Sophy β-Glucan from the Black Yeast Aureobasidium pullulans Attenuates Salmonella-Induced Intestinal Epithelial Barrier Injury in Caco-2 Cell Monolayers via Exerting Anti-Oxidant and Anti-Inflammatory Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Caco-2 Cells Culture
2.3. Cell Viability
2.4. Sophy β-Glucan Pre-Treatment, Salmonella Infection In Vitro and Samples Collection
2.5. Bacterial Adhesion and Invasion Assay
2.6. Measurement of Trans-Epithelial Electrical Resistance (TER) and Permeability
2.7. Supernatants Cytokines
2.8. Antioxidant Indices
2.9. Quantitative Real-Time PCR Analysis
2.10. Western Blotting
2.11. Statistical Analyses
3. Results
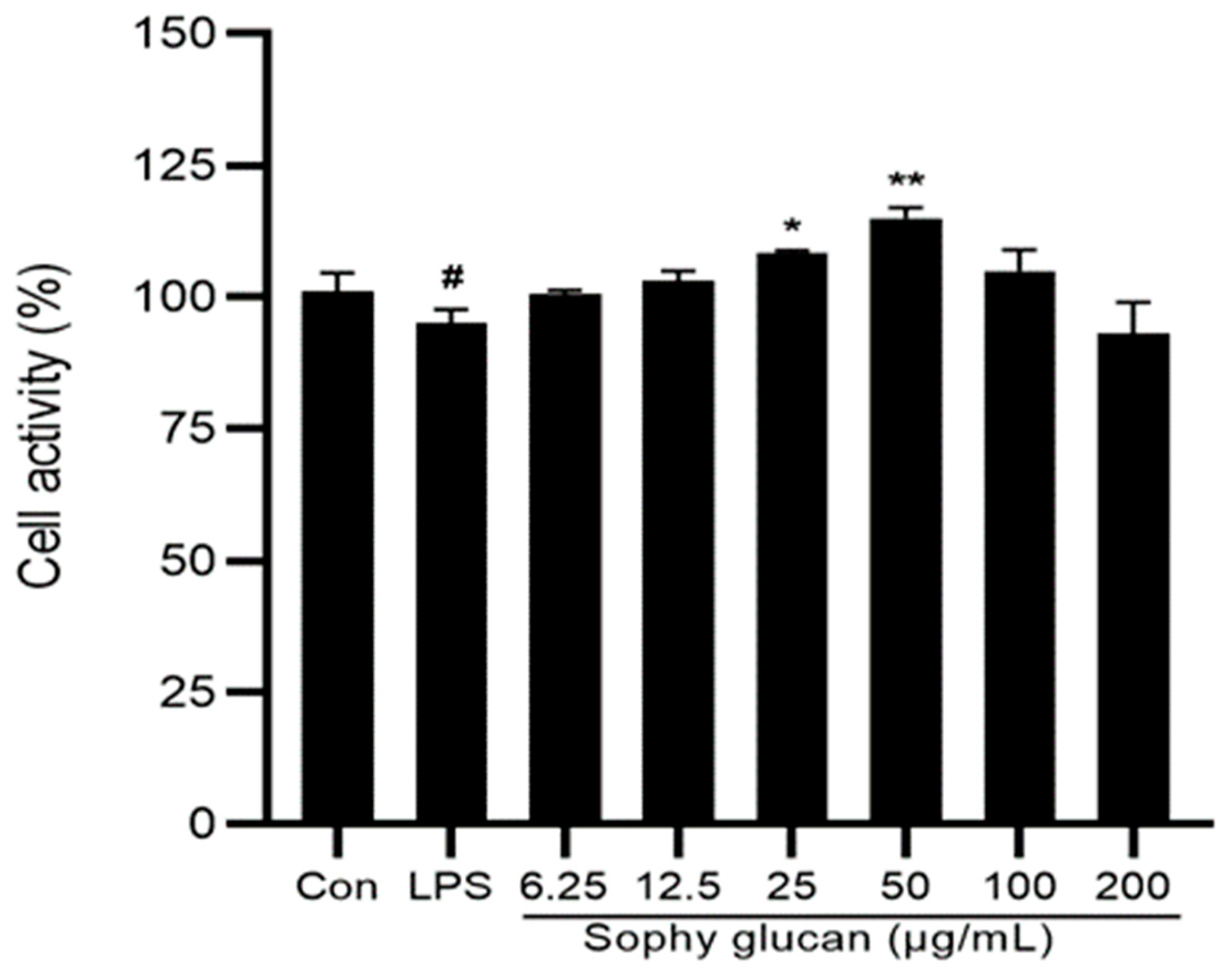
3.1. Effect of Sophy β-Glucan on the Cell Viability of Caco-2 Cells
3.2. Sophy β-Glucan Reduced the Adhesion and Invasion of SE into the Caco-2 Cell Monolayer
3.3. Sophy β-Glucan Ameliorates the Intestinal Epithelial Barrier Dysfunction Induced by SE
3.4. Sophy β-Glucan Prevents SE-Induced Disruption of TJ in Caco-2 Cell Monolayer
3.5. Sophy β-Glucan Suppressed the SE-Induced Pro-Inflammatory Status of the Caco-2 Cell
3.6. Sophy β-Glucan Enhanced Anti-Oxidative Functions in SE-Stimulated Caco-2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal barrier in human health and disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef] [PubMed]
- Artis, D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 2008, 8, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhang, Z.; Hang, X.; Jiang, Y. L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol. 2009, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Eng, S.-K.; Pusparajah, P.; Ab Mutalib, N.-S.; Ser, H.-L.; Chan, K.-G.; Lee, L.-H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. Salmonella control in poultry flocks and its public health impact. EFSA J. 2019, 17, e05596. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Im, J.; Lee, J.-S.; Jeon, H.J.; Mogeni, O.D.; Kim, J.H.; Rakotozandrindrainy, R.; Baker, S.; Marks, F. The global burden and epidemiology of invasive non-typhoidal Salmonella infections. Hum. Vaccin. Immunother. 2019, 15, 1421–1426. [Google Scholar] [CrossRef]
- Rychlik, I.; Elsheimer-Matulova, M.; Kyrova, K. Gene expression in the chicken caecum in response to infections with non-typhoid Salmonella. Vet. Res. 2014, 45, 119. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Hess, C.; Hess, M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Mkangara, M. Prevention and control of human Salmonella enterica infections: An implication in food safety. Int. J. Food Sci. 2023, 2023, 8899596. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Human intestinal barrier: Effects of stressors, diet, prebiotics, and probiotics. Clin. Transl. Gastroenterol. 2021, 12, e00308. [Google Scholar] [CrossRef] [PubMed]
