Effects of Fisetin Treatment on Cellular Senescence of Various Tissues and Organs of Old Sheep
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Use Ethics
2.2. Histology
2.3. Senescent-Associated β-Galactosidase (SA-β-Gal) Staining
2.4. GL13 Staining Using SenTraGor Reagent for Detection of Senescent Cells
2.5. Immunofluorescence Staining
2.6. Brain Cortex, Heart, Spleen, Lung, Liver Tissue, and Bone Marrow Harvesting and Homogenization
2.7. RNA Isolation, cDNA Synthesis, and Semi-Quantitative and Quantitative-PCR (Q-PCR)
2.8. Plasma Malondialdehyde (MDA) and S100 Calcium-Binding Protein B (S100B) Measurement
2.9. Statistical Analysis
3. Results
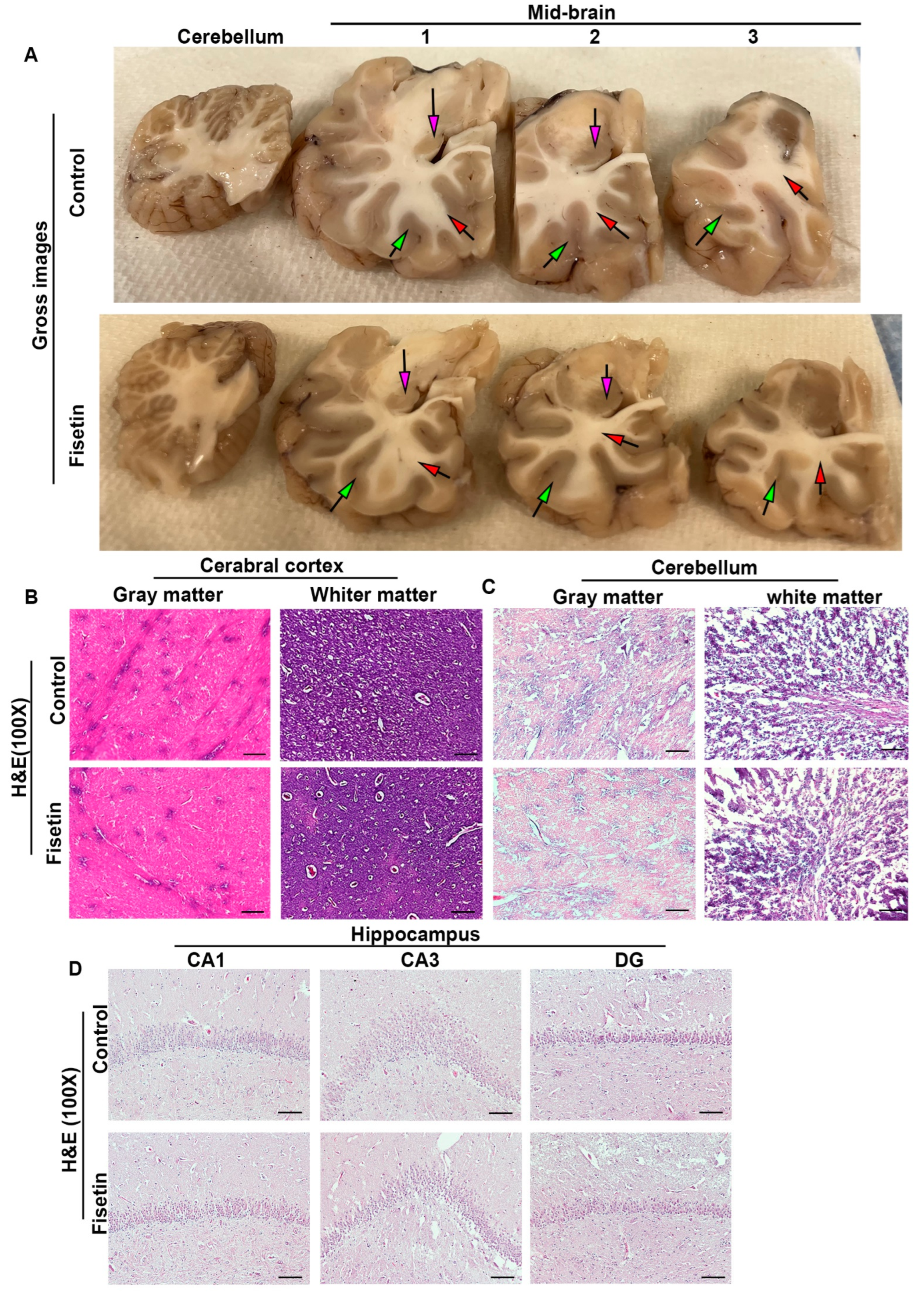
3.1. Effects of Fisetin Treatment on the General Morphology of Old Sheep Brains
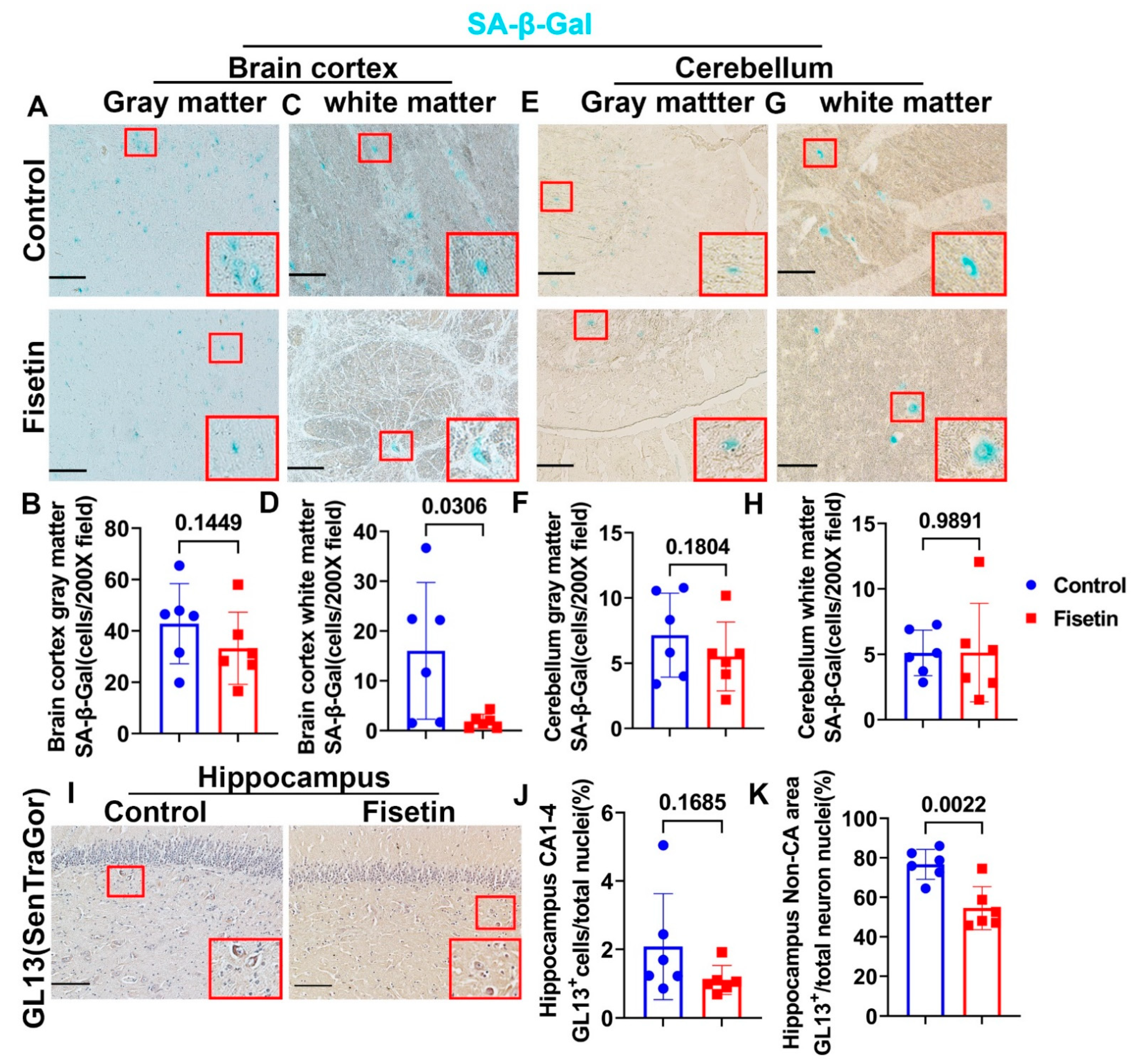
3.2. Effecta of Fisetin Treatment on the Cellular Senescence of Different Brain Regions of Old Sheep
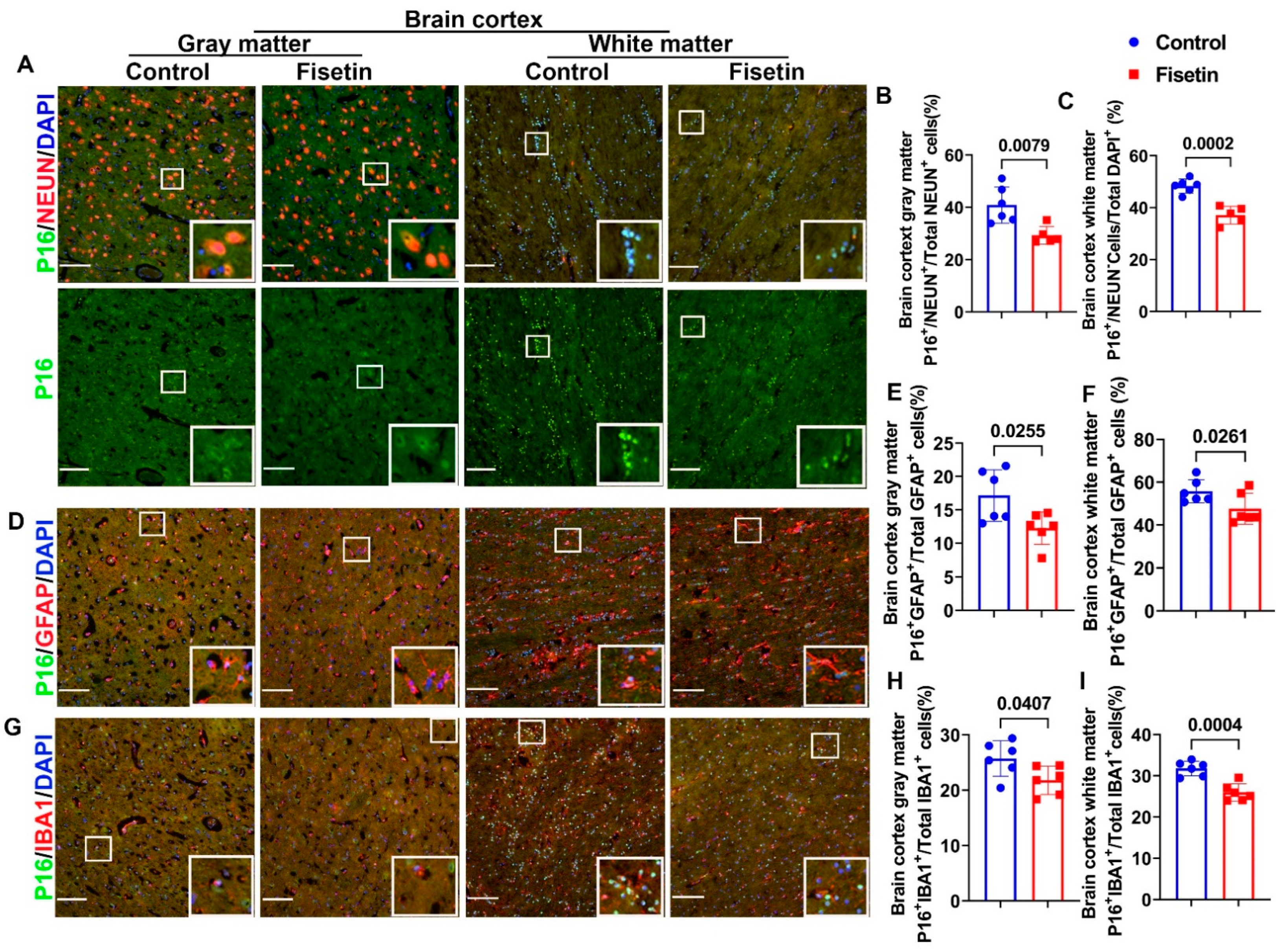
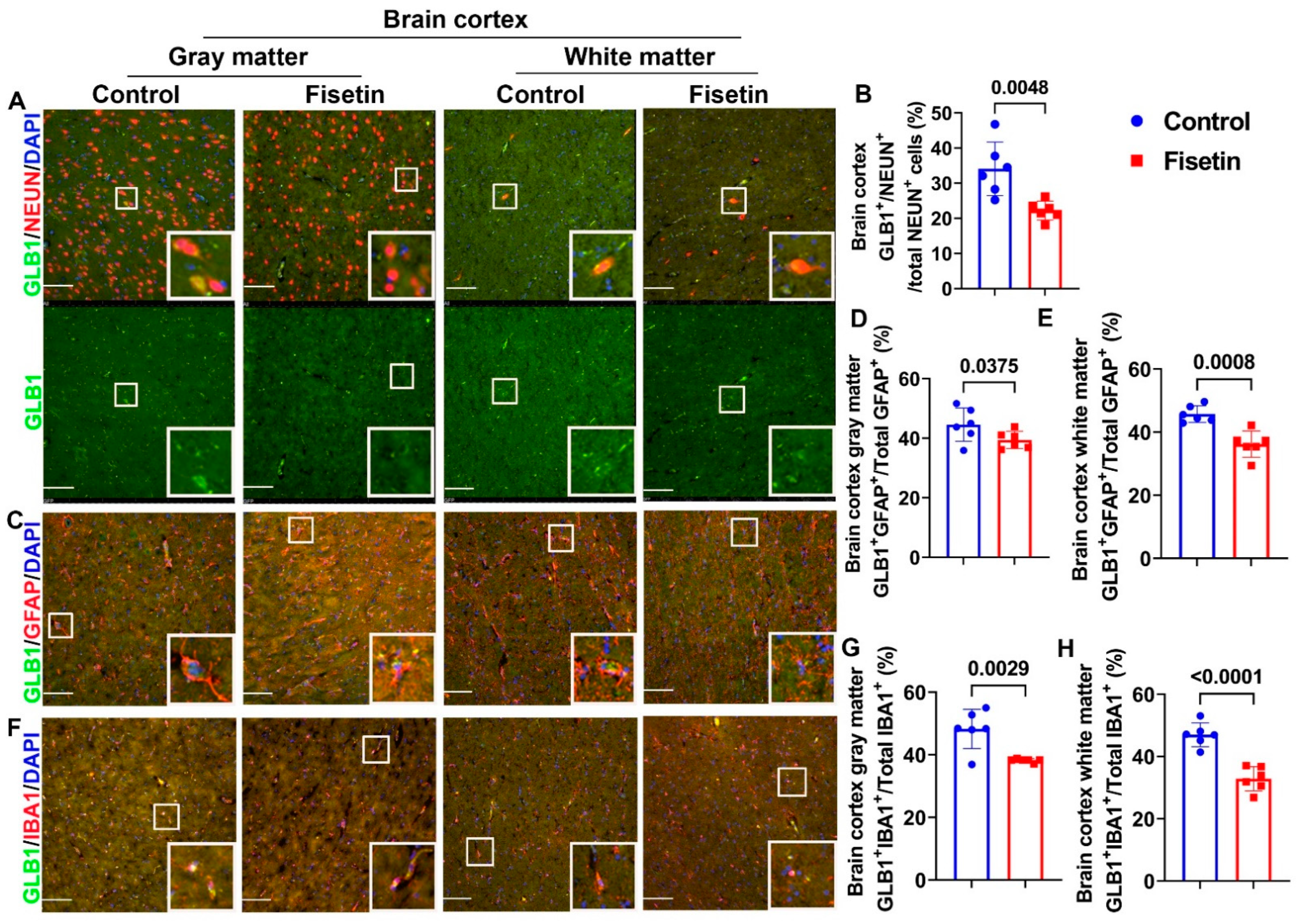
3.3. Effects of Fisetin Treatment on Cellular Senescence in Neurons, Astrocytes, and Microglial Cells of the Cerebral Cortex
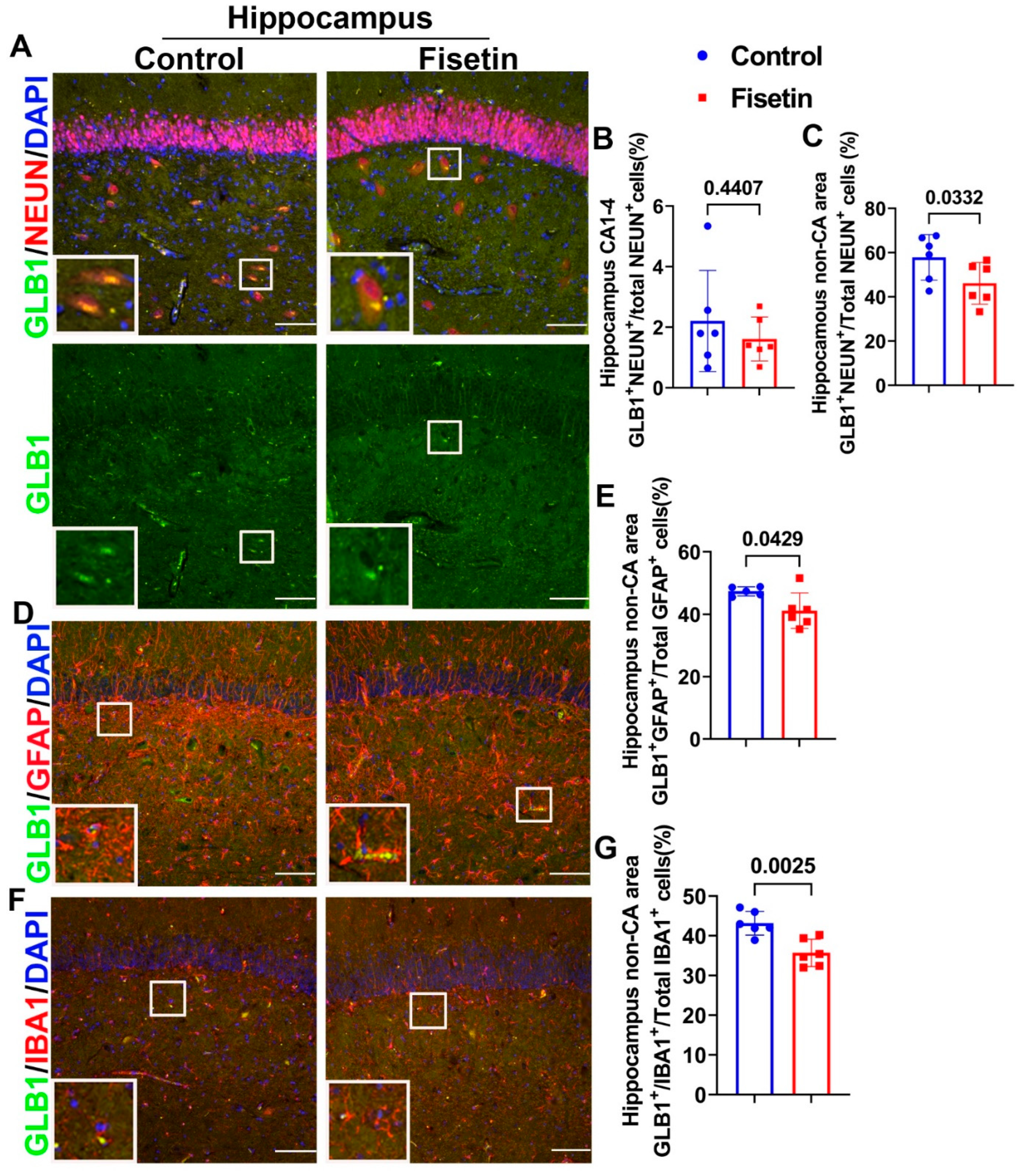
3.4. Effects of Fisetin Treatments on Cellular Senescence of Neurons, Astrocytes, and Microglial Cells of the Hippocampus
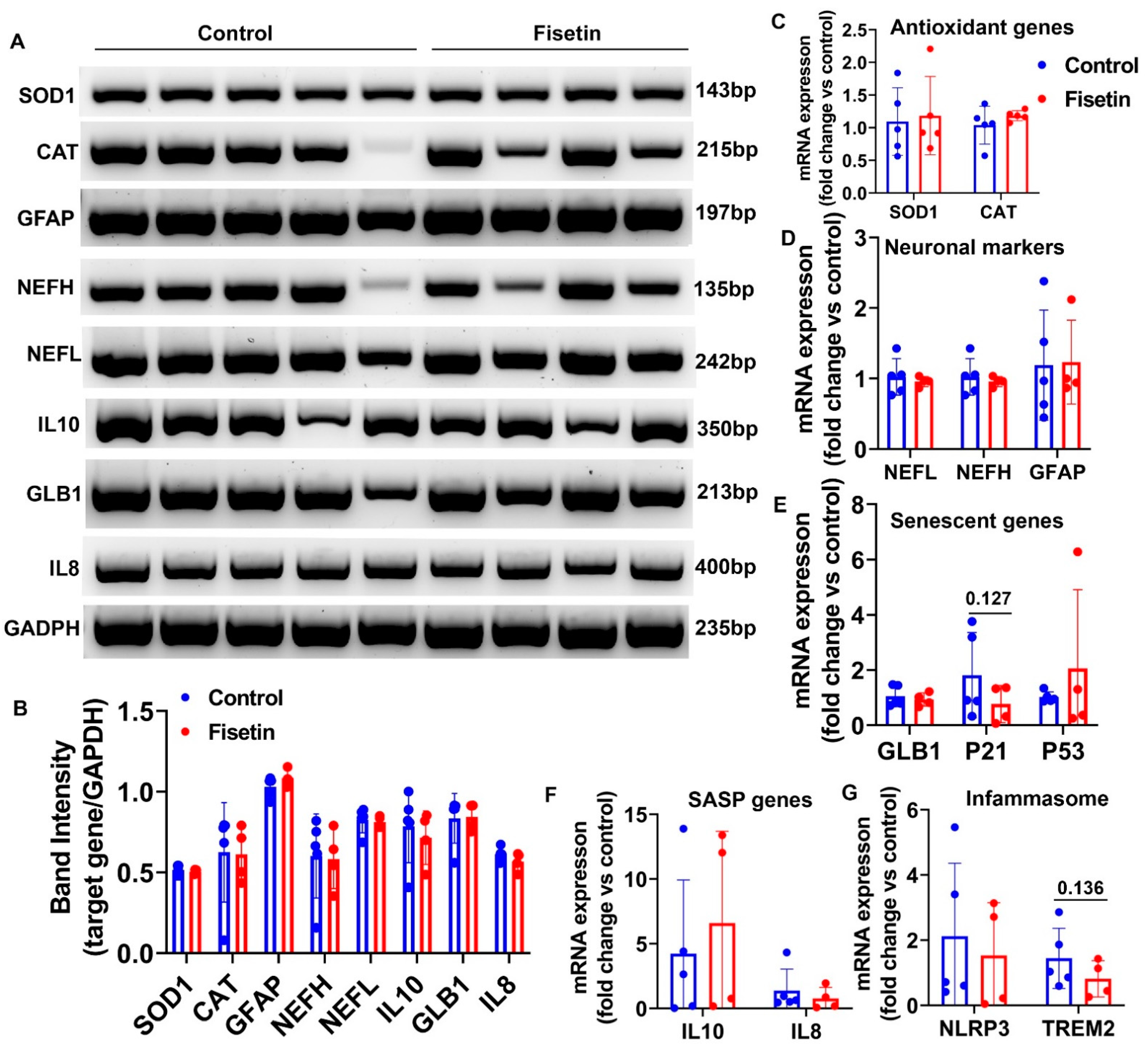
3.5. Effects of Fisetin Treatment on Gene Expression of Sheep Brain Cortex
3.6. Effect of Fisetin Treatment on Gene Expression in Heart Tissues
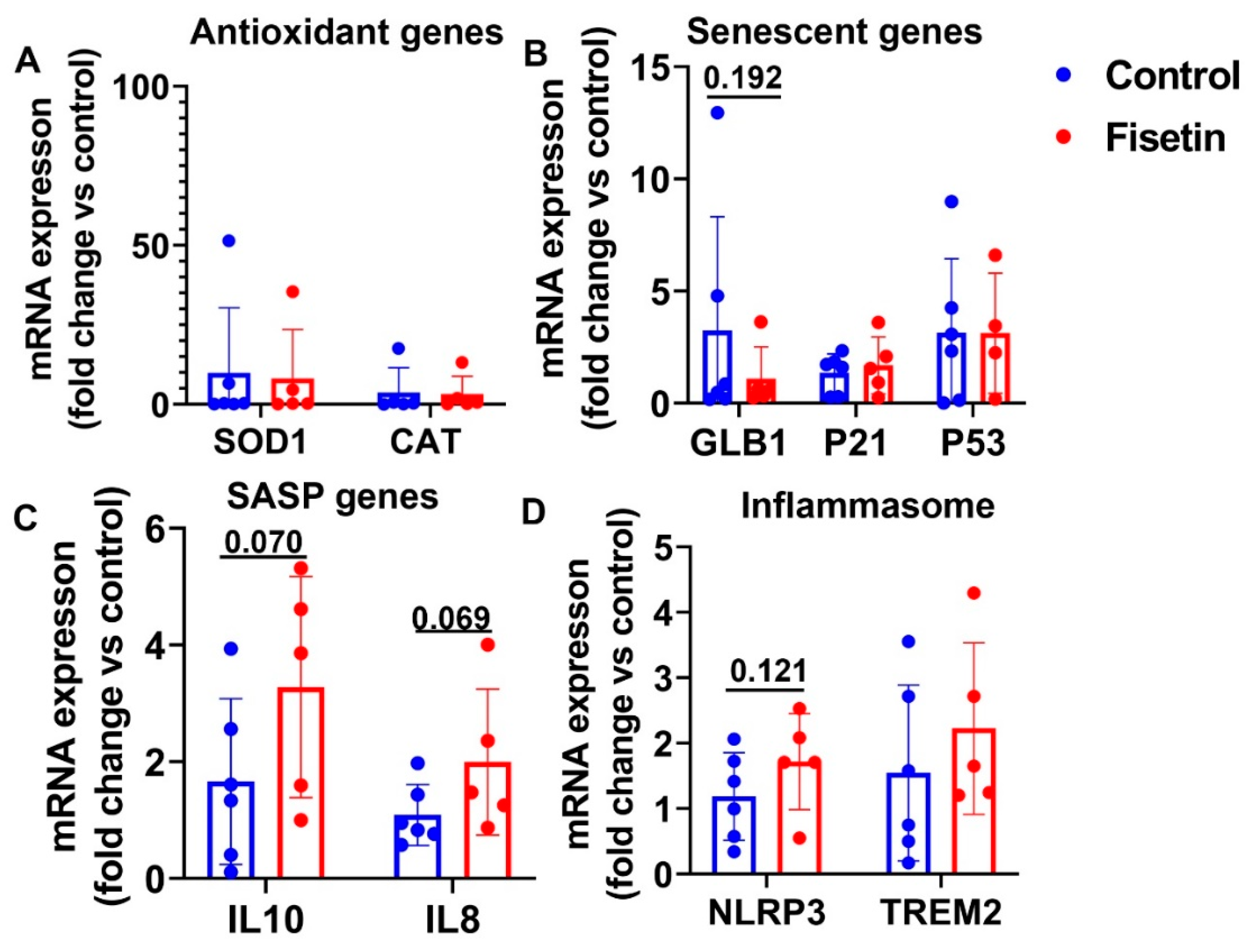
3.7. Effects of Fisetin Treatment on Sheep Spleen Tissue Gene Expression
3.8. Effects of Fisetin Treatment on Gene Expression of Bone Marrow Nucleated Cells
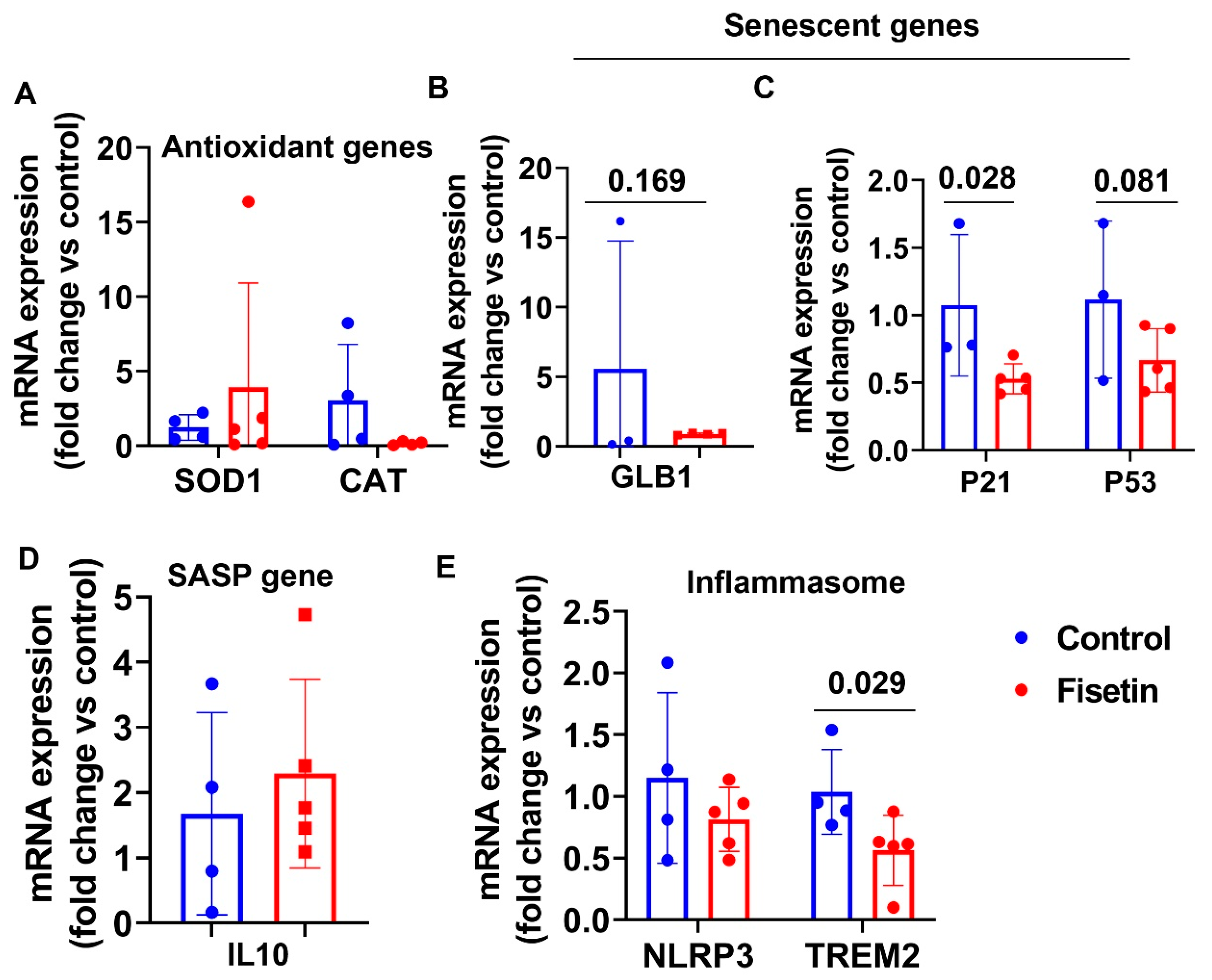
3.9. Effects of Fisetin Treatment on Cellular Gene Expression in Lung Tissues
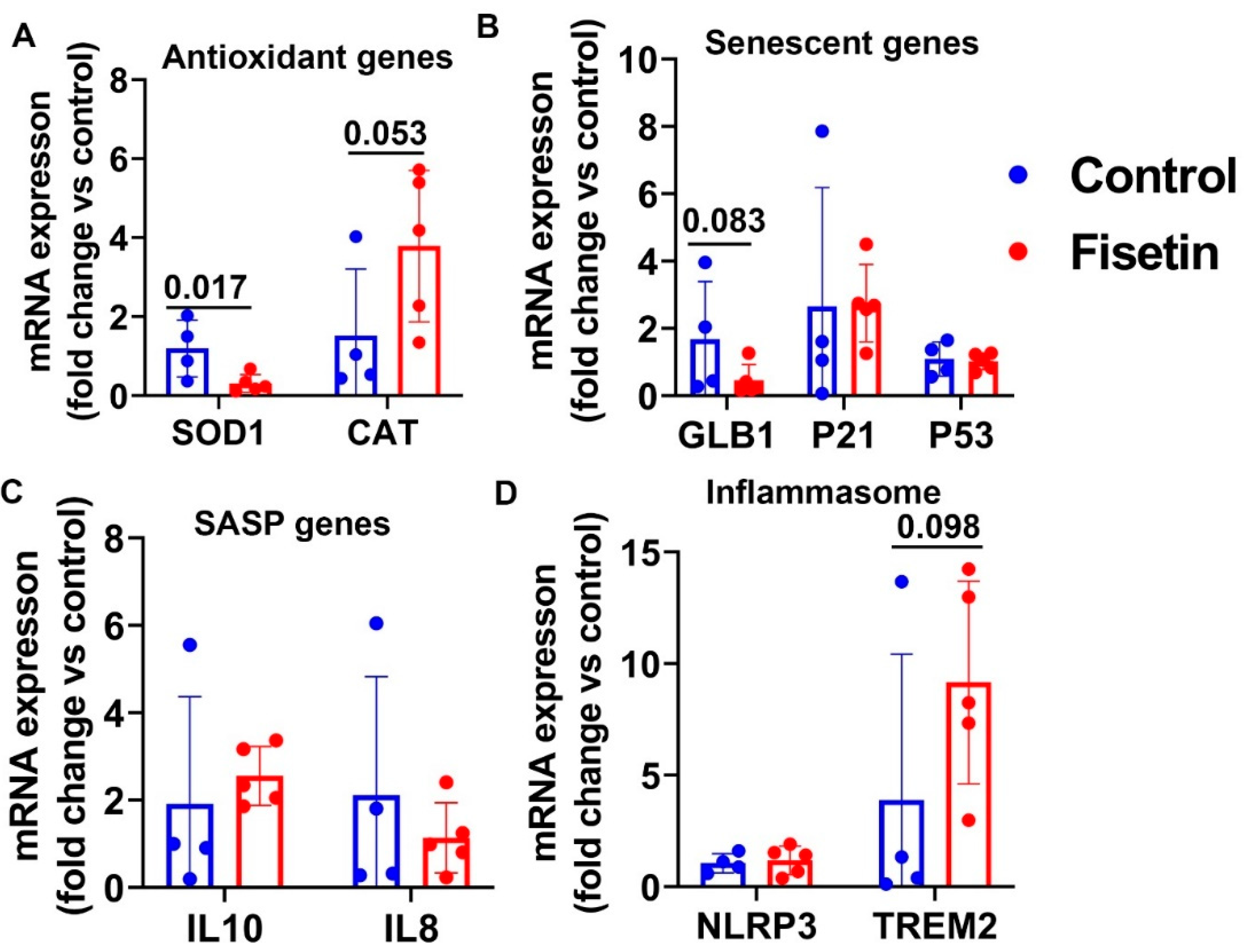
3.10. Effects of Fisetin Treatment on the Gene Expression of the Liver of Old Sheep
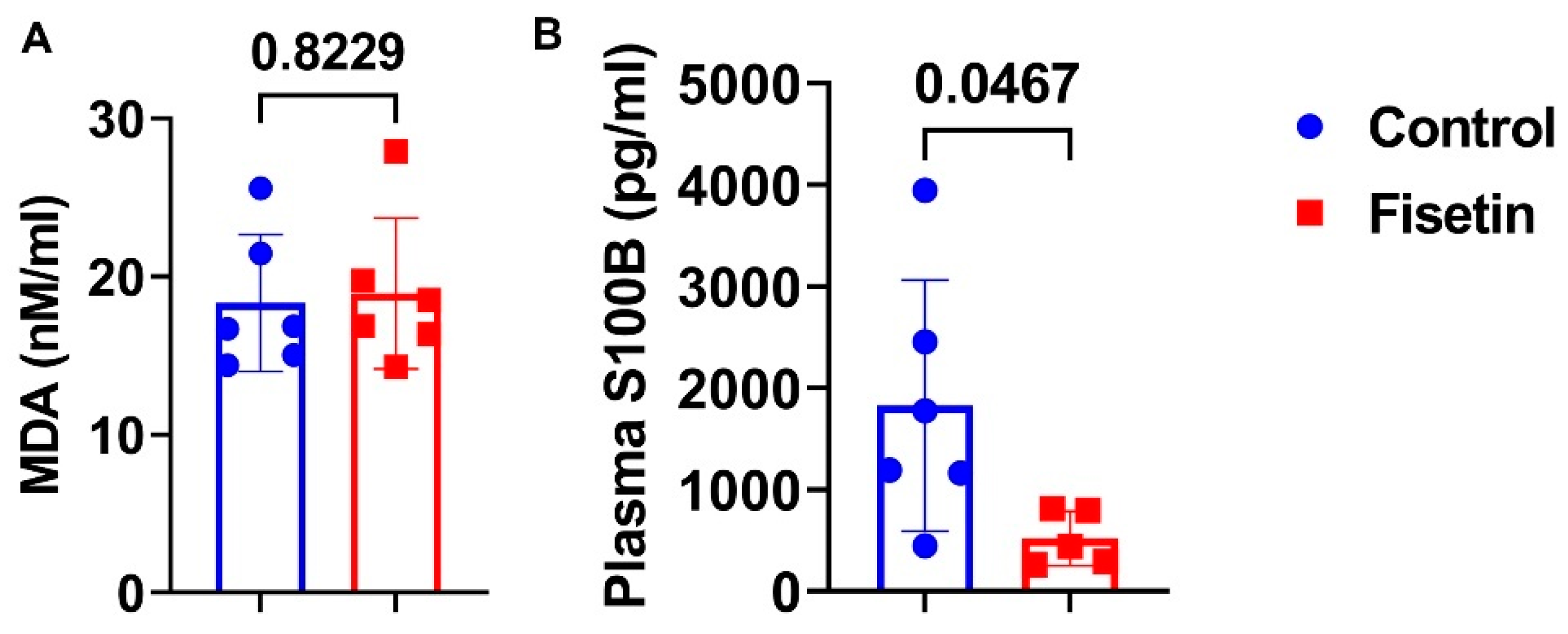
3.11. Effects of Fisetin Treatment on Plasma MDA and S100B
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.; Kim, B.K.; Park, S.K. Effects of Fisetin, a Plant-Derived Flavonoid, on Response to Oxidative Stress, Aging, and Age-Related Diseases in Caenorhabditis elegans. Pharmaceuticals 2022, 15, 1528. [Google Scholar] [CrossRef]
