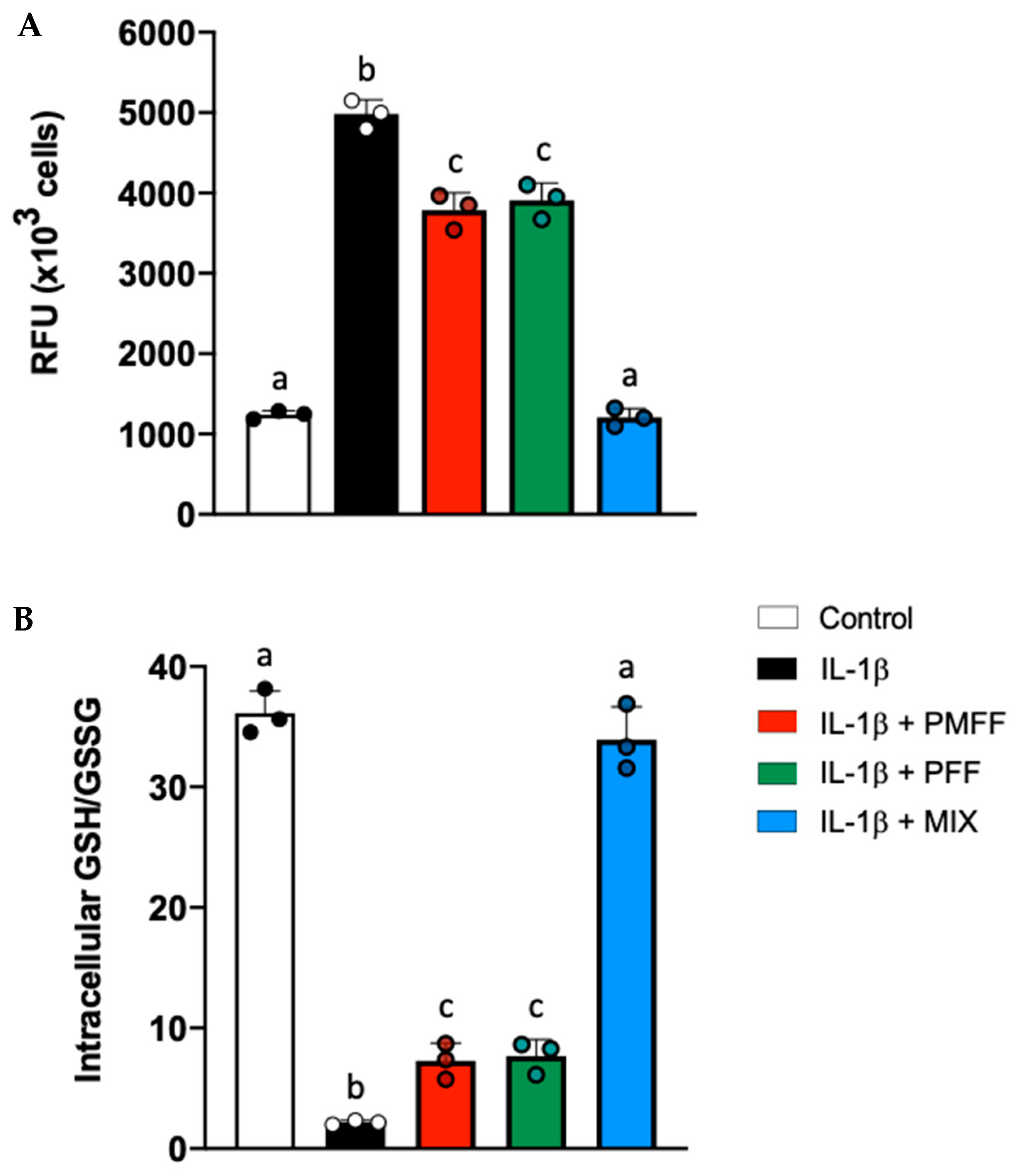
3.7. PMFF and PFF in Combination Enhances the Antioxidative Response in Differentiated, IL-1β-Activated Caco-2 Cells
3.8. NOB and XTM in Combination Synergistically Counteract Endocellular Redox Unbalance in Differentiated, IL-1β-Activated Caco-2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ko, J.; Auyeung, K. Inflammatory Bowel Disease: Etiology, Pathogenesis and Current Therapy. Curr. Pharm. Des. 2014, 20, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yang, M.F.; Liang, Y.J.; Xu, J.; Xu, H.M.; Nie, Y.Q.; Wang, L.S.; Yao, J.; Li, D.F. Immunology of Inflammatory Bowel Disease: Molecular Mechanisms and Therapeutics. J. Inflamm. Res. 2022, 15, 1825–1844. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Moura, F.A.; Goulart, M.O.F.; Campos, S.B.G.; da Paz Martins, A.S. The Close Interplay of Nitro-Oxidative Stress, Advanced Glycation End Products and Inflammation in Inflammatory Bowel Diseases. Curr. Med. Chem. 2018, 27, 2059–2076. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Y.R. Oxidative Stress and Redox Signaling Mechanisms of Inflammatory Bowel Disease: Updated Experimental and Clinical Evidence. Exp. Biol. Med. 2012, 237, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Dziąbowska-Grabias, K.; Sztanke, M.; Zając, P.; Celejewski, M.; Kurek, K.; Szkutnicki, S.; Korga, P.; Bulikowski, W.; Sztanke, K. Antioxidant Therapy in Inflammatory Bowel Diseases. Antioxidants 2021, 10, 412. [Google Scholar] [CrossRef]
- Piotrowska, M.; Swierczynski, M.; Fichna, J.; Piechota-Polanczyk, A. The Nrf2 in the Pathophysiology of the Intestine: Molecular Mechanisms and Therapeutic Implications for Inflammatory Bowel Diseases. Pharmacol. Res. 2021, 163, 105243. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-KappaB in Inflammatory Bowel Disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of Reactive Oxygen Species and NF-ΚB Signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Ligumsky, M. Role of Interleukin 1 in Inflammatory Bowel Disease-Enhanced Production during Active Disease. Gut 1990, 31, 686–689. [Google Scholar] [CrossRef]
- Reinecker, H.C.; Steffen, M.; Witthoeft, T.; Pflueger, I.; Schreiber, S.; MacDermott, R.P.; Raedler, A. Enhanced Secretion of Tumour Necrosis Factor-Alpha, IL-6, and IL-1β by Isolated Lamina Propria Mononuclear Cells from Patients with Ulcerative Colitis and Crohn’s Disease. Clin. Exp. Immunol. 1993, 94, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Van De Walle, J.; Hendrickx, A.; Romier, B.; Larondelle, Y.; Schneider, Y.J. Inflammatory Parameters in Caco-2 Cells: Effect of Stimuli Nature, Concentration, Combination and Cell Differentiation. Toxicol. In Vitro 2010, 24, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Schuerer-Maly, C.C.; Eckmann, L.; Kagnoff, M.F.; Falco, M.T.; Maly, F.E. Colonic Epithelial Cell Lines as a Source of Interleukin-8: Stimulation by