- Prasongsuk, S.; Lotrakul, P.; Ali, I.; Bankeeree, W.; Punnapayak, H. The current status of Aureobasidium pullulans in biotechnology. Folia Microbiol. 2018, 63, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Kondo, N.; Isono, T.; Ogata, M.; Hirabayashi, K. Characterization of the secondary structure and order-disorder transition of a β-(1 → 3, 1 → 6)-glucan from Aureobasidium pullulans. Int. J. Biol. Macromol. 2020, 154, 1382–1391. [Google Scholar] [CrossRef]
- Kono, H.; Kondo, N.; Hirabayashi, K.; Ogata, M.; Totani, K.; Ikematsu, S.; Osada, M. NMR spectroscopic structural characterization of a water-soluble β-(1→3, 1→6)-glucan from Aureobasidium pullulans. Carbohydr. Polym. 2017, 174, 876–886. [Google Scholar] [CrossRef]
- Muramatsu, D.; Okabe, M.; Takaoka, A.; Kida, H.; Iwai, A. Aureobasidium pullulans produced β-glucan is effective to enhance Kurosengoku soybean extract induced Thrombospondin-1 expression. Sci. Rep. 2017, 7, 2831. [Google Scholar] [CrossRef]
- No, H.; Kim, J.; Seo, C.-R.; Lee, D.E.; Kim, J.H.; Kuge, T.; Mori, T.; Kimoto, H.; Kim, J.-K. Anti-inflammatory effects of β-1,3-1,6-glucan derived from black yeast Aureobasidium pullulans in RAW264.7 cells. Int. J. Biol. Macromol. 2021, 193, 592–600. [Google Scholar] [CrossRef]
- Muramatsu, D.; Iwai, A.; Aoki, S.; Uchiyama, H.; Kawata, K.; Nakayama, Y.; Nikawa, Y.; Kusano, K.; Okabe, M.; Miyazaki, T. β-Glucan derived from Aureobasidium pullulans is effective for the prevention of influenza in mice. PLoS ONE 2012, 7, e41399. [Google Scholar] [CrossRef]
- Suzuki, T.; Kusano, K.; Kondo, N.; Nishikawa, K.; Kuge, T.; Ohno, N. Biological activity of high-purity β-1,3-1,6-glucan derived from the black yeast Aureobasidium pullulans: A literature review. Nutrients 2021, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Xia, Q.; Liu, L.; Wu, Z.; Pan, D. Recent advances of cereal β-glucan on immunity with gut microbiota regulation functions and its intelligent gelling application. Crit. Rev. Food Sci. Nutr. 2021, 63, 3895–3911. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; de Vos, W.M.; Satokari, R. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef] [PubMed]
- Thanou, M.M.; Kotzé, A.F.; Scharringhausen, T.; Lueßen, H.L.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal Caco-2 cell monolayers. J. Control. Release 2000, 64, 15–25. [Google Scholar] [CrossRef]
- Duizer, E.; Penninks, A.H.; Stenhuis, W.H.; Groten, J.P. Comparison of permeability characteristics of the human colonic Caco-2 and rat small intestinal IEC-18 cell lines. J. Control. Release 1997, 49, 39–49. [Google Scholar] [CrossRef]
- Ménard, S.; Lacroix-Lamandé, S.; Ehrhardt, K.; Yan, J.; Grassl, G.; Wiedemann, A. Cross-talk between the intestinal epithelium and Salmonella Typhimurium. Front. Microbiol. 2022, 13, 906238. [Google Scholar] [CrossRef] [PubMed]