- Maher, P.; Akaishi, T.; Abe, K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc. Natl. Acad. Sci. USA 2006, 103, 16568–16573. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. A comparison of the neurotrophic activities of the flavonoid fisetin and some of its derivatives. Free Radic. Res. 2006, 40, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. The flavonoid fisetin promotes nerve cell survival from trophic factor withdrawal by enhancement of proteasome activity. Arch. Biochem. Biophys. 2008, 476, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. Modulation of multiple pathways involved in the maintenance of neuronal function during aging by fisetin. Genes Nutr. 2009, 4, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Ehren, J.L.; Maher, P. Concurrent regulation of the transcription factors Nrf2 and ATF4 mediates the enhancement of glutathione levels by the flavonoid fisetin. Biochem. Pharmacol. 2013, 85, 1816–1826. [Google Scholar] [CrossRef]
- Currais, A.; Prior, M.; Dargusch, R.; Armando, A.; Ehren, J.; Schubert, D.; Quehenberger, O.; Maher, P. Modulation of p25 and inflammatory pathways by fisetin maintains cognitive function in Alzheimer’s disease transgenic mice. Aging Cell 2014, 13, 379–390. [Google Scholar] [CrossRef]
- Currais, A.; Farrokhi, C.; Dargusch, R.; Armando, A.; Quehenberger, O.; Schubert, D.; Maher, P. Fisetin Reduces the Impact of Aging on Behavior and Physiology in the Rapidly Aging SAMP8 Mouse. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 299–307. [Google Scholar] [CrossRef]
- Cho, I.; Song, H.O.; Cho, J.H. Flavonoids mitigate neurodegeneration in aged Caenorhabditis elegans by mitochondrial uncoupling. Food Sci. Nutr. 2020, 8, 6633–6642. [Google Scholar] [CrossRef]
- Das, J.; Singh, R.; Ladol, S.; Nayak, S.K.; Sharma, D. Fisetin prevents the aging-associated decline in relative spectral power of alpha, beta and linked MUA in the cortex and behavioral alterations. Exp. Gerontol. 2020, 138, 111006. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, Y.; Wu, Q.; Yang, J.; Chen, J.; Zhong, S.; Eliezer, D.; Tan, Q.; Wu, C. Fisetin inhibits tau aggregation by interacting with the protein and preventing the formation of beta-strands. Int. J. Biol. Macromol. 2021, 178, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, A.K.; Garg, G.; Rizvi, S.I. Fisetin as a caloric restriction mimetic protects rat brain against aging induced oxidative stress, apoptosis and neurodegeneration. Life Sci 2018, 193, 171–179. [Google Scholar] [CrossRef]
