Role of Oxidative Stress, Methionine Oxidation and Methionine Sulfoxide Reductases (MSR) in Alzheimer’s Disease
Abstract
1. Alzheimer’s Disease: Occurrence, Etiology and Pathophysiology
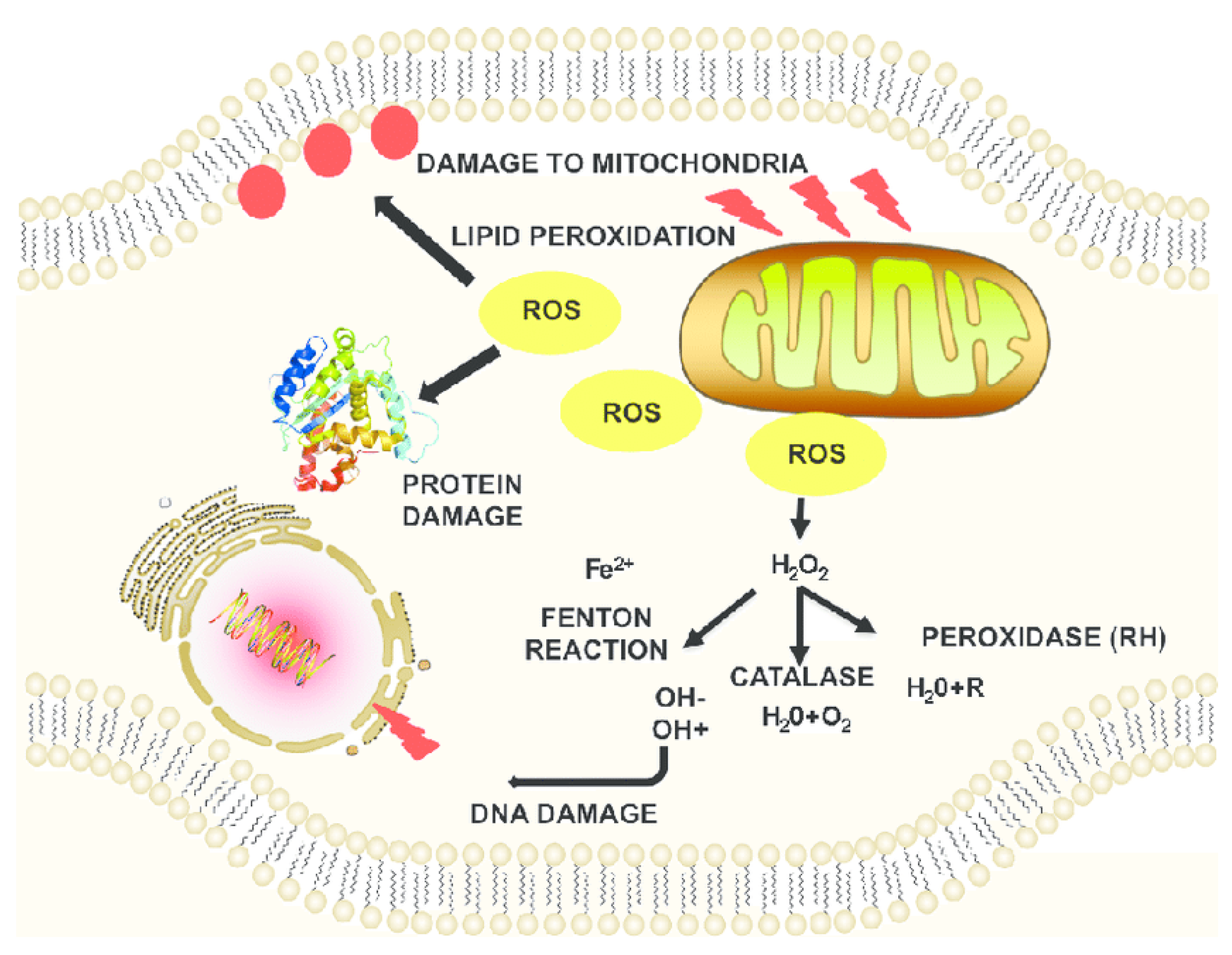
2. Oxidative Stress Due to ROS-Induced Mechanisms
3. Interplay between Oxidative Stress and Various Molecular Mechanisms of AD
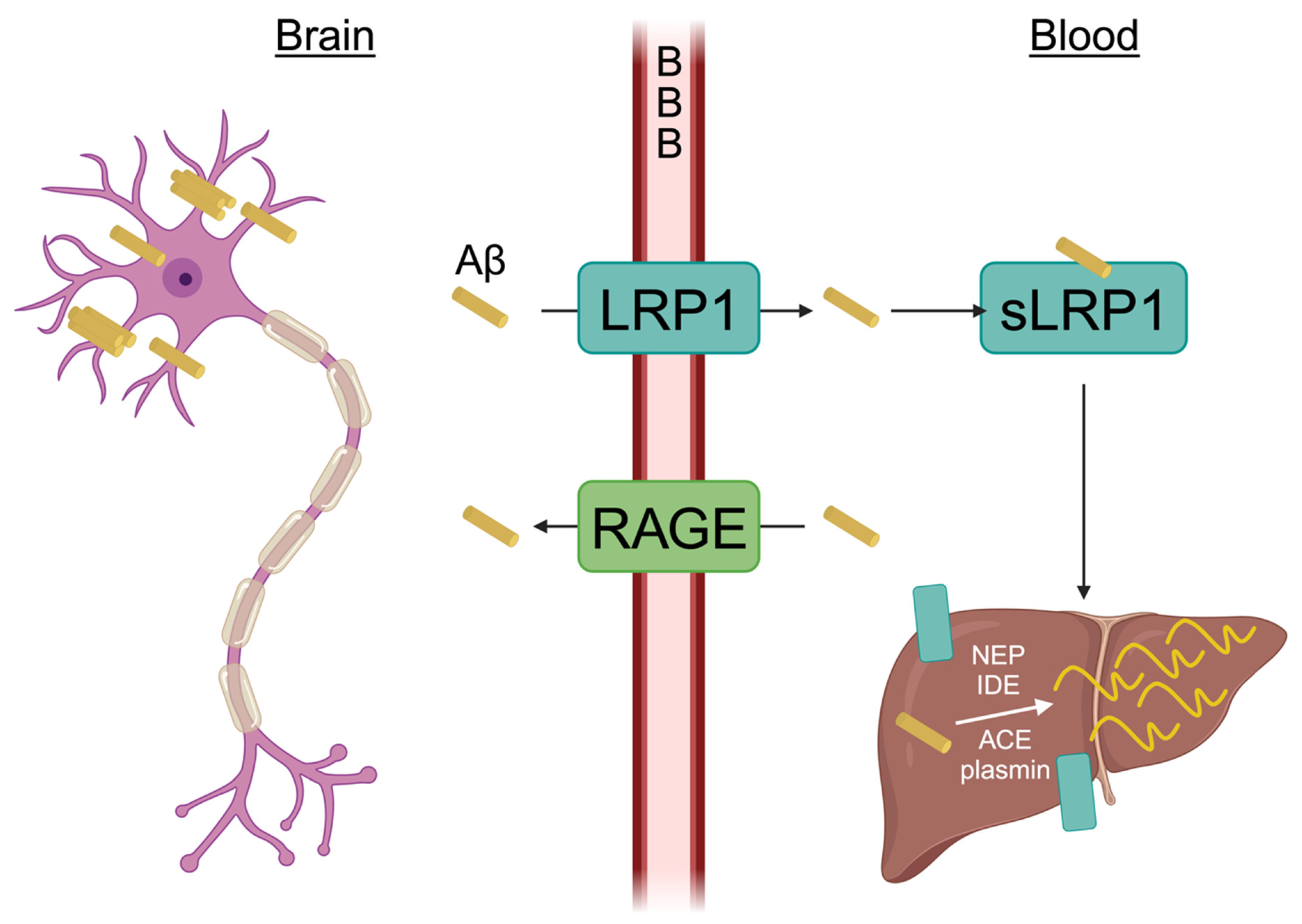
3.1. Aβ Induced Oxidative Stress
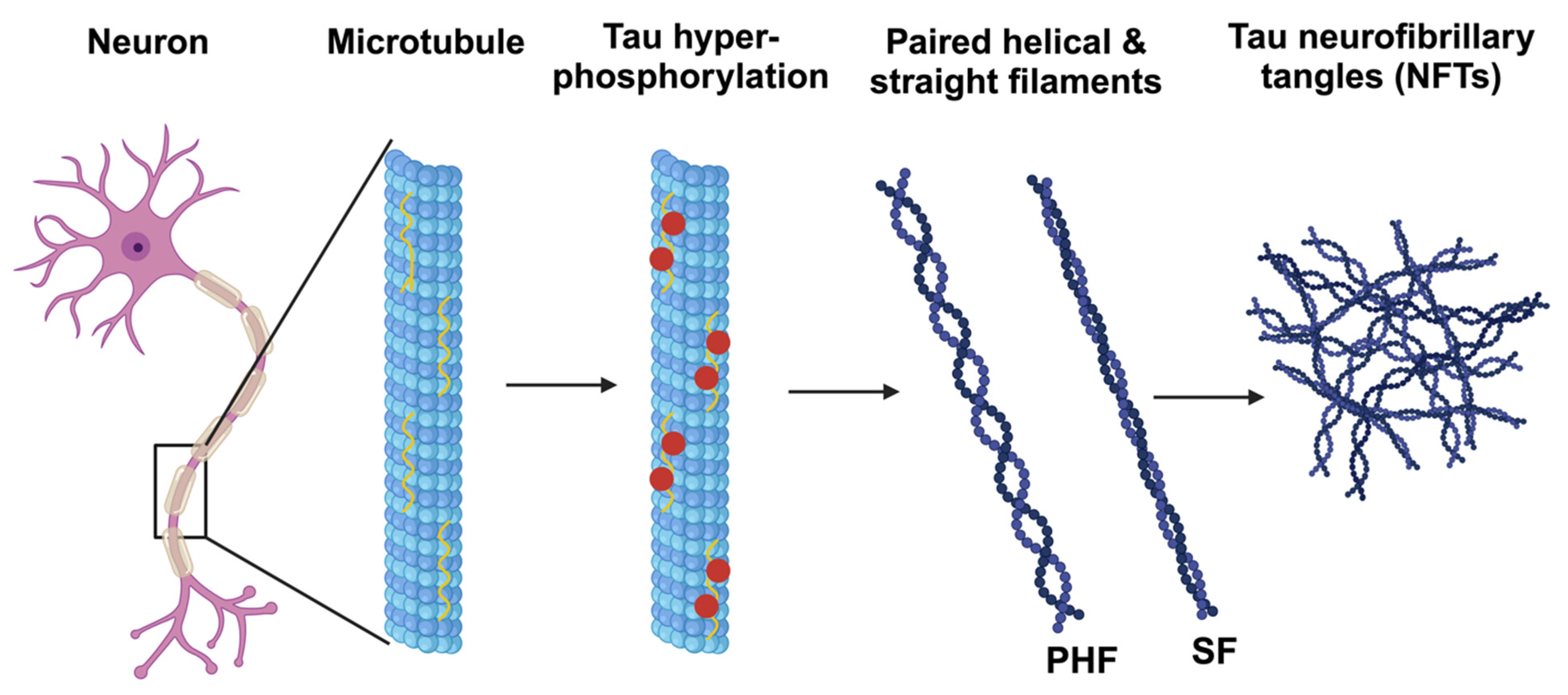
3.2. Oxidative Stress and Tau Phosphorylation
4. Role of Methionine and MSR in Aβ Aggregation, Mitochondrial Dysfunction and Neurotoxicity
5. Role of Methionine and MSR in the Intracellular Calcium (Ca2+) Homeostasis
6. Role of Methionine and MSR on Apolipoprotein A-I (apoA-I) Levels and Its Protective Mechanism
7. Role of Methionine 35 (Met35) Residue of Aβ42 in the Neurotoxicity of the AD Brain
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Korolev, I.O. Alzheimer’s Disease: A Clinical and Basic Science Review. Med. Stud. Res. J. 2014, 4, 24–33. [Google Scholar]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s Disease Hypothesis and Related Therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Srivastava, P.; Seth, A.; Tripathi, P.N.; Banerjee, A.G.; Shrivastava, S.K. Comprehensive Review of Mechanisms of Pathogenesis Involved in Alzheimer’s Disease and Potential Therapeutic Strategies. Prog. Neurobiol. 2018, 174, 53–89. [Google Scholar] [CrossRef] [PubMed]
- International, A.D. World Alzheimer Report 2019: Attitudes to Dementia; Alzheimer’s Disease Internationals: London, UK, 2019. [Google Scholar]
- Fisar, Z. Linking the Amyloid, Tau, and Mitochondrial Hypotheses of Alzheimer’s Disease and Identifying Promising Drug Targets. Biomolecules 2022, 12, 1676. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative Stress, Amyloid-Βpeptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1345–1367. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a Central Mechanism in Alzheimer’s Disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.H.; Yu, J.T.; Tan, L. The Role of SORL1 in Alzheimer’s Disease. Mol. Neurobiol. 2015, 51, 909–918. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, A.; Keegan, R.M.; Deshmukh, R. Recent Advances in the Neurobiology and Neuropharmacology of Alzheimer’s Disease. Biomed. Pharmacother. 2018, 98, 297–307. [Google Scholar] [CrossRef]
- Gu, Y.; Scarmeas, N. Dietary Patterns in Alzheimer’s Disease and Cognitive Aging. Curr. Alzheimer Res. 2011, 8, 510–519. [Google Scholar] [CrossRef]
- Delacourte, A. Tauopathies: Recent Insights into Old Diseases. Folia Neuropathol. 2005, 43, 244–257. [Google Scholar]
- Verwilst, P.; Kim, H.S.; Kim, S.; Kang, C.; Kim, J.S. Shedding Light on Tau Protein Aggregation: The Progress in Developing Highly Selective Fluorophores. Chem. Soc. Rev. 2018, 47, 2249–2265. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal Transduction by Reactive Oxygen Species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA Damage and Disease: Induction, Repair and Significance. Mutat. Res. Rev. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Höhn, A.; Tramutola, A.; Cascella, R. Proteostasis Failure in Neurodegenerative Diseases: Focus on Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 5497046. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative Stress, Mitochondrial Dysfunction, and Aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef]
- Sharma, C.; Kim, S.R. Linking Oxidative Stress and Proteinopathy in Alzheimer’s Disease. Antioxidants 2021, 10, 1231. [Google Scholar] [CrossRef]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef]
- Praticò, D. Oxidative Stress Hypothesis in Alzheimer’s Disease: A Reappraisal. Trends Pharmacol. Sci. 2008, 29, 609–615. [Google Scholar] [CrossRef]
- Mazumder, A.; Diederich, M. Natural Compound-Generated Oxidative Stress: From Bench to Bedside. In Free Radicals and Diseases; IntechOpen: London, UK, 2016; pp. 88–112. [Google Scholar]
- Schmidt, A.M.; Du Yan, S.; Yan, S.F.; Stern, D.M. The Biology of the Receptor for Advanced Glycation End Products and Its Ligands. Biochim. Biophys. Acta 2000, 1498, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Gkogkolou, P.; Böhm, M. Advanced Glycation End Products: Key Players in Skin Aging? Dermatoendocrinology 2012, 4, 259. [Google Scholar] [CrossRef] [PubMed]