Inflammatory Cytokines and Bacterial Lipopolysaccharide. Immunology 1994, 81, 85–91. [Google Scholar]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-Inflammatory Effects of Phytochemicals from Fruits, Vegetables, and Food Legumes: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic Anti-Inflammatory Effects and Mechanisms of Combined Phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, D.; Yu, C.; Lv, B.; Peng, J.; Wang, J.; Lin, Y. Citrus Nobiletin Ameliorates Experimental Colitis by Reducing Inflammation and Restoring Impaired Intestinal Barrier Function. Mol. Nutr. Food Res. 2015, 59, 829–842. [Google Scholar] [CrossRef]
- Hagenlocher, Y.; Gommeringer, S.; Held, A.; Feilhauer, K.; Köninger, J.; Bischoff, S.C.; Lorentz, A. Nobiletin Acts Anti-Inflammatory on Murine IL-10−/− Colitis and Human Intestinal Fibroblasts. Eur. J. Nutr. 2019, 58, 1391–1401. [Google Scholar] [CrossRef]
- Cho, J.M.; Yun, S.M.; Choi, Y.H.; Heo, J.; Kim, N.J.; Kim, S.H.; Kim, E.H. Xanthohumol Prevents Dextran Sulfate Sodium-Induced Colitis via Inhibition of IKKβ/NF-ΚB Signaling in Mice. Oncotarget 2018, 9, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Guo, L.; Zhao, H.; Ho, C.T. Chemistry and Bioactivity of Nobiletin and Its Metabolites. J. Funct. Foods 2014, 6, 2–10. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological Profile of Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef] [PubMed]
- Whitman, S.C.; Kurowska, E.M.; Manthey, J.A.; Daugherty, A. Nobiletin, a Citrus Flavonoid Isolated from Tangerines, Selectively Inhibits Class A Scavenger Receptor-Mediated Metabolism of Acetylated LDL by Mouse Macrophages. Atherosclerosis 2005, 178, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Raciti, G.A.; Fiory, F.; Campitelli, M.; Desiderio, A.; Spinelli, R.; Longo, M.; Nigro, C.; Pepe, G.; Sommella, E.; Campiglia, P.; et al. Citrus aurantium L. Dry Extracts Promote C/ Ebpβ Expression and Improve Adipocyte Differentiation in 3T3-L1 Cells. PLoS ONE 2018, 13, e0193704. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, W.; Zeng, S.L.; Li, P.; Liu, E.H. Chemical Structures, Bioactivities and Molecular Mechanisms of Citrus Polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Walle, T. Methoxylated Flavones, a Superior Cancer Chemopreventive Flavonoid Subclass? Semin. Cancer Biol. 2007, 17, 354–362. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, S.; Ho, C.T.; Huang, Q. Citrus Polymethoxyflavones as Regulators of Metabolic Homoeostasis: Recent Advances for Possible Mechanisms. Trends Food Sci. Technol. 2021, 110, 743–753. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, H.; Chen, J.; Cao, J.; Chen, Q.; Li, X.; Sun, C. Polymethoxyflavones from Citrus Inhibited Gastric Cancer Cell Proliferation through Inducing Apoptosis by Upregulating RARβ, Both in Vitro and in Vivo. Food Chem. Toxicol. 2020, 146, 111811. [Google Scholar] [CrossRef]