- Singh, S.; Garg, G.; Singh, A.K.; Bissoyi, A.; Rizvi, S.I. Fisetin, a potential caloric restriction mimetic, attenuates senescence biomarkers in rat erythrocytes. Biochem Cell Biol 2019, 97, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, A.; Ali, W.; Jo, M.H.; Park, J.; Ikram, M.; Kim, M.O. Fisetin Rescues the Mice Brains Against D-Galactose-Induced Oxidative Stress, Neuroinflammation and Memory Impairment. Front Pharmacol 2021, 12, 612078. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Noh, J.S.; Jung, Y.; Leem, S.H.; Hyun, J.W.; Chang, Y.C.; Kwon, T.K.; Kim, G.Y.; Lee, H.; Choi, Y.H. Fisetin Attenuated Oxidative Stress-Induced Cellular Damage in ARPE-19 Human Retinal Pigment Epithelial Cells Through Nrf2-Mediated Activation of Heme Oxygenase-1. Front Pharmacol 2022, 13, 927898. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Cha, H.J.; Kim, D.H.; Kwon, C.Y.; Park, S.H.; Hong, S.H.; Bang, E.; Cheong, J.; Kim, G.Y.; Choi, Y.H. Fisetin Protects C2C12 Mouse Myoblasts from Oxidative Stress-Induced Cytotoxicity through Regulation of the Nrf2/HO-1 Signaling. J. Microbiol. Biotechnol. 2023, 33, 591–599. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Elsallabi, O.; Patruno, A.; Pesce, M.; Cataldi, A.; Carradori, S.; Gallorini, M. Fisetin as a Senotherapeutic Agent: Biopharmaceutical Properties and Crosstalk between Cell Senescence and Neuroprotection. Molecules 2022, 27, 738. [Google Scholar] [CrossRef]
- Liu, L.; Yue, X.; Sun, Z.; Hambright, W.S.; Feng, Q.; Cui, Y.; Huard, J.; Robbins, P.D.; Wang, Z.; Mu, X. Senolytic elimination of senescent macrophages restores muscle stem cell function in severely dystrophic muscle. Aging 2022, 14, 7650–7661. [Google Scholar] [CrossRef]
- Liu, L.; Yue, X.; Sun, Z.; Hambright, W.S.; Wei, J.; Li, Y.; Matre, P.; Cui, Y.; Wang, Z.; Rodney, G.; et al. Reduction of senescent fibro-adipogenic progenitors in progeria-aged muscle by senolytics rescues the function of muscle stem cells. J. Cachexia Sarcopenia Muscle 2022, 13, 3137–3148. [Google Scholar] [CrossRef] [PubMed]