- Maillard, L.C. Action of Amino Acids on Sugars. Formation of Melanoidins in a Methodical Way. Compte-Rendu L’academie Sci. 1912, 154, 66–68. [Google Scholar]
- Smith, M.A.; Taneda, S.; Richey, P.L.; Miyata, S.; Yan, S.D.; Stern, D.; Sayre, L.M.; Monnier, V.M.; Perry, G. Advanced Maillard Reaction End Products Are Associated with Alzheimer Disease Pathology. Proc. Natl. Acad. Sci. USA 1994, 91, 5710–5714. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.K.; Ghanbari, H.; Wataya, T.; Shimohama, S. Oxidative Damage Is the Earliest Event in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, H.A.; Ghanbari, K.; Harris, P.L.R.; Jones, P.K.; Kubat, Z.; Castellani, R.J.; Wolozin, B.L.; Smith, M.A.; Perry, G. Oxidative Damage in Cultured Human Olfactory Neurons from Alzheimer’s Disease Patients. Aging Cell 2004, 3, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Migliore, L.; Fontana, I.; Trippi, F.; Colognato, R.; Coppedè, F.; Tognoni, G.; Nucciarone, B.; Siciliano, G. Oxidative DNA Damage in Peripheral Leukocytes of Mild Cognitive Impairment and AD Patients. Neurobiol. Aging 2005, 26, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Harris, P.L.R.; Zhu, X.; Santos, M.S.; Oliveira, C.R.; Smith, M.A.; Perry, G. Lipoic Acid and N-Acetyl Cysteine Decrease Mitochondrial-Related Oxidative Stress in Alzheimer Disease Patient Fibroblasts. J. Alzheimer’s Dis. 2007, 12, 195–206. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid Precursor Protein Processing and Alzheimer’s Disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Takalo, M.; Salminen, A.; Soininen, H.; Hiltunen, M.; Haapasalo, A. Protein Aggregation and Degradation Mechanisms in Neurodegenerative Diseases. Am. J. Neurodegener. Dis. 2013, 2, 1. [Google Scholar]
- Butterfield, D.A.; Swomley, A.M.; Sultana, S. Amyloid β-Peptide (1–42)-Induced Oxidative Stress in Alzheimer Disease: Importance in Disease Pathogenesis and Progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Di Domenico, F.; Swomley, A.M.; Head, E.; Perluigi, M. Redox Proteomics Analysis to Decipher the Neurobiology of Alzheimer-like Neurodegeneration: Overlaps in Down’s Syndrome and Alzheimer’s Disease Brain. Biochem. J. 2014, 463, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Theuns, J.; Van Broeckhoven, C. Transcriptional Regulation of Alzheimer’s Disease Genes: Implications for Susceptibility. Hum. Mol. Genet. 2000, 9, 2383–2394. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.W.; Chernak, J.M. DNA Binding and Regulatory Effects of Transcription Factors SP1 and USF at the Rat Amyloid Precursor Protein Gene Promoter. Nucleic Acids Res. 1995, 23, 2229–2235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Picone, P.; Nuzzo, D.; Caruana, L.; Messina, E.; Barera, A.; Vasto, S.; Di Carlo, M. Metformin Increases APP Expression and Processing via Oxidative Stress, Mitochondrial Dysfunction and NF-κB Activation: Use of Insulin to Attenuate Metformin’s Effect. Biochim. Biophys. Acta 2015, 1853, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.Y.; Lin, Y.P.; Lin, Y.S.; Chang, S.S. Advanced Glycation End Products Enhance Amyloid Precursor Protein Expression by Inducing Reactive Oxygen Species. Free Radic. Biol. Med. 2010, 49, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Randall, J.D.; Cahill, C.M.; Eder, P.S.; Huang, X.; Gunshin, H.; Leiter, L.; McPhee, J.; Sarang, S.S.; Utsuki, T.; et al. An Iron-Responsive Element Type II in the 5′-Untranslated Region of the Alzheimer’s Amyloid Precursor Protein Transcript. J. Biol. Chem. 2002, 277, 45518–45528. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Moir, R.D.; Tanzi, R.E.; Bush, A.I.; Rogers, J.T. Redox-Active Metals, Oxidative Stress, and Alzheimer’s Disease Pathology. Ann. N. Acad. Sci. 2004, 1012, 153–163. [Google Scholar] [CrossRef]
- Chen, X.F.; Zhang, Y.W.; Xu, H.; Bu, G. Transcriptional Regulation and Its Misregulation in Alzheimer’s Disease. Mol. Brain 2013, 6, 44. [Google Scholar] [CrossRef]
- Chami, L.; Checler, F. BACE1 Is at the Crossroad of a Toxic Vicious Cycle Involving Cellular Stress and β-Amyloid Production in Alzheimer’s Disease. Mol. Neurodegener. 2012, 7, 52. [Google Scholar] [CrossRef]
