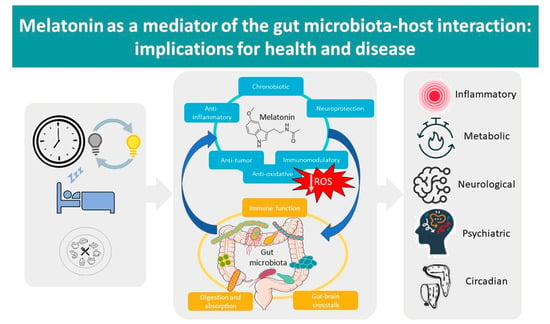
Melatonin as a Mediator of the Gut Microbiota–Host Interaction: Implications for Health and Disease
Abstract
:1. Introduction
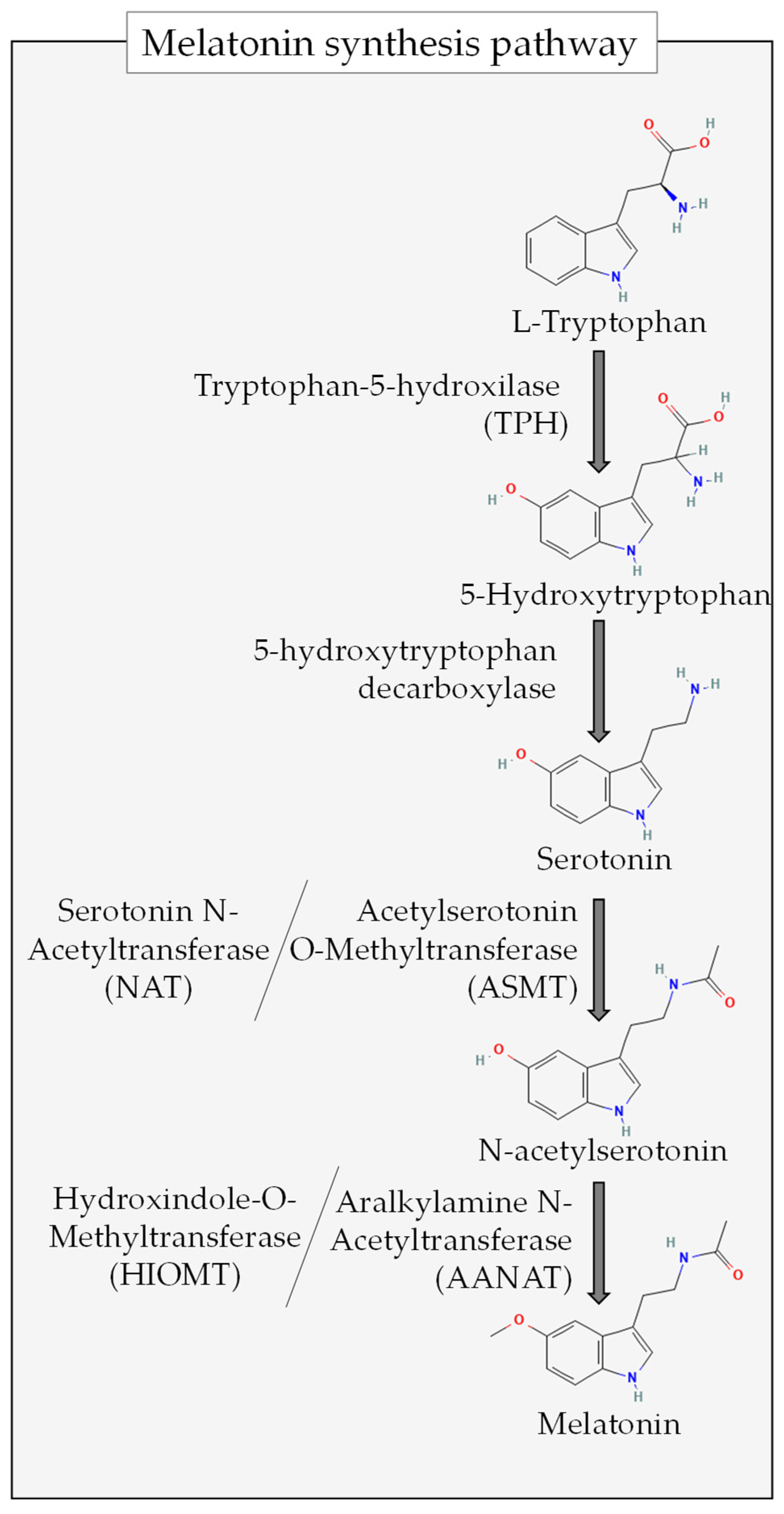
2. Antioxidant Properties of Melatonin
3. ROS in the Gut
Gut Melatonin and ROS
4. Melatonin Effects on Gut Microbiota Composition
- ➢
- Effects of exogenous melatonin
| Study | Animal Model | Treatment to Alter Gut Microbiota Composition | Melatonin Treatment/Intervention (Route, Dosage, Time Period)/ Measurement | Effects of Melatonin Treatment |
|---|---|---|---|---|
| Xu et al., 2017 [62] | Mouse | High-fat diet | By gavage 50 mg/kg body weight Once daily, 10 weeks | Revert 14/69 OTUs altered ↓ Firmicutes/Bacteroidetes ↑ Akkermansia |
| Yildirim et al., 2019 [63] | Rat | High-fat diet | In drinking water 4 mg/kg per day 2 weeks | ↑ Clostridiales ↑ Bacteroidales ↑ Enterobacteriales |
| Yin et al., 2018 [64] | Mouse | High-fat diet | In drinking water 0.4 mL/mL 2 weeks | ↑ Bacteroides ↑ Alistipes |
| Ren et al., 2018 [12] | Mouse | Weanling mouse model | In drinking water 0.2 mg/mL 2 weeks | ↑ Richness ↑ Lactobacillus ↓ Escherichia coli |
| Jing et al., 2019 [67] | Mouse | Mouse model of spinal cord injury | Intraperitoneal injection 10 mg/kg Twice a day | ↑ Lactobacillales ↑ Lactobacillus ↓ Clostridiales |
| Li et al., 2021 [70] | Sheep | Brucella infection | Overexpression of ASMT in transgenic sheep | ↓ Abundance of microbes related to infectious diseases |
| Zhang et al., 2022 [71] | Mouse | - | Endogenous melatonin reduction (Aanat-knockout (Aanat−/−)) | Microbiota dysbiosis ↑ Gut permeability ↑ Systemic inflammation |
| Zhao et al., 2022 [68] | Mouse | Induced colitis (oxazolone) | 50 mg/kg body weight By gavage 1 week before induction of colitis | ↑ Bifidobacterium ↓ Desulfovibrio ↓ Peptococcaceae ↓ Lachnospiraceae |
| Xia et al., 2022 [69] | Sucking piglets | Healthy | 10 mL oral melatonin solution (1 mg/mL) 21 days | ↑ Actinobacteria ↓ Selenomonadales |
| Ouyang et al., 2021 [72] | Lactating cows | Healthy | Ruminal melatonin In vitro | (+) Muribaculaceae, Succinivibrionaceae, Rikenellaceae, unidentified Cyanobacteria, Defluviitaleaceae, Veillonellaceae, Spirochaetaceae and Prevotellaceae ↑ Prevotellaceae ↑ Muribaculaceae ↓ Succinivibrionaceae ↓ Veillonellaceae |
| Yin et al., 2020 [65] | Mouse | High-fat diet | ↑ Bacteroidetes ↓ Firmicutes |
- ➢
- Manipulated endogenous melatonin production
5. Effects of Melatonin on Rhythmic Variations of Microbiota
6. Effects of Melatonin on Other Aspects of Gut Microbiota
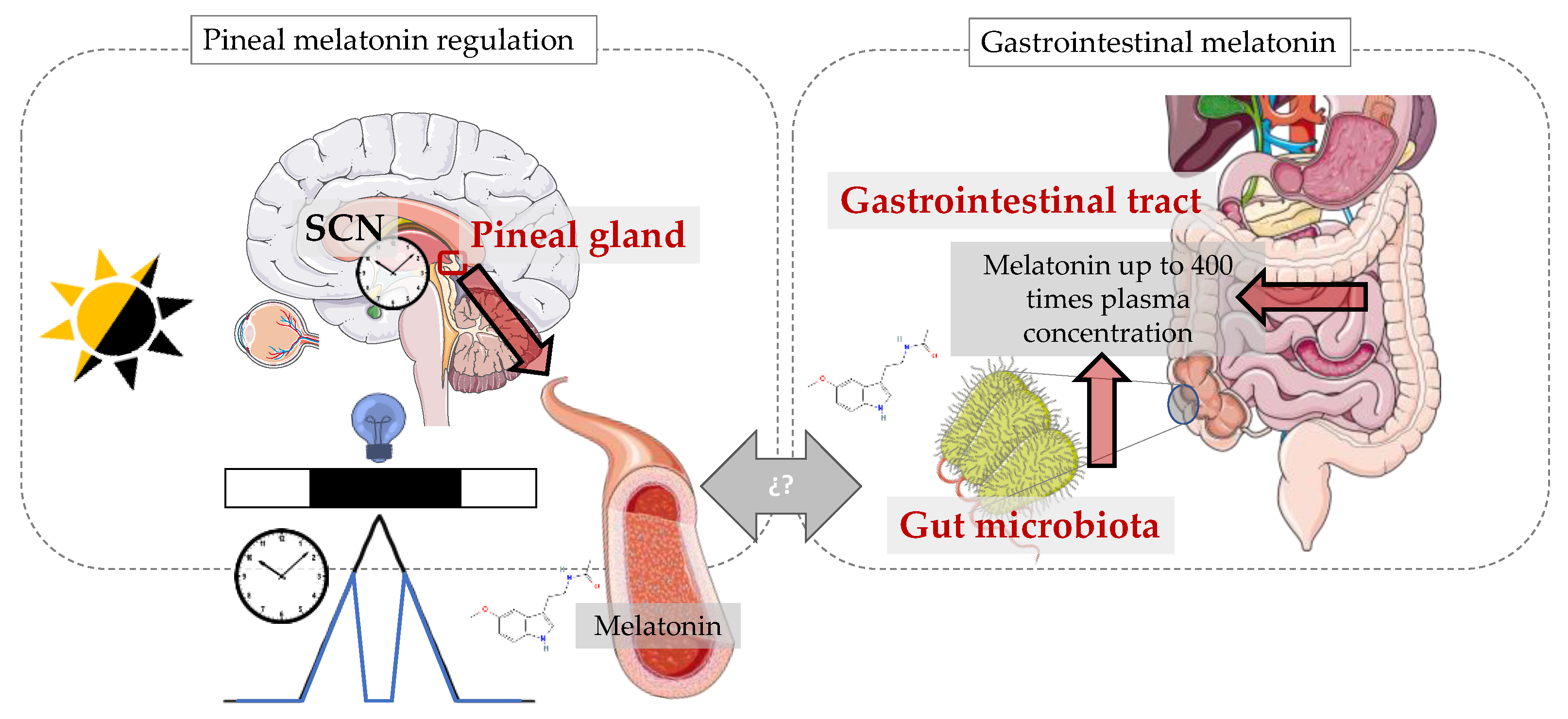
7. Gut Microbiota as an Extrapineal Source of Melatonin
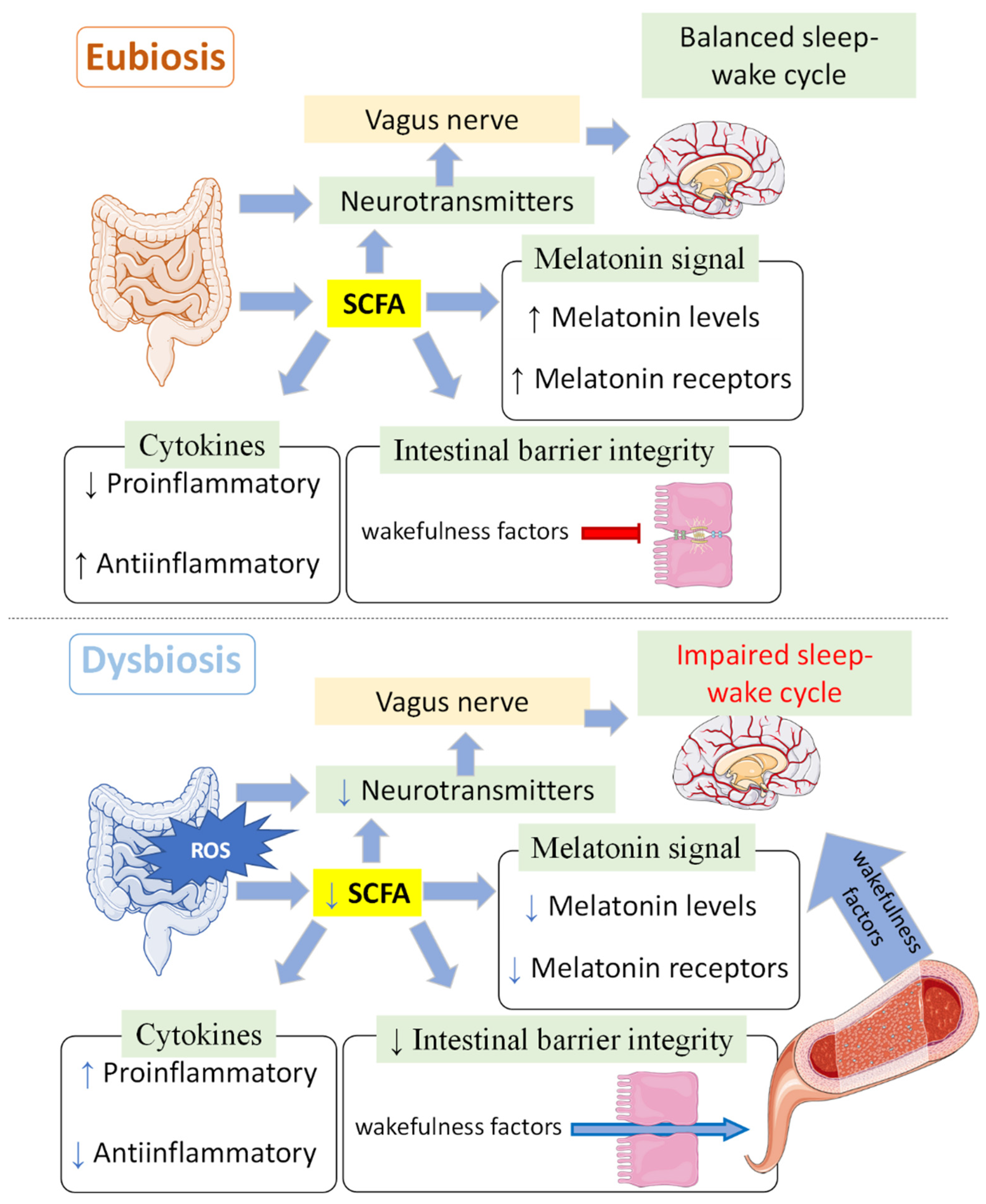
8. Sleep, Melatonin and Microbiota
9. Circadian System, Melatonin and Gut Microbiota
- ➢
- Influence of circadian cues on microbiota composition and effects on (and of) melatonin
| Study | Circadian Cue | Animal Model | Effects on Gut Microbiota | Other Effects | Effects of Melatonin Treatment |
|---|---|---|---|---|---|
| Hong et al., 2020 [11] | Constant light | Mouse | ↓ Blautia ↓ Ruminiclostridium ↓ Lachnospiraceae ↓ Lactobacillus ↓ Eubacterium ↓ Roseburia ↓ Bacteroides | ↑ Weight ↑ Insulin resistance ↑ Lipid influx | Reversion of dysbiosis and ↓ Anaerotruncus ↓ Alloprevotella ↓ Faecalibaculum |
| Cui et al., 2022 [109] | Long/short photoperiods | Laying ducks (diurnal) | 20 Light:4 Darkness ↓ Microbiota α-diversity 16 Light:8 Darkness Relative abundance of: = Actinobacteria = Fusobacteria = Proteobacteria = Fusobacterium = Clostridium_sensu_stricto_1 = Pectobacterium | 16 Light:8 Darkness ↑ Acetate ↑ Propionate, ↑ Butyrate and ↑ Total SCFA (Ileal chyme) ↓ Melatonin with increasing photoperiods. | - |
| Short et al., 2020 [111] | Long/short photoperiods | Hamster | Pinealectomised, short-day photoperiod (compared to sham-pinealectomised) ↑ Prevotella ↑ Clostridium ↓ Desulfovibrio Pinealectomised, long-day photoperiod (compared to sham-pinealectomised) ↑ Hungatella (Treatments did not uniformly affect OTU abundances—see paper for details). | In the presence of the pineal gland, animals with short-day photoperiods lost more weight than those with long-day photoperiods | - |
| Rong et al., 2021 [110] | Induced jet lag | Mouse | ↑ Escherichia coli (↑ LPS) | ↑ Lipid uptake ↑ Fat accumulation in white adipose tissue ↓ Angiopoietin-like 4 | Reversed those phenotypes through gut microbiota (only in non-microbiota-depleted animals). |
| Wang et al., 2021 [117] | Feeding time night-restricted | Piglets (diurnal) | Night-restricted ↑ Log Catenibacterium/Butyrivibrio ↑ Log Streptococcus/Butyrivibrio | Night-restricted ↓ Melatonin (day and night) ↓ Ghrelin ↓ Dopamine ↓ Serotonin | - |
10. Melatonin, Gut Microbiota and Disease
10.1. Inflammatory Bowel Disease
10.2. Melatonin, Gut Microbiota and Metabolic Disorders
10.2.1. Breastfeeding, Melatonin and Microbiota
10.2.2. Obesity
10.2.3. Other Metabolic Alterations
10.3. Oxidative Stress, Gut Microbiota and Neurological Disorders: Possible Role of Melatonin
10.3.1. Migraines
10.3.2. Multiple Sclerosis
10.3.3. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
10.3.4. Autism Spectrum Disorder
10.3.5. Bipolar Disorder
10.3.6. Alzheimer’s and Parkinson’s Diseases
10.3.7. Stress
10.3.8. Chronic Pain
10.4. Reproductive System and Development
11. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bonmati-Carrion, M.-A.; Tomas-Loba, A. Melatonin and Cancer: A Polyhedral Network Where the Source Matters. Antioxidants 2021, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Bonmati-Carrion, M.A.; Arguelles-Prieto, R.; Martinez-Madrid, M.J.; Reiter, R.; Hardeland, R.; Rol, M.A.; Madrid, J.A. Protecting the Melatonin Rhythm through Circadian Healthy Light Exposure. Int. J. Mol. Sci. 2014, 15, 23448–23500. [Google Scholar] [CrossRef] [PubMed]
- Raikhlin, N.T.; Kvetnoy, I.M.; Tolkachev, V.N. Melatonin May Be Synthesised in Enterochromaffin Cells. Nature 1975, 255, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Kim, S.H.; Park, J.W.; Kho, Y.; Seok, P.R.; Shin, J.H.; Choi, Y.J.; Jun, J.H.; Jung, H.C.; Kim, E.K. Melatonin in the Colon Modulates Intestinal Microbiota in Response to Stress and Sleep Deprivation. Intest. Res. 2020, 18, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin Attenuates Microbiota Dysbiosis of Jejunum in Short-Term Sleep Deprived Mice. J. Microbiol. 2020, 58, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of Melatonin in Sleep Deprivation-Induced Intestinal Barrier Dysfunction in Mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Melatonin-Mediated Colonic Microbiota Metabolite Butyrate Prevents Acute Sleep Deprivation-Induced Colitis in Mice. Int. J. Mol. Sci. 2021, 22, 11894. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin Ameliorates Corticosterone-Mediated Oxidative Stress-Induced Colitis in Sleep-Deprived Mice Involving Gut Microbiota. Oxid. Med. Cell. Longev. 2021, 2021, 9981480. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin Prevents the Dysbiosis of Intestinal Microbiota in Sleep-Restricted Mice by Improving Oxidative Stress and Inhibiting Inflammation. Saudi J. Gastroenterol. 2022, 28, 209–217. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. The Role of Aeromonas-Goblet Cell Interactions in Melatonin-Mediated Improvements in Sleep Deprivation-Induced Colitis. Oxid. Med. Cell. Longev. 2022, 2022, e8133310. [Google Scholar] [CrossRef]