- Mullen, M.; Nelson, A.L.; Goff, A.; Billings, J.; Kloser, H.; Huard, C.; Mitchell, J.; Hambright, W.S.; Ravuri, S.; Huard, J. Fisetin Attenuates Cellular Senescence Accumulation During Culture Expansion of Human Adipose-Derived Stem Cells. Stem. Cells 2023, 41, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Livingston, M.J.; Ma, Z.; Hu, X.; Wen, L.; Ding, H.F.; Zhou, D.; Dong, Z. Tubular cell senescence promotes maladaptive kidney repair and chronic kidney disease after cisplatin nephrotoxicity. JCI Insight 2023, 8, e166643. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.Y.; Kim, J.; Han, S.J. The flavonoid fisetin ameliorates renal fibrosis by inhibiting SMAD3 phosphorylation, oxidative damage, and inflammation in ureteral obstructed kidney in mice. Kidney Res. Clin. Pract. 2023, 42, 325–339. [Google Scholar] [CrossRef]
- Chen, Y.P.; Sivalingam, K.; Shibu, M.A.; Peramaiyan, R.; Day, C.H.; Shen, C.Y.; Lai, C.H.; Chen, R.J.; Viswanadha, V.P.; Chen, Y.F.; et al. Protective effect of Fisetin against angiotensin II-induced apoptosis by activation of IGF-IR-PI3K-Akt signaling in H9c2 cells and spontaneous hypertension rats. Phytomedicine 2019, 57, 1–8. [Google Scholar] [CrossRef]
- Yan, L.; Jia, Q.; Cao, H.; Chen, C.; Xing, S.; Huang, Y.; Shen, D. Fisetin ameliorates atherosclerosis by regulating PCSK9 and LOX-1 in apoE(-/-) mice. Exp. Ther. Med. 2021, 21, 25. [Google Scholar] [CrossRef]
- Kim, S.G.; Sung, J.Y.; Kang, Y.J.; Choi, H.C. Fisetin alleviates cellular senescence through PTEN mediated inhibition of PKCdelta-NOX1 pathway in vascular smooth muscle cells. Arch. Gerontol. Geriatr. 2023, 108, 104927. [Google Scholar] [CrossRef]
- Evangelou, K.; Lougiakis, N.; Rizou, S.V.; Kotsinas, A.; Kletsas, D.; Munoz-Espin, D.; Kastrinakis, N.G.; Pouli, N.; Marakos, P.; Townsend, P.; et al. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 2017, 16, 192–197. [Google Scholar] [CrossRef]
- Gao, X.; Usas, A.; Proto, J.D.; Lu, A.; Cummins, J.H.; Proctor, A.; Chen, C.W.; Huard, J. Role of donor and host cells in muscle-derived stem cell-mediated bone repair: Differentiation vs. paracrine effects. FASEB J. 2014, 28, 3792–3809. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3--new capabilities and interfaces. Nucleic. Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Beausejour, C.M.; Krtolica, A.; Galimi, F.; Narita, M.; Lowe, S.W.; Yaswen, P.; Campisi, J. Reversal of human cellular senescence: Roles of the p53 and p16 pathways. EMBO J. 2003, 22, 4212–4222. [Google Scholar] [CrossRef] [PubMed]