- Guglielmotto, M.; Aragno, M.; Autelli, R.; Giliberto, L.; Novo, E.; Colombatto, S.; Danni, O.; Parola, M.; Smith, M.A.; Perry, G.; et al. The Up-Regulation of BACE1 Mediated by Hypoxia and Ischemic Injury: Role of Oxidative Stress and HIF1alpha. J. Neurochem. 2009, 108, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Kaur, U.; Banerjee, P.; Bir, A.; Sinha, M.; Biswas, A.; Chakrabarti, S. Reactive Oxygen Species, Redox Signaling and Neuroinflammation in Alzheimer’s Disease: The NF-κB Connection. Curr. Top. Med. Chem. 2015, 15, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhou, W.; Fung, V.; Christensen, M.A.; Qing, H.; Sun, X.; Song, W. Oxidative Stress Potentiates BACE1 Gene Expression and Abeta Generation. J. Neural Transm. 2004, 112, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Bardini, P.; Obbili, A.; Vitali, A.; Borghi, R.; Zaccheo, D.; Pronzato, M.A.; Danni, O.; Smith, M.A.; Perry, G.; et al. Oxidative Stress Increases Expression and Activity of BACE in NT2 Neurons. Neurobiol. Dis. 2002, 10, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Parola, M.; Bardini, P.; Piccini, A.; Borghi, R.; Guglielmotto, M.; Santoro, G.; Davit, A.; Danni, O.; Smith, M.A.; et al. Beta-Site APP Cleaving Enzyme up-Regulation Induced by 4-Hydroxynonenal Is Mediated by Stress-Activated Protein Kinases Pathways. J. Neurochem. 2005, 92, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Guglielmotto, M.; Aragno, M.; Borghi, R.; Autelli, R.; Giliberto, L.; Muraca, G.; Danni, O.; Zhu, X.; Smith, M.A.; et al. Oxidative Stress Activates a Positive Feedback between the γ- and β-Secretase Cleavages of the β-Amyloid Precursor Protein. J. Neurochem. 2008, 104, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Mouton-Liger, F.; Paquet, C.; Dumurgier, J.; Bouras, C.; Pradier, L.; Gray, F.; Hugon, J. Oxidative Stress Increases BACE1 Protein Levels through Activation of the PKR-eIF2α Pathway. Biochem. Biophys. Acta 2012, 1822, 885–896. [Google Scholar] [CrossRef]
- Misonou, H.; Morishima-kawashima, M.; Ihara, Y. Oxidative Stress Induces Intracellular Accumulation of Amyloid Beta-Protein (Abeta) in Human Neuroblastoma Cells. Biochemistry 2000, 39, 6951–6959. [Google Scholar] [CrossRef]
- Deane, R.; Bell, R.D.; Sagare, A.; Zlokovic, B.V. Clearance of Amyloid-Beta Peptide across the Blood-Brain Barrier: Implication for Therapies in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2009, 8, 16–30. [Google Scholar] [CrossRef]
- Bates, K.A.; Verdile, G.; Li, Q.X.; Ames, D.; Hudson, P.; Masters, C.L.; Martins, R.N. Clearance Mechanisms of Alzheimer’s Amyloid-β Peptide: Implications for Therapeutic Design and Diagnostic Tests. Mol. Psychiatr. 2008, 14, 469–486. [Google Scholar] [CrossRef]
- Ramanathan, A.; Nelson, A.R.; Sagare, A.P.; Zlokovic, B.V. Impaired Vascular-Mediated Clearance of Brain Amyloid Beta in Alzheimer’s Disease: The Role, Regulation and Restoration of LRP1. Front. Aging Neurosci. 2015, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Dries, D.R.; Yu, G.; Herz, J. Extracting β-Amyloid from Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2012, 109, 3199–3200. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, H.; Nishitoh, H.; Urano, F.; Sadamitsu, C.; Matsuzawa, A.; Takeda, K.; Masutani, H.; Yodoi, J.; Urano, Y.; Nagano, T.; et al. Amyloid β Induces Neuronal Cell Death through ROS-Mediated ASK1 Activation. Cell Death Differ. 2005, 12, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Behl, C. Hydrogen Peroxide Mediates Amyloid β Protein Toxicity. Cell 1994, 77, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Sinha, M.; Thakurta, I.G.; Banerjee, P.; Chattopadhyay, M. Oxidative Stress and Amyloid Beta Toxicity in Alzheimer’s Disease: Intervention in a Complex Relationship by Antioxidants. Curr. Med. Chem. 2013, 20, 4648–4664. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Di Domenico, F.; Barone, E.; Butterfield, D.A. mTOR in Alzheimer Disease and Its Earlier Stages: Links to Oxidative Damage in the Progression of This Dementing Disorder. Free Radic. Biol. Med. 2021, 169, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Tzvetkov, N.T.; Georgieva, M.G.; Ognyanov, I.V.; Kordos, K.; Jozwik, A.; Kuhl, T.; Perry, G.; Petralia, M.C.; Mazzon, E.; et al. Reactive Oxygen Species and Their Impact in Neurodegenerative Diseases: Literature Landscape Analysis. Antioxid. Redox Signal 2021, 34, 402–420. [Google Scholar] [CrossRef]