- Ke, Z.; Zhao, Y.; Tan, S.; Chen, H.; Li, Y.; Zhou, Z.; Huang, C. Citrus Reticulata Blanco Peel Extract Ameliorates Hepatic Steatosis, Oxidative Stress and Inflammation in HF and MCD Diet-Induced NASH C57BL/6 J Mice. J. Nutr. Biochem. 2020, 83, 108426. [Google Scholar] [CrossRef]
- Ambrož, M.; Lněničková, K.; Matoušková, P.; Skálová, L.; Boušová, I. Antiproliferative Effects of Hop-Derived Prenylflavonoids and Their Influence on the Efficacy of Oxaliplatine, 5-Fluorouracil and Irinotecan in Human ColorectalC Cells. Nutrients 2019, 11, 879. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated Flavonoids, Promising Nutraceuticals with Impressive Biological Activities. Trends Food Sci. Technol. 2015, 44, 93–104. [Google Scholar] [CrossRef]
- Mukai, R. Prenylation Enhances the Biological Activity of Dietary Flavonoids by Altering Their Bioavailability. Biosci. Biotechnol. Biochem. 2018, 82, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.L.; Stevens, J.F.; Ivanov, V.; Mccall, M.; Frei, B.; Deinzer, M.L.; Buhler, D.R. Antioxidant and Prooxidant Actions of Prenylated and Nonprenylated Chalcones and Flavanones in Vitro. J. Agric. Food Chem. 2000, 48, 3876–3884. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.M.; Silva, A.M.S. The Antioxidant Activity of Prenylflavonoids. Molecules 2020, 25, 696. [Google Scholar] [CrossRef] [PubMed]
- Dorn, C.; Massinger, S.; Wuzik, A.; Heilmann, J.; Hellerbrand, C. Xanthohumol Suppresses Inflammatory Response to Warm Ischemia–Reperfusion Induced Liver Injury. Exp. Mol. Pathol. 2013, 94, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Legette, L.L.; Moreno Luna, A.Y.; Reed, R.L.; Miranda, C.L.; Bobe, G.; Proteau, R.R.; Stevens, J.F. Xanthohumol Lowers Body Weight and Fasting Plasma Glucose in Obese Male Zucker Fa/Fa Rats. Phytochemistry 2013, 91, 236–241. [Google Scholar] [CrossRef]
- Gerhäuser, C. Broad Spectrum Antiinfective Potential of Xanthohumol from Hop (Humulus lupulus L.) in Comparison with Activities of Other Hop Constituents and Xanthohumol Metabolites. Mol. Nutr. Food Res. 2005, 49, 827–831. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The Inhibitory Effects of Flavonoids on α-Amylase and α-Glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef]
- Tronina, T.; Popłonski, J.; Bartmanska, A. Flavonoids as Phytoestrogenic Components of Hops and Beer. Molecules 2020, 25, 4201. [Google Scholar] [CrossRef]
- Lv, H.W.; Wang, Q.L.; Luo, M.; Zhu, M.D.; Liang, H.M.; Li, W.J.; Cai, H.; Zhou, Z.B.; Wang, H.; Tong, S.Q.; et al. Phytochemistry and Pharmacology of Natural Prenylated Flavonoids. Arch. Pharm. Res. 2023, 46, 207–272. [Google Scholar] [CrossRef]
- Shi, S.; Li, J.; Zhao, X.; Liu, Q.; Song, S.J. A Comprehensive Review: Biological Activity, Modification and Synthetic Methodologies of Prenylated Flavonoids. Phytochemistry 2021, 191, 112895. [Google Scholar] [CrossRef] [PubMed]