- Wissler Gerdes, E.O.; Zhu, Y.; Weigand, B.M.; Tripathi, U.; Burns, T.C.; Tchkonia, T.; Kirkland, J.L. Cellular senescence in aging and age-related diseases: Implications for neurodegenerative diseases. Int. Rev. Neurobiol. 2020, 155, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef]
- Song, S.; Tchkonia, T.; Jiang, J.; Kirkland, J.L.; Sun, Y. Targeting Senescent Cells for a Healthier Aging: Challenges and Opportunities. Adv. Sci. 2020, 7, 2002611. [Google Scholar] [CrossRef]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Xu, M.; Tchkonia, T.; Ding, H.; Ogrodnik, M.; Lubbers, E.R.; Pirtskhalava, T.; White, T.A.; Johnson, K.O.; Stout, M.B.; Mezera, V.; et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. USA 2015, 112, E6301–E6310. [Google Scholar] [CrossRef]
- Xu, M.; Palmer, A.K.; Ding, H.; Weivoda, M.M.; Pirtskhalava, T.; White, T.A.; Sepe, A.; Johnson, K.O.; Stout, M.B.; Giorgadze, N.; et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 2015, 4, e12997. [Google Scholar] [CrossRef]
- Farr, J.N.; Xu, M.; Weivoda, M.M.; Monroe, D.G.; Fraser, D.G.; Onken, J.L.; Negley, B.A.; Sfeir, J.G.; Ogrodnik, M.B.; Hachfeld, C.M.; et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 2017, 23, 1072–1079. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Maher, P.A. Using Plants as a Source of Potential Therapeutics for the Treatment of Alzheimer’s Disease. Yale. J. Biol. Med. 2020, 93, 365–373. [Google Scholar]
- Maher, P. Preventing and Treating Neurological Disorders with the Flavonol Fisetin. Brain. Plast. 2021, 6, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Ravula, A.R.; Teegala, S.B.; Kalakotla, S.; Pasangulapati, J.P.; Perumal, V.; Boyina, H.K. Fisetin, potential flavonoid with multifarious targets for treating neurological disorders: An updated review. Eur. J. Pharmacol. 2021, 910, 174492. [Google Scholar] [CrossRef]
