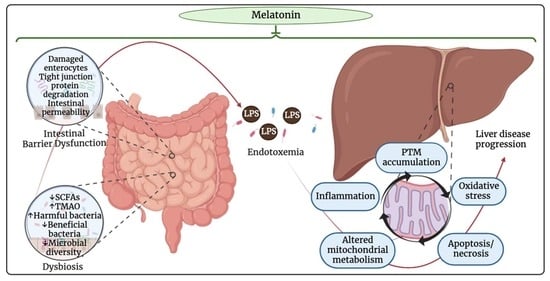
Melatonin Prevents Alcohol- and Metabolic Dysfunction- Associated Steatotic Liver Disease by Mitigating Gut Dysbiosis, Intestinal Barrier Dysfunction, and Endotoxemia
Abstract
:1. Introduction: MT
1.1. Synthesis of MT
1.2. Contributing Factors of ALD within the Gut–Liver Axis
1.3. Contributing Factors of MASLD via the Gut–Liver Axis
1.4. Functions of MT in the Gut–Liver Axis
1.4.1. Intestinal Barrier Dysfunction
1.4.2. Oxidative Stress and PTMs in Mitochondria
1.4.3. Gut–Mitochondria Axis
2. Melatonin in the Gut–Liver Axis of ALD
Translational Research on the Effects of MT on the Progression of ALD
3. MT in the Gut–Liver Axis of MASLD
Translational Research on the Effects of MT in MASLD
4. Acute Toxicity in the Gut–Liver Axis: MT and Septic Hepatotoxicity
5. Concluding Remarks
5.1. Suggestion of Behavioral Changes That May Increase MT to Prevent or Treat Liver Diseases
5.2. Discussion of Future Studies and Considerations
5.3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Baekelandt, S.; Mandiki, S.N.M.; Kestemont, P. Are cortisol and melatonin involved in the immune modulation by the light environment in pike perch Sander lucioperca? J. Pineal Res. 2019, 67, e12573. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A.; Brown, G.M. Pinealectomy reduces melatonin levels in the serum but not in the gastrointestinal tract of rats. Neurosignals 1997, 6, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Konturek, S.J.; Konturek, P.C.; Brzozowski, T.; Bubenik, G.A. Role of melatonin in upper gastrointestinal tract. J. Physiol. Pharmacol. 2007, 58 (Suppl. S6), 23–52. [Google Scholar] [PubMed]
- Bubenik, G.A. Gastrointestinal melatonin: Localization, function, and clinical relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free. Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2020, 22, 181. [Google Scholar] [CrossRef]
- Sangsopha, J.; Johns, N.P.; Johns, J.; Moongngarm, A. Dietary sources of melatonin and benefits from production of high melatonin pasteurized milk. J. Food Sci. Technol. 2020, 57, 2026–2037. [Google Scholar] [CrossRef]
- Zuraikat, F.M.; Wood, R.A.; Barragán, R.; St-Onge, M.P. Sleep and Diet: Mounting Evidence of a Cyclical Relationship. Annu. Rev. Nutr. 2021, 41, 309–332. [Google Scholar] [CrossRef]
- Mukherjee, S.; Maitra, S.K. Gut Melatonin in Vertebrates: Chronobiology and Physiology. Front. Endocrinol. 2015, 6, 112. [Google Scholar] [CrossRef]
- Yasmin, F.; Sutradhar, S.; Das, P.; Mukherjee, S. Gut melatonin: A potent candidate in the diversified journey of melatonin research. Gen. Comp. Endocrinol. 2021, 303, 113693. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Markus, R.P.; Sousa, K.S.; da Silveira Cruz-Machado, S.; Fernandes, P.A.; Ferreira, Z.S. Possible Role of Pineal and Extra-Pineal Melatonin in Surveillance, Immunity, and First-Line Defense. Int. J. Mol. Sci. 2021, 22, 12143. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhang, J.; Reiter, R.J.; Ma, X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: A therapeutic target to reduce intestinal inflammation. Med. Res. Rev. 2020, 40, 606–632. [Google Scholar] [CrossRef] [PubMed]
- Terziev, D.; Terzieva, D. Experimental Data on the Role of Melatonin in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Biomedicines 2023, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jiao, J.; Fan, X.; Sun, H.; Zhang, Y.; Jiang, J.; Liu, C. Endophytic Bacterium Pseudomonas fluorescens RG11 May Transform Tryptophan to Melatonin and Promote Endogenous Melatonin Levels in the Roots of Four Grape Cultivars. Front. Plant Sci. 2016, 7, 2068. [Google Scholar] [CrossRef]
- Jiao, J.; Ma, Y.; Chen, S.; Liu, C.; Song, Y.; Qin, Y.; Yuan, C.; Liu, Y. Melatonin-Producing Endophytic Bacteria from Grapevine Roots Promote the Abiotic Stress-Induced Production of Endogenous Melatonin in Their Hosts. Front. Plant Sci. 2016, 7, 1387. [Google Scholar] [CrossRef]
- Danilovich, M.E.; Alberto, M.R.; Juárez Tomás, M.S. Microbial production of beneficial indoleamines (serotonin and melatonin) with potential application to biotechnological products for human health. J. Appl. Microbiol. 2021, 131, 1668–1682. [Google Scholar] [CrossRef]
- Arnao, M.B.; Giraldo-Acosta, M.; Castejón-Castillejo, A.; Losada-Lorán, M.; Sánchez-Herrerías, P.; El Mihyaoui, A.; Cano, A.; Hernández-Ruiz, J. Melatonin from Microorganisms, Algae, and Plants as Possible Alternatives to Synthetic Melatonin. Metabolites 2023, 13, 72. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Pang, S.F. The role of serotonin and melatonin in gastrointestinal physiology: Ontogeny, regulation of food intake, and mutual serotonin-melatonin feedback. J. Pineal Res. 1994, 16, 91–99. [Google Scholar] [CrossRef]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Kurhaluk, N. Alcohol and melatonin. Chronobiol. Int. 2021, 38, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol. Res. 2017, 38, 147–161. [Google Scholar]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Ceni, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 20, 17756–17772. [Google Scholar] [CrossRef] [PubMed]
- Vatsalya, V.; Kong, M.; Cave, M.C.; Liu, N.; Schwandt, M.L.; George, D.T.; Ramchandani, V.A.; McClain, C.J. Association of serum zinc with markers of liver injury in very heavy drinking alcohol-dependent patients. J. Nutr. Biochem. 2018, 59, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.; Assiri, M.A.; Harris, P.S.; Michel, C.R.; Yun, Y.; Marentette, J.O.; Huynh, F.K.; Orlicky, D.J.; Shearn, C.T.; Saba, L.M.; et al. Quantifying Competition among Mitochondrial Protein Acylation Events Induced by Ethanol Metabolism. J. Proteome Res. 2019, 18, 1513–1531. [Google Scholar] [CrossRef]
- Cho, Y.E.; Yu, L.R.; Abdelmegeed, M.A.; Yoo, S.H.; Song, B.J. Apoptosis of enterocytes and nitration of junctional complex proteins promote alcohol-induced gut leakiness and liver injury. J. Hepatol. 2018, 69, 142–153. [Google Scholar] [CrossRef]
- Addolorato, G.; Ponziani, F.R.; Dionisi, T.; Mosoni, C.; Vassallo, G.A.; Sestito, L.; Petito, V.; Picca, A.; Marzetti, E.; Tarli, C.; et al. Gut microbiota compositional and functional fingerprint in patients with alcohol use disorder and alcohol-associated liver disease. Liver Int. 2020, 40, 878–888. [Google Scholar] [CrossRef]
- Harjumäki, R.; Pridgeon, C.S.; Ingelman-Sundberg, M. CYP2E1 in Alcoholic and Non-Alcoholic Liver Injury. Roles of ROS, Reactive Intermediates and Lipid Overload. Int. J. Mol. Sci. 2021, 22, 8221. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Hou, X.; Yang, L.; Chu, H. The Role of Gut Bacteria and Fungi in Alcohol-Associated Liver Disease. Front. Med. 2022, 9, 840752. [Google Scholar] [CrossRef] [PubMed]