- Sychrová, A.; Škovranová, G.; Čulenová, M.; Bittner Fialová, S. Prenylated Flavonoids in Topical Infections and Wound Healing. Molecules 2022, 27, 4491. [Google Scholar] [CrossRef] [PubMed]
- Carbone, K.; Gervasi, F. An Updated Review of the Genus Humulus: A Valuable Source of Bioactive Compounds for Health and Disease Prevention. Plants 2022, 11, 3434. [Google Scholar] [CrossRef]
- Turdo, A.; Glaviano, A.; Pepe, G.; Calapà, F.; Raimondo, S.; Fiori, M.E.; Carbone, D.; Basilicata, M.G.; Di Sarno, V.; Ostacolo, C.; et al. Nobiletin and Xanthohumol Sensitize Colorectal Cancer Stem Cells to Standard Chemotherapy. Cancers 2021, 13, 3927. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Barreca, M.M.; Raimondo, S.; Diana, P.; Pepe, G.; Basilicata, M.G.; Conigliaro, A.; Alessandro, R. Nobiletin and Xanthohumol Counteract the TNFα-Mediated Activation of Endothelial Cells through the Inhibition of the NF-ΚB Signaling Pathway. Cell Biol. Int. 2023, 47, 634–647. [Google Scholar] [CrossRef]
- Allegra, M.; de Cicco, P.; Ercolano, G.; Attanzio, A.; Busà, R.; Cirino, G.; Tesoriere, L.; Livrea, M.A.; Ianaro, A. Indicaxanthin from Opuntia Ficus Indica (L. Mill) Impairs Melanoma Cell Proliferation, Invasiveness, and Tumor Progression. Phytomedicine 2018, 50, 19–24. [Google Scholar] [CrossRef]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R. Quantitation of Nitrate and Nitrite in Extracellular Fluids. Methods Enzymol. 1996, 268, 237–246. [Google Scholar]
- Chou, T.C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Allegra, M.; D’Acquisto, F.; Tesoriere, L.; Attanzio, A.; Livrea, M.A. Pro-Oxidant Activity of Indicaxanthin from Opuntia Ficus Indica Modulates Arachidonate Metabolism and Prostaglandin Synthesis through Lipid Peroxide Production in LPS-Stimulated RAW 264.7 Macrophages. Redox Biol. 2014, 2, 892–900. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Seubwai, W.; Wongkham, C.; Puapairoj, A.; Khuntikeo, N.; Pugkhem, A.; Hahnvajanawong, C.; Chaiyagool, J.; Umezawa, K.; Okada, S.; Wongkham, S. Aberrant Expression of NF-ΚB in Liver Fluke Associated Cholangiocarcinoma: Implications for Targeted Therapy. PLoS ONE 2014, 9, e106056. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Valatas, V.; Ward, S.G. Nitric Oxide in Inflammatory Bowel Disease: A Universal Messenger in an Unsolved Puzzle. Immunology 2004, 113, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Sheibanie, A.F.; Yen, J.-H.; Khayrullina, T.; Emig, F.; Zhang, M.; Tuma, R.; Ganea, D. The Proinflammatory Effect of Prostaglandin E2 in Experimental Inflammatory Bowel Disease Is Mediated through the IL-23→IL-17 Axis. J. Immunol. 2007, 178, 8138–8147. [Google Scholar] [CrossRef]
- Dodson, M.; Redmann, M.; Rajasekaran, N.S.; Darley-Usmar, V.; Zhang, J. KEAP1-NRF2 Signalling and Autophagy in Protection against Oxidative and Reductive Proteotoxicity. Biochem. J. 2015, 469, 347–355. [Google Scholar] [CrossRef]