- Gruendler, R.; Hippe, B.; Sendula Jengic, V.; Peterlin, B.; Haslberger, A.G. Nutraceutical Approaches of Autophagy and Neuroinflammation in Alzheimer’s Disease: A Systematic Review. Molecules 2020, 25, 6018. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.; Gonzales, M.; Garbarino, V.; Kautz, T.; Palavicini, J.; Lopez-Cruzan, M.; Dehkordi, S.K.; Mathews, J.; Zare, H.; Xu, P.; et al. Senolytic therapy to modulate the progression of Alzheimer’s Disease (SToMP-AD)-Outcomes from the first clinical trial of senolytic therapy for Alzheimer’s disease. Res. Sq. 2023, preprint. [Google Scholar] [CrossRef]
- Piechota, M.; Sunderland, P.; Wysocka, A.; Nalberczak, M.; Sliwinska, M.A.; Radwanska, K.; Sikora, E. Is senescence-associated beta-galactosidase a marker of neuronal senescence? Oncotarget 2016, 7, 81099–81109. [Google Scholar] [CrossRef]
- Michetti, F.; Clementi, M.E.; Di Liddo, R.; Valeriani, F.; Ria, F.; Rende, M.; Di Sante, G.; Romano Spica, V. The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker. Int. J. Mol. Sci. 2023, 24, 9605. [Google Scholar] [CrossRef]
- Michetti, F.; D’Ambrosi, N.; Toesca, A.; Puglisi, M.A.; Serrano, A.; Marchese, E.; Corvino, V.; Geloso, M.C. The S100B story: From biomarker to active factor in neural injury. J. Neurochem. 2019, 148, 168–187. [Google Scholar] [CrossRef]
- Antignano, I.; Liu, Y.; Offermann, N.; Capasso, M. Aging microglia. Cell. Mol. Life Sci. 2023, 80, 126. [Google Scholar] [CrossRef]
- Ng, P.Y.; McNeely, T.L.; Baker, D.J. Untangling senescent and damage-associated microglia in the aging and diseased brain. FEBS J. 2023, 290, 1326–1339. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.Y.; Chang, P.C.; Shen, Y.C.; Lin, C.; Tsai, C.F.; Chen, J.H.; Yeh, W.L.; Wu, L.H.; Lin, H.Y.; Liu, Y.S.; et al. Regulatory effects of fisetin on microglial activation. Molecules 2014, 19, 8820–8839. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yao, L.; Cui, J.; Liu, B. Fisetin Protects against Intracerebral Hemorrhage-Induced Neuroinflammation in Aged Mice. Cerebrovasc Dis. 2018, 45, 154–161. [Google Scholar] [CrossRef]
- Cordaro, M.; D’Amico, R.; Fusco, R.; Peritore, A.F.; Genovese, T.; Interdonato, L.; Franco, G.; Arangia, A.; Gugliandolo, E.; Crupi, R.; et al. Discovering the Effects of Fisetin on NF-kappaB/NLRP-3/NRF-2 Molecular Pathways in a Mouse Model of Vascular Dementia Induced by Repeated Bilateral Carotid Occlusion. Biomedicines 2022, 10, 1448. [Google Scholar] [CrossRef]
