Identification and Validation of PTGS2 Gene as an Oxidative Stress-Related Biomarker for Arteriovenous Fistula Failure
Abstract
:1. Background
2. Methods
2.1. Data Source
2.2. Identification of Oxidative Stress-Related Differentially Expressed Genes (OSDEGs)
2.3. Functional Enrichment Analysis of OSDEGs
2.4. Construction of a Protein–Protein Interaction (PPI) Network
2.5. Transcription Factor (TF) and miRNA Regulatory Network Analysis of OSDEGs
2.6. Construction of Weighted Gene Co-Expression Network Analysis (WGCNA)
2.7. Machine Learning
2.8. Human Tissue Collection
2.9. Animal Study
2.10. Doppler Ultrasound
2.11. Serum Creatinine and Blood Urea
2.12. Morphometric and Immunohistochemical Analysis
2.13. Statistical Analyses
3. Results
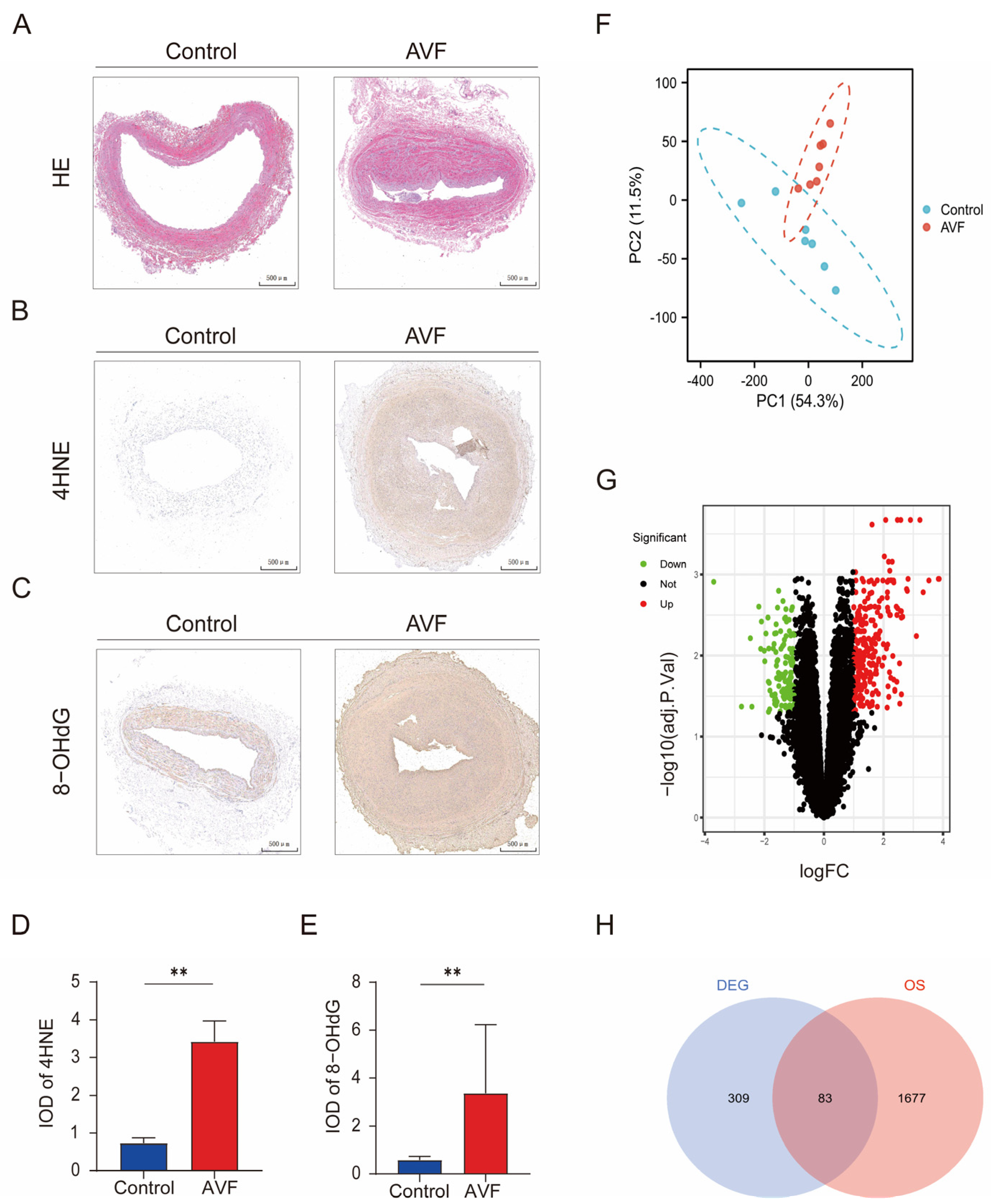
3.1. Oxidative Stress Is Elevated in AVF Failure Patients and Identification of OSDEGs
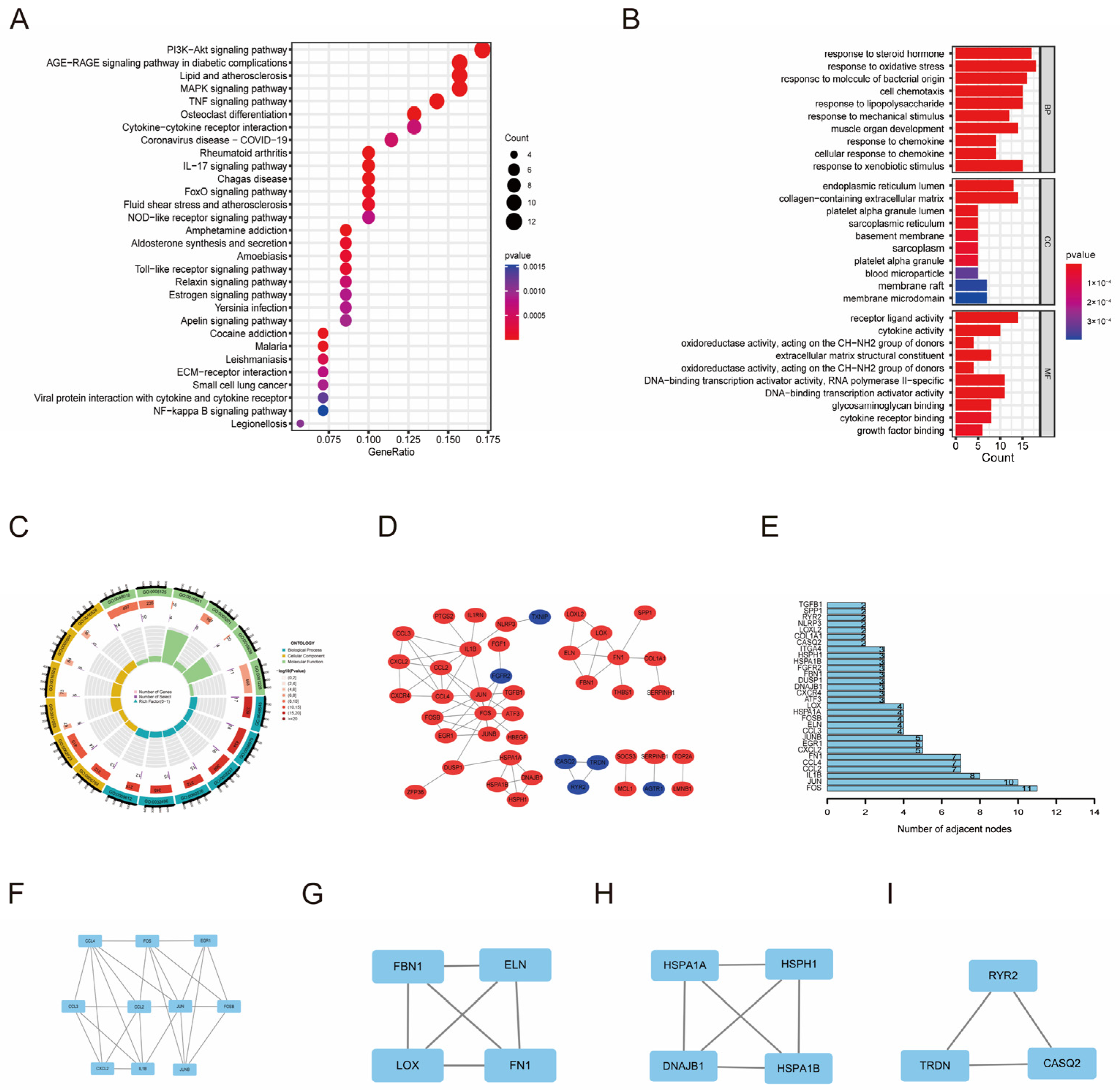
3.2. Functional Enrichment Analysis and Regulatory Network of OSDEGs
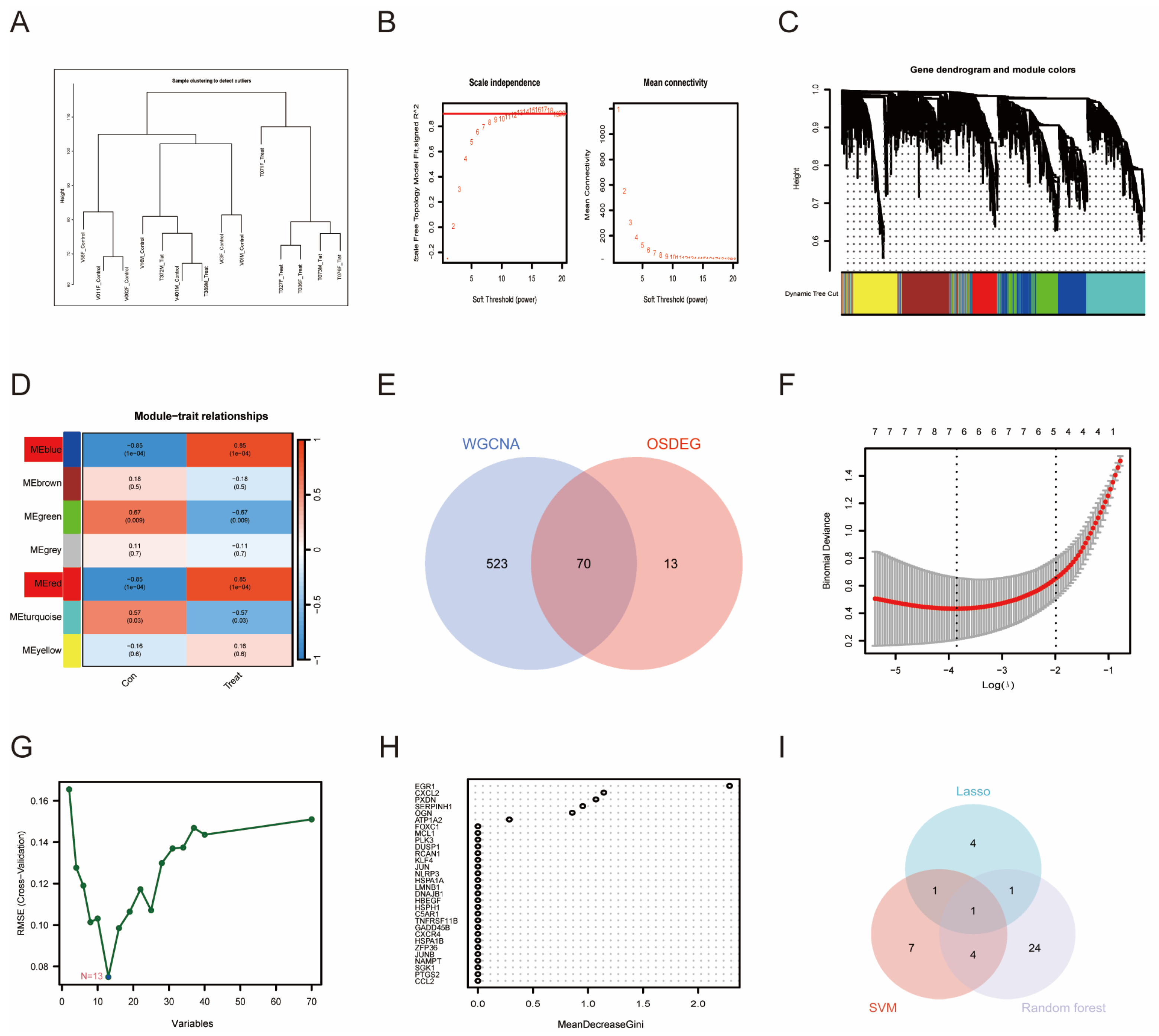
3.3. WGCNA and Machine Learning-Identified PTGS2 as an Essential Biomarker
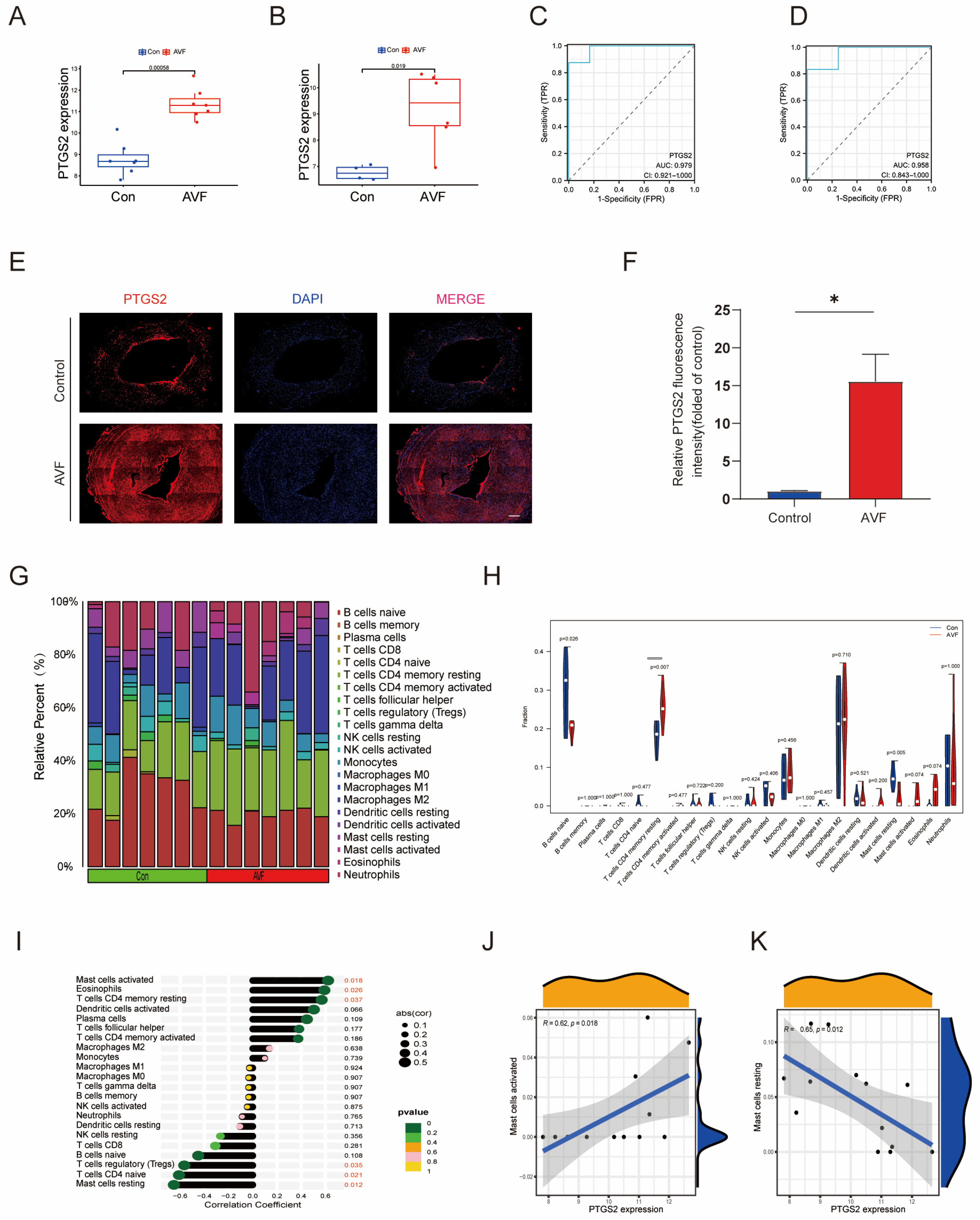