- Namachivayam, A.; Valsala Gopalakrishnan, A. A review on molecular mechanism of alcoholic liver disease. Life Sci. 2021, 274, 119328. [Google Scholar] [CrossRef]
- Butura, A.; Nilsson, K.; Morgan, K.; Morgan, T.R.; French, S.W.; Johansson, I.; Schuppe-Koistinen, I.; Ingelman-Sundberg, M. The impact of CYP2E1 on the development of alcoholic liver disease as studied in a transgenic mouse model. J. Hepatol. 2009, 50, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, C.; Dai, S.; Liu, Y.; Zhang, F.; Peng, C.; Li, Y. Quercetin Protects Ethanol-Induced Hepatocyte Pyroptosis via Scavenging Mitochondrial ROS and Promoting PGC-1α-Regulated Mitochondrial Homeostasis in L02 Cells. Oxid. Med. Cell. Longev. 2022, 2022, 4591134. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, S.; Wang, J.; Renukuntla, J.; Sirimulla, S.; Chen, J. A comprehensive review of cytochrome P450 2E1 for xenobiotic metabolism. Drug Metab. Rev. 2019, 51, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K. The role of cytochrome P4502E1 in the pathogenesis of alcoholic liver disease and carcinogenesis. Chem. Biol. Interact. 2020, 316, 108918. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Farhadi, A.; Forsyth, C.B.; Rangan, J.; Jakate, S.; Shaikh, M.; Banan, A.; Fields, J.Z. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J. Hepatol. 2009, 50, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Dubinkina, V.B.; Tyakht, A.V.; Odintsova, V.Y.; Yarygin, K.S.; Kovarsky, B.A.; Pavlenko, A.V.; Ischenko, D.S.; Popenko, A.S.; Alexeev, D.G.; Taraskina, A.Y.; et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 2017, 5, 141. [Google Scholar] [CrossRef]
- Baraona, E.; Julkunen, R.; Tannenbaum, L.; Lieber, C.S. Role of intestinal bacterial overgrowth in ethanol production and metabolism in rats. Gastroenterology 1986, 90, 103–110. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Giordano, M.; Nunnari, G.; Bertino, G.; Malaguarnera, M. Gut microbiota in alcoholic liver disease: Pathogenetic role and therapeutic perspectives. World J. Gastroenterol. 2014, 20, 16639–16648. [Google Scholar] [CrossRef]
- Anderson, G. Linking the biological underpinnings of depression: Role of mitochondria interactions with melatonin, inflammation, sirtuins, tryptophan catabolites, DNA repair and oxidative and nitrosative stress, with consequences for classification and cognition. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Maes, M. Gut Dysbiosis Dysregulates Central and Systemic Homeostasis via Suboptimal Mitochondrial Function: Assessment, Treatment and Classification Implications. Curr. Top Med. Chem. 2020, 20, 524–539. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Duan, Y.; Lang, S.; Jiang, L.; Wang, Y.; Llorente, C.; Liu, J.; Mogavero, S.; Bosques-Padilla, F.; Abraldes, J.G.; et al. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J. Hepatol. 2020, 72, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Jew, M.H.; Hsu, C.L. Alcohol, the gut microbiome, and liver disease. J. Gastroenterol. Hepatol. 2023, 38, 1205–1210. [Google Scholar] [CrossRef]
- Maccioni, L.; Gao, B.; Leclercq, S.; Pirlot, B.; Horsmans, Y.; De Timary, P.; Leclercq, I.; Fouts, D.; Schnabl, B.; Stärkel, P. Intestinal permeability, microbial translocation, changes in duodenal and fecal microbiota, and their associations with alcoholic liver disease progression in humans. Gut Microbes 2020, 12, 1782157. [Google Scholar] [CrossRef]
- Betrapally, N.S.; Gillevet, P.M.; Bajaj, J.S. Changes in the Intestinal Microbiome and Alcoholic and Nonalcoholic Liver Diseases: Causes or Effects? Gastroenterology 2016, 150, 1745–1755.e3. [Google Scholar] [CrossRef]
- Chaudhry, K.K.; Samak, G.; Shukla, P.K.; Mir, H.; Gangwar, R.; Manda, B.; Isse, T.; Kawamoto, T.; Salaspuro, M.; Kaihovaara, P.; et al. ALDH2 Deficiency Promotes Ethanol-Induced Gut Barrier Dysfunction and Fatty Liver in Mice. Alcohol. Clin. Exp. Res. 2015, 39, 1465–1475. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Lin, Y.; Wang, X.; Kawamoto, T.; Chidambaram, S.B.; Song, B.J. ALDH2 deficiency increases susceptibility to binge alcohol-induced gut leakiness, endotoxemia, and acute liver injury in mice through the gut-liver axis. Redox Biol.. 2023, 59, 102577. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. Cytochrome P450s and Alcoholic Liver Disease. Curr. Pharm. Des. 2018, 24, 1502–1517. [Google Scholar] [CrossRef]
- Moon, K.H.; Hood, B.L.; Kim, B.J.; Hardwick, J.P.; Conrads, T.P.; Veenstra, T.D.; Song, B.J. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology 2006, 44, 1218–1230. [Google Scholar] [CrossRef]
- Moon, K.H.; Hood, B.L.; Mukhopadhyay, P.; Rajesh, M.; Abdelmegeed, M.A.; Kwon, Y.I.; Conrads, T.P.; Veenstra, T.D.; Song, B.J.; Pacher, P. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology 2008, 135, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I. Role of CYP2E1 in ethanol-induced oxidant stress, fatty liver and hepatotoxicity. Dig. Dis. 2010, 28, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Song, B.J. Ethanol-inducible cytochrome P450 (CYP2E1): Biochemistry, molecular biology and clinical relevance: 1996 update. Alcohol. Clin. Exp. Res. 1996, 20, 138a–146a. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.; French, S.W.; Morgan, T.R. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology 2002, 36, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, D.; Wang, X.; Ward, S.C.; Cederbaum, A.I. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free. Radic. Biol. Med. 2010, 49, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I.; Yang, L.; Wang, X.; Wu, D. CYP2E1 Sensitizes the Liver to LPS- and TNF α-Induced Toxicity via Elevated Oxidative and Nitrosative Stress and Activation of ASK-1 and JNK Mitogen-Activated Kinases. Int. J. Hepatol. 2012, 2012, 582790. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Voigt, R.M.; Keshavarzian, A. Intestinal CYP2E1: A mediator of alcohol-induced gut leakiness. Redox Biol. 2014, 3, 40–46. [Google Scholar] [CrossRef]
- Roberts, B.J.; Song, B.J.; Soh, Y.; Park, S.S.; Shoaf, S.E. Ethanol induces CYP2E1 by protein stabilization. Role of ubiquitin conjugation in the rapid degradation of CYP2E1. J. Biol. Chem. 1995, 270, 29632–29635. [Google Scholar] [CrossRef]
- Roberts, B.J.; Shoaf, S.E.; Jeong, K.S.; Song, B.J. Induction of CYP2E1 in liver, kidney, brain and intestine during chronic ethanol administration and withdrawal: Evidence that CYP2E1 possesses a rapid phase half-life of 6 hours or less. Biochem. Biophys. Res. Commun. 1994, 205, 1064–1071. [Google Scholar] [CrossRef]