- Hong, F.; Pan, S.; Xu, P.; Xue, T.; Wang, J.; Guo, Y.; Jia, L.; Qiao, X.; Li, L.; Zhai, Y. Melatonin Orchestrates Lipid Homeostasis through the Hepatointestinal Circadian Clock and Microbiota during Constant Light Exposure. Cells 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wang, P.; Yan, J.; Liu, G.; Zeng, B.; Hussain, T.; Peng, C.; Yin, J.; Tan, B.; Li, T.; et al. Melatonin Alleviates Weanling Stress in Mice: Involvement of Intestinal Microbiota. J. Pineal Res. 2018, 64, e12448. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.M.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.J. The Changing Biological Roles of Melatonin during Evolution: From an Antioxidant to Signals of Darkness, Sexual Selection and Fitness. Biol. Rev. Camb. Philos. Soc. 2010, 85, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal Melatonin: Sources, Regulation, and Potential Functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.; Tan, D.-X.; Cabrera, J.; D’Arpa, D.; Sainz, R.; Mayo, J.; Ramos, S. The Oxidant/Antioxidant Network: Role of Melatonin. Neurosignals 1999, 8, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Mahal, H.S.; Sharma, H.S.; Mukherjee, T. Antioxidant Properties of Melatonin: A Pulse Radiolysis Study. Free Radic. Biol. Med. 1999, 26, 557–565. [Google Scholar] [CrossRef]
- Reiter, R.J.; Calvo, J.R.; Karbownik, M.; Qi, W.; Tan, D.X. Melatonin and Its Relation to the Immune System and Inflammation. Ann. N. Y. Acad. Sci. 2000, 917, 376–386. [Google Scholar] [CrossRef]
- Galano, A.; Reiter, R.J. Melatonin and Its Metabolites vs Oxidative Stress: From Individual Actions to Collective Protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef]
- Gultekin, F.; Delibas, N.; Yasar, S.; Kilinc, I. In Vivo Changes in Antioxidant Systems and Protective Role of Melatonin and a Combination of Vitamin C and Vitamin E on Oxidative Damage in Erythrocytes Induced by Chlorpyrifos-Ethyl in Rats. Arch. Toxicol. 2001, 75, 88–96. [Google Scholar] [CrossRef]
- Montilla, P.; Cruz, A.; Padillo, F.J.; Túnez, I.; Gascon, F.; Muñoz, M.C.; Gómez, M.; Pera, C. Melatonin versus Vitamin E as Protective Treatment against Oxidative Stress after Extra-Hepatic Bile Duct Ligation in Rats. J. Pineal Res. 2001, 31, 138–144. [Google Scholar] [CrossRef]
- Matuszak, Z.; Bilska, M.A.; Reszka, K.J.; Chignell, C.F.; Bilski, P. Interaction of Singlet Molecular Oxygen with Melatonin and Related Indoles. Photochem. Photobiol. 2003, 78, 449. [Google Scholar] [CrossRef]
- Cagnoli, C.M.; Atabay, C.; Kharlamova, E.; Manev, H. Melatonin Protects Neurons from Singlet Oxygen-induced Apoptosis. J. Pineal Res. 1995, 18, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Limson, J.; Nyokong, T.; Daya, S. The Interaction of Melatonin and Its Precursors with Aluminium, Cadmium, Copper, Iron, Lead, and Zinc: An Adsorptive Voltammetric Study. J. Pineal Res. 1998, 24, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.; Limson, J.; Nyokong, T.; Daya, S. Melatonin Protects against Copper-Mediated Free Radical Damage. J. Pineal Res. 2002, 32, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Natarajan, M.; Lopez-Burillo, S.; Reiter, R.J. Protection against Oxidative Protein Damage Induced by Metal-Catalyzed Reaction or Alkylperoxyl Radicals: Comparative Effects of Melatonin and Other Antioxidants. Biochim. Et Biophys. Acta Gen. Subj. 2003, 1620, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Ramos, E.; De Los Ríos, C.; Egea, J.; Del Pino, J.; Reiter, R.J. A Review of Metal-Catalyzed Molecular Damage: Protection by Melatonin. J. Pineal Res. 2014, 56, 343–370. [Google Scholar] [CrossRef] [PubMed]
- Poeggeler, B.; Reiter, R.J.; Hardeland, R.; Sewerynek, E.; Melchiorri, D.; Barlow-Walden, L.R. Melatonin, a Mediator of Electron Transfer and Repair Reactions, Acts Synergistically with the Chain-Breaking Antioxidants Ascorbate, Trolox and Glutathione. Neuroendocrinol. Lett. 1995, 17, 87–92. [Google Scholar]
- Gitto, E.; Tan, D.-X.; Reiter, R.J.; Karbownik, M.; Manchester, L.C.; Cuzzocrea, S.; Fulia, F.; Barberi, I. Individual and Synergistic Antioxidative Actions of Melatonin: Studies with Vitamin E, Vitamin C, Glutathione and Desferrrioxamine (Desferoxamine) in Rat Liver Homogenates. J. Pharm. Pharmacol. 2001, 53, 1393–1401. [Google Scholar] [CrossRef]
- Sliwinski, T.; Rozej, W.; Morawiec-Bajda, A.; Morawiec, Z.; Reiter, R.; Blasiak, J. Protective Action of Melatonin against Oxidative DNA Damage-Chemical Inactivation versus Base-Excision Repair. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 634, 220–227. [Google Scholar] [CrossRef]
- Davanipour, Z.; Poulsen, H.E.; Weimann, A.; Sobel, E. Endogenous Melatonin and Oxidatively Damaged Guanine in DNA. BMC Endocr. Disord. 2009, 9, 22. [Google Scholar] [CrossRef]
- Ferreira, S.G.; Peliciari-Garcia, R.A.; Takahashi-Hyodo, S.A.; Rodrigues, A.C.; Amaral, F.G.; Berra, C.M.; Bordin, S.; Curi, R.; Cipolla-Neto, J. Effects of Melatonin on DNA Damage Induced by Cyclophosphamide in Rats. Braz. J. Med. Biol. Res. 2013, 46, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Fu, A.; Hoffman, A.E.; Zheng, T.; Zhu, Y. Melatonin Enhances DNA Repair Capacity Possibly by Affecting Genes Involved in DNA Damage Responsive Pathways. BMC Cell Biol. 2013, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, M.; Shirazi, A.; Izadi, P.; Tavakkoly Bazzaz, J.; Rezaeejam, H. Expression Levels of Two Dna Repair-Related Genes under 8 Gy Ionizing Radiation and 100 Mg/Kg Melatonin Delivery in Rat Peripheral Blood. J. Biomed. Phys. Eng. 2017, 7, 27–36. [Google Scholar] [PubMed]
- Santoro, R.; Marani, M.; Blandino, G.; Muti, P.; Strano, S. Melatonin Triggers P53 Ser Phosphorylation and Prevents DNA Damage Accumulation. Oncogene 2012, 31, 2931–2942. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Zapico, C.; Coto-Montes, A. A Proposed Mechanism to Explain the Stimulatory Effect of Melatonin on Antioxidative Enzymes. J. Pineal Res. 2005, 39, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Liu, C.; Duan, W.X.; Xu, S.C.; He, M.D.; Chen, C.H.; Wang, Y.; Zhou, Z.; Yu, Z.P.; Zhang, L.; et al. Melatonin Ameliorates Bisphenol A-Induced DNA Damage in the Germ Cells of Adult Male Rats. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 752, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Franco, M.; Planells, E.; Quintero, B.; Acuña-Castroviejo, D.; Rusanova, I.; Escames, G.; Molina-López, J. Effect of Melatonin Supplementation on Antioxidant Status and DNA Damage in High Intensity Trained Athletes. Int. J. Sports Med. 2017, 38, 1117–1125. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Koppisepi, S. Medical Implications of Melatonin: Receptor-Mediated and Receptor-Independent Actions. Adv. Med. Sci. 2007, 52, 11–28. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Osuna, C.; Gitto, E. Actions of Melatonin in the Reduction of Oxidative Stress: A Review. J. Biomed. Sci. 2000, 7, 444–458. [Google Scholar] [CrossRef]
- Barlow-Walden, L.R.; Reiter, R.J.; Abe, M.; Pablos, M.; Menendez-Pelaez, A.; Chen, L.D.; Poeggeler, B. Melatonin Stimulates Brain Glutathione Peroxidase Activity. Neurochem. Int. 1995, 26, 497–502. [Google Scholar] [CrossRef]