3.4. Validation of PTGS2 and Immune Infiltration Analysis
3.5. PTGS2 Inhibition Alleviates Lumen Stenosis in the AVF Mouse Model
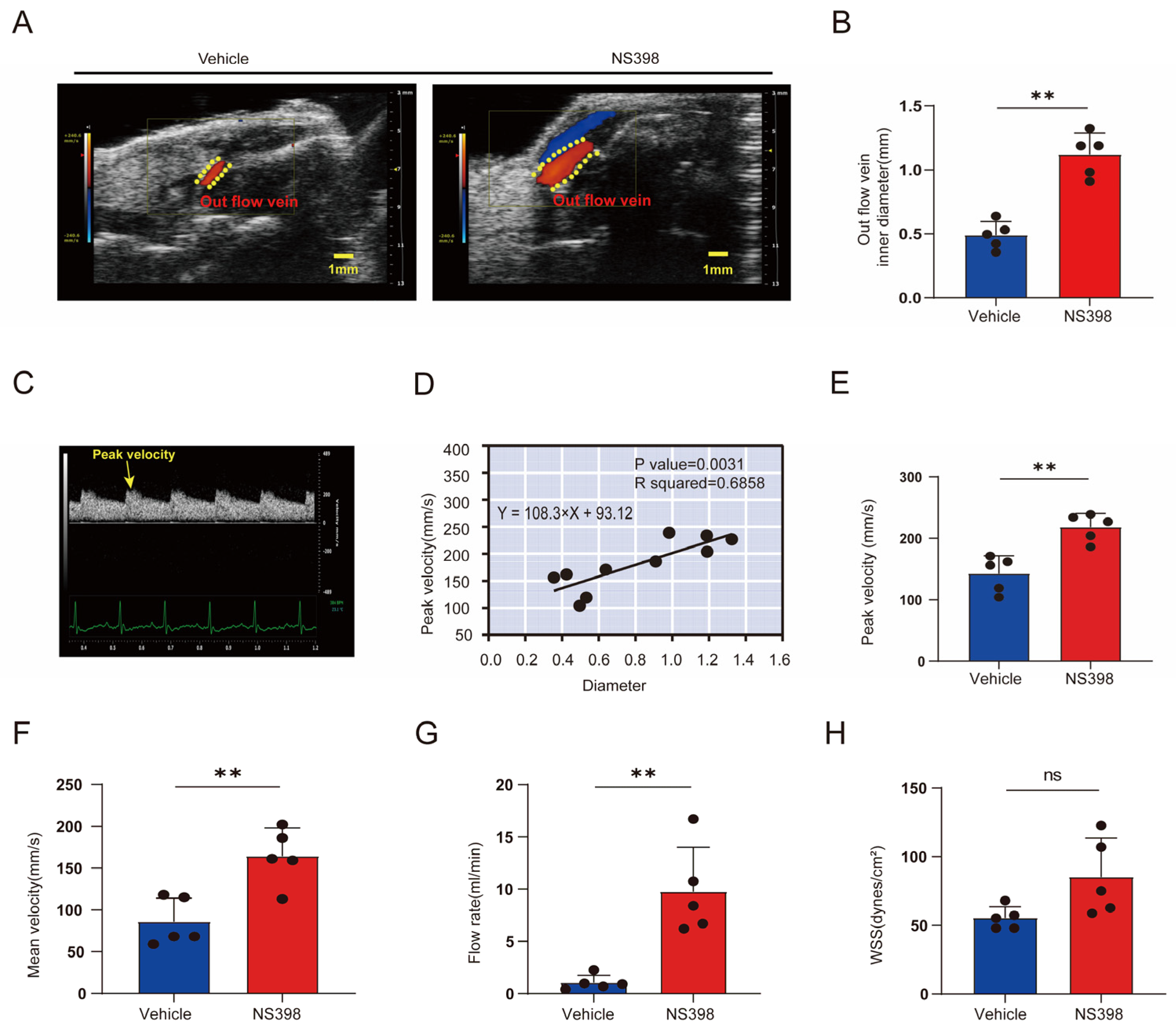
3.6. Hemodynamic Changes after NS398 Administration Compared with Controls
3.7. The Administration of NS398 Can Attenuate the Proliferation and Migration of Smooth Muscle Cells in AVF
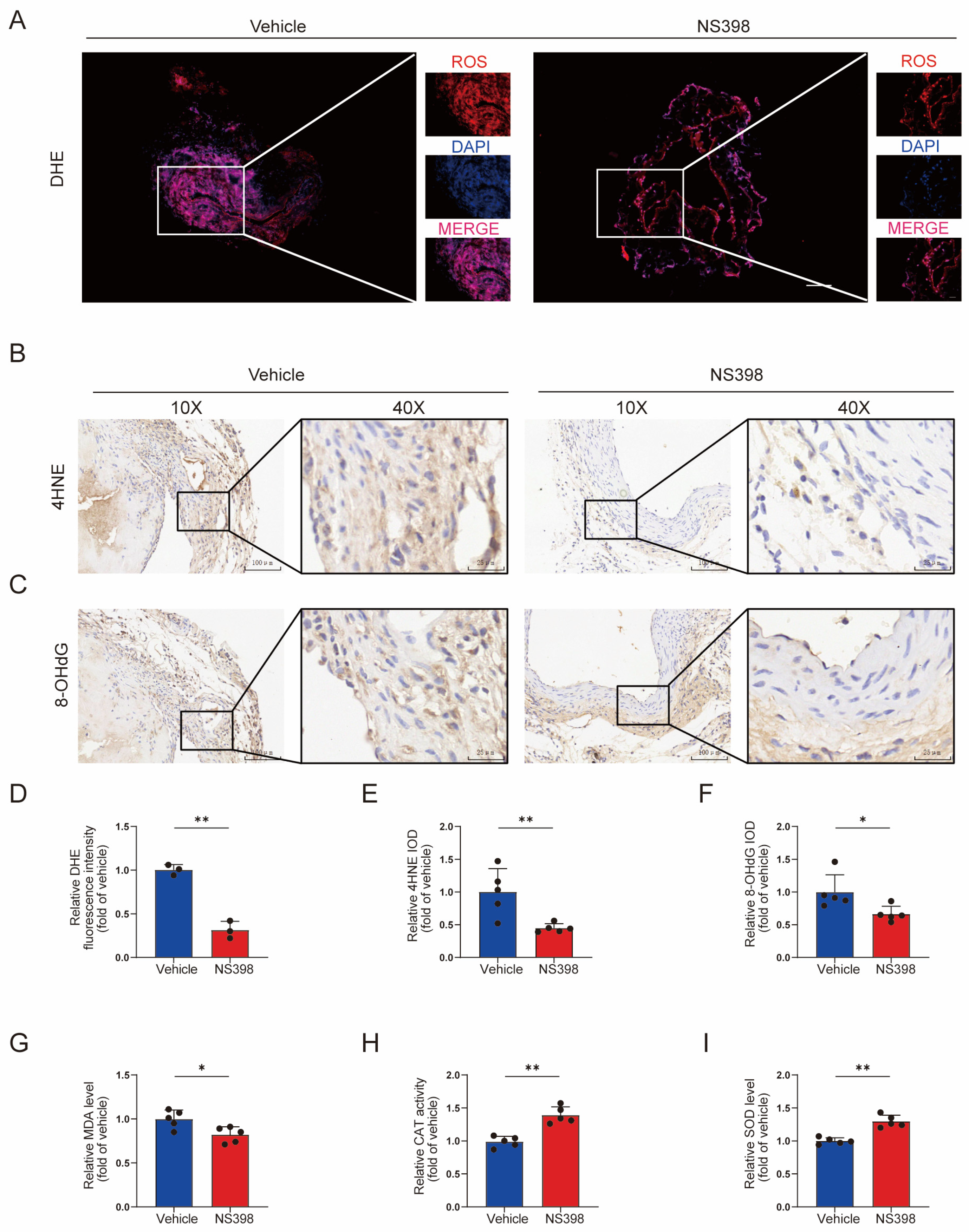
3.8. NS398 Can Reduce Oxidative Stress in AVF
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Agarwal, A.K.; Haddad, N.J.; Vachharajani, T.J.; Asif, A. Innovations in vascular access for hemodialysis. Kidney Int. 2019, 95, 1053–1063. [Google Scholar] [CrossRef]
- Manns, B.; Tonelli, M.; Yilmaz, S.; Lee, H.; Laupland, K.; Klarenbach, S.; Radkevich, V.; Murphy, B. Establishment and maintenance of vascular access in incident hemodialysis patients: A prospective cost analysis. J. Am. Soc. Nephrol. 2005, 16, 201–209. [Google Scholar] [CrossRef]
- Santoro, D.; Benedetto, F.; Mondello, P.; Pipitò, N.; Barillà, D.; Spinelli, F.; Ricciardi, C.A.; Cernaro, V.; Buemi, M. Vascular access for hemodialysis: Current perspectives. Int. J. Nephrol. Renov. Dis. 2014, 7, 281–294. [Google Scholar] [CrossRef]