- Hansen, J.; Cherwitz, D.L.; Allen, J.I. The role of tumor necrosis factor-alpha in acute endotoxin-induced hepatotoxicity in ethanol-fed rats. Hepatology 1994, 20, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Song, B.J.; Akbar, M.; Abdelmegeed, M.A.; Byun, K.; Lee, B.; Yoon, S.K.; Hardwick, J.P. Mitochondrial dysfunction and tissue injury by alcohol, high fat, nonalcoholic substances and pathological conditions through post-translational protein modifications. Redox Biol. 2014, 3, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.E.; Kim, D.K.; Seo, W.; Gao, B.; Yoo, S.H.; Song, B.J. Fructose Promotes Leaky Gut, Endotoxemia, and Liver Fibrosis Through Ethanol-Inducible Cytochrome P450-2E1-Mediated Oxidative and Nitrative Stress. Hepatology 2021, 73, 2180–2195. [Google Scholar] [CrossRef] [PubMed]
- Drożdż, K.; Nabrdalik, K.; Hajzler, W.; Kwiendacz, H.; Gumprecht, J.; Lip, G.Y.H. Metabolic-Associated Fatty Liver Disease (MAFLD), Diabetes, and Cardiovascular Disease: Associations with Fructose Metabolism and Gut Microbiota. Nutrients 2021, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Karkucinska-Wieckowska, A.; Simoes, I.C.M.; Kalinowski, P.; Lebiedzinska-Arciszewska, M.; Zieniewicz, K.; Milkiewicz, P.; Górska-Ponikowska, M.; Pinton, P.; Malik, A.N.; Krawczyk, M.; et al. Mitochondria, oxidative stress and nonalcoholic fatty liver disease: A complex relationship. Eur. J. Clin. Investig. 2022, 52, e13622. [Google Scholar] [CrossRef]
- Abdelmegeed, M.A.; Ha, S.K.; Choi, Y.; Akbar, M.; Song, B.J. Role of CYP2E1 in Mitochondrial Dysfunction and Hepatic Injury by Alcohol and Non-Alcoholic Substances. Curr. Mol. Pharmacol. 2017, 10, 207–225. [Google Scholar] [CrossRef]
- Abdelmegeed, M.A.; Banerjee, A.; Yoo, S.H.; Jang, S.; Gonzalez, F.J.; Song, B.J. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J. Hepatol. 2012, 57, 860–866. [Google Scholar] [CrossRef]
- Kathirvel, E.; Chen, P.; Morgan, K.; French, S.W.; Morgan, T.R. Oxidative stress and regulation of anti-oxidant enzymes in cytochrome P4502E1 transgenic mouse model of non-alcoholic fatty liver. J. Gastroenterol. Hepatol. 2010, 25, 1136–1143. [Google Scholar] [CrossRef]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef]
- Song, B.J.; Abdelmegeed, M.A.; Henderson, L.E.; Yoo, S.H.; Wan, J.; Purohit, V.; Hardwick, J.P.; Moon, K.H. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver disease. Oxid. Med. Cell. Longev. 2013, 2013, 781050. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Song, Z.; Li, S.; Hu, M.; Shaukat, H.; Qin, H. Protective Effects of Sesamol against Liver Oxidative Stress and Inflammation in High-Fat Diet-Induced Hepatic Steatosis. Nutrients 2021, 13, 4484. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.S.; Yun, C.H.; Ahn, T. Extracts from Erythronium japonicum and Corylopsis coreana Uyeki reduce 1,3-dichloro-2-propanol-mediated oxidative stress in human hepatic cells. Food Sci. Biotechnol. 2019, 28, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Roque Bravo, R.; Carmo, H.; Valente, M.J.; Silva, J.P.; Carvalho, F.; Bastos, M.L.; Dias da Silva, D. 4-Fluoromethamphetamine (4-FMA) induces in vitro hepatotoxicity mediated by CYP2E1, CYP2D6, and CYP3A4 metabolism. Toxicology 2021, 463, 152988. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kaur, R.; Sharma, V.L. Ameliorative potential of Adhatoda vasica against anti-tubercular drugs induced hepatic impairments in female Wistar rats in relation to oxidative stress and xeno-metabolism. J. Ethnopharmacol. 2021, 270, 113771. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhao, Y.; Ma, L.; Piao, R. Orientin reverses acetaminophen-induced acute liver failure by inhibiting oxidative stress and mitochondrial dysfunction. J. Pharmacol. Sci. 2022, 149, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.D.; Chen, J.F.; Liu, J.J.; Xie, J.H.; Zhang, Z.B.; Gu, J.Y.; Zhuo, J.Y.; Huang, S.; Su, Z.R.; Sun, Z.H. Tetrahydrocurcumin and octahydrocurcumin, the primary and final hydrogenated metabolites of curcumin, possess superior hepatic-protective effect against acetaminophen-induced liver injury: Role of CYP2E1 and Keap1-Nrf2 pathway. Food Chem. Toxicol. 2019, 123, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Si, C.; Gao, P.; Cederbaum, A.I.; Xiong, H.; Lu, Y. The role of CYP2A5 in liver injury and fibrosis: Chemical-specific difference. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 33–43. [Google Scholar] [CrossRef]
- Jang, S.; Yu, L.R.; Abdelmegeed, M.A.; Gao, Y.; Banerjee, A.; Song, B.J. Critical role of c-jun N-terminal protein kinase in promoting mitochondrial dysfunction and acute liver injury. Redox Biol. 2015, 6, 552–564. [Google Scholar] [CrossRef]
- Stading, R.; Couroucli, X.; Lingappan, K.; Moorthy, B. The role of cytochrome P450 (CYP) enzymes in hyperoxic lung injury. Expert Opin. Drug Metab. Toxicol. 2021, 17, 171–178. [Google Scholar] [CrossRef]
- Zong, H.; Armoni, M.; Harel, C.; Karnieli, E.; Pessin, J.E. Cytochrome P-450 CYP2E1 knockout mice are protected against high-fat diet-induced obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E532–E539. [Google Scholar] [CrossRef] [PubMed]
- Aubert, J.; Begriche, K.; Knockaert, L.; Robin, M.A.; Fromenty, B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: Mechanisms and pathophysiological role. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, M.A.; Choi, Y.; Godlewski, G.; Ha, S.K.; Banerjee, A.; Jang, S.; Song, B.J. Cytochrome P450-2E1 promotes fast food-mediated hepatic fibrosis. Sci. Rep. 2017, 7, 39764. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Clare, K.; Dillon, J.F.; Brennan, P.N. Reactive Oxygen Species and Oxidative Stress in the Pathogenesis of MAFLD. J. Clin. Transl. Hepatol. 2022, 10, 939–946. [Google Scholar] [CrossRef]
- Dallio, M.; Sangineto, M.; Romeo, M.; Villani, R.; Romano, A.D.; Loguercio, C.; Serviddio, G.; Federico, A. Immunity as Cornerstone of Non-Alcoholic Fatty Liver Disease: The Contribution of Oxidative Stress in the Disease Progression. Int. J. Mol. Sci. 2021, 22, 436. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free. Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Malhi, H.; Bronk, S.F.; Werneburg, N.W.; Gores, G.J. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 2006, 281, 12093–12101. [Google Scholar] [CrossRef]