- Pablos, M.I.; Agapito, M.T.; Gutierrez, R.; Recio, J.M.; Reiter, R.J.; Barlow-Walden, L.; Acuña-Castroviejo, D.; Menendez-Pelaez, A. Melatonin Stimulates the Activity of the Detoxifying Enzyme Glutathione Peroxidase in Several Tissues of Chicks. J. Pineal Res. 1995, 19, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Laothong, U.; Pinlaor, P.; Hiraku, Y.; Boonsiri, P.; Prakobwong, S.; Khoontawad, J.; Pinlaor, S. Protective Effect of Melatonin against Opisthorchis Viverrini-Induced Oxidative and Nitrosative DNA Damage and Liver Injury in Hamsters. J. Pineal Res. 2010, 49, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Sokolovic, D.; Djordjevic, B.; Kocic, G.; Stoimenov, T.J.; Stanojkovic, Z.; Sokolovic, D.M.; Veljkovic, A.; Ristic, G.; Despotovic, M.; Milisavljevic, D.; et al. The Effects of Melatonin on Oxidative Stress Parameters and DNA Fragmentation in Testicular Tissue of Rats Exposed to Microwave Radiation. Adv. Clin. Exp. Med. 2015, 24, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Ballard, J.W.O.; Towarnicki, S.G. Mitochondria, the Gut Microbiome and ROS. Cell. Signal. 2020, 75, 109737. [Google Scholar] [CrossRef]
- Yardeni, T.; Tanes, C.E.; Bittinger, K.; Mattei, L.M.; Schaefer, P.M.; Singh, L.N.; Wu, G.D.; Murdock, D.G.; Wallace, D.C. Host Mitochondria Influence Gut Microbiome Diversity: A Role for ROS. Sci. Signal. 2019, 12, eaaw3159. [Google Scholar] [CrossRef]
- Ahlawat, S.; Kumar, P.; Mohan, H.; Goyal, S.; Sharma, K.K. Inflammatory Bowel Disease: Tri-Directional Relationship between Microbiota, Immune System and Intestinal Epithelium. Crit. Rev. Microbiol. 2021, 47, 254–273. [Google Scholar] [CrossRef]
- Hu, Q.; Ren, J.; Li, G.; Wu, J.; Wu, X.; Wang, G.; Gu, G.; Ren, H.; Hong, Z.; Li, J. The Mitochondrially Targeted Antioxidant MitoQ Protects the Intestinal Barrier by Ameliorating Mitochondrial DNA Damage via the Nrf2/ARE Signaling Pathway. Cell Death Dis. 2018, 9, 403. [Google Scholar] [CrossRef]
- Berg, R.D.; Garlington, A.W. Translocation of Certain Indigenous Bacteria from the Gastrointestinal Tract to the Mesenteric Lymph Nodes and Other Organs in a Gnotobiotic Mouse Model. Infect. Immun. 1979, 23, 403–411. [Google Scholar] [CrossRef]
- Wiest, R.; Garcia-Tsao, G. Bacterial Translocation (BT) in Cirrhosis. Hepatology 2005, 41, 422–433. [Google Scholar] [CrossRef]
- Iovine, N.M.; Pursnani, S.; Voldman, A.; Wasserman, G.; Blaser, M.J.; Weinrauch, Y. Reactive Nitrogen Species Contribute to Innate Host Defense against Campylobacter Jejuni. Infect. Immun. 2008, 76, 986–993. [Google Scholar] [CrossRef]
- Check, J.; Byrd, C.L.; Menio, J.; Rippe, R.A.; Hines, I.N.; Wheeler, M.D. Src Kinase Participates in LPS-Induced Activation of NADPH Oxidase. Mol. Immunol. 2010, 47, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-N.; Lin, C.-C.; Cheng, H.-Y.; Yang, C.-M. Regulation of Cyclooxygenase-2 and Cytosolic Phospholipase A2 Gene Expression by Lipopolysaccharide through the RNA-Binding Protein HuR: Involvement of NADPH Oxidase, Reactive Oxygen Species and Mitogen-Activated Protein Kinases. Br. J. Pharmacol. 2011, 163, 1691–1706. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Luo, L.; Ardita, C.S.; Richardson, A.N.; Kwon, Y.M.; Mercante, J.W.; Alam, A.; Gates, C.L.; Wu, H.; Swanson, P.A.; et al. Symbiotic Lactobacilli Stimulate Gut Epithelial Proliferation via Nox-Mediated Generation of Reactive Oxygen Species. EMBO J. 2013, 32, 3017–3028. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, O.S.; Elesawy, R.O.; Eltokhy, A.K.; Al-Shenawy, H.A.; Ghanem, H.B.; Rizk, F.H.; Barhoma, R.A.; Shalaby, R.H.; Abdelsattar, A.M.; Mashal, S.S.; et al. Moderating Gut Microbiome/Mitochondrial Axis in Oxazolone Induced Ulcerative Colitis: The Evolving Role of β-Glucan and/or, Aldose Reductase Inhibitor, Fidarestat. Int. J. Mol. Sci. 2023, 24, 2711. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.K.; Sarkar, S.; Chattopadhyay, A.; Tan, D.X.; Bandyopadhyay, D. Enterochromaffin Cells as the Source of Melatonin: Key Findings and Functional Relevance in Mammals. Melatonin Res. 2019, 2, 61–82. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, Y.; Wu, S.; Yang, X.; Huang, Q.; Dong, Y.; Xu, D.; Huang, Z. Prolonged Darkness Attenuates Imidacloprid Toxicity through the Brain-Gut-Microbiome Axis in Zebrafish, Danio Rerio. Sci Total Environ. 2023, 881, 163481. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin Improves Skin Barrier Damage Caused by Sleep Restriction through Gut Microbiota. J. Pineal Res. 2023, 75, e12874. [Google Scholar] [CrossRef]
- Zhu, D.; Ma, Y.; Ding, S.; Jiang, H.; Fang, J. Effects of Melatonin on Intestinal Microbiota and Oxidative Stress in Colitis Mice. BioMed Res. Int. 2018, 2018, 6. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T.; et al. An Integrated Catalog of Reference Genes in the Human Gut Microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef]
- Hugon, P.; Dufour, J.C.; Colson, P.; Fournier, P.E.; Sallah, K.; Raoult, D. A Comprehensive Repertoire of Prokaryotic Species Identified in Human Beings. Lancet Infect. Dis. 2015, 15, 1211–1219. [Google Scholar] [CrossRef]
- Degruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, J.; Hong, F.; Wang, S.; Jin, X.; Xue, T.; Jia, L.; Zhai, Y. Melatonin Prevents Obesity through Modulation of Gut Microbiota in Mice. J. Pineal Res. 2017, 62, e12399. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Arabacl Tamer, S.; Sahin, D.; Bagriacik, F.; Kahraman, M.M.; Onur, N.D.; Cayirli, Y.B.; Cilingir Kaya, Ö.T.; Aksu, B.; Akdeniz, E.; et al. The Effects of Antibiotics and Melatonin on Hepato-Intestinal Inflammation and Gut Microbial Dysbiosis Induced by a Short-Term High-Fat Diet Consumption in Rats. Br. J. Nutr. 2019, 122, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Han, H.; Chen, S.; Gao, J.; Liu, G.; Wu, X.; Deng, J.; Yu, Q.; Huang, X.; et al. Melatonin Reprogramming of Gut Microbiota Improves Lipid Dysmetabolism in High-Fat Diet-Fed Mice. J. Pineal Res. 2018, 65, e12524. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Han, H.; Ma, J.; Liu, G.; Wu, X.; Huang, X.; Fang, R.; Baba, K.; Bin, P.; et al. Administration of Exogenous Melatonin Improves the Diurnal Rhythms of the Gut Microbiota in Mice Fed a High-Fat Diet. mSystems 2020, 5, e00002-20. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.G.; Sourjik, V. Chemotaxis of Escherichia Coli to Major Hormones and Polyamines Present in Human Gut. ISME J. 2018, 12, 2736. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Yang, D.; Bai, F.; Zhang, C.; Qin, C.; Li, D.; Wang, L.; Yang, M.; Chen, Z.; Li, J. Melatonin Treatment Alleviates Spinal Cord Injury-Induced Gut Dysbiosis in Mice. J. Neurotrauma 2019, 36, 2646–2664. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Yuan, X.; Cui, Y.Y.; Liu, J.; Shen, J.; Jin, B.Y.; Feng, B.C.; Zhai, Y.J.; Zheng, M.Q.; Kou, G.J.; et al. Melatonin Mitigates Oxazolone-Induced Colitis in Microbiota-Dependent Manner. Front. Immunol. 2022, 12, 783806. [Google Scholar] [CrossRef]
- Xia, S.; Gao, W.; Li, Y.; Ma, J.; Gong, S.; Gao, Z.; Tang, W.; Tian, W.; Tang, S. Effects of Melatonin on Intestinal Function and Bacterial Compositions in Sucking Piglets. J. Anim. Physiol. Anim. Nutr. 2022, 106, 1139–1148. [Google Scholar] [CrossRef]
- Li, G.; Lv, D.; Yao, Y.; Wu, H.; Wang, J.; Deng, S.; Song, Y.; Guan, S.; Wang, L.; Ma, W.; et al. Overexpression of ASMT Likely Enhances the Resistance of Transgenic Sheep to Brucellosis by Influencing Immune-Related Signaling Pathways and Gut Microbiota. FASEB Journal Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21783. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, T.; Cao, M.; Yuan, C.; Reiter, R.J.; Zhao, Z.; Zhao, Y.; Chen, L.; Fan, W.; Wang, X.; et al. Gut Microbiota Dysbiosis Induced by Decreasing Endogenous Melatonin Mediates the Pathogenesis of Alzheimer’s Disease and Obesity. Front. Immunol. 2022, 13, 900132. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Wang, M.; Bu, D.; Ma, L.; Liu, F.; Xue, C.; Du, C.; Aboragah, A.; Loor, J.J. Ruminal Microbes Exhibit a Robust Circadian Rhythm and Are Sensitive to Melatonin. Front. Nutr. 2021, 8, 760578. [Google Scholar] [CrossRef] [PubMed]