- Dember, L.M.; Beck, G.J.; Allon, M.; Delmez, J.A.; Dixon, B.S.; Greenberg, A.; Himmelfarb, J.; Vazquez, M.A.; Gassman, J.J.; Greene, T.; et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 2008, 299, 2164–2171. [Google Scholar] [CrossRef]
- Weiss, M.F.; Scivittaro, V.; Anderson, J.M. Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2001, 37, 970–980. [Google Scholar] [CrossRef]
- Sharma, K. Obesity, oxidative stress, and fibrosis in chronic kidney disease. Kidney Int. Suppl. 2014, 4, 113–117. [Google Scholar] [CrossRef]
- Hu, K.; Guo, Y.; Li, Y.; Lu, C.; Cai, C.; Zhou, S.; Ke, Z.; Li, Y.; Wang, W. Oxidative stress: An essential factor in the process of arteriovenous fistula failure. Front. Cardiovasc. Med. 2022, 9, 984472. [Google Scholar] [CrossRef]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Chen, W.; Zhong, Y.; Feng, N.; Guo, Z.; Wang, S.; Xing, D. New horizons in the roles and associations of COX-2 and novel natural inhibitors in cardiovascular diseases. Mol. Med. (Camb. Mass.) 2021, 27, 123. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Li, M.; Xu, J.; Howell, D.C.; Li, Z.; Chen, F.E. Recent development on COX-2 inhibitors as promising anti-inflammatory agents: The past 10 years. Acta Pharm. Sin. B 2022, 12, 2790–2807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, F.; Tang, J.; Zhang, Q.; Gong, Y.; Wang, Q.; Shen, Y.; Xiong, L.; Breyer, R.M.; Lazarus, M.; et al. Cyclooxygenase-2-derived prostaglandin E2 promotes injury-induced vascular neointimal hyperplasia through the E-prostanoid 3 receptor. Circ. Res. 2013, 113, 104–114. [Google Scholar] [CrossRef]
- Choi, H.C.; Kim, H.S.; Lee, K.Y.; Chang, K.C.; Kang, Y.J. NS-398, a selective COX-2 inhibitor, inhibits proliferation of IL-1beta-stimulated vascular smooth muscle cells by induction of HO-1. Biochem. Biophys. Res. Commun. 2008, 376, 753–757. [Google Scholar] [CrossRef]
- Roan, J.N.; Tsai, Y.C.; Chen, I.W.; Chang, S.W.; Huang, C.C.; Lam, C.F. Inhibition of cyclooxygenase-2 modulates phenotypic switching of vascular smooth muscle cells during increased aortic blood flow. Heart Vessel. 2012, 27, 307–315. [Google Scholar] [CrossRef]