- Shum, M.; Ngo, J.; Shirihai, O.S.; Liesa, M. Mitochondrial oxidative function in NAFLD: Friend or foe? Mol. Metab. 2021, 50, 101134. [Google Scholar] [CrossRef]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, M.A.; Banerjee, A.; Jang, S.; Yoo, S.H.; Yun, J.W.; Gonzalez, F.J.; Keshavarzian, A.; Song, B.J. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free. Radic. Biol. Med. 2013, 65, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.D.; Wang, Y.H.; Chang, C.; Gershwin, M.E.; Lian, Z.X. The intestinal microbiota and microenvironment in liver. Autoimmun. Rev. 2015, 14, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H. Role of Gut Dysbiosis in Liver Diseases: What Have We Learned So Far? Diseases 2019, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Cope, K.; Risby, T.; Diehl, A.M. Increased gastrointestinal ethanol production in obese mice: Implications for fatty liver disease pathogenesis. Gastroenterology 2000, 119, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Engstler, A.J.; Aumiller, T.; Degen, C.; Dürr, M.; Weiss, E.; Maier, I.B.; Schattenberg, J.M.; Jin, C.J.; Sellmann, C.; Bergheim, I. Insulin resistance alters hepatic ethanol metabolism: Studies in mice and children with non-alcoholic fatty liver disease. Gut 2016, 65, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef]
- Ferro, D.; Baratta, F.; Pastori, D.; Cocomello, N.; Colantoni, A.; Angelico, F.; Del Ben, M. New Insights into the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Gut-Derived Lipopolysaccharides and Oxidative Stress. Nutrients 2020, 12, 2762. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression From NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Taxon- and Site-Specific Melatonin Catabolism. Molecules 2017, 22, 2015. [Google Scholar] [CrossRef] [PubMed]
- Popović, B.; Velimirović, M.; Stojković, T.; Brajović, G.; De Luka, S.R.; Milovanović, I.; Stefanović, S.; Nikolić, D.; Ristić-Djurović, J.L.; Petronijević, N.D.; et al. The influence of ageing on the extrapineal melatonin synthetic pathway. Exp. Gerontol. 2018, 110, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Zubero, E.; López-Pingarrón, L.; Alatorre-Jiménez, M.A.; Ochoa-Moneo, P.; Buisac-Ramón, C.; Rivas-Jiménez, M.; Castán-Ruiz, S.; Antoñanzas-Lombarte, Á.; Tan, D.X.; García, J.J.; et al. Melatonin’s role as a co-adjuvant treatment in colonic diseases: A review. Life Sci. 2017, 170, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Corral, S.A.; Reiter, R.J.; Tan, D.-X.; Manchester, L.C.; Liu, X. Chapter 18: Antioxidant and Anti-Inflammatory Role of Melatonin in Alzheimer’s Neurodegeneration; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Hardeland, R. Melatonin metabolism in the central nervous system. Curr. Neuropharmacol. 2010, 8, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; León, J.; Carazo, A.; Khaldy, H. Mitochondrial regulation by melatonin and its metabolites. Adv. Exp. Med. Biol. 2003, 527, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, B.; Yavari, R.; Badalzadeh, R.; Mahmoodpoor, A. An Overview on Mitochondrial-Based Therapies in Sepsis-Related Myocardial Dysfunction: Mitochondrial Transplantation as a Promising Approach. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3277274. [Google Scholar] [CrossRef] [PubMed]
- Swanson, G.R.; Siskin, J.; Gorenz, A.; Shaikh, M.; Raeisi, S.; Fogg, L.; Forsyth, C.; Keshavarzian, A. Disrupted diurnal oscillation of gut-derived Short chain fatty acids in shift workers drinking alcohol: Possible mechanism for loss of resiliency of intestinal barrier in disrupted circadian host. Transl. Res. 2020, 221, 97–109. [Google Scholar] [CrossRef]
- Engen, P.A.; Green, S.J.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. The Gastrointestinal Microbiome: Alcohol Effects on the Composition of Intestinal Microbiota. Alcohol. Res. 2015, 37, 223–236. [Google Scholar]
- Tran, L.; Jochum, S.B.; Shaikh, M.; Wilber, S.; Zhang, L.; Hayden, D.M.; Forsyth, C.B.; Voigt, R.M.; Bishehsari, F.; Keshavarzian, A.; et al. Circadian misalignment by environmental light/dark shifting causes circadian disruption in colon. PLoS ONE 2021, 16, e0251604. [Google Scholar] [CrossRef]
- Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Circadian rhythms: A regulator of gastrointestinal health and dysfunction. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Swanson, G.R.; Gorenz, A.; Shaikh, M.; Desai, V.; Forsyth, C.; Fogg, L.; Burgess, H.J.; Keshavarzian, A. Decreased melatonin secretion is associated with increased intestinal permeability and marker of endotoxemia in alcoholics. Am. J. Physiol. Gastrointest Liver Physiol. 2015, 308, G1004–G1011. [Google Scholar] [CrossRef] [PubMed]
- Kurhaluk, N.; Tkachenko, H. Melatonin and alcohol-related disorders. Chronobiol. Int. 2020, 37, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Mitochondrial bioenergetics decay in aging: Beneficial effect of melatonin. Cell. Mol. Life Sci. 2017, 74, 3897–3911. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.; Rosales-Corral, S.; de Campos Zuccari, D.A.P.; de Almeida Chuffa, L.G. Melatonin: A mitochondrial resident with a diverse skill set. Life Sci. 2022, 301, 120612. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, N.; Yan, S.; Lu, Y.; Miao, X.; Gu, Z.; Shao, Y. Melatonin attenuates renal fibrosis in diabetic mice by activating the AMPK/PGC1α signaling pathway and rescuing mitochondrial function. Mol. Med. Rep. 2019, 19, 1318–1330. [Google Scholar] [CrossRef]
- Chen, W.R.; Zhou, Y.J.; Sha, Y.; Wu, X.P.; Yang, J.Q.; Liu, F. Melatonin attenuates vascular calcification by inhibiting mitochondria fission via an AMPK/Drp1 signalling pathway. J. Cell Mol. Med. 2020, 24, 6043–6054. [Google Scholar] [CrossRef]
- Robin, M.A.; Sauvage, I.; Grandperret, T.; Descatoire, V.; Pessayre, D.; Fromenty, B. Ethanol increases mitochondrial cytochrome P450 2E1 in mouse liver and rat hepatocytes. FEBS Lett. 2005, 579, 6895–6902. [Google Scholar] [CrossRef]
- Neve, E.P.; Ingelman-Sundberg, M. Molecular basis for the transport of cytochrome P450 2E1 to the plasma membrane. J. Biol. Chem. 2000, 275, 17130–17135. [Google Scholar] [CrossRef] [PubMed]
- Neve, E.P.; Ingelman-Sundberg, M. A soluble NH(2)-terminally truncated catalytically active form of rat cytochrome P450 2E1 targeted to liver mitochondria(1). FEBS Lett. 1999, 460, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. Cytochrome P-4502E1: Its physiological and pathological role. Physiol. Rev. 1997, 77, 517–544. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Cederbaum, A.I. Overexpression of CYP2E1 in mitochondria sensitizes HepG2 cells to the toxicity caused by depletion of glutathione. J. Biol. Chem. 2006, 281, 5128–5136. [Google Scholar] [CrossRef] [PubMed]
- Solís-Muñoz, P.; Solís-Herruzo, J.A.; Fernández-Moreira, D.; Gómez-Izquierdo, E.; García-Consuegra, I.; Muñoz-Yagüe, T.; García Ruiz, I. Melatonin improves mitochondrial respiratory chain activity and liver morphology in ob/ob mice. J. Pineal Res. 2011, 51, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Q.; Fichna, J.; Bashashati, M.; Li, Y.Y.; Storr, M. Distribution, function and physiological role of melatonin in the lower gut. World J. Gastroenterol. 2011, 17, 3888–3898. [Google Scholar] [CrossRef]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef]