- Paulose, J.K.; Wright, J.M.; Patel, A.G.; Cassone, V.M. Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. PLoS ONE 2016, 11, e0146643. [Google Scholar] [CrossRef] [PubMed]
- Paulose, J.K.; Cassone, V.M. The Melatonin-Sensitive Circadian Clock of the Enteric Bacterium Enterobacter Aerogenes. Gut Microbes 2016, 7, 424. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Swanson, G.R.; Siskin, J.; Gorenz, A.; Shaikh, M.; Raeisi, S.; Fogg, L.; Forsyth, C.; Keshavarzian, A. Disrupted Diurnal Oscillation of Gut-Derived Short Chain Fatty Acids in Shift Workers Drinking Alcohol: Possible Mechanism for Loss of Resiliency of Intestinal Barrier in Disrupted Circadian Host. Transl. Res. 2020, 221, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Poeggeler, B.; Alvares, F.L.; Ogden, G.B.; Reiter, R.J. Melatonin Immunoreactivity in the Photosynthetic Prokaryote Rhodospirillum Rubrum: Implications for an Ancient Antioxidant System. Cell Mol. Biol. Res. 1995, 41, 391–395. [Google Scholar] [PubMed]
- Pan, J.; Li, F.; Wang, C.; Li, X.; Zhang, S.; Zhang, W.; Zhao, G.; Ma, C.; Liu, G.; Yang, K. Effects of Duodenal 5-Hydroxytryptophan Perfusion on Melatonin Synthesis in GI Tract of Sheep. Molecules 2021, 26, 5275. [Google Scholar] [CrossRef]
- Anderson, G. Gut Dysbiosis Dysregulates Central and Systemic Homeostasis via Decreased Melatonin and Suboptimal Mitochondria Functioning: Pathoetiological and Pathophysiological Implications. Melatonin Res. 2019, 2, 70–85. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Zhu, S.W.; Zhang, J.D.; Duan, L.P. Short Chain Fatty Acids Contribute to Gut Microbiota-Induced Promotion of Colonic Melatonin Receptor Expression. J. Biol. Regul. Homeost. Agents 2019, 33, 767–771. [Google Scholar]
- Noguti, J.; Andersen, M.L.; Cirelli, C.; Ribeiro, D.A. Oxidative Stress, Cancer, and Sleep Deprivation: Is There a Logical Link in This Association? Sleep Breath 2013, 17, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, L.; Gulyani, S.; Nienhuis, R.; Siegel, J.M. Sleep Deprivation Decreases Superoxide Dismutase Activity in Rat Hippocampus and Brainstem. Neuroreport 2002, 13, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Neculicioiu, V.S.; Colosi, I.A.; Costache, C.; Toc, D.A.; Sevastre-Berghian, A.; Colosi, H.A.; Clichici, S. Sleep Deprivation-Induced Oxidative Stress in Rat Models: A Scoping Systematic Review. Antioxidants 2023, 12, 1600. [Google Scholar] [CrossRef] [PubMed]
- Atrooz, F.; Salim, S. Sleep Deprivation, Oxidative Stress and Inflammation. Adv. Protein Chem. Struct. Biol. 2020, 119, 309–336. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, S.; Swetha Kumari, A.; Nimesh, A.; Soundararaghavan, S.; Ananthanarayanan, P.H.; Dhiman, P. Markers of Oxidative Stress in Pregnant Women with Sleep Disturbances. Oman Med. J. 2015, 30, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Grubač, Ž.; Šutulović, N.; Šuvakov, S.; Jerotić, D.; Puškaš, N.; Macut, D.; Rašić-Marković, A.; Simić, T.; Stanojlović, O.; Hrnčić, D. Anxiogenic Potential of Experimental Sleep Fragmentation Is Duration-Dependent and Mediated via Oxidative Stress State. Oxid. Med. Cell. Longev. 2021, 2021, 2262913. [Google Scholar] [CrossRef]
- Gulec, M.; Ozkol, H.; Selvi, Y.; Tuluce, Y.; Aydin, A.; Besiroglu, L.; Ozdemir, P.G. Oxidative Stress in Patients with Primary Insomnia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 37, 247–251. [Google Scholar] [CrossRef]
- Bin Heyat, M.B.; Akhtar, F.; Sultana, A.; Tumrani, S.; Teelhawod, B.N.; Abbasi, R.; Amjad Kamal, M.; Muaad, A.Y.; Lai, D.; Wu, K. Role of Oxidative Stress and Inflammation in Insomnia Sleep Disorder and Cardiovascular Diseases: Herbal Antioxidants and Anti-Inflammatory Coupled with Insomnia Detection Using Machine Learning. Curr. Pharm. Des. 2022, 28, 3618–3636. [Google Scholar] [CrossRef]
- Duan, W.; Ye, P.; Leng, Y.-Q.; Liu, D.-H.; Sun, J.-C.; Tan, X.; Wang, W.-Z. Oxidative Stress in the RVLM Mediates Sympathetic Hyperactivity Induced by Circadian Disruption. Neurosci. Lett. 2022, 791, 136917. [Google Scholar] [CrossRef]
- Zisapel, N. New Perspectives on the Role of Melatonin in Human Sleep, Circadian Rhythms and Their Regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Claustrat, B.; Leston, J. Melatonin: Physiological Effects in Humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Kräuchi, K.; Wirz-Justice, A. Role of Melatonin in the Regulation of Human Circadian Rhythms and Sleep. J. Neuroendocr. 2003, 15, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Fatemeh, G.; Sajjad, M.; Niloufar, R.; Neda, S.; Leila, S.; Khadijeh, M. Effect of Melatonin Supplementation on Sleep Quality: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Neurol. 2022, 269, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-Chain Fatty Acids Stimulate Colonic Transit via Intraluminal 5-HT Release in Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1269–R1276. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.V.-A.; Foster, K.R. Why Does the Microbiome Affect Behaviour? Nat. Rev. Microbiol. 2018, 16, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Simkin, D.R. Microbiome and Mental Health, Specifically as It Relates to Adolescents. Curr. Psychiatry Rep. 2019, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Imeri, L.; Opp, M.R. How (and Why) the Immune System Makes Us Sleep. Nat. Rev. Neurosci. 2009, 10, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Haarhuis, J.E.; Kardinaal, A.; Kortman, G.A.M. Probiotics, Prebiotics and Postbiotics for Better Sleep Quality: A Narrative Review. Benef. Microbes 2022, 13, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Yamazaki, M.; Sukigara, H.; Motohashi, H.; Sasaki, H.; Miyakawa, H.; Haraguchi, A.; Ikeda, Y.; Fukuda, S.; Shibata, S. Gut Microbiota-Derived Short Chain Fatty Acids Induce Circadian Clock Entrainment in Mouse Peripheral Tissue. Sci. Rep. 2018, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Foley, M.H.; O’Flaherty, S.; Barrangou, R.; Theriot, C.M. Bile Salt Hydrolases: Gatekeepers of Bile Acid Metabolism and Host-Microbiome Crosstalk in the Gastrointestinal Tract. PLoS Pathog 2019, 15, e1007581. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, K.; MacSharry, J.; Casey, P.G.; Shanahan, F.; Joyce, S.A.; Gahan, C.G.M. Unconjugated Bile Acids Influence Expression of Circadian Genes: A Potential Mechanism for Microbe-Host Crosstalk. PLoS ONE 2016, 11, e0167319. [Google Scholar] [CrossRef]
- Bowers, S.J.; Vargas, F.; González, A.; He, S.; Jiang, P.; Dorrestein, P.C.; Knight, R.; Wright, K.P.; Lowry, C.A.; Fleshner, M.; et al. Repeated Sleep Disruption in Mice Leads to Persistent Shifts in the Fecal Microbiome and Metabolome. PLoS ONE 2020, 15, e0229001. [Google Scholar] [CrossRef]
- Cui, Y.M.; Wang, J.; Zhang, H.J.; Qi, G.H.; Qiao, H.Z.; Gan, L.P.; Wu, S.G. Effect of Changes in Photoperiods on Melatonin Expression and Gut Health Parameters in Laying Ducks. Front. Microbiol. 2022, 13, 819427. [Google Scholar] [CrossRef]
- Rong, B.; Wu, Q.; Reiter, R.J.; Sun, C. The Mechanism of Oral Melatonin Smeliorates Intestinal and Adipose Lipid Dysmetabolism through Reducing Escherichia Coli-Derived Lipopolysaccharide. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1643–1667. [Google Scholar] [CrossRef]