- Smith, D.G.; Cappai, R.; Barnham, K.J. The Redox Chemistry of the Alzheimer’s Disease Amyloid β Peptide. Biochim. Biophys. Acta 2007, 1768, 1976–1990. [Google Scholar] [CrossRef]
- Sinha, M.; Bhowmick, P.; Banerjee, A.; Chakrabarti, S. Antioxidant Role of Amyloid β Protein in Cell-Free and Biological Systems: Implication for the Pathogenesis of Alzheimer Disease. Free Radic. Biol. Med. 2013, 56, 184–192. [Google Scholar] [CrossRef]
- Shelat, P.B.; Chalimoniuk, M.; Wang, J.H.; Strosznajder, J.B.; Lee, J.C.; Sun, A.Y.; Simonyi, A.; Sun, G.Y. Amyloid Beta Peptide and NMDA Induce ROS from NADPH Oxidase and AA Release from Cytosolic Phospholipase A2 in Cortical Neurons. J. Neurochem. 2008, 106, 45–55. [Google Scholar] [CrossRef]
- Hu, H.; Li, M. Mitochondria-Targeted Antioxidant Mitotempo Protects Mitochondrial Function against Amyloid Beta Toxicity in Primary Cultured Mouse Neurons. Biochem. Biophys. Res. Commun. 2016, 478, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, Y.; Cooper, C.; Liu, B.; Wilson, B.; Hong, J.-S. Microglia Enhance β-Amyloid Peptide-Induced Toxicity in Cortical and Mesencephalic Neurons by Producing Reactive Oxygen Species. J. Neurochem. 2002, 83, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Cartier, L.; Dubois-dauphin, M.; Li, B.; Serrander, L.; Krause, K.H. A Key Role for the Microglial NADPH Oxidase in APP-Dependent Killing of Neurons. Neurobiol. Aging 2006, 27, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Doens, D.; Fernández, P.L. Microglia Receptors and Their Implications in the Response to Amyloid β for Alzheimer’s Disease Pathogenesis. J. Neuroinflamm. 2014, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hu, X.; Qian, L.; Chen, S.H.; Zhou, H.; Wilson, B.; Miller, D.S.; Hong, J.S. Microglial MAC1 Receptor and PI3K Are Essential in Mediating β-Amyloid Peptide-Induced Microglial Activation and Subsequent Neurotoxicity. J. Neuroinflamm. 2011, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Mondragón-Rodríguez, S.; Perry, G.; Luna-Muñoz, J.; Acevedo-Aquino, M.C.; Williams, S. Phosphorylation of Tau Protein at Sites Ser (396-404) Is One of the Earliest Events in Alzheimer’s Disease and Down Syndrome. Neuropathol. Appl. Neurobiol. 2014, 40, 121–135. [Google Scholar] [CrossRef]
- Alavi Naini, S.M.; Soussi-Yanicostas, N. Tau Hyperphosphorylation and Oxidative Stress, a Critical Vicious Circle in Neurodegenerative Tauopathies? Oxid. Med. Cell. Longev. 2015, 2015, 151979. [Google Scholar] [CrossRef]
- Su, B.; Wang, X.; Lee, H.G.; Tabaton, M.; Perry, G.; Smith, M.A.; Zhu, X. Chronic Oxidative Stress Causes Increased Tau Phosphorylation in M17 Neuroblastoma Cells. Neurosci. Lett. 2010, 468, 267–271. [Google Scholar] [CrossRef]
- Giraldo, E.; Lloret, A.; Fuchsberger, T.; Viña, J. Aβ and Tau Toxicities in Alzheimer’s Are Linked via Oxidative Stress-Induced P38 Activation: Protective Role of Vitamin E. Redox Biol. 2014, 2, 873–877. [Google Scholar] [CrossRef]
- Lovell, M.A.; Xiong, S.; Xie, C.; Davies, P.; Markesbery, W.R. Induction of Hyperphosphorylated Tau in Primary Rat Cortical Neuron Cultures Mediated by Oxidative Stress and Glycogen Synthase Kinase-3. J. Alzheimer’s Dis. 2004, 6, 659–671. [Google Scholar] [CrossRef]
- Egaña, J.T.; Zambrano, C.; Nuñez, M.T.; Gonzalez-Billault, C.; Maccioni, R.B. Iron-Induced Oxidative Stress Modify Tau Phosphorylation Patterns in Hippocampal Cell Cultures. Biometals 2003, 16, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, C.A.; Egaña, J.T.; Núñez, M.T.; Maccioni, R.B.; González-Billault, C. Oxidative Stress Promotes Tau Dephosphorylation in Neuronal Cells: The Roles of Cdk5 and PP1. Free Radic. Biol. Med. 2004, 36, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Boutte, A.M.; Woltjer, R.L.; Zimmerman, L.J.; Stamer, S.L.; Montine, K.S.; Manno, M.V.; Cimino, P.J.; Liebler, D.C.; Montine, T.J. Selectively Increased Oxidative Modifications Mapped to Detergent-Insoluble Forms of Aβ and β-III Tubulin in Alzheimer’s Disease. FASEB J. 2006, 20, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Atwood, C.S.; Anderson, V.E.; Siedlak, S.L.; Smith, M.A.; Perry, G.; Carey, P.R. Metal Binding and Oxidation of Amyloid-Beta within Isolated Senile Plaque Cores: Raman Microscopic Evidence. Biochemistry 2003, 42, 2768–2773. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.M.; Kokjohn, T.A.; Beach, T.G.; Sue, L.I.; Brune, D.; Lopez, J.C.; Kalback, W.M.; Abramowski, D.; Sturchler-Pierrat, C.; Staufenbiel, M.; et al. Comparative Analysis of Amyloid-Beta Chemical Structure and Amyloid Plaque Morphology of Transgenic Mouse and Alzheimer’s Disease Brains. J. Biol. Chem. 2001, 276, 12991–12998. [Google Scholar] [CrossRef] [PubMed]