- Zhao, L.; An, R.; Yang, Y.; Yang, X.; Liu, H.; Yue, L.; Li, X.; Lin, Y.; Reiter, R.J.; Qu, Y. Melatonin alleviates brain injury in mice subjected to cecal ligation and puncture via attenuating inflammation, apoptosis, and oxidative stress: The role of SIRT1 signaling. J. Pineal Res. 2015, 59, 230–239. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, J.; Bu, S.; Li, B.; Zhang, Q.; Wang, Q.; Lai, D. Melatonin protects against chronic stress-induced oxidative meiotic defects in mice MII oocytes by regulating SIRT1. Cell Cycle 2020, 19, 1677–1695. [Google Scholar] [CrossRef]
- Chandramowlishwaran, P.; Vijay, A.; Abraham, D.; Li, G.; Mwangi, S.M.; Srinivasan, S. Role of Sirtuins in Modulating Neurodegeneration of the Enteric Nervous System and Central Nervous System. Front. Neurosci. 2020, 14, 614331. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Deng, Y.J.; Xie, Q.Q.; Ren, E.H.; Ma, Z.J.; He, X.G.; Gao, Y.C.; Kang, X.W. Sirtuins and intervertebral disc degeneration: Roles in inflammation, oxidative stress, and mitochondrial function. Clin. Chim. Acta 2020, 508, 33–42. [Google Scholar] [CrossRef]
- Jiang, J.; Liang, S.; Zhang, J.; Du, Z.; Xu, Q.; Duan, J.; Sun, Z. Melatonin ameliorates PM(2.5) -induced cardiac perivascular fibrosis through regulating mitochondrial redox homeostasis. J. Pineal Res. 2021, 70, e12686. [Google Scholar] [CrossRef]
- Ma, S.; Chen, J.; Feng, J.; Zhang, R.; Fan, M.; Han, D.; Li, X.; Li, C.; Ren, J.; Wang, Y.; et al. Melatonin Ameliorates the Progression of Atherosclerosis via Mitophagy Activation and NLRP3 Inflammasome Inhibition. Oxid Med. Cell. Longev. 2018, 2018, 9286458. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Y.; Gao, Y.; Wang, Z.; Ma, J. Melatonin Attenuates Anoxia/Reoxygenation Injury by Inhibiting Excessive Mitophagy Through the MT2/SIRT3/FoxO3a Signaling Pathway in H9c2 Cells. Drug Des. Devel. Ther. 2020, 14, 2047–2060. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Jou, M.J.; Acuna-Castroviejo, D. Melatonin Mitigates Mitochondrial Meltdown: Interactions with SIRT3. Int. J. Mol. Sci. 2018, 19, 2439. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, Y.; Gao, Y.; Lin, D.; Wang, Z.; Ma, J. Melatonin postconditioning ameliorates anoxia/reoxygenation injury by regulating mitophagy and mitochondrial dynamics in a SIRT3-dependent manner. Eur. J. Pharmacol. 2021, 904, 174157. [Google Scholar] [CrossRef]
- Yu, L.M.; Dong, X.; Xue, X.D.; Xu, S.; Zhang, X.; Xu, Y.L.; Wang, Z.S.; Wang, Y.; Gao, H.; Liang, Y.X.; et al. Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia-reperfusion injury by improving mitochondrial quality control: Role of SIRT6. J. Pineal Res. 2021, 70, e12698. [Google Scholar] [CrossRef]
- Kim, H.G.; Huang, M.; Xin, Y.; Zhang, Y.; Zhang, X.; Wang, G.; Liu, S.; Wan, J.; Ahmadi, A.R.; Sun, Z.; et al. The epigenetic regulator SIRT6 protects the liver from alcohol-induced tissue injury by reducing oxidative stress in mice. J. Hepatol. 2019, 71, 960–969. [Google Scholar] [CrossRef]
- Zeng, C.; Chen, M. Progress in Nonalcoholic Fatty Liver Disease: SIRT Family Regulates Mitochondrial Biogenesis. Biomolecules 2022, 12, 1079. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Qin, X.; Turdi, S.; Sun, D.; Culver, B.; Reiter, R.J.; Wang, X.; Zhou, H.; Ren, J. ALDH2 contributes to melatonin-induced protection against APP/PS1 mutation-prompted cardiac anomalies through cGAS-STING-TBK1-mediated regulation of mitophagy. Signal Transduct. Target. Ther. 2020, 5, 119. [Google Scholar] [CrossRef]
- Zhou, H.; Du, W.; Li, Y.; Shi, C.; Hu, N.; Ma, S.; Wang, W.; Ren, J. Effects of melatonin on fatty liver disease: The role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J. Pineal Res. 2018, 64, e12450. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Favero, G.; Rodella, L.F. Impact of Melatonin on Skeletal Muscle and Exercise. Cells 2020, 9, 288. [Google Scholar] [CrossRef]
- Chen, C.; Yang, C.; Wang, J.; Huang, X.; Yu, H.; Li, S.; Li, S.; Zhang, Z.; Liu, J.; Yang, X.; et al. Melatonin ameliorates cognitive deficits through improving mitophagy in a mouse model of Alzheimer’s disease. J. Pineal Res. 2021, 71, e12774. [Google Scholar] [CrossRef]
- Doğanlar, Z.B.; Doğanlar, O.; Kurtdere, K.; Güçlü, H.; Chasan, T.; Turgut, E. Melatonin prevents blood-retinal barrier breakdown and mitochondrial dysfunction in high glucose and hypoxia-induced in vitro diabetic macular edema model. Toxicol. Vitr. 2021, 75, 105191. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, F.; Dou, Y.; Tian, X.; Liu, C.; Li, H.; Shen, H.; Chen, G. Melatonin Alleviates Intracerebral Hemorrhage-Induced Secondary Brain Injury in Rats via Suppressing Apoptosis, Inflammation, Oxidative Stress, DNA Damage, and Mitochondria Injury. Transl. Stroke Res. 2018, 9, 74–91. [Google Scholar] [CrossRef]
- Yapislar, H.; Haciosmanoglu, E.; Sarioglu, T.; Ekmekcioglu, C. The melatonin MT(2) receptor is involved in the anti-apoptotic effects of melatonin in rats with type 2 diabetes mellitus. Tissue Cell 2022, 76, 101763. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Ballway, J.W.; Song, B.J. Translational Approaches with Antioxidant Phytochemicals against Alcohol-Mediated Oxidative Stress, Gut Dysbiosis, Intestinal Barrier Dysfunction, and Fatty Liver Disease. Antioxidants 2021, 10, 384. [Google Scholar] [CrossRef]
- Kim, D.H.; Sim, Y.; Hwang, J.H.; Kwun, I.S.; Lim, J.H.; Kim, J.; Kim, J.I.; Baek, M.C.; Akbar, M.; Seo, W.; et al. Ellagic Acid Prevents Binge Alcohol-Induced Leaky Gut and Liver Injury through Inhibiting Gut Dysbiosis and Oxidative Stress. Antioxidants 2021, 10, 1386. [Google Scholar] [CrossRef]
- Whitfield, C.; Trent, M.S. Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 2014, 83, 99–128. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Voigt, R.M.; Shaikh, M.; Tang, Y.; Cederbaum, A.I.; Turek, F.W.; Keshavarzian, A. Role for intestinal CYP2E1 in alcohol-induced circadian gene-mediated intestinal hyperpermeability. Am. J. Physiol. Gastrointest Liver Physiol. 2013, 305, G185–G195. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Voigt, R.M.; Burgess, H.J.; Swanson, G.R.; Keshavarzian, A. Circadian rhythms, alcohol and gut interactions. Alcohol 2015, 49, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Swanson, G.R.; Gorenz, A.; Shaikh, M.; Desai, V.; Kaminsky, T.; Van Den Berg, J.; Murphy, T.; Raeisi, S.; Fogg, L.; Vitaterna, M.H.; et al. Night workers with circadian misalignment are susceptible to alcohol-induced intestinal hyperpermeability with social drinking. Am. J. Physiol. Gastrointest Liver Physiol. 2016, 311, G192–G201. [Google Scholar] [CrossRef] [PubMed]
- Fonzi, S.; Solinas, G.P.; Costelli, P.; Parodi, C.; Murialdo, G.; Bo, P.; Albergati, A.; Montalbetti, L.; Savoldi, F.; Polleri, A. Melatonin and cortisol circadian secretion during ethanol withdrawal in chronic alcoholics. Chronobiologia 1994, 21, 109–112. [Google Scholar]