- Shor, E.K.; Brown, S.P.; Freeman, D.A. A Novel Role for the Pineal Gland: Regulating Seasonal Shifts in the Gut Microbiota of Siberian Hamsters. J. Pineal Res. 2020, 69, e12696. [Google Scholar] [CrossRef] [PubMed]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential Adaptation of Human Gut Microbiota to Bariatric Surgery–Induced Weight Loss: Links with Metabolic and Low-Grade Inflammation Markers. Diabetes 2010, 59, 3049. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.M.; Lee, E.J.; Kotter, C.V.; Austin, G.L.; Gianella, S.; Siewe, B.; Smith, D.M.; Landay, A.L.; McManus, M.C.; Robertson, C.E.; et al. Gut Dendritic Cell Activation Links an Altered Colonic Microbiome to Mucosal and Systemic T-Cell Activation in Untreated HIV-1 Infection. Mucosal Immunol. 2016, 9, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of Intestinal Prevotella Copri Correlates with Enhanced Susceptibility to Arthritis. eLife 2013, 2013, e01202. [Google Scholar] [CrossRef]
- Ohara, T. Identification of the Microbial Diversity after Fecal Microbiota Transplantation Therapy for Chronic Intractable Constipation Using 16s rRNA Amplicon Sequencing. PLoS ONE 2019, 14, e0214085. [Google Scholar] [CrossRef]
- Wang, Q.J.; Guo, Y.; Yao, C.Y.; Zhang, K.H.; Li, Q.; Shan, C.H.; Liu, P.; Wang, M.Z.; Zhu, F.; An, L.; et al. Loss of Diurnal Behavioral Rhythms and Impaired Lipid Metabolism in Growing Pigs with Mistimed Feeding. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21972. [Google Scholar] [CrossRef]
- Walensky, R.P.; Houry, D. Centers for Disease Control and Prevention 2022. Available online: https://www.cdc.gov/ibd/data-and-statistics/prevalence.html (accessed on 1 September 2023).
- Ilott, N.E.; Bollrath, J.; Danne, C.; Schiering, C.; Shale, M.; Adelmann, K.; Krausgruber, T.; Heger, A.; Sims, D.; Powrie, F. Defining the Microbial Transcriptional Response to Colitis through Integrated Host and Microbiome Profiling. ISME J. 2016, 10, 2389–2404. [Google Scholar] [CrossRef]
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front. Microbiol. 2016, 7, 1081. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, J.T.; Zhang, H.Q.; Song, Q.H.; Xu, G.H.; Cai, L.; Tang, X.D.; Zhang, H.F.; Liu, F.-E.; Jia, Z.S.; et al. Melatonin Attenuates Noise Stress-Induced Gastrointestinal Motility Disorder and Gastric Stress Ulcer: Role of Gastrointestinal Hormones and Oxidative Stress in Rats. J. Neurogastroenterol. Motil. 2015, 21, 189–199. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of Gut Bacteria on Human Health and Diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between Intestinal Microbiota and Ulcerative Colitis: Mechanisms and Clinical Application of Probiotics and Fecal Microbiota Transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of Inflammation-Driven Bacterial Dysbiosis in the Gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef] [PubMed]
- de Vrieze, J. Medical Research. The Promise of Poop. Science 2013, 341, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Pigneur, B.; Sokol, H. Fecal Microbiota Transplantation in Inflammatory Bowel Disease: The Quest for the Holy Grail. Mucosal Immunol. 2016, 9, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Dudzińska, E.; Gryzinska, M.; Ognik, K.; Gil-Kulik, P.; Kocki, J. Oxidative Stress and Effect of Treatment on the Oxidation Product Decomposition Processes in IBD. Oxid. Med. Cell. Longev. 2018, 2018, 7918261. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Grácio, D.; Teixeira, J.P.; Magro, F. Oxidative Stress and DNA Damage: Implications in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Song, G.H.; Leng, P.H.; Gwee, K.A.; Moochhala, S.M.; Ho, K.Y. Melatonin Improves Abdominal Pain in Irritable Bowel Syndrome Patients Who Have Sleep Disturbances: A Randomised, Double Blind, Placebo Controlled Study. Gut 2005, 54, 1402. [Google Scholar] [CrossRef]
- Lu, W.Z.; Gwee, K.A.; Moochhalla, S.; Ho, K.Y. Melatonin Improves Bowel Symptoms in Female Patients with Irritable Bowel Syndrome: A Double-Blind Placebo-Controlled Study. Aliment. Pharmacol. Ther. 2005, 22, 927–934. [Google Scholar] [CrossRef]
- Jing, W.; Zhu, M.; Wang, F.; Zhao, X.; Dong, S.; Xu, Y.; Wang, S.; Yang, J.; Wang, K.; Liu, W. Hyaluronic Acid-Melatonin Nanoparticles Improve the Dysregulated Intestinal Barrier, Microbiome and Immune Response in Mice with Dextran Sodium Sulfate-Induced Colitis. J. Biomed. Nanotechnol. 2022, 18, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, S.; Son, M.; Cheon, J.H.; Park, Y.S. Melatonin Controls Microbiota in Colitis by Goblet Cell Differentiation and Antimicrobial Peptide Production through Toll-like Receptor 4 Signalling. Sci. Rep. 2020, 10, 2232. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yamashita, R.; Kawashima, J.; Mori, H. Omics Profiles of Fecal and Oral Microbiota Change in Irritable Bowel Syndrome Patients with Diarrhea and Symptom Exacerbation. J. Gastroenterol. 2022, 57, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, S.; Liu, Z.; Wei, H.; Zhang, L.; He, M.; Pei, F.; Zhang, J.; Sun, Q.; Duan, L. Increased Expression of Colonic Mucosal Melatonin in Patients with Irritable Bowel Syndrome Correlated with Gut Dysbiosis. Genom. Proteom. Bioinform. 2020, 18, 708. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A.; Pang, S.F. The Role of Serotonin and Melatonin in Gastrointestinal Physiology: Ontogeny, Regulation of Food Intake, and Mutual Serotonin-Melatonin Feedback. J. Pineal Res. 1994, 16, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.Y.; Lam, K.L.; Li, X.; Kong, A.P.S.; Cheung, P.C.K. Circadian Disruption-Induced Metabolic Syndrome in Mice Is Ameliorated by Oat β-Glucan Mediated by Gut Microbiota. Carbohydr. Polym. 2021, 267, 118216. [Google Scholar] [CrossRef] [PubMed]
- Buonfiglio, D.; Parthimos, R.; Dantas, R.; Silva, R.C.; Gomes, G.; Andrade-Silva, J.; Ramos-Lobo, A.; Amaral, F.G.; Matos, R.; Sinésio, J.; et al. Melatonin Absence Leads to Long-Term Leptin Resistance and Overweight in Rats. Front. Endocrinol. 2018, 9, 122. [Google Scholar] [CrossRef]
- López-Olmeda, J.F.; Madrid, J.A.; Sánchez-Vázquez, F.J. Melatonin Effects on Food Intake and Activity Rhythms in Two Fish Species with Different Activity Patterns: Diurnal (Goldfish) and Nocturnal (Tench). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 180–187. [Google Scholar] [CrossRef]
- Piccinetti, C.C.; Migliarini, B.; Olivotto, I.; Simoniello, M.P.; Giorgini, E.; Carnevali, O. Melatonin and Peripheral Circuitries: Insights on Appetite and Metabolism in Danio Rerio. Zebrafish 2013, 10, 275–282. [Google Scholar] [CrossRef]
- Piccinetti, C.C.; Migliarini, B.; Olivotto, I.; Coletti, G.; Amici, A.; Carnevali, O. Appetite Regulation: The Central Role of Melatonin in Danio Rerio. Horm. Behav. 2010, 58, 780–785. [Google Scholar] [CrossRef]
- Angelakis, E.; Yasir, M.; Bachar, D.; Azhar, E.I.; Lagier, J.C.; Bibi, F.; Jiman-Fatani, A.A.; Alawi, M.; Bakarman, M.A.; Robert, C.; et al. Gut Microbiome and Dietary Patterns in Different Saudi Populations and Monkeys. Sci. Rep. 2016, 6, 32191. [Google Scholar] [CrossRef] [PubMed]
- Telle-Hansen, V.H.; Holven, K.B.; Ulven, S.M. Impact of a Healthy Dietary Pattern on Gut Microbiota and Systemic Inflammation in Humans. Nutrients 2018, 10, 1783. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhong, Y.; Luo, X.M.; Ma, Y.; Liu, J.; Wang, H. Intermittent Fasting Reshapes the Gut Microbiota and Metabolome and Reduces Weight Gain More Effectively Than Melatonin in Mice. Front. Nutr. 2021, 8, 784681. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H.B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, C.; Gökmen, V. Neuroactive Compounds in Foods: Occurrence, Mechanism and Potential Health Effects. Food Res. Int. 2020, 128, 108744. [Google Scholar] [CrossRef] [PubMed]