- Weng, S.X.; Sui, M.H.; Chen, S.; Wang, J.A.; Xu, G.; Ma, J.; Shan, J.; Fang, L. Parthenolide inhibits proliferation of vascular smooth muscle cells through induction of G0/G1 phase cell cycle arrest. J. Zhejiang Univ. Sci. B 2009, 10, 528–535. [Google Scholar] [CrossRef]
- Aguado, A.; Galán, M.; Zhenyukh, O.; Wiggers, G.A.; Roque, F.R.; Redondo, S.; Peçanha, F.; Martín, A.; Fortuño, A.; Cachofeiro, V.; et al. Mercury induces proliferation and reduces cell size in vascular smooth muscle cells through MAPK, oxidative stress and cyclooxygenase-2 pathways. Toxicol. Appl. Pharmacol. 2013, 268, 188–200. [Google Scholar] [CrossRef]
- Martinez, L.; Rojas, M.G.; Tabbara, M.; Pereira-Simon, S.; Santos Falcon, N.; Rauf, M.A.; Challa, A.; Zigmond, Z.M.; Griswold, A.J.; Duque, J.C.; et al. The Transcriptomics of the Human Vein Transformation After Arteriovenous Fistula Anastomosis Uncovers Layer-Specific Remodeling and Hallmarks of Maturation Failure. Kidney Int. Rep. 2023, 8, 837–850. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Fornes, O.; Castro-Mondragon, J.A.; Khan, A.; van der Lee, R.; Zhang, X.; Richmond, P.A.; Modi, B.P.; Correard, S.; Gheorghe, M.; Baranašić, D.; et al. JASPAR 2020: Update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2020, 48, D87–D92. [Google Scholar] [CrossRef] [PubMed]