- Che, Z.; Song, Y.; Xu, C.; Li, W.; Dong, Z.; Wang, C.; Ren, Y.; So, K.F.; Tipoe, G.L.; Wang, F.; et al. Melatonin alleviates alcoholic liver disease via EGFR-BRG1-TERT axis regulation. Acta Pharm. Sin. B 2023, 13, 100–112. [Google Scholar] [CrossRef]
- Arioz, B.I.; Tarakcioglu, E.; Olcum, M.; Genc, S. The Role of Melatonin on NLRP3 Inflammasome Activation in Diseases. Antioxidants 2021, 10, 1020. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Najafi, M.; Kavyiani, N.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Anti-Inflammatory Activity of Melatonin: A Focus on the Role of NLRP3 Inflammasome. Inflammation 2021, 44, 1207–1222. [Google Scholar] [CrossRef]
- Lee, S.E.; Koh, H.; Joo, D.J.; Nedumaran, B.; Jeon, H.J.; Park, C.S.; Harris, R.A.; Kim, Y.D. Induction of SIRT1 by melatonin improves alcohol-mediated oxidative liver injury by disrupting the CRBN-YY1-CYP2E1 signaling pathway. J. Pineal Res. 2020, 68, e12638. [Google Scholar] [CrossRef]
- Mishra, A.; Paul, S.; Swarnakar, S. Downregulation of matrix metalloproteinase-9 by melatonin during prevention of alcohol-induced liver injury in mice. Biochimie 2011, 93, 854–866. [Google Scholar] [CrossRef]
- Ullah, U.; Badshah, H.; Malik, Z.; Uddin, Z.; Alam, M.; Sarwar, S.; Aman, A.; Khan, A.U.; Shah, F.A. Hepatoprotective effects of melatonin and celecoxib against ethanol-induced hepatotoxicity in rats. Immunopharmacol. Immunotoxicol. 2020, 42, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.M.; Lee, Y.H.; Kim, J.W.; Ham, D.S.; Kang, E.S.; Cha, B.S.; Lee, H.C.; Lee, B.W. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy 2015, 11, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Rui, B.B.; Chen, H.; Jang, L.; Li, Z.; Yang, J.M.; Xu, W.P.; Wei, W. Melatonin Upregulates the Activity of AMPK and Attenuates Lipid Accumulation in Alcohol-induced Rats. Alcohol Alcohol. 2016, 51, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Meng, X.; Li, Y.; Zhou, Y.; Xu, D.P.; Li, S.; Li, H.B. Effects of Melatonin on Liver Injuries and Diseases. Int. J. Mol. Sci. 2017, 18, 673. [Google Scholar] [CrossRef] [PubMed]
- Kurhaluk, N.; Tkachenko, H.; Lukash, O. Melatonin modulates oxidative phosphorylation, hepatic and kidney autophagy-caused subclinical endotoxemia and acute ethanol-induced oxidative stress. Chronobiol. Int. 2020, 37, 1709–1724. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Hwang, S.L.; Lee, E.J.; Kim, H.M.; Chung, M.J.; Elfadl, A.K.; Lee, S.E.; Nedumaran, B.; Harris, R.A.; Jeong, K.S. Melatonin ameliorates alcohol-induced bile acid synthesis by enhancing miR-497 expression. J. Pineal Res. 2017, 62, e12386. [Google Scholar] [CrossRef] [PubMed]
- Teunis, C.; Nieuwdorp, M.; Hanssen, N. Interactions between Tryptophan Metabolism, the Gut Microbiome and the Immune System as Potential Drivers of Non-Alcoholic Fatty Liver Disease (NAFLD) and Metabolic Diseases. Metabolites 2022, 12, 514. [Google Scholar] [CrossRef]
- Genario, R.; Cipolla-Neto, J.; Bueno, A.A.; Santos, H.O. Melatonin supplementation in the management of obesity and obesity-associated disorders: A review of physiological mechanisms and clinical applications. Pharmacol. Res. 2021, 163, 105254. [Google Scholar] [CrossRef]
- Halpern, B.; Mancini, M.C.; Bueno, C.; Barcelos, I.P.; de Melo, M.E.; Lima, M.S.; Carneiro, C.G.; Sapienza, M.T.; Buchpiguel, C.A.; do Amaral, F.G.; et al. Melatonin Increases Brown Adipose Tissue Volume and Activity in Patients With Melatonin Deficiency: A Proof-of-Concept Study. Diabetes 2019, 68, 947–952. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.M.; Jung, J.; Kim, Y.J.; Moon, K.C.; Seo, J.H.; Ha, T.K.; Ha, E. Pinealectomy increases thermogenesis and decreases lipogenesis. Mol. Med. Rep. 2020, 22, 4289–4297. [Google Scholar] [CrossRef]
- Sohrabi, M.; Gholami, A.; Amirkalali, B.; Taherizadeh, M.; Kolahdoz, M.; SafarnezhadTameshkel, F.; Aghili, S.; Hajibaba, M.; Zamani, F.; Nasiri Toosi, M.; et al. Is melatonin associated with pro-inflammatory cytokine activity and liver fibrosis in non-alcoholic fatty liver disease (NAFLD) patients? Gastroenterol. Hepatol. Bed Bench 2021, 14, 229–236. [Google Scholar] [PubMed]
- Sohrabi, M.; Ajdarkosh, H.; Gholami, A.; Amirkalali, B.; Mansorian, M.R.; Aten, S.; Sohrabi, M.; Nasiri-Toosi, M.; Zamani, F.; Keyvani, H. Association between Melatonin Value and Interleukins1B, -18, and -33 Levels in Patients with Different Stages of Non-Alcoholic Fatty Liver Disease. Middle East J. Dig. Dis. 2022, 14, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Lane, E.A.; Moss, H.B. Pharmacokinetics of melatonin in man: First pass hepatic metabolism. J. Clin. Endocrinol. Metab. 1985, 61, 1214–1216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, A.; Liu, X.; Ma, Y.; Zhao, F.; Wang, M.; Loor, J.J.; Wang, H. Melatonin ameliorates ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. J. Hazard. Mater. 2021, 407, 124489. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Arabacı Tamer, S.; Sahin, D.; Bagriacik, F.; Kahraman, M.M.; Onur, N.D.; Cayirli, Y.B.; Cilingir Kaya Ö, T.; Aksu, B.; Akdeniz, E.; et al. The effects of antibiotics and melatonin on hepato-intestinal inflammation and gut microbial dysbiosis induced by a short-term high-fat diet consumption in rats. Br. J. Nutr. 2019, 122, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kang, W.; Mao, X.; Ge, L.; Du, H.; Li, J.; Hou, L.; Liu, D.; Yin, Y.; Liu, Y.; et al. Melatonin mitigates aflatoxin B1-induced liver injury via modulation of gut microbiota/intestinal FXR/liver TLR4 signaling axis in mice. J. Pineal Res. 2022, 73, e12812. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Melgarejo, F.J.; Caro-Díaz, C.; Cabello-Guzmán, G. Potential Crosstalk between Fructose and Melatonin: A New Role of Melatonin-Inhibiting the Metabolic Effects of Fructose. Int. J. Endocrinol. 2018, 2018, 7515767. [Google Scholar] [CrossRef]
- Pafili, K.; Roden, M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol. Metab. 2021, 50, 101122. [Google Scholar] [CrossRef]
- Caballero, M.E.; Berlanga, J.; Ramirez, D.; Lopez-Saura, P.; Gozalez, R.; Floyd, D.N.; Marchbank, T.; Playford, R.J. Epidermal growth factor reduces multiorgan failure induced by thioacetamide. Gut 2001, 48, 34–40. [Google Scholar] [CrossRef]
- Shin, S.K.; Cho, H.W.; Song, S.E.; Bae, J.H.; Im, S.S.; Hwang, I.; Ha, H.; Song, D.K. Ablation of catalase promotes non-alcoholic fatty liver via oxidative stress and mitochondrial dysfunction in diet-induced obese mice. Pflugers Arch. 2019, 471, 829–843. [Google Scholar] [CrossRef]