- Johns, N.P.; Johns, J. Bioavailability of Dietary Phytomelatonin in Animals and Humans. In Serotonin and Melatonin; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-315-36933-4. [Google Scholar]
- Johns, N.P.; Johns, J.; Porasuphatana, S.; Plaimee, P.; Sae-Teaw, M. Dietary Intake of Melatonin from Tropical Fruit Altered Urinary Excretion of 6-Sulfatoxymelatonin in Healthy Volunteers. J. Agric. Food Chem. 2013, 61, 913–919. [Google Scholar] [CrossRef]
- Maldonado, M.D.; Moreno, H.; Calvo, J.R. Melatonin Present in Beer Contributes to Increase the Levels of Melatonin and Antioxidant Capacity of the Human Serum. Clin. Nutr. 2009, 28, 188–191. [Google Scholar] [CrossRef]
- Reiter, R.J.; Manchester, L.C.; Tan, D.X. Melatonin in Walnuts: Influence on Levels of Melatonin and Total Antioxidant Capacity of Blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef]
- Wirtz, P.H.; Spillmann, M.; Bärtschi, C.; Ehlert, U.; Von Känel, R. Oral Melatonin Reduces Blood Coagulation Activity: A Placebo-Controlled Study in Healthy Young Men. J. Pineal Res. 2008, 44, 127–133. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; Van Montfrans, G.A.; Van Someren, E.J.W.; Mairuhu, G.; Buijs, R.M. Daily Nighttime Melatonin Reduces Blood Pressure in Male Patients with Essential Hypertension. Hypertension 2004, 43, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Monaco, M.H.; Donovan, S.M. Impact of Early Gut Microbiota on Immune and Metabolic Development and Function. Semin. Fetal Neonatal Med. 2016, 21, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Gombert, M.; Codoñer-Franch, P. Melatonin in Early Nutrition: Long-Term Effects on Cardiovascular System. Int. J. Mol. Sci. 2021, 22, 6809. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Vaillancourt, C.; Maes, M.; Reiter, R.J. Breast Feeding and Melatonin: Implications for Improving Perinatal Health. J. Breastfeed. Biol. 2016, 1, 8–20. [Google Scholar] [CrossRef]
- Anderson, G.; Vaillancourt, C.; Maes, M.; Reiter, R.J. Breastfeeding and the Gut-Brain Axis: Is There a Role for Melatonin? Biomol. Concepts 2017, 8, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Obesity and Oxidative Stress: Potential Roles of Melatonin as Antioxidant and Metabolic Regulator. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 159–168. [Google Scholar] [CrossRef]
- Ivanov, D.O.; Evsyukova, I.I.; Mazzoccoli, G.; Anderson, G.; Polyakova, V.O.; Kvetnoy, I.M.; Carbone, A.; Nasyrov, R.A. The Role of Prenatal Melatonin in the Regulation of Childhood Obesity. Biology 2020, 9, 72. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Favero, G.; Rodella, L.F. Impact of Melatonin on Skeletal Muscle and Exercise. Cells 2020, 9, 288. [Google Scholar] [CrossRef]
- Fielding, R.A.; Reeves, A.R.; Jasuja, R.; Liu, C.; Barrett, B.B.; Lustgarten, M.S. Muscle Strength Is Increased in Mice That Are Colonized with Microbiota from High-Functioning Older Adults. Exp. Gerontol. 2019, 127, 110722. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, S.; Huang, H.; Liang, J.; Wu, Y.; Li, C.; Yuan, H.; Zhao, X.; Lai, X.; Hou, S. Influence of Excessive Exercise on Immunity, Metabolism, and Gut Microbial Diversity in an Overtraining Mice Model. Scand. J. Med. Sci. Sports 2018, 28, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, F.; Zhang, C.; Zhang, Q.; Su, X.; Zhu, X.; Liu, A.; Shi, W.; Lin, W.; Jin, Z.; et al. Melatonin Alleviates Titanium Nanoparticles Induced Osteolysis via Activation of Butyrate/GPR109A Signaling Pathway. J. Nanobiotechnology 2021, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.R.; Meerlo, P.; Grønli, J.; Johan Van Hasselt, S.; Mrdalj, J.; Pallesen, S.; Pedersen, T.T.; Henriksen, E.G.; Skrede, S. Shift in Food Intake and Changes in Metabolic Regulation and Gene Expression during Simulated Night-Shift Work: A Rat Model. Nutrients 2016, 8, 712. [Google Scholar] [CrossRef] [PubMed]
- Voigt, R.M.; Forsyth, C.B.; Green, S.J.; Mutlu, E.; Engen, P.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. Circadian Disorganization Alters Intestinal Microbiota. PLoS ONE 2014, 9, e97500. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Karamitri, A.; Jockers, R. Melatonin in Type 2 Diabetes Mellitus and Obesity. Nat. Rev. Endocrinol. 2019, 15, 105–125. [Google Scholar] [CrossRef]
- Huang, X.; Qiu, Y.; Gao, Y.; Zhou, R.; Hu, Q.; He, Z.; Lv, Y.; Wang, X.; Chen, W.; Deng, Y.; et al. Gut Microbiota Mediate Melatonin Signalling in Association with Type 2 Diabetes. Diabetologia 2022, 65, 1627–1641. [Google Scholar] [CrossRef]
- Garaulet, M.; Qian, J.; Florez, J.C.; Arendt, J.; Saxena, R.; Scheer, F.A.J.L. Melatonin Effects on Glucose Metabolism: Time to Unlock the Controversy. Trends Endocrinol. Metab. 2020, 31, 192–204. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Popescu-Olaru, I.; Cozma, L.; Tulbă, D.; Hinescu, M.E.; Ceafalan, L.C.; Gherghiceanu, M.; Popescu, B.O. Oxidative Stress and the Microbiota-Gut-Brain Axis. Oxid. Med. Cell. Longev. 2018, 2018, 2406594. [Google Scholar] [CrossRef]
- Ravcheev, D.A.; Thiele, I. Systematic Genomic Analysis Reveals the Complementary Aerobic and Anaerobic Respiration Capacities of the Human Gut Microbiota. Front. Microbiol. 2014, 5, 674. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Mercante, J.W.; Neish, A.S. Reactive Oxygen Production Induced by the Gut Microbiota: Pharmacotherapeutic Implications. Curr. Med. Chem. 2012, 19, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Tse, J.K.Y. Gut Microbiota, Nitric Oxide, and Microglia as Prerequisites for Neurodegenerative Disorders. ACS Chem Neurosci 2017, 8, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Fernandes, B.S.; Puri, B.K.; Walker, A.J.; Carvalho, A.F.; Berk, M. Leaky Brain in Neurological and Psychiatric Disorders: Drivers and Consequences. Aust. N. Z. J. Psychiatry 2018, 52, 924–948. [Google Scholar] [CrossRef] [PubMed]
- Boutrid, N.; Rahmoune, H. “3M”: Migraine, Microbiota and Melatonin. Med. Hypotheses 2019, 127, 90. [Google Scholar] [CrossRef]
- Anderson, G. Integrating Pathophysiology in Migraine: Role of the Gut Microbiome and Melatonin. Curr. Pharm. Des. 2019, 25, 3550–3562. [Google Scholar] [CrossRef]
- Anderson, G.; Rodriguez, M.; Reiter, R.J. Multiple Sclerosis: Melatonin, Orexin, and Ceramide Interact with Platelet Activation Coagulation Factors and Gut-Microbiome-Derived Butyrate in the Circadian Dysregulation of Mitochondria in Glia and Immune Cells. Int. J. Mol. Sci. 2019, 20, 5500. [Google Scholar] [CrossRef]
- Muñoz-Jurado, A.; Escribano, B.M.; Caballero-Villarraso, J.; Galván, A.; Agüera, E.; Santamaría, A.; Túnez, I. Melatonin and Multiple Sclerosis: Antioxidant, Anti-Inflammatory and Immunomodulator Mechanism of Action. Inflammopharmacology 2022, 30, 1569–1596. [Google Scholar] [CrossRef]
- Cobb, C.A.; Cole, M.P. Oxidative and Nitrative Stress in Neurodegeneration. Neurobiol. Dis. 2015, 84, 4–21. [Google Scholar] [CrossRef]
- Giloteaux, L.; Hanson, M.R.; Keller, B.A. A Pair of Identical Twins Discordant for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Differ in Physiological Parameters and Gut Microbiome Composition. Am. J. Case Rep. 2016, 17, 720–729. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Mitochondria and Immunity in Chronic Fatigue Syndrome. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 103, 109976. [Google Scholar] [CrossRef] [PubMed]