- Coutinho de Almeida, R.; Ramos, Y.F.M.; Mahfouz, A.; den Hollander, W.; Lakenberg, N.; Houtman, E.; van Hoolwerff, M.; Suchiman, H.E.D.; Rodríguez Ruiz, A.; Slagboom, P.E.; et al. RNA sequencing data integration reveals an miRNA interactome of osteoarthritis cartilage. Ann. Rheum. Dis. 2019, 78, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Horvath, S. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 2005, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, R.; Guo, X.; Zou, Y.; Chen, S.; Zhou, K.; Chen, Y.; Li, Y.; Gao, S.; Wu, Y. Identification of TYR, TYRP1, DCT and LARP7 as related biomarkers and immune infiltration characteristics of vitiligo via comprehensive strategies. Bioengineered 2021, 12, 2214–2227. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, C.; Zhang, Y.; Su, B.; Fan, M.; Wei, H. Selecting Feature Subsets Based on SVM-RFE and the Overlapping Ratio with Applications in Bioinformatics. Molecules 2017, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Alakwaa, F.M.; Chaudhary, K.; Garmire, L.X. Deep Learning Accurately Predicts Estrogen Receptor Status in Breast Cancer Metabolomics Data. J. Proteome Res. 2018, 17, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.H.; Luo, W.J.; Yan, Z.G.; Liu, W.P. FAM171B as a Novel Biomarker Mediates Tissue Immune Microenvironment in Pulmonary Arterial Hypertension. Mediat. Inflamm. 2022, 2022, 1878766. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhou, H.; Hu, P.; Pan, Y.; Wang, S.; Liu, L.; Liu, X. Identification and validation of immune and oxidative stress-related diagnostic markers for diabetic nephropathy by WGCNA and machine learning. Front. Immunol. 2023, 14, 1084531. [Google Scholar] [CrossRef]
- Yang, B.; Vohra, P.K.; Janardhanan, R.; Misra, K.D.; Misra, S. Expression of profibrotic genes in a murine remnant kidney model. J. Vasc. Interv. Radiol. 2011, 22, 1765–1772.e1. [Google Scholar] [CrossRef]
- Cai, C.; Kilari, S.; Zhao, C.; Simeon, M.L.; Misra, A.; Li, Y.; van Wijnen, A.J.; Mukhopadhyay, D.; Misra, S. Therapeutic Effect of Adipose Derived Mesenchymal Stem Cell Transplantation in Reducing Restenosis in a Murine Angioplasty Model. J. Am. Soc. Nephrol. 2020, 31, 1781–1795. [Google Scholar] [CrossRef]