- Rishi, P.; Bharrhan, S.; Bhalla, M.P.; Koul, A.; Chopra, K. Inhibition of endotoxin-induced hepatotoxicity by melatonin in rats. Int. J. Biomed. Sci. 2008, 4, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, C.; Zhao, M.; Shi, C.E.; Zhu, R.M.; Wang, H.; Zhao, H.; Wei, W.; Li, J.B.; Xu, D.X. Melatonin alleviates lipopolysaccharide-induced hepatic SREBP-1c activation and lipid accumulation in mice. J. Pineal Res. 2011, 51, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Yang, L.; Li, Y.; Chen, J.; Zhang, X.; Wang, H.; Zhai, S.; Jiang, X.; Meca, G.; Wang, S.; et al. Melatonin alleviates Ochratoxin A-induced liver inflammation involved intestinal microbiota homeostasis and microbiota-independent manner. J. Hazard. Mater. 2021, 413, 125239. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, L.; Wu, J.; An, S.; Fang, H.; Han, Y.; Huang, Q.; Chen, Z.; Zeng, Z. Melatonin Attenuates Sepsis-Induced Small-Intestine Injury by Upregulating SIRT3-Mediated Oxidative-Stress Inhibition, Mitochondrial Protection, and Autophagy Induction. Front. Immunol. 2021, 12, 625627. [Google Scholar] [CrossRef] [PubMed]
- Kleber, A.; Kubulus, D.; Rössler, D.; Wolf, B.; Volk, T.; Speer, T.; Fink, T. Melatonin modifies cellular stress in the liver of septic mice by reducing reactive oxygen species and increasing the unfolded protein response. Exp. Mol. Pathol. 2014, 97, 565–571. [Google Scholar] [CrossRef]
- Borrelli, A.; Bonelli, P.; Tuccillo, F.M.; Goldfine, I.D.; Evans, J.L.; Buonaguro, F.M.; Mancini, A. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: Current and innovative therapeutic approaches. Redox Biol. 2018, 15, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Buxton, O.M.; L’Hermite-Balériaux, M.; Hirschfeld, U.; Cauter, E. Acute and delayed effects of exercise on human melatonin secretion. J. Biol. Rhythms. 1997, 12, 568–574. [Google Scholar] [CrossRef]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef]
- Croci, S.; D’Apolito, L.I.; Gasperi, V.; Catani, M.V.; Savini, I. Dietary Strategies for Management of Metabolic Syndrome: Role of Gut Microbiota Metabolites. Nutrients 2021, 13, 1389. [Google Scholar] [CrossRef]
- Wrzosek, L.; Ciocan, D.; Hugot, C.; Spatz, M.; Dupeux, M.; Houron, C.; Lievin-Le Moal, V.; Puchois, V.; Ferrere, G.; Trainel, N.; et al. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut 2021, 70, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Fuke, N.; Nagata, N.; Suganuma, H.; Ota, T. Regulation of Gut Microbiota and Metabolic Endotoxemia with Dietary Factors. Nutrients 2019, 11, 2277. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D.; Collin, F. Melatonin: Action as antioxidant and potential applications in human disease and aging. Toxicology 2010, 278, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Mazzola, M.; Leone, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Gerges Geagea, A.; Jurjus, A.; et al. Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2016, 160, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Suhail Kasim, B.Z.; Wafa Harrouk, K.A. Melatonin: Pharmacy Compounding Advisory Committee Meeting; Food and Drug Administration: Silver Spring, MD, USA, 2021. [Google Scholar]
- Kuehn, B.M. Climbing Melatonin Use for Insomnia Raises Safety Concerns. JAMA 2022, 328, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F.; Lowes, D.A.; Allen, L.; Cameron, G.; Aucott, L.S.; Webster, N.R. Melatonin as a potential therapy for sepsis: A phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J. Pineal Res. 2014, 56, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Sawhney, G.; Pandhi, P. The therapeutic potential of melatonin: A review of the science. MedGenMed 2004, 6, 46. [Google Scholar]
- Nickkholgh, A.; Schneider, H.; Sobirey, M.; Venetz, W.P.; Hinz, U.; Pelzl le, H.; Gotthardt, D.N.; Cekauskas, A.; Manikas, M.; Mikalauskas, S.; et al. The use of high-dose melatonin in liver resection is safe: First clinical experience. J. Pineal Res. 2011, 50, 381–388. [Google Scholar] [CrossRef]
- Seabra, M.L.; Bignotto, M.; Pinto, L.R., Jr.; Tufik, S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J. Pineal Res. 2000, 29, 193–200. [Google Scholar] [CrossRef]
- Lissoni, P.; Barni, S.; Rovelli, F.; Brivio, F.; Ardizzoia, A.; Tancini, G.; Conti, A.; Maestroni, G.J. Neuroimmunotherapy of advanced solid neoplasms with single evening subcutaneous injection of low-dose interleukin-2 and melatonin: Preliminary results. Eur. J. Cancer 1993, 29, 185–189. [Google Scholar] [CrossRef]
- Arendt, J.; Aldhous, M.; Marks, V. Alleviation of jet lag by melatonin: Preliminary results of controlled double blind trial. Br. Med. J. (Clin. Res. Ed.) 1986, 292, 1170. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Waldhauser, F.; Garfield, G.; Lynch, H.J.; Wurtman, R.J. Effects of melatonin on human mood and performance. Brain Res. 1984, 323, 201–207. [Google Scholar] [CrossRef] [PubMed]
- James, S.P.; Sack, D.A.; Rosenthal, N.E.; Mendelson, W.B. Melatonin administration in insomnia. Neuropsychopharmacology 1990, 3, 19–23. [Google Scholar] [PubMed]
- Samel, A.; Wegmann, H.M.; Vejvoda, M.; Maass, H.; Gundel, A.; Schütz, M. Influence of melatonin treatment on human circadian rhythmicity before and after a simulated 9-hr time shift. J. Biol. Rhythms. 1991, 6, 235–248. [Google Scholar] [CrossRef]
- Gramajo, A.L.; Marquez, G.E.; Torres, V.E.; Juárez, C.P.; Rosenstein, R.E.; Luna, J.D. Therapeutic benefit of melatonin in refractory central serous chorioretinopathy. Eye 2015, 29, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Menczel Schrire, Z.; Phillips, C.L.; Chapman, J.L.; Duffy, S.L.; Wong, G.; D’Rozario, A.L.; Comas, M.; Raisin, I.; Saini, B.; Gordon, C.J.; et al. Safety of higher doses of melatonin in adults: A systematic review and meta-analysis. J. Pineal Res. 2022, 72, e12782. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.P.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef]
- López-González, A.; Álvarez-Sánchez, N.; Lardone, P.J.; Cruz-Chamorro, I.; Martínez-López, A.; Guerrero, J.M.; Reiter, R.J.; Carrillo-Vico, A. Melatonin treatment improves primary progressive multiple sclerosis: A case report. J. Pineal Res. 2015, 58, 173–177. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Sainz, R.M.; Mayo, J.C.; Lopez-Burillo, S. Melatonin: Reducing the toxicity and increasing the efficacy of drugs. J. Pharm. Pharmacol. 2002, 54, 1299–1321. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Gunes, A.; Dahl, M.L. Variation in CYP1A2 activity and its clinical implications: Influence of environmental factors and genetic polymorphisms. Pharmacogenomics 2008, 9, 625–637. [Google Scholar] [CrossRef] [PubMed]

| Stage of ALD and Model | Species Used | MT Dose and Treatment Duration | Signaling Pathway Affected by MT | Consequences of MT Effects | Reference |
|---|---|---|---|---|---|