- Rana, T. Influence and Implications of the Molecular Paradigm of Nitric Oxide Underlying Inflammatory Reactions of the Gastrointestinal Tract of Dog: A Major Hallmark of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 28, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Grishin, A.; Bowling, J.; Bell, B.; Wang, J.; Ford, H.R. Roles of Nitric Oxide and Intestinal Microbiota in the Pathogenesis of Necrotizing Enterocolitis. J. Pediatr. Surg. 2016, 51, 13–17. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ko, H.C.; Ko, S.Y.; Hwang, J.H.; Park, J.G.; Kang, S.H.; Han, S.H.; Yun, S.H.; Kim, S.J. Correlation between Flavonoid Content and the NO Production Inhibitory Activity of Peel Extracts from Various Citrus Fruits. Biol. Pharm. Bull. 2007, 30, 772–778. [Google Scholar] [CrossRef]
- Choi, S.Y.; Hwang, J.H.; Ko, H.C.; Park, J.G.; Kim, S.J. Nobiletin from Citrus Fruit Peel Inhibits the DNA-Binding Activity of NF-ΚB and ROS Production in LPS-Activated RAW 264.7 Cells. J. Ethnopharmacol. 2007, 113, 149–155. [Google Scholar] [CrossRef]
- Rong, X.; Xu, J.; Jiang, Y.; Li, F.; Chen, Y.; Dou, Q.P.; Li, D. Citrus Peel Flavonoid Nobiletin Alleviates Lipopolysaccharide-Induced Inflammation by Activating IL-6/STAT3/FOXO3a-Mediated Autophagy. Food Funct. 2021, 12, 1305–1317. [Google Scholar] [CrossRef]
- Funaro, A.; Wu, X.; Song, M.; Zheng, J.; Guo, S.; Rakariyatham, K.; Rodriguez-Estrada, M.T.; Xiao, H. Enhanced Anti-Inflammatory Activities by the Combination of Luteolin and Tangeretin. J. Food Sci. 2016, 81, H1320–H1327. [Google Scholar] [CrossRef]
- Laavola, M.; Nieminen, R.; Yam, M.; Sadikun, A.; Asmawi, M.; Basir, R.; Welling, J.; Vapaatalo, H.; Korhonen, R.; Moilanen, E. Flavonoids Eupatorin and Sinensetin Present in Orthosiphon Stamineus Leaves Inhibit Inflammatory Gene Expression and STAT1 Activation. Planta Med. 2012, 78, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Tohnai, N. Cyclooxygenase Isozymes and Their Gene Structures and Expression. Prostaglandins Other Lipid. Mediat. 2002, 68–69, 95–114. [Google Scholar] [CrossRef]
- Martin, G.R.; Wallace, J.L. Gastrointestinal Inflammation: A Central Component of Mucosal Defense and Repair. Exp. Biol. Med. 2006, 231, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lagunas, M.J.; Martín-Venegas, R.; Moreno, J.J.; Ferrer, R. PGE2 Promotes Ca2+-Mediated Epithelial Barrier Disruption through EP1 and EP4 Receptors in Caco-2 Cell Monolayers. Am. J. Physiol. Cell Physiol. 2010, 299, C324–C334. [Google Scholar] [CrossRef]
- Stenson, W.F. The Universe of Arachidonic Acid Metabolites in Inflammatory Bowel Disease: Can We Tell the Good from the Bad? Curr. Opin. Gastroenterol. 2014, 30, 347–351. [Google Scholar] [CrossRef]
- Yang, L.; Wu, G.; Wu, Q.; Peng, L.; Yuan, L. METTL3 Overexpression Aggravates LPS-Induced Cellular Inflammation in Mouse Intestinal Epithelial Cells and DSS-Induced IBD in Mice. Cell Death. Discov. 2022, 8, 62. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. The Role of COX-2 in Intestinal Inflammation and Colorectal Cancer. Oncogene 2010, 29, 781–788. [Google Scholar] [CrossRef]