- Maher, P. Modulation of the Neuroprotective and Anti-inflammatory Activities of the Flavonol Fisetin by the Transition Metals Iron and Copper. Antioxidants 2020, 9, 1113. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Liu, C.; Xue, R.; Wang, Y.; Sun, Y.; Liang, Z.; Fan, W.; Jiang, J.; Zhao, J.; Su, Q.; et al. Fisetin inhibits cardiac hypertrophy by suppressing oxidative stress. J. Nutr. Biochem. 2018, 62, 221–229. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Athapaththu, A.; Choi, Y.H.; Park, C.; Jin, C.Y.; Kang, C.H.; Lee, M.H.; Kim, G.Y. Fisetin Inhibits NLRP3 Inflammasome by Suppressing TLR4/MD2-Mediated Mitochondrial ROS Production. Antioxidants 2021, 10, 1215. [Google Scholar] [CrossRef]



| Accession # | Gene ID | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Product Size (bp) |
|---|---|---|---|---|
| XM_018065254.1 | Goat GFAP | caggatctgctcaacgtcaa | atctccacggtcttcaccac | 197 |
| XM_015102219.3 | Sheep GLB1 | agtccccctacctacgcact | ggtcgaagtcaccaggatgt | 213 |
| NM_001145185.2 | Sheep SOD1 | ggttccacgtccatcagttt | tttgtcagccttcacattgc | 143 |
| XM_005690077.3 | Goat CAT | ctcgtccaggatgtggtttt | gagcctgattctccagcaac | 215 |
| XM_042234806.1 | Sheep NEFH | acatcgcatcctaccaggag | cagcgatctcaatgtccaga | 135 |
| XM_015093090.3 | Sheep NEFL | aagcgcatagacagcctgat | ctctcggtcagcacagtgaa | 242 |
| HM043737.1 | Sheep GAPDH | acagtcaaggcagagaacgg | ggttcacgcccatcacaaac | 235 |
| X78306.1 | Sheep IL8 | tcgatgccaatgcataaaaa | ttggggtctaagcacacctc | 147 |
| NM_001009327.1 | Sheep IL10 | tgttgacccagtctctgctg | ttcacgtgctccttgatgtc | 136 |
| FJ943992.1 | Sheep P21 | gagagcgatggaacttcgac | agtggtcctcctgagacgtg | 186 |
| FJ855223.1 | Sheep P53 | cctgctcccgtactcagaag | ctggcagaacagcttgttga | 247 |
| XM_042250404.1 | Sheep NLRP3 | ctgtgcacacggtggtattc | ctctgagtcccaaggctcac | 157 |
| XM_004018807.5 | Sheep TREM2 | agcctttcggaagaggagag | agctggtaacctgggttgtg | 179 |
| Staining Methods | Brain Cortex Gray Matter (p Value) | Brain Cortex White Matter (p Value) | Hippocampus CA1-4 Area (p Value) | Hippocampus (Non-CA Area) (p Value) | Cerebellum Gray Matter (p Value) | Cerebellum White Matter (p Value) |
|---|---|---|---|---|---|---|
| SA-β-Gal staining | 0.1449 | 0.0306 | N/D | N/D | 0.1804 | 0.9891 |
| GL13 staining | N/D | N/D | 0.1685 | 0.0022 | N/D | N/D |