- Kuda, T.; Kosaka, M.; Hirano, S.; Kawahara, M.; Sato, M.; Kaneshima, T.; Nishizawa, M.; Takahashi, H.; Kimura, B. Effect of sodium-alginate and laminaran on Salmonella Typhimurium infection in human enterocyte-like HT-29-Luc cells and BALB/c mice. Carbohydr. Polym. 2015, 125, 113–119. [Google Scholar] [CrossRef]
- Shao, Y.; Guo, Y.; Wang, Z. β-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poult. Sci. 2013, 92, 1764–1773. [Google Scholar] [CrossRef]
- Martel, J.; Chang, S.H.; Ko, Y.F.; Hwang, T.L.; Young, J.D.; Ojcius, D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. 2022, 33, 247–265. [Google Scholar] [CrossRef]
- Nie, Y.; Cao, M.; Wu, D.; Li, N.; Peng, J.; Yi, S.; Yang, X.; Zhang, M.; Hu, G.; Zhao, J. KH-type splicing regulatory protein is regulated by nuclear factor-κB signaling to mediate innate immunity in Caco-2 cells infected by Salmonella Enteritidis. Folia Microbiol. 2018, 63, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Lépine, A.F.P.; de Wit, N.; Oosterink, E.; Wichers, H.; Mes, J.; de Vos, P. Lactobacillus acidophilus Attenuates Salmonella-induced stress of epithelial cells by modulating tight-junction genes and cytokine responses. Front. Microbiol. 2018, 9, 1439. [Google Scholar] [CrossRef] [PubMed]
- Malago, J.J.; Koninkx, J.F.J.G.; Tooten, P.C.J.; Van Liere, E.A.; Van Dijk, J.E. Anti-inflammatory properties of heat shock protein 70 and butyrate on Salmonella-induced interleukin-8 secretion in enterocyte-like Caco-2 cells. Clin. Exp. Immunol. 2005, 141, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Malago, J.J.; Tooten, P.C.; Koninkx, J.F. Anti-inflammatory properties of probiotic bacteria on Salmonella-induced IL-8 synthesis in enterocyte-like Caco-2 cells. Benef. Microbes 2010, 1, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, Y.; Zou, S.; Duan, B.; Sun, M.; Xu, X. Yeast β-glucan suppresses the chronic inflammation and improves the microenvironment in adipose tissues of ob/ob Mice. J. Agric. Food Chem. 2018, 66, 621–629. [Google Scholar] [CrossRef]
- Walachowski, S.; Tabouret, G.; Fabre, M.; Foucras, G. Molecular analysis of a short-term model of β-glucans-trained immunity highlights the accessory contribution of GM-CSF in priming mouse macrophages response. Front. Immunol. 2017, 8, 1089. [Google Scholar] [CrossRef]
- Kawata, K.; Iwai, A.; Muramatsu, D.; Aoki, S.; Uchiyama, H.; Okabe, M.; Hayakawa, S.; Takaoka, A.; Miyazaki, T. Stimulation of macrophages with the β-glucan produced by Aureobasidium pullulans promotes the secretion of tumor necrosis factor-related apoptosis inducing ligand (TRAIL). PLoS ONE 2015, 10, e0124809. [Google Scholar] [CrossRef]
- Tada, R.; Yoshikawa, M.; Kuge, T.; Tanioka, A.; Ishibashi, K.; Adachi, Y.; Tsubaki, K.; Ohno, N. A highly branched 1,3-beta-D-glucan extracted from Aureobasidium pullulans induces cytokine production in DBA/2 mouse-derived splenocytes. Int. Immunopharmacol. 2009, 9, 1431–1436. [Google Scholar] [CrossRef]