- Dudhgaonkar, S.P.; Tandan, S.K.; Kumar, D.; Raviprakash, V.; Kataria, M. Influence of Simultaneous Inhibition of Cyclooxygenase-2 and Inducible Nitric Oxide Synthase in Experimental Colitis in Rats. Inflammopharmacology 2007, 15, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kohno, H.; Suzuki, R.; Sugie, S.; Tanaka, T. Suppression of Colitis-Related Mouse Colon Carcinogenesis by a COX-2 Inhibitor and PPAR Ligands. BMC Cancer 2005, 5, 46. [Google Scholar] [CrossRef]
- Eaden, J. Review Article: The Data Supporting a Role for Aminosalicylates in the Chemoprevention of Colorectal Cancer in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2003, 18, 15–21. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Eaden, J.; Steinhart, A.H.; Munkholm, P.; Gordon, P.H. Cancer Prevention in Inflammatory Bowel Disease and the Chemoprophylactic Potential of 5-Aminosalicylic Acid. Inflamm. Bowel Dis. 2002, 8, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Chun, K.S.; Cha, H.H.; Han, S.S.; Keum, Y.S.; Park, K.K.; Lee, S.S. Molecular Mechanisms Underlying Chemopreventive Activities of Anti-Inflammatory Phytochemicals: Down-Regulation of COX-2 and INOS through Suppression of NF-ΚB Activation. Mutat. Res. 2001, 480–481, 243–268. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Freitas, V.; Almeida, L.; Laranjinha, J. Red Wine Extract Preserves Tight Junctions in Intestinal Epithelial Cells under Inflammatory Conditions: Implications for Intestinal Inflammation. Food Funct. 2019, 10, 1364–1374. [Google Scholar] [CrossRef]
- Nunes, C.; Almeida, L.; Barbosa, R.M.; Laranjinha, J. Luteolin Suppresses the JAK/STAT Pathway in a Cellular Model of Intestinal Inflammation. Food Funct. 2017, 8, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Bernotti, S.; Seidman, E.; Sinnett, D.; Brunet, S.; Dionne, S.; Delvin, E.; Levy, E. Inflammatory Reaction without Endogenous Antioxidant Response in Caco-2 Cells Exposed to Iron/Ascorbate-Mediated Lipid Peroxidation. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G898–G906. [Google Scholar] [CrossRef]
- Zhi, Z.; Tang, X.; Wang, Y.; Chen, R.; Ji, H. Sinensetin Attenuates Amyloid Beta25-35-Induced Oxidative Stress, Inflammation, and Apoptosis in SH-SY5Y Cells Through the TLR4/NF-ΚB Signaling Pathway. Neurochem. Res. 2021, 46, 3012–3024. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, R.; Wang, R.; Dai, J.; Chen, H.; Wang, J.; Li, X. Sinensetin Attenuates IL-1β-Induced Cartilage Damage and Ameliorates Osteoarthritis by Regulating SERPINA3. Food Funct. 2022, 13, 9973–9987. [Google Scholar] [CrossRef]
- Hagenlocher, Y.; Feilhauer, K.; Schäffer, M.; Bischoff, S.C.; Lorentz, A. Citrus Peel Polymethoxyflavones Nobiletin and Tangeretin Suppress LPS- and IgE-Mediated Activation of Human Intestinal Mast Cells. Eur. J. Nutr. 2017, 56, 1609–1620. [Google Scholar] [CrossRef]