- Nakatomi, Y.; Mizuno, K.; Ishii, A.; Wada, Y.; Tanaka, M.; Tazawa, S.; Onoe, K.; Fukuda, S.; Kawabe, J.; Takahashi, K.; et al. Neuroinflammation in Patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: An 11C-(R)-PK11195 PET Study. J. Nucl. Med. 2014, 55, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Pathoetiology and Pathophysiology of Borderline Personality: Role of Prenatal Factors, Gut Microbiome, Mu- and Kappa-Opioid Receptors in Amygdala-PFC Interactions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 98, 109782. [Google Scholar] [CrossRef] [PubMed]
- Averina, O.V.; Kovtun, A.S.; Polyakova, S.I.; Savilova, A.M.; Rebrikov, D.V.; Danilenko, V.N. The Bacterial Neurometabolic Signature of the Gut Microbiota of Young Children with Autism Spectrum Disorders. J. Med. Microbiol. 2020, 69, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Dong, Y.; He, C.; Zhao, M.; He, Q. The Gut Microbiota and Oxidative Stress in Autism Spectrum Disorders (ASD). Oxid. Med. Cell. Longev. 2020, 2020, 8396708. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Anderson, G.M.; Bellissant, E.; Botbol, M.; Charbuy, H.; Camus, F.; Graignic, R.; Kermarrec, S.; Fougerou, C.; Cohen, D.; et al. Day and Nighttime Excretion of 6-Sulphatoxymelatonin in Adolescents and Young Adults with Autistic Disorder. Psychoneuroendocrinology 2012, 37, 1990–1997. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Huang, S.-D.; Zou, J.-J.; Wang, Q.-X.; Naveed, M.; Bao, H.-N.; Wang, W.; Fukunaga, K.; Han, F. Autism Spectrum Disorder (ASD): Disturbance of the Melatonin System and Its Implications. Biomed. Pharmacother. 2020, 130, 110496. [Google Scholar] [CrossRef]
- Pagan, C.; Delorme, R.; Callebert, J.; Goubran-Botros, H.; Amsellem, F.; Drouot, X.; Boudebesse, C.; Le Dudal, K.; Ngo-Nguyen, N.; Laouamri, H.; et al. The Serotonin-N-Acetylserotonin-Melatonin Pathway as a Biomarker for Autism Spectrum Disorders. Transl. Psychiatry 2014, 4, e479. [Google Scholar] [CrossRef]
- Han, P.P.; Zou, M.Y.; Yang, X.L.; Liu, X.C.; Liang, S.; Sun, C.H.; Xia, W.; Wu, L.J. Sleep problems and the association with the levels of 6-sulfatoxymelatonin in children with autism spectrum disorder. Zhonghua Er Ke Za Zhi 2017, 55, 911–915. [Google Scholar] [CrossRef]
- Hua, X.; Zhu, J.; Yang, T.; Guo, M.; Li, Q.; Chen, J.; Li, T. The Gut Microbiota and Associated Metabolites Are Altered in Sleep Disorder of Children with Autism Spectrum Disorders. Front. Psychiatry 2020, 11, 855. [Google Scholar] [CrossRef]
- Frye, R.E.; Slattery, J.C.; Quadros, E.V. Folate Metabolism Abnormalities in Autism: Potential Biomarkers. Biomark. Med. 2017, 11, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef] [PubMed]
- Coretti, L.; Paparo, L.; Riccio, M.P.; Amato, F.; Cuomo, M.; Natale, A.; Borrelli, L.; Corrado, G.; De Caro, C.; Comegna, M.; et al. Gut Microbiota Features in Young Children with Autism Spectrum Disorders. Front. Microbiol. 2018, 9, 3146. [Google Scholar] [CrossRef] [PubMed]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A.M. Urinary Metabolomics of Young Italian Autistic Children Supports Abnormal Tryptophan and Purine Metabolism. Mol. Autism 2016, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Rossi, M.; Sousa, I.; Blasi, F.; Bacchelli, E.; Alen, R.; Vanhala, R.; Monaco, A.P.; Järvelä, I.; Maestrini, E.; et al. Is ASMT a Susceptibility Gene for Autism Spectrum Disorders? A Replication Study in European Populations. Mol. Psychiatry 2007, 12, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Ruan, Y.; Lu, T.; Liu, C.; Jia, M.; Yue, W.; Liu, J.; Bourgeron, T.; Zhang, D. Sequencing ASMT Identifies Rare Mutations in Chinese Han Patients with Autism. PLoS ONE 2013, 8, e53727. [Google Scholar] [CrossRef] [PubMed]
- Melke, J.; Goubran Botros, H.; Chaste, P.; Betancur, C.; Nygren, G.; Anckarsäter, H.; Rastam, M.; Ståhlberg, O.; Gillberg, I.C.; Delorme, R.; et al. Abnormal Melatonin Synthesis in Autism Spectrum Disorders. Mol. Psychiatry 2008, 13, 90–98. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Melatonin in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Dev. Med. Child Neurol. 2011, 53, 783–792. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Melatonin in Autism Spectrum Disorders. Curr. Clin. Pharmacol. 2014, 9, 326–334. [Google Scholar] [CrossRef]
- Díaz-Román, A.; Zhang, J.; Delorme, R.; Beggiato, A.; Cortese, S. Sleep in Youth with Autism Spectrum Disorders: Systematic Review and Meta-Analysis of Subjective and Objective Studies. Evid. Based Ment. Health 2018, 21, 146–154. [Google Scholar] [CrossRef]
- Morgan, B.; Nageye, F.; Masi, G.; Cortese, S. Sleep in Adults with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis of Subjective and Objective Studies. Sleep Med. 2020, 65, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Garstang, J.; Wallis, M. Randomized Controlled Trial of Melatonin for Children with Autistic Spectrum Disorders and Sleep Problems. Child Care Health Dev. 2006, 32, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Beresford, B.; Dawson, V.; Elphick, H.; Fairhurst, C.; Hewitt, C.; Scantlebury, A.; Spiers, G.; Thomas, M.; Wright, K.; et al. Oral Melatonin for Non-Respiratory Sleep Disturbance in Children with Neurodisabilities: Systematic Review and Meta-Analyses. Dev. Med. Child Neurol. 2019, 61, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.; Sims, D.; Smart, S.; Alwazeer, A.; Alderson-Day, B.; Allgar, V.; Whitton, C.; Tomlinson, H.; Bennett, S.; Jardine, J.; et al. Melatonin versus Placebo in Children with Autism Spectrum Conditions and Severe Sleep Problems Not Amenable to Behaviour Management Strategies: A Randomised Controlled Crossover Trial. J. Autism Dev. Disord. 2011, 41, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; Turner, K.S.; Foldes, E.; Brooks, M.M.; Kronk, R.; Wiggs, L. Behavioral Parent Training to Address Sleep Disturbances in Young Children with Autism Spectrum Disorder: A Pilot Trial. Sleep Med. 2013, 14, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Malow, B.; Adkins, K.W.; McGrew, S.G.; Wang, L.; Goldman, S.E.; Fawkes, D.; Burnette, C. Melatonin for Sleep in Children with Autism: A Controlled Trial Examining Dose, Tolerability, and Outcomes. J. Autism Dev. Disord. 2012, 42, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, F.; Giannotti, F.; Sebastiani, T.; Panunzi, S.; Valente, D. Controlled-Release Melatonin, Singly and Combined with Cognitive Behavioural Therapy, for Persistent Insomnia in Children with Autism Spectrum Disorders: A Randomized Placebo-Controlled Trial. J. Sleep Res. 2012, 21, 700–709. [Google Scholar] [CrossRef]
- Gernert, C.; Falkai, P.; Falter-Wagner, C.M. The Generalized Adaptation Account of Autism. Front. Neurosci. 2020, 14, 534218. [Google Scholar] [CrossRef]
- Maruani, J.; Anderson, G.; Etain, B.; Lejoyeux, M.; Bellivier, F.; Geoffroy, P.A. The Neurobiology of Adaptation to Seasons: Relevance and Correlations in Bipolar Disorders. Chronobiol. Int. 2018, 35, 1335–1353. [Google Scholar] [CrossRef]
- Manchia, M.; Squassina, A.; Pisanu, C.; Congiu, D.; Garzilli, M.; Guiso, B.; Suprani, F.; Paribello, P.; Pulcinelli, V.; Novella Iaselli, M.; et al. Investigating the Relationship between Melatonin Levels, Melatonin System, Microbiota Composition and Bipolar Disorder Psychopathology across the Different Phases of the Disease. Int. J. Bipolar Disord. 2019, 7, 27. [Google Scholar] [CrossRef]