- Tada, R.; Yoshikawa, M.; Ikeda, F.; Adachi, Y.; Kato, Y.; Kuge, T.; Tanioka, A.; Ishibashi, K.-I.; Tsubaki, K.; Ohno, N. Induction of IFN-γ by a highly branched 1,3-β-d-glucan from Aureobasidium pullulans in mouse-derived splenocytes via dectin-1-independent pathways. Biochem. Biophys. Res. Commun. 2011, 404, 1105–1110. [Google Scholar] [CrossRef]
- Tada, R.; Yoshikawa, M.; Kuge, T.; Tanioka, A.; Ishibashi, K.; Adachi, Y.; Tsubaki, K.; Ohno, N. Granulocyte macrophage colony-stimulating factor is required for cytokine induction by a highly 6-branched 1,3-β-D-glucan from Aureobasidium pullulans in mouse-derived splenocytes. Immunopharmacol. Immunotoxicol. 2011, 33, 302–308. [Google Scholar] [CrossRef]
- Raupach, B.; Peuschel, K.; Monack, A. Caspase-1-mediated activation of interleukin-1beta (IL-1beta) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2006, 74, 4922–4926. [Google Scholar] [CrossRef] [PubMed]
- Billiet, T.; Rutgeerts, P.; Ferrante, M.; Van, S. Targeting TNF-α for the treatment of inflammatory bowel disease. Expert. Opin. Biol. Ther. 2014, 14, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Breese, J.; Michie, A.; Nicholls, W.; Murch, H.; Williams, B.; Domizio, P.; Walker, A.; Macdonald, T. Tumor necrosis factor α-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology 1994, 106, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, K.; Fujino, M.; Kusano, K.; Yi, S.-Q.; Iwai, A.; Li, X.-K. The effects of oral administration of Aureobasidium pullulans-cultured fluid containing β-glucan on concanavalin A injected mice. Heliyon 2021, 7, e07277. [Google Scholar] [CrossRef]
- Hayashi, N.; Shoubayashi, Y.; Kondo, N.; Fukudome, K. Hydrothermal processing of β-glucan from Aureobasidium pullulans produces a low molecular weight reagent that regulates inflammatory responses induced by TLR ligands. Biochem. Biophs. Res. Commun. 2019, 511, 318–322. [Google Scholar] [CrossRef]
- Elmaksoud, H.A.A.; Motawea, M.H.; Desoky, A.A.; Elharrif, M.G.; Ibrahimi, A. Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed. Pharmacother. 2021, 142, 112073. [Google Scholar] [CrossRef]
- Rao, R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. FBL 2008, 13, 7210–7226. [Google Scholar] [CrossRef]
- Ookawara, T.; Imazeki, N.; Matsubara, O.; Kizaki, T.; Oh-Ishi, S.; Nakao, C.; Sato, Y.; Ohno, H. Tissue distribution of immunoreactive mouse extracellular superoxide dismutase. Am. J. Physiol. 1998, 275, C840–C847. [Google Scholar] [CrossRef]