- Chang, S.N.; Dey, D.K.; Oh, S.T.; Kong, W.H.; Cho, K.H.; Al-Olayan, E.M.; Hwang, B.S.; Kang, S.C.; Park, J.G. Phorbol 12-Myristate 13-Acetate Induced Toxicity Study and the Role of Tangeretin in Abrogating Hif-1α-Nf-Κb Crosstalk in Vitro and in Vivo. Int. J. Mol. Sci. 2020, 21, 9261. [Google Scholar] [CrossRef]
- Eun, S.H.; Woo, J.T.; Kim, D.H. Tangeretin Inhibits IL-12 Expression and NF-ΚB Activation in Dendritic Cells and Attenuates Colitis in Mice. Planta Med. 2017, 83, 527–533. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase II Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Zhang, C.; Bai, X.; Zhang, J.; Zhao, X.; Chen, L.; Wang, L.; Zhu, C.; Cui, L.; et al. Nobiletin Promotes Antioxidant and Anti-Inflammatory Responses and Elicits Protection against Ischemic Stroke in Vivo. Brain Res. 2016, 1636, 130–141. [Google Scholar] [CrossRef]
- He, Z.; Li, X.; Chen, H.; He, K.; Liu, Y.; Gong, J.; Gong, J. Nobiletin Attenuates Lipopolysaccharide/D-Galactosamine-Induced Liver Injury in Mice by Activating the Nrf2 Antioxidant Pathway and Subsequently Inhibiting NF-ΚB-Mediated Cytokine Production. Mol. Med. Rep. 2016, 14, 5595–5600. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yao, Y.; Huang, H.; Hao, H.; Ying, M. Xanthohumol Attenuates Cisplatin-Induced Nephrotoxicity through Inhibiting NF-ΚB and Activating Nrf2 Signaling Pathways. Int. Immunopharmacol. 2018, 61, 277–282. [Google Scholar] [CrossRef]
- Rahman, S.U.; Ali, T.; Hao, Q.; He, K.; Li, W.; Ullah, N.; Zhang, Z.; Jiang, Y.; Li, S. Xanthohumol Attenuates Lipopolysaccharide-Induced Depressive Like Behavior in Mice: Involvement of NF-ΚB/Nrf2 Signaling Pathways. Neurochem. Res. 2021, 46, 3135–3148. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol Ameliorates Lipopolysaccharide (LPS)-Induced Acute Lung Injury via Induction of AMPK/GSK3β-Nrf2 Signal Axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef]
- Lv, C.; Li, Y.; Liang, R.; Huang, W.; Xiao, Y.; Ma, X.; Wang, Y.; Zou, H.; Qin, F.; Sun, C.; et al. Characterization of Tangeretin as an Activator of Nuclear Factor Erythroid 2-Related Factor 2/Antioxidant Response Element Pathway in HEK293T Cells. Curr. Res. Food Sci. 2023, 6, 100459. [Google Scholar] [CrossRef]
- Liang, F.; Fang, Y.; Cao, W.; Zhang, Z.; Pan, S.; Xu, X. Attenuation of Tert-Butyl Hydroperoxide (t-BHP)-Induced Oxidative Damage in HepG2 Cells by Tangeretin: Relevance of the Nrf2-ARE and MAPK Signaling Pathways. J. Agric. Food Chem. 2018, 66, 6317–6325. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Li, S.; Wu, Y.; Yu, C.; Ni, L.B.; Xiao, J.; Shao, Z.; Zhu, H.; Wang, J.; et al. Tangeretin Suppresses Osteoarthritis Progression via the Nrf2/NF-ΚB and MAPK/NF-ΚB Signaling Pathways. Phytomedicine 2022, 98, 153928. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Huang, Q.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Directly Interact with Keap1 and LPS Is Involved in the Anti-Inflammatory Mechanisms of (-)-Epicatechin-3-Gallate in LPS-Induced Macrophages and Endotoxemia. Free Radic. Biol. Med. 2016, 94, 1–16. [Google Scholar] [CrossRef]