- Näslund, J.; Schierhorn, A.; Hellman, U.; Lannfelt, L.; Roses, A.D.; Tjernberg, L.O.; Silberring, J.; Gandy, S.E.; Winblad, B.; Greengard, P. Relative Abundance of Alzheimer A Beta Amyloid Peptide Variants in Alzheimer Disease and Normal Aging. Proc. Natl. Acad. Sci. USA 1994, 91, 8378–8382. [Google Scholar] [CrossRef] [PubMed]
- Oien, D.B.; Moskovitz, J. Substrates of the Methionine Sulfoxide Reductase System and Their Physiological Relevance. Curr. Top. Dev. Biol. 2008, 80, 93–133. [Google Scholar]
- Moskovitz, J.; Weissbach, H.; Brot, N. Cloning the Expression of a Mammalian Gene Involved in the Reduction of Methionine Sulfoxide Residues in Proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 2095–2099. [Google Scholar] [CrossRef]
- Hansel, A.; Kuschel, L.; Hehl, S.; Lemke, C.; Agricola, H.J.; Hoshi, T.; Heinemann, S.H. Mitochondrial Targeting of the Human Peptide Methionine Sulfoxide Reductase (MSRA), an Enzyme Involved in the Repair of Oxidized Proteins. FASEB J. 2002, 16, 911–913. [Google Scholar] [CrossRef]
- Moskovitz, J.; Bar-Noy, S.; Williams, W.M.; Requena, J.; Berlett, B.S.; Stadtman, E.R. Methionine Sulfoxide Reductase (MsrA) Is a Regulator of Antioxidant Defense and Lifespan in Mammals. Proc. Natl. Acad. Sci. USA 2001, 98, 12920–12925. [Google Scholar] [CrossRef]
- Pal, R.; Oien, D.B.; Ersen, F.Y.; Moskovitz, J. Elevated Levels of Brain-Pathologies Associated with Neurodegenerative Diseases in the Methionine Sulfoxide Reductase A Knockout Mouse. Exp. Brain Res. 2007, 180, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Kim, A.K.; Jung, S.A.; Kim, S.W.; Yu, K.; Lee, J.H. The Drosophila Homolog of Methionine Sulfoxide Reductase A Extends Lifespan and Increases Nuclear Localization of FOXO. FEBS Lett. 2010, 584, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Rahman, M.A.; Strassman, J.; Yancey, S.O.; Kushner, S.R.; Brot, N.; Weissbach, H. Escherichia Coli Peptide Methionine Sulfoxide Reductase Gene: Regulation of Expression and Role in Protecting against Oxidative Damage. J. Bacteriol. 1995, 177, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Flescher, E.; Berlett, B.S.; Azare, J.; Poston, J.M.; Stadtman, E.R. Overexpression of Peptide-Methionine Sulfoxide Reductase in Saccharomyces Cerevisiae and Human T Cells Provides Them with High Resistance to Oxidative Stress. Proc. Natl. Acad. Sci. USA 1998, 95, 14071–14075. [Google Scholar] [CrossRef] [PubMed]
- Misiti, F.; Clementi, M.E.; Giardina, B. Oxidation of Methionine 35 Reduces Toxicity of the Amyloid Beta-Peptide(1–42) in Neuroblastoma Cells (IMR-32) via Enzyme Methionine Sulfoxide Reductase A Expression and Function. Neurochem. Int. 2010, 56, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Maiti, P.; Lopes, D.H.; Oien, D.B.; Attar, A.; Liu, T.; Mittal, S.; Hayes, J.; Bitan, G. Induction of Methionine-Sulfoxide Reductases Protects Neurons from Amyloid β-Protein Insults in Vitro and in Vivo. Biochemistry 2011, 50, 10687–10697. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Lee, E.H.; Chung, W.S.; Lee, S.J.; Shin, S.H.; Joo, S.H.; Kim, S.K.; Lee, J.H. Increased Viability of PC12 Cells Exposed to Amyloid-Beta Peptide by Transduction with Human TAT-Methionine Sulfoxide Reductase. Neuroreport 2003, 14, 2349–2353. [Google Scholar] [CrossRef]
- Petropoulos, I.; Mary, J.; Perichon, M.; Friguet, B. Rat Peptide Methionine Sulphoxide Reductase: Cloning of the cDNA, and down-Regulation of Gene Expression and Enzyme Activity during Aging. Biochem. J. 2001, 355, 819–825. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Moskovitz, J.; Berlett, B.S.; Levine, R.L. Cyclic Oxidation and Reduction of Protein Methionine Residues Is an Important Antioxidant Mechanism. Mol. Cell Biochem. 2002, 234, 3–9. [Google Scholar] [CrossRef]