- Sottero, B.; Rossin, D.; Poli, G.; Biasi, F. Lipid oxidation products in the pathogenesis of inflammation-related gut diseases. Curr. Med. Chem. 2018, 25, 1311–1326. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, Y.; Tan, C.; Bai, J.; Zhu, Y.; Zhang, J.; Zhou, X.; Zhao, Y. Barley β-glucan resist oxidative stress of Caenorhabditis elegans via daf-2/daf-16 pathway. Int. J. Biol. Macromol. 2021, 193, 1021–1031. [Google Scholar] [CrossRef]


| Target Genes | Primer Sequence (5′~3′) | Accession NO. |
|---|---|---|
| GAPDH | F: GCACCGTCAAGGCTGAGAAC | NM_002046.7 |
| R: ATGGTGGTGAAGACGCCAGT | ||
| TNF-α | F: TAGCCCATGTTGTAGCAAACC | NM_000594.3 |
| R: ATGAGGTACAGGCCCTCTGAT | ||
| IL-1β | F: AGTGGCAATGAGGATGACTTGT | NM_000576.2 |
| R: AGATGAAGGGAAAGAAGGTGCT | ||
| IL-8 | F: TTGCCAAGGAGTGCTAAAGAA | NM_000584.3 |
| R: GCCCTCTTCAAAAACTTCTCC | ||
| IL-10 | F: TTTAAGGGTTACCTGGGTTGC | NM_000572.2 |
| R: TTGATGTCTGGGTCTTGGTTC | ||
| TGF-β | F: GCCAGAGTGGTTATCTTTTGATG | NM_00060.4 |
| R: AGTGTGTTATCCCTGCTGTCAC | ||
| Claudin-1 | F: AGAAGATGAGGATGGCTGTCA | NM_021101.4 |
| R: TTGGTGTTGGGTAAGAGGTTG | ||
| Claudin-2 | F: TTCTTCCCTGTTCTCCCTGAT | NM_020384.3 |
| R: CCCCTGGTTCTTCACACATAC | ||
| Claudin-4 | F: TATGGATGAACTGCGTGGTG | NM_001305.3 |
| R: CACGATGAACTGCGTGGTG | ||
| Occludin | F: CCTTCACCCCCATCTGACTAT | NM_002538 |
| R: CTTTGACCTTCCTGCTCTTCC | ||
| Zo-1 | F: GGATGTTTATCGTCGCATTGTA | NM_003257.3 |
| R: AAGAGCCCAGTTTTCCATTGTA |
| Items | Sophy β-Glucan Levels, μg/mL | SEM | p-Values | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 50 | |||||||
| Saline | SE | Saline | SE | β-glucan | SE | Interaction | ||
| Tight junction protein genes expression | ||||||||
| Claudin-1 | 1.00 b | 1.49 a | 1.00 b | 1.00 b | 0.023 | 0.017 | 0.012 | 0.001 |
| Claudin-4 | 1.00 c | 2.19 a | 1.24 b | 1.10 bc | 0.052 | 0.001 | 0.001 | 0.001 |
| Occludin | 1.00 cd | 1.01 bc | 1.55 a | 0.74 d | 0.056 | 0.030 | 0.001 | 0.001 |
| Zo-1 | 1.00 d | 1.29 bc | 1.48 a | 1.23 cd | 0.049 | 0.670 | 0.579 | 0.001 |
| Pro-inflammatory-related genes expressions | ||||||||
| IL-1β | 1.00 c | 5.62 a | 3.60 b | 1.08 c | 0.860 | 0.001 | 0.001 | 0.010 |
| IL-8 | 1.00 c | 7.08 a | 1.24 c | 4.53 b | 0.079 | 0.001 | 0.001 | 0.001 |
| TNF-α | 1.00 c | 6.93 a | 1.44 c | 4.88 b | 0.243 | 0.005 | 0.001 | 0.001 |
| Anti-inflammatory-related genes | ||||||||
| IL-10 | 1.00 c | 3.48 b | 3.67 b | 9.84 a | 0.244 | 0.001 | 0.001 | 0.001 |
| TGF-β | 1.00 ab | 0.67 cd | 0.85 bc | 1.08 a | 0.073 | 0.106 | 0.513 | 0.001 |
| Pro-inflammatory cytokines levels | ||||||||
| IL-1β (pg/mL) | 8.56 d | 62.8 b | 45.5 c | 82.4 a | 2.48 | 0.001 | <0.001 | 0.001 |
| IL-8 (pg/mL) | 36.8 d | 259.6 b | 98.1 c | 339.6 a | 10.6 | <0.001 | <0.001 | 0.001 |
| TNF-α (pg/mL) | 32.7 c | 114.5 a | 49.6 c | 72.9 b | 11.5 | 0.002 | <0.001 | 0.004 |
| Anti-inflammatory-cytokines level | ||||||||
| IL-10 (pg/mL) | 45.3 b | 23.5 c | 106.9 a | 148.3 a | 4.54 | <0.001 | 0.033 | 0.002 |
| Items | Sophy β-Glucan Levels, μg/mL | SEM | p-Values | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 50 | |||||||