- Rummel, N.G.; Butterfield, D.A. Altered Metabolism in Alzheimer Disease Brain: Role of Oxidative Stress. Antioxid Redox Signal 2022, 36, 1289–1305. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Guo, J.; Ye, X.-Y.; Xie, Y.; Xie, T. Oxidative Stress: The Core Pathogenesis and Mechanism of Alzheimer’s Disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Oxidative Stress in Alzheimer’s Disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging Roles of Oxidative Stress in Brain Aging and Alzheimer’s Disease. Neurobiol Aging 2021, 107, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Plascencia-Villa, G.; Perry, G. Preventive and Therapeutic Strategies in Alzheimer’s Disease: Focus on Oxidative Stress, Redox Metals, and Ferroptosis. Antioxid. Redox Signal 2021, 34, 591–610. [Google Scholar] [CrossRef]
- Pohanka, M. Oxidative Stress in Alzheimer Disease as a Target for Therapy. Bratisl. Lek. Listy 2018, 119, 535–543. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Anderson, G.; Chirumbolo, S.; Maes, M. Preventive Treatments to Slow Substantia Nigra Damage and Parkinson’s Disease Progression: A Critical Perspective Review. Pharmacol. Res. 2020, 161, 105065. [Google Scholar] [CrossRef]
- Anderson, G.; Seo, M.; Berk, M.; Carvalho, A.F.; Maes, M. Gut Permeability and Microbiota in Parkinson’s Disease: Role of Depression, Tryptophan Catabolites, Oxidative and Nitrosative Stress and Melatonergic Pathways. Curr. Pharm. Des. 2016, 22, 6142–6151. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Wang, Z.; Cao, J.; Gao, T.; Dong, Y.; Chen, Y. Role of Melatonin in Murine “Restraint Stress”-Induced Dysfunction of Colonic Microbiota. J. Microbiol. 2021, 59, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, H.; Rafiqi, S.I.; Ahmad, S.; Jinna, S.; Khan, S.A.; Karim, T.; Qureshi, O.; Zahid, Z.A.; Elhai, J.D.; Levine, J.C.; et al. Hyperinsulinemia Associated Depression. Clin. Med. Insights Endocrinol. Diabetes 2022, 15, 117955142210902. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, Ł.; Pollis, M.; Żółtowska, A.; Manfredini, D. Gut Bless Your Pain—Roles of the Gut Microbiota, Sleep, and Melatonin in Chronic Orofacial Pain and Depression. Biomedicines 2022, 10, 1528. [Google Scholar] [CrossRef] [PubMed]
- Haddad, H.W.; Mallepalli, N.R.; Scheinuk, J.E.; Bhargava, P.; Cornett, E.M.; Urits, I.; Kaye, A.D. The Role of Nutrient Supplementation in the Management of Chronic Pain in Fibromyalgia: A Narrative Review. Pain Ther. 2021, 10, 827–848. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Cao, M.; Gong, Y.; Tang, L.; Jiang, X.; Li, Y.; Yang, M.; Xu, S.; Li, J.; Che, L.; et al. Gut Microbial Metabolism of Dietary Fibre Protects against High Energy Feeding Induced Ovarian Follicular Atresia in a Pig Model. Br. J. Nutr. 2021, 125, 38–49. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, P.; Hu, Y.; Shan, L.; Geng, Q.; Gong, Y.; Fan, H.; Zhang, T.; Zhou, Y. Elevated Testicular Apoptosis Is Associated with Elevated Sphingosine Driven by Gut Microbiota in Prediabetic Sheep. BMC Biol. 2022, 20, 121. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Zheng, Y.; Zha, X.; Elsabagh, M.; Zhang, Y.; Ma, Y.; Loor, J.J.; Wang, M.; Wang, H. Effects of the Maternal Gut Microbiome and Gut-Placental Axis on Melatonin Efficacy in Alleviating Cadmium-Induced Fetal Growth Restriction. Ecotoxicol. Environ. Saf. 2022, 237, 113550. [Google Scholar] [CrossRef]
- Messman, R.D.; Contreras-Correa, Z.E.; Paz, H.A.; Lemley, C.O. Melatonin-Induced Changes in the Bovine Vaginal Microbiota during Maternal Nutrient Restriction. J. Anim. Sci. 2021, 99, skab098. [Google Scholar] [CrossRef]
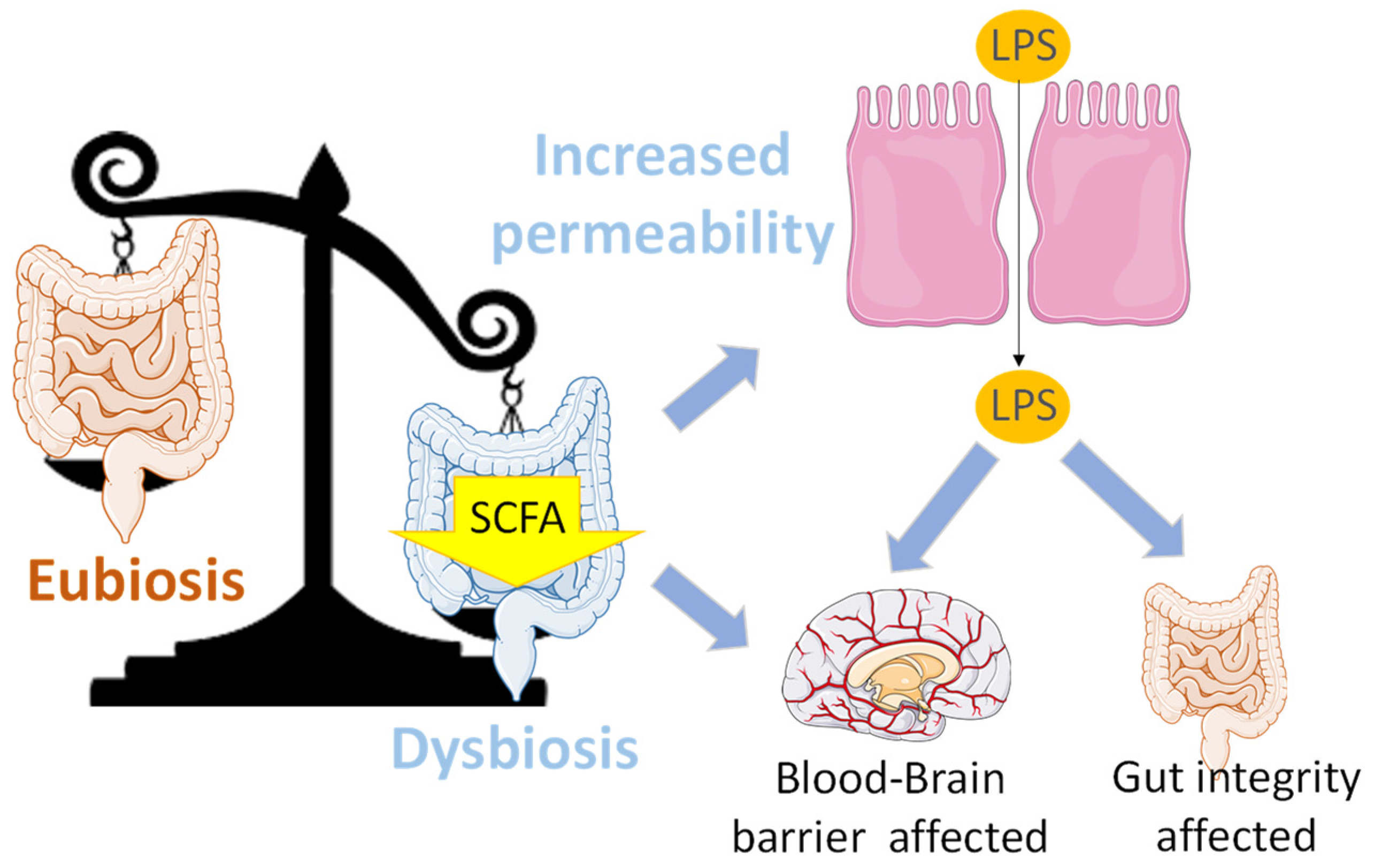
| Study | Animal Model/ Duration of Sleep Deprivation (SD) | Effect of SD on the Gastrointestinal Tract | Effect of SD on Gut Microbiota | Other Effects | Melatonin Treatment (Route, Dosage, Time Period)/ Measurement | Effects of Melatonin Treatment |
|---|---|---|---|---|---|---|
| Gao et al., 2019 [6] and Gao et al., 2020 [5] | Mouse 72 h | Colitis | ↓ Akkermansia ↓ Bacteroides ↓ Faecalibacterium (probiotics) ↑ Aeromonas | ↑ Norepinephrine ↓ Plasma melatonin ↓ Antioxidant ability ↓ Anti-inflammatory cytokines ↑ Proinflammatory cytokines | 20 and 40 mg/kg Intraperitoneal injections once 60 min before SD, and a single dose per day at 7:00 am for a total of 3 days | Reverse changes Improve mucosal injury and dysbiosis ↑ Plasma melatonin ↑ OTUs ↑ Diversity and richness ↑ Bacteroidaceae ↑ Prevotellaceae ↑ Firmicutes/Bacteroidetes ↑ Moraxellaceae ↑ Aeromonadaceae ↑ Anti-inflammatory cytokines ↓ Proinflammatory cytokines ↓ ROS |
| Wang et al., 2022 [9] | Mouse 20 h/day for 28 days | - | ↑ α-diversity ↑ OTUs ↑ Helicobacter ↑ Clostridium ↓ Bacteroidetes ↓ Lactobacillus | ↓ Plasma melatonin (48.91%) ↓ Antioxidant enzymes ↓ Total antioxidant capacity in intestinal tissues ↓ Anti-inflammatory cytokines (IL10, IFNγ), ↑ Glucose ↑ Norepinephrine ↑ Corticosterone ↑ Proinflammatory cytokines (IL6 and TNFα) | 10−5 mol/L, drinking water | Reverse changes ↓ Oxidative stress ↓ Inflammatory response ↓ Dysbiosis |
| Gao et al., 2021 [7] | Mouse, 72 h | Mucosa injury | ↓ Faecalibacterium | ↓ Plasma melatonin ↓ Card9 expression ↓ Butyrate | 20 mg/kg. Intraperitoneal injections once 60 min before SD, and a single dose per day at 7:00 am for a total of 3 days. | Reverse effects |
| Gao et al., 2022 [10] | Mouse 72-h No SD—effects mimicked by: Aeromonas veronii LPS supplementation | Mucosa injury | - | ↓ Goblet cells ↓ Mucin protein ↓ Villin ↓ Tff3 mRNA ↑ TLR4 ↑ MyD 88↓ p-GSK-3β ↓ β-catenin | 20 and 40 mg/kg Intraperitoneal injections once 60 min before SD, and a single dose per day at 7:00 am for a total of 3 days | Reverse effects (through MT2) of SD |
| Study | Animal Model | Chemical to Induce Colitis/IBD | Melatonin Treatment | Effects of Melatonin Treatment on Microbiota Composition | Effects of Melatonin Treatment on Colitis Symptoms |
|---|---|---|---|---|---|
| Zhu et al., 2018 [58] | Mouse | Dextran Sulphate Sodium (DSS) | Drinking water 0.2 mg/L | = Diversity = Abundance = Coverage ↑ Firmicutes ↑ Bacteroidetes (with no melatonin) | Increased antioxidant capability |
| Jing et al., 2022 [132] | Mouse | Dextran Sulphate Sodium (DSS) | Melatonin + hyaluronic acid (aggregates) | Alleviate dysbiosis Restore Firmicutes/Bacteroidetes ↑ Richness ↑ Diversity ↑ Lactobacillus ↓ Bacteroides, Blautia, Streptococcus | Restoration of the intestinal barrier Inhibition of colon inflammation |
| Zhao et al., 2022 [68] | Mouse | Oxazolone | Via gavage 50 mg/kg | ↓ Richness at the OTU level ↓ Diversity at the OTU level ↑ Bifidobacterium ↓ Desulfovibrio ↓ Peptococcaceae ↓ Lachnospiraceae | Counteracting body weight loss Counteracting colon shortening Neutrophil infiltration Suppression of type 2 immune response |
| Kim et al., 2020 [133] | Mouse (wild type and TLR4 knockout) | DSS | Oral and rectal 10 mg/kg/day | Revert dysbiosis ↑ Richness ↑ Diversity ↓ Proteobacteria ↑ Ruminococcaceae ↓= Bacteroidetes ↑= Firmicutes | ↓ Disease activity index Alleviation of the shortening of colon and histopathologic features |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonmatí-Carrión, M.-Á.; Rol, M.-A. Melatonin as a Mediator of the Gut Microbiota–Host Interaction: Implications for Health and Disease. Antioxidants 2024, 13, 34. https://doi.org/10.3390/antiox13010034
Bonmatí-Carrión M-Á, Rol M-A. Melatonin as a Mediator of the Gut Microbiota–Host Interaction: Implications for Health and Disease. Antioxidants. 2024; 13(1):34. https://doi.org/10.3390/antiox13010034
Chicago/Turabian StyleBonmatí-Carrión, María-Ángeles, and Maria-Angeles Rol. 2024. "Melatonin as a Mediator of the Gut Microbiota–Host Interaction: Implications for Health and Disease" Antioxidants 13, no. 1: 34. https://doi.org/10.3390/antiox13010034