- Vinokur, V.; Grinberg, L.; Berenshtein, E.; Gross, M.; Moskovitz, J.; Reznick, A.Z.; Chevion, M.; Eliashar, R. Methionine-Centered Redox Cycle in Organs of the Aero-Digestive Tract of Young and Old Rats. Biogerontology 2009, 10, 43–52. [Google Scholar] [CrossRef]
- Gabbita, S.P.; Aksenov, M.Y.; Lovell, M.A.; Markesbery, W.R. Decrease in Peptide Methionine Sulfoxide Reductase in Alzheimer’s Disease Brain. J. Neurochem. 1999, 73, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Du, F.; Bowman, C.F.; Yan, S.S. Methionine Sulfoxide Reductase A Affects β-Amyloid Solubility and Mitochondrial Function in a Mouse Model of Alzheimer’s Disease. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E388–E393. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.M.; Roberts, B.R.; McColl, G.; Hare, D.J.; Doble, P.A.; Li, Q.X.; Lind, M.; Roberts, A.M.; Mertens, H.D.; Kirby, N.; et al. Stabilization of Nontoxic Aβ-Oligomers: Insights into the Mechanism of Action of Hydroxyquinolines in Alzheimer’s Disease. J. Neurosci. 2015, 35, 2871–2884. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-Beta Protein Dimers Isolated Directly from Alzheimer’s Brains Impair Synaptic Plasticity and Memory. Nat. Med. 2003, 14, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.M.; Carrasco, G.A.; Moskovitz, J. Induction of Methionine Sulfoxide Reductase Activity by Pergolide, Pergolide Sulfoxide, and S-Adenosyl-Methionine in Neuronal Cells. Neurosci. Lett. 2003, 533, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Amano, A.; Sanjo, N.; Araki, W.; Anraku, Y.; Nakakido, M.; Matsubara, E.; Tomiyama, T.; Nagata, T.; Tsumoto, K.; Kataoka, K.; et al. Peripheral Administration of Nanomicelle-Encapsulated Anti-Aβ Oligomer Fragment Antibody Reduces Various Toxic Aβ Species in the Brain. J. Nanobiotechnol. 2023, 21, 36. [Google Scholar] [CrossRef]
- Bosetti, F.; Brizzi, F.; Barogi, S.; Mancuso, M.; Siciliano, G.; Tendi, E.A.; Murri, L.; Rapoport, S.I.; Solaini, G. Cytochrome c Oxidase and Mitochondrial F1F0-ATPase (ATP Synthase) Activities in Platelets and Brain from Patients with Alzheimer’s Disease. Neurobiol. Aging 2002, 23, 371–376. [Google Scholar] [CrossRef]
- Muirhead, K.E.; Borger, E.; Aitken, L.; Conway, S.J.; Gunn-Moore, F.J. The Consequences of Mitochondrial Amyloid Beta-Peptide in Alzheimer’s Disease. Biochem. J. 2010, 426, 255–270. [Google Scholar] [CrossRef]
- Xiang, X.J.; Song, L.; Deng, X.J.; Tang, Y.; Min, Z.; Luo, B.; Wen, Q.X.; Li, K.Y.; Chen, J.; Ma, Y.L.; et al. Mitochondrial Methionine Sulfoxide Reductase B2 Links Oxidative Stress to Alzheimer’s Disease-like Pathology. Exp. Neurol. 2019, 318, 145–156. [Google Scholar] [CrossRef]
- LaFerla, F.M. Calcium Dyshomeostasis and Intracellular Signalling in Alzheimer’s Disease. Nat. Rev. Neurosci. 2002, 3, 862–872. [Google Scholar] [CrossRef]
- Bezprozvanny, I.; Mattson, M.P. Neuronal Calcium Mishandling and the Pathogenesis of Alzheimer’s Disease. Trends Neurosci. 2008, 31, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Cheng, B.; Davis, D.; Bryant, K.; Lieberburg, I.; Rydel, R.E. Beta-Amyloid Peptides Destabilize Calcium Homeostasis and Render Human Cortical Neurons Vulnerable to Excitotoxicity. J. Neurosci. 1992, 12, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Demuro, A.; Parker, I.; Stutzmann, G.E. Calcium Signaling and Amyloid Toxicity in Alzheimer Disease. J. Biol. Chem. 2010, 285, 12463–12468. [Google Scholar] [CrossRef]
- Michaelis, M.L.; Bigelow, D.J.; Schoneich, C.; Williams, T.D.; Ramonda, L.; Yin, D.; Huhmer, A.F.; Yao, Y.; Gao, J.; Squier, T.C. Decreased Plasma Membrane Calcium Transport Activity in Aging Brain. Life Sci. 1996, 59, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yin, D.; Yao, Y.; Williams, T.D.; Squier, T.C. Progressive Decline in the Ability of Calmodulin Isolated from Aged Brain to Activate the Plasma Membrane Ca-ATPase. Biochemistry 1998, 37, 9536–9548. [Google Scholar] [CrossRef] [PubMed]
- Sharov, V.S.; Schöneich, C. Diastereoselective Protein Methionine Oxidation by Reactive Oxygen Species and Diastereoselective Repair by Methionine Sulfoxide Reductase. Free Radic. Biol. Med. 2000, 29, 986–994. [Google Scholar] [CrossRef]
- Sharov, V.S.; Ferrington, D.A.; Squier, T.C.; Schöneich, C. Diastereoselective Reduction of Protein-Bound Methionine Sulfoxide by Methionine Sulfoxide Reductase. FEBS Lett. 1999, 455, 247–250. [Google Scholar] [CrossRef]