- Keleku-Lukwete, N.; Suzuki, M.; Yamamoto, M. An Overview of the Advantages of KEAP1-NRF2 System Activation during Inflammatory Disease Treatment. Antioxid. Redox Signal. 2018, 29, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting Molecular Cross-Talk between Nrf2 and NF-ΚB Response Pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. The Effects of Stress and Aging on Glutathione Metabolism. Ageing Res. Rev. 2005, 4, 288–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Yang, J.J.; Ni, W. Immunomodulatory Effects of Sinensetin on Macrophage and Cyclophosphamide-Induced Immunosuppression in Mice. Pharmazie 2022, 77, 147–151. [Google Scholar] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restivo, I.; Basilicata, M.G.; Giardina, I.C.; Massaro, A.; Pepe, G.; Salviati, E.; Pecoraro, C.; Carbone, D.; Cascioferro, S.; Parrino, B.; et al. A Combination of Polymethoxyflavones from Citrus sinensis and Prenylflavonoids from Humulus lupulus Counteracts IL-1β-Induced Differentiated Caco-2 Cells Dysfunction via a Modulation of NF-κB/Nrf2 Activation. Antioxidants 2023, 12, 1621. https://doi.org/10.3390/antiox12081621
Restivo I, Basilicata MG, Giardina IC, Massaro A, Pepe G, Salviati E, Pecoraro C, Carbone D, Cascioferro S, Parrino B, et al. A Combination of Polymethoxyflavones from Citrus sinensis and Prenylflavonoids from Humulus lupulus Counteracts IL-1β-Induced Differentiated Caco-2 Cells Dysfunction via a Modulation of NF-κB/Nrf2 Activation. Antioxidants. 2023; 12(8):1621. https://doi.org/10.3390/antiox12081621
Chicago/Turabian StyleRestivo, Ignazio, Manuela Giovanna Basilicata, Ilenia Concetta Giardina, Alessandro Massaro, Giacomo Pepe, Emanuela Salviati, Camilla Pecoraro, Daniela Carbone, Stella Cascioferro, Barbara Parrino, and et al. 2023. "A Combination of Polymethoxyflavones from Citrus sinensis and Prenylflavonoids from Humulus lupulus Counteracts IL-1β-Induced Differentiated Caco-2 Cells Dysfunction via a Modulation of NF-κB/Nrf2 Activation" Antioxidants 12, no. 8: 1621. https://doi.org/10.3390/antiox12081621
APA StyleRestivo, I., Basilicata, M. G., Giardina, I. C., Massaro, A., Pepe, G., Salviati, E., Pecoraro, C., Carbone, D., Cascioferro, S., Parrino, B., Diana, P., Ostacolo, C., Campiglia, P., Attanzio, A., D’Anneo, A., Pojero, F., Allegra, M., & Tesoriere, L. (2023). A Combination of Polymethoxyflavones from Citrus sinensis and Prenylflavonoids from Humulus lupulus Counteracts IL-1β-Induced Differentiated Caco-2 Cells Dysfunction via a Modulation of NF-κB/Nrf2 Activation. Antioxidants, 12(8), 1621. https://doi.org/10.3390/antiox12081621