| Acute Ethanol-Induced Stress | Male 2–3-month-old white mice | 10 mg/kg for 10 days | ↑ Mitochondrial function ↑ Mitochondrial respiration ↑ RCR, ADP/O, and Vph | Modulates oxidative phosphorylation; mitigates subclinical endotoxemia and oxidative stress | [166] |
| Steatosis/hepatitis | AML-12 cells and 7-week-old C57BL wild-type mice | 10 μmol/L in cell model and 5 mg/kg for 10 days in Gao Binge Model | EGFR-BRG1-TERT pathway | Downstream effects of MT | [157] |
| Hepatotoxicity | Adult Male Rats | 50 mg/kg for 11 consecutive days | ↓ Serum transaminases ↓ ALP ↑ GSH, GST ↓ NO ↓ TNF-α, p-NF-κB, COX2 ↓ Hepatic cellular apoptosis | Decreased EtOH- induced apoptosis and inflammation via JNK and TNF-α signaling cascades | [162] |
| Steatosis | Mice and human samples | 10 mg/kg orally for last 2 weeks of 4-week alcohol exposure Once daily for 7 days via tail-vein | CRBN-YY1-CYP2E1 ↓ CYP2E1 ↓ ROS ↓ Serum AST and ↓ALT ↓ IL-6 and ↓TNF-α ↓ Hepatic TGs ↓ Hepatic cholesterol | Induction of SIRT1 acts via the CRBN- YY1-CYP2E1 pathway to mitigate oxidative stress, improve liver function, prevent hepatic fat accumulation and inflammation | [160] |
| Fat accumulation | Adult male Sprague Dawley rats | 20 and 40 mg/kg administration | ↓ ALT, AST, and serum and hepatic TG ↑ SOD ↓ MDA ↑ p-AMPK ↑ MT1R expression | ↑ AMPK ↓ Lipid accumulation | [164] |
| Steatosis | Female Balb/C Mice | 15 mg/kg via i.p. prior to ethanol for 3 days | ↓ MMP-9 activity, which then prevented NF-κB translocation to the nucleus after EtOH exposure ↓ Total pathology score but no significant effect on transaminases | Prevented inflammation | [161] |
| Chronic ALD | Mouse model | 10 mg/kg daily oral gavage for last 2 weeks of 4 weeks of ethanol | ↑ miR-497 expression | Ameliorates alcohol- induced bile acid synthesis by up- regulating miR-497 expression and attenuating the BTG2-YY1 pathway | [167] |
| Stage of MASLD or Liver Injury | Species Used in Study | MT Dose/ Treatment Duration | Signaling Pathway Affected by MT | MT Effects | Reference |
|---|---|---|---|---|---|
| Endotoxin- induced Hepatotoxicity | Female Wistar rats | 10 mg/kg MT 30 min before LPS and 2 h after LPS | ↓ LPS ↑ GSH levels ↑ SOD activity ↑ Catalase activity ↓ Serum nitrite NO2 ↓ TNF-α ↓ Hepatic necrosis | Mitigates endotoxin- induced hepatotoxicity ↑ Antioxidant stores ↓ Oxidative stress ↓ Hepatic inflammation and cellular death pathways | [182] |
| MASLD (Steatosis) ±HFD | Catalase-KO mice (CKO) and HepG2 cells | 500 μg/kg/day MT for 6 weeks | ↓ Liver weight ↓ Fat accumulation Restored Aspect Ratio (AR) and Form Factor (FF) values as measures of mitochondrial function ↑ mRNA expression of FOXO1, PGC1β, and PPAR-γ Improved mitochon- drial morphology ↑ mRNA expression of CPT1, CPT2, COX1, FGF21, Lcad, Mcad, Aconitase, IDH, SDH, MDH ↑ Hepatic [ATP] | Ablation of catalase plays a role in MASLD, and MT supports the function of mitochondria; HFD-exposed CKO mice exhibited cellular lipid accumulation and decreased mitochon- drial biogenesis, which was recovered with MT; MT prevented fatty liver development and main- tained mitochondrial in- tegrity in hepatocytes; Mitigated oxidative stress-induced mito- chondrial dysfunction and progression of MASLD | [181] |
| HFD-mediated MASLD lipogenesis and fibrosis |
| 20 mg/kg/day MT via i.p. for 12 weeks after 12 weeks HFD or LFD | In HFD-fed mice, MT ↓ NR4A1 level ↓ Hepatocyte vacu- olization, steatosis, and fibrosis ↓ MMP9 ↓ VCAM1 ↓ IL-6 ↓ TNF-α ↓ TGF-β ↓ Mitochondrial ROS production ↓ Mitochondrial PTP opening ↑ ΔΨm mitochon- drial inner mem- brane potential | NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy; Prevented fat accumula- tion and fibrosis by inhibiting NR4A1. NR4A1 then activates Drp-1-mediated mito- chondrial fission, and repressing BNIP3-medi- ated mitophagy, which protects mitochondria; MT mitigated oxidative stress and calcium over- load by suppressing fission | [142] |
| MASH | Male 6–8-week-old CD-1 mice on a regular diet | 5 mg/kg MT 30 min before 2 mg/kg LPS then 5 mg/kg 150 min after LPS | ↓ LPS-induced acti- vation of SREBP-1c ↓ expression of SREBP-1c genes ↓ serum and hepatic TG levels | Prevents LPS-induced fat accumulation | [183] |
| Hepatitis | Male 1-day-old Cherry Valley ducklings | 0.2 mg/mL MT supplemented in drinking water for 2 weeks | ↓ Bacteriodetes induced by Ochratoxin A (OTA) ↑ Firmicutes ↓ Bacteriodetes/Firmi- cutes Ratio ↓ Bacteroides ↓ Bacteroides uniformis ↓ Turicibacter sangui- nis↓ Serum LPS levels ↑ Protein expression of Occludin and tight junction pro- tein-1 (TJP-1) ↑ Villi height and crypt depth ↑ Villi height/crypt depth ratio Restored gut histology ↓ TLR4, MyD88, p- -IKBα, p-IKBα/IKBα ratio, p-p65, liver IL-1β level, liver IL- 6, liver TNF-α ↑ Liver IL-10 ↓ Percentage of inflammatory liver cells in histology quantified | Anti-inflammatory Restored the physical barrier of gut Restored liver function and inflammatory markers | [184] |
| HFD-induced hepato-intestinal dysfunction and inflammation | Male Sprague Dawley rats | 4 mg/kg/day for 2 weeks | ↓ Perirenal fat ↓ Blood glucose ↑ TG levels by 3–4x ↓ Intestinal motility ↓ Liver weight ↑ GSH levels ↓ Myeloperoxidase (MPO) activity ↓ Serum ALT levels ↑ Hypertrophic goblet cell levels, epithelium, and brush border ↓ Vacuolization of hepatocytes Kept mitochondria intact | Restored Liver function and prevented fat accumulation Improved antioxidant levels and prevented oxidative stress Improved intestinal function and motility | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
LeFort, K.R.; Rungratanawanich, W.; Song, B.-J. Melatonin Prevents Alcohol- and Metabolic Dysfunction- Associated Steatotic Liver Disease by Mitigating Gut Dysbiosis, Intestinal Barrier Dysfunction, and Endotoxemia. Antioxidants 2024, 13, 43. https://doi.org/10.3390/antiox13010043
LeFort KR, Rungratanawanich W, Song B-J. Melatonin Prevents Alcohol- and Metabolic Dysfunction- Associated Steatotic Liver Disease by Mitigating Gut Dysbiosis, Intestinal Barrier Dysfunction, and Endotoxemia. Antioxidants. 2024; 13(1):43. https://doi.org/10.3390/antiox13010043
Chicago/Turabian StyleLeFort, Karli R., Wiramon Rungratanawanich, and Byoung-Joon Song. 2024. "Melatonin Prevents Alcohol- and Metabolic Dysfunction- Associated Steatotic Liver Disease by Mitigating Gut Dysbiosis, Intestinal Barrier Dysfunction, and Endotoxemia" Antioxidants 13, no. 1: 43. https://doi.org/10.3390/antiox13010043
APA StyleLeFort, K. R., Rungratanawanich, W., & Song, B.-J. (2024). Melatonin Prevents Alcohol- and Metabolic Dysfunction- Associated Steatotic Liver Disease by Mitigating Gut Dysbiosis, Intestinal Barrier Dysfunction, and Endotoxemia. Antioxidants, 13(1), 43. https://doi.org/10.3390/antiox13010043