| P16+/NEUN+ (%) | 0.0079 | N/A | 0.7446 | N/D | N/D | N/D |
| P16+/NEUN−/DAPI (%) | N/A | 0.0002 | N/D | N/D | N/D | N/D |
| P16+/GFAP+ (%) | 0.0255 | 0.0261 | N/A | 0.1076 | N/D | N/D |
| P16+/IBA1+ (%) | 0.0407 | 0.0004 | N/A | 0.0102 | N/D | N/D |
| GLB1+/NEUN+ (%) | 0.0048 | N/A | 0.4407 | 0.0332 | N/D | N/D |
| GLB1+/GFAP+ (%) | 0.0375 | 0.0008 | N/A | 0.0429 | N/D | N/D |
| GLB1+/IBA1+ (%) | 0.0029 | <0.0001 | N/A | 0.0025 | N/D | N/D |
| Genes Name | Brain Cortex (p Value) | Heart (p Value) | Spleen (p Value) | Bone Marrow (p Value) | Lung (p Value) | Liver (p Value) |
|---|---|---|---|---|---|---|
| SOD1 | NS | NS | 0.017 | 0.105 | NS | 0.028 |
| CAT | NS | NS | 0.053 | NS | NS | 0.090 |
| GLB1 | NS | 0.192 | 0.083 | NS | 0.169 | 0.016 |
| P21 | 0.127 | NS | NS | NS | 0.028 | 0.033 |
| P53 | NS | NS | NS | 0.046 | 0.081 | 0.081 |
| IL10 | NS | 0.070 | NS | NS | NS | 0.030 |
| IL8 | NS | 0.069 | NS | NS | N/A | 0.037 |
| NLRP3 | NS | 0.121 | NS | NS | NS | 0.017 |
| TREM2 | 0.136 | NS | 0.098 | 0.073 | 0.029 | 0.082 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huard, C.A.; Gao, X.; Dey Hazra, M.E.; Dey Hazra, R.-O.; Lebsock, K.; Easley, J.T.; Millett, P.J.; Huard, J. Effects of Fisetin Treatment on Cellular Senescence of Various Tissues and Organs of Old Sheep. Antioxidants 2023, 12, 1646. https://doi.org/10.3390/antiox12081646
Huard CA, Gao X, Dey Hazra ME, Dey Hazra R-O, Lebsock K, Easley JT, Millett PJ, Huard J. Effects of Fisetin Treatment on Cellular Senescence of Various Tissues and Organs of Old Sheep. Antioxidants. 2023; 12(8):1646. https://doi.org/10.3390/antiox12081646
Chicago/Turabian StyleHuard, Charles A., Xueqin Gao, Maria E. Dey Hazra, Rony-Orijit Dey Hazra, Kimberly Lebsock, Jeremiah T. Easley, Peter J. Millett, and Johnny Huard. 2023. "Effects of Fisetin Treatment on Cellular Senescence of Various Tissues and Organs of Old Sheep" Antioxidants 12, no. 8: 1646. https://doi.org/10.3390/antiox12081646
APA StyleHuard, C. A., Gao, X., Dey Hazra, M. E., Dey Hazra, R.-O., Lebsock, K., Easley, J. T., Millett, P. J., & Huard, J. (2023). Effects of Fisetin Treatment on Cellular Senescence of Various Tissues and Organs of Old Sheep. Antioxidants, 12(8), 1646. https://doi.org/10.3390/antiox12081646