| Saline | SE | Saline | SE | β-Glucan | SE | Interaction | ||
| Cell culture supernatant 3 h post-infection | ||||||||
| MDA (nmol/mL) | 0.53 | 0.45 | 0.45 | 0.31 | 0.025 | 0.002 | 0.002 | 0.256 |
| T-AOC (U/mL) | 0.44 c | 0.94 b | 1.42 a | 1.36 a | 0.122 | 0.001 | 0.019 | 0.006 |
| SOD (U/mL) | 28.51 | 44.26 | 32.86 | 47.47 | 2.366 | 0.001 | 0.001 | 0.189 |
| GSH (U/mL) | 17.21 b | 25.42 a | 12.12 c | 24.38 a | 1.661 | 0.002 | 0.001 | 0.017 |
| 12 h post-infection | ||||||||
| MDA (nmol/mL) | 0.37 b | 0.61 a | 0.39 b | 0.35 b | 0.030 | 0.011 | 0.004 | 0.001 |
| T-AOC (U/mL) | 0.67 c | 1.14 b | 0.69 c | 1.95 a | 0.157 | 0.001 | 0.001 | 0.001 |
| SOD (U/mL) | 21.85 | 39.77 | 33.66 | 47.50 | 2.717 | 0.006 | 0.001 | 0.927 |
| GSH (U/mL) | 17.50 b | 20.33 a | 11.08 c | 21.08 a | 1.194 | 0.001 | 0.001 | 0.001 |
| Cell lysates 3 h post-infection | ||||||||
| SOD (U/mgpro) | 47.69 b | 43.82 c | 48.31 a | 46.51 b | 0.276 | 0.364 | 0.001 | 0.022 |
| GSH (U/mg) | 9.57 | 7.59 | 9.67 | 7.22 | 0.368 | 0.694 | 0.001 | 0.515 |
| 12 h post-infection | ||||||||
| SOD (U/mg) | 42.86 | 26.23 | 45.49 | 29.93 | 2.518 | 0.001 | 0.001 | 0.927 |
| GSH (U/mg) | 9.20 cd | 9.48 bc | 10.61 ab | 8.16 d | 0.307 | 0.901 | 0.019 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, F.; Liu, H.; Li, X.; Hu, Z.; Huang, J.; Bi, R.; Abbas, W.; Guo, Y.; Wang, Z. Sophy β-Glucan from the Black Yeast Aureobasidium pullulans Attenuates Salmonella-Induced Intestinal Epithelial Barrier Injury in Caco-2 Cell Monolayers via Exerting Anti-Oxidant and Anti-Inflammatory Properties. Antioxidants 2024, 13, 48. https://doi.org/10.3390/antiox13010048
Guo F, Liu H, Li X, Hu Z, Huang J, Bi R, Abbas W, Guo Y, Wang Z. Sophy β-Glucan from the Black Yeast Aureobasidium pullulans Attenuates Salmonella-Induced Intestinal Epithelial Barrier Injury in Caco-2 Cell Monolayers via Exerting Anti-Oxidant and Anti-Inflammatory Properties. Antioxidants. 2024; 13(1):48. https://doi.org/10.3390/antiox13010048
Chicago/Turabian StyleGuo, Fangshen, Hongbin Liu, Xiaomin Li, Zeqiong Hu, Jia Huang, Ruichen Bi, Waseem Abbas, Yuming Guo, and Zhong Wang. 2024. "Sophy β-Glucan from the Black Yeast Aureobasidium pullulans Attenuates Salmonella-Induced Intestinal Epithelial Barrier Injury in Caco-2 Cell Monolayers via Exerting Anti-Oxidant and Anti-Inflammatory Properties" Antioxidants 13, no. 1: 48. https://doi.org/10.3390/antiox13010048
APA StyleGuo, F., Liu, H., Li, X., Hu, Z., Huang, J., Bi, R., Abbas, W., Guo, Y., & Wang, Z. (2024). Sophy β-Glucan from the Black Yeast Aureobasidium pullulans Attenuates Salmonella-Induced Intestinal Epithelial Barrier Injury in Caco-2 Cell Monolayers via Exerting Anti-Oxidant and Anti-Inflammatory Properties. Antioxidants, 13(1), 48. https://doi.org/10.3390/antiox13010048