- Gandy, S.; Czernik, A.J.; Greengard, P. Phosphorylation of Alzheimer Disease Amyloid Precursor Peptide by Protein Kinase C and Ca2+/Calmodulin-Dependent Protein Kinase II. Proc. Natl. Acad. Sci. USA 1988, 85, 6218–6221. [Google Scholar] [CrossRef]
- McKee, A.C.; Kosik, K.S.; Kennedy, M.B.; Kowall, N.W. Hippocampal Neurons Predisposed to Neurofibrillary Tangle Formation Are Enriched in Type II Calcium/Calmodulin-Dependent Protein Kinase. J. Neuropathol. Exp. Neurol. 1990, 49, 49–63. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chen, G.H.; Hu, X.Y.; Lu, Y.P.; Zhou, J.N.; Liu, R.Y. The Expression of Calcium/Calmodulin-Dependent Protein Kinase II-Alpha in the Hippocampus of Patients with Alzheimer’s Disease and Its Links with AD-Related Pathology. Brain Res. 2005, 1031, 101–108. [Google Scholar] [CrossRef]
- Ramelot, T.A.; Nicholson, L.K. Phosphorylation-Induced Structural Changes in the Amyloid Precursor Protein Cytoplasmic Tail Detected by NMR. J. Mol. Biol. 2001, 307, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.I.; Rebelo, S.; Esselmann, H.; Wiltfang, J.; Lah, J.; Lane, R.; Small, S.A.; Gandy, S.; da Cruz E Silva, E.F.; da Cruz E Silva, O.A. Retrieval of the Alzheimer’s Amyloid Precursor Protein from the Endosome to the TGN Is S655 Phosphorylation State-Dependent and Retromer-Mediated. Mol. Neurodegener. 2010, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Reese, L.C.; Laezza, F.; Woltjer, R.; Taglialatela, G. Dysregulated Phosphorylation of Ca(2+)/Calmodulin-Dependent Protein Kinase II-Alpha in the Hippocampus of Subjects with Mild Cognitive Impairment and Alzheimer’s Disease. J. Neurochem. 2011, 119, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Liu, W.; Yan, Z. Beta-Amyloid Impairs AMPA Receptor Trafficking and Function by Reducing Ca2+/Calmodulin-Dependent Protein Kinase II Synaptic Distribution. J. Biol. Chem. 2009, 284, 10639–10649. [Google Scholar] [CrossRef] [PubMed]
- Simonian, N.A.; Elvhage, T.; Czernik, A.J.; Greengard, P.; Hyman, B.T. Calcium/Calmodulin-Dependent Protein Kinase II Immunostaining Is Preserved in Alzheimer’s Disease Hippocampal Neurons. Brain Res. 1994, 657, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Mah, V.H.; Eskin, T.A.; Kazee, A.M.; Lapham, L.; Higgins, G.A. In Situ Hybridization of Calcium/Calmodulin Dependent Protein Kinase II and Tau mRNAs; Species Differences and Relative Preservation in Alzheimer’s Disease. Brain Res. Mol. Brain Res. 1992, 12, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Perry, G.; Troncoso, J.; Monteiro, M.J. Alpha-Calcium-Calmodulin-Dependent Kinase II Is Associated with Paired Helical Filaments of Alzheimer’s Disease. J. Neuropathol. Exp. Neurol. 1996, 55, 954–963. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, H.; Hiragami, Y.; Murayama, M.; Ishizuka, K.; Kawahara, M.; Takashima, A. Phosphorylation of Tau at Serine 416 by Ca2+/Calmodulin-Dependent Protein Kinase II in Neuronal Soma in Brain. J. Neurochem. 2005, 94, 1438–1447. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Ichinose, T.; Yamauchi, T. Phosphorylation of Tau Protein to Sites Found in Alzheimer’s Disease Brain Is Catalyzed by Ca2+/Calmodulin-Dependent Protein Kinase II as Demonstrated Tandem Mass Spectrometry. Neurosci. Lett. 2003, 353, 185–188. [Google Scholar] [CrossRef]
- Singh, T.J.; Wang, J.Z.; Novak, M.; Kontzekova, E.; Grundke-Iqbal, I.; Iqbal, K. Calcium/Calmodulin-Dependent Protein Kinase II Phosphorylates Tau at Ser-262 but Only Partially Inhibits Its Binding to Microtubules. FEBS Lett. 1996, 387, 145–148. [Google Scholar] [CrossRef]
- Erickson, J.R.; Joiner, M.L.; Guan, X.; Kutschke, W.; Yang, J.; Oddis, C.V.; Bartlett, R.K.; Lowe, J.S.; O’Donnell, S.E.; Aykin-Burns, N.; et al. A Dynamic Pathway for Calcium-Independent Activation of Camkii by Methionine Oxidation. Cell 2008, 133, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Lefterov, I.; Fitz, N.F.; Cronican, A.A.; Fogg, A.; Lefterov, P.; Kodali, R.; Wetzel, R.; Koldamova, R. Apolipoprotein A-I Deficiency Increases Cerebral Amyloid Angiopathy and Cognitive Deficits in APP/PS1DeltaE9 Mice. J. Biol. Chem. 2010, 285, 36945–36957. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.L.; Cao, D.; Lu, H.; Mans, R.A.; Su, Y.R.; Jungbauer, L.; Linton, M.F.; Fazio, S.; LaDu, M.J.; Li, L. Overexpression of Human Apolipoprotein A-I Preserves Cognitive Function and Attenuates Neuroinflammation and Cerebral Amyloid Angiopathy in a Mouse Model of Alzheimer. J. Biol. Chem. 2010, 285, 36958–36968. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Song, H.; Xu, J.; Huang, J.; Hu, M.; Gu, X.; Chen, J.; Zheng, G.; Chen, H.; Gao, X. Biomimetic ApoE-Reconstituted High Density Lipoprotein Nanocarrier for Blood-Brain Barrier Penetration and Amyloid Beta-Targeting Drug Delivery. Mol. Pharm. 2016, 13, 3976–3987. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.Q.; Binger, K.J.; Howlett, G.J.; Griffin, M.D.W. Methionine Oxidation Induces Amyloid Fibril Formation by Full-Length Apolipoprotein A-I. Proc. Natl. Acad. Sci. USA 2010, 107, 1977–1982. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Du, F.; Meng, B.; Xie, G.-H.; Cao, J.; Fan, D.; Yu, H. Hepatic Overexpression of Methionine Sulfoxide Reductase A Reduces Atherosclerosis in Apolipoprotein E-Deficient Mice. J. Lipid Res. 2015, 56, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Sultana, R. Methionine-35 of Aβ (1–42): Importance for Oxidative Stress in Alzheimer Disease. J. Amino Acid. 2011, 2011, 198430. [Google Scholar] [CrossRef]
- Kanski, J.; Aksenova, M.; Butterfield, D.A. The Hydrophobic Environment of Met35 of Alzheimer’s Aβ(1–42) Is Important for the Neurotoxic and Oxidative Properties of the Peptide. Neurotox. Res. 2002, 4, 219–223. [Google Scholar] [CrossRef]
- Barnham, K.J.; Ciccotosto, G.D.; Tickler, A.K.; Ali, F.E.; Smith, D.G.; Williamson, N.A.; Lam, Y.H.; Carrington, D.; Tew, D.; Kocak, G.; et al. Neurotoxic, Redox-Competent Alzheimer’s Beta-Amyloid Is Released from Lipid Membrane by Methionine Oxidation. J. Biol. Chem. 2003, 278, 42959–42965. [Google Scholar] [CrossRef]
- Yatin, S.M.; Varadarajan, S.; Link, C.D.; Butterfield, D.A. In Vitro and in Vivo Oxidative Stress Associated with Alzheimer’s Amyloid β-Peptide (1–42). Neurobiol. Aging 1999, 20, 325–330. [Google Scholar]
- Friedemann, M.; Helk, E.; Tiiman, A.; Zovo, K.; Palumaa, P.; Tõugu, V. Effect of Methionine-35 Oxidation on the Aggregation of Amyloid-β Peptide. Biochem. Biophys. Rep. 2015, 3, 94–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walker, E.J.; Bettinger, J.Q.; Welle, K.A.; Hryhorenko, J.R.; Ghaemmaghami, S. Global Analysis of Methionine Oxidation Provides a Census of Folding Stabilities for the Human Proteome. Proc. Natl. Acad. Sci. USA 2019, 116, 6081–6090. [Google Scholar] [CrossRef] [PubMed]
- Cannizzo, E.S.; Clement, C.C.; Morozova, K.; Valdor, R.; Kaushik, S.; Almeida, L.N.; Follo, C.; Sahu, R.; Cuervo, A.M.; Macian, F.; et al. Age-Related Oxidative Stress Compromises Endosomal Proteostasis. Cell Rep. 2012, 2, 136–149. [Google Scholar] [CrossRef]
- Jones, D.P. Redox Theory of Aging. Redox Biol. 2015, 5, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Basisty, N.; Holtz, A.; Schilling, B. Accumulation of “Old Proteins” and the Critical Need for MS-Based Protein Turnover Measurements in Aging and Longevity. Proteomics 2020, 20, e1800403. [Google Scholar] [CrossRef] [PubMed]
- Basisty, N.; Meyer, J.G.; Schilling, B. Protein Turnover in Aging and Longevity. Proteomics 2018, 18, e1700108. [Google Scholar] [CrossRef]
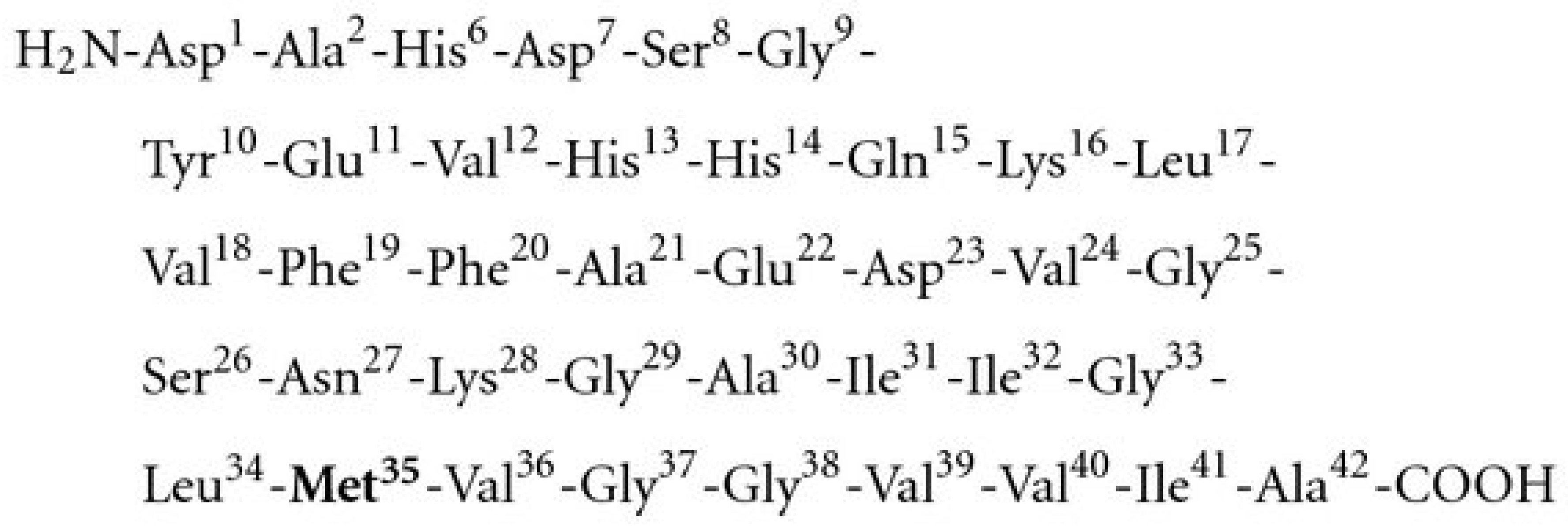
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandran, S.; Binninger, D. Role of Oxidative Stress, Methionine Oxidation and Methionine Sulfoxide Reductases (MSR) in Alzheimer’s Disease. Antioxidants 2024, 13, 21. https://doi.org/10.3390/antiox13010021
Chandran S, Binninger D. Role of Oxidative Stress, Methionine Oxidation and Methionine Sulfoxide Reductases (MSR) in Alzheimer’s Disease. Antioxidants. 2024; 13(1):21. https://doi.org/10.3390/antiox13010021
Chicago/Turabian StyleChandran, Sanjana, and David Binninger. 2024. "Role of Oxidative Stress, Methionine Oxidation and Methionine Sulfoxide Reductases (MSR) in Alzheimer’s Disease" Antioxidants 13, no. 1: 21. https://doi.org/10.3390/antiox13010021
APA StyleChandran, S., & Binninger, D. (2024). Role of Oxidative Stress, Methionine Oxidation and Methionine Sulfoxide Reductases (MSR) in Alzheimer’s Disease. Antioxidants, 13(1), 21. https://doi.org/10.3390/antiox13010021