- Kilari, S.; Cai, C.; Zhao, C.; Sharma, A.; Chernogubova, E.; Simeon, M.; Wu, C.C.; Song, H.L.; Maegdefessel, L.; Misra, S. The Role of MicroRNA-21 in Venous Neointimal Hyperplasia: Implications for Targeting miR-21 for VNH Treatment. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 1681–1693. [Google Scholar] [CrossRef]
- Janardhanan, R.; Yang, B.; Kilari, S.; Leof, E.B.; Mukhopadhyay, D.; Misra, S. The Role of Repeat Administration of Adventitial Delivery of Lentivirus-shRNA-Vegf-A in Arteriovenous Fistula to Prevent Venous Stenosis Formation. J. Vasc. Interv. Radiol. 2016, 27, 576–583. [Google Scholar] [CrossRef]
- Stachowicz, K.; Sowa-Kućma, M.; Pańczyszyn-Trzewik, P.; Misztak, P.; Marciniak, M.; Bobula, B.; Tokarski, K. Behavioral consequences of co-administration of MTEP and the COX-2 inhibitor NS398 in mice. Part 2. Neurosci. Lett. 2021, 741, 135435. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Yim, H.J.; Hwang, J.W.; Kim, M.J.; Lee, Y.S.; Jung, Y.K.; Yim, H.; Kim, B.H.; Park, H.C.; Seo, Y.S.; et al. Improved anti-fibrotic effects by combined treatments of simvastatin and NS-398 in experimental liver fibrosis models. Korean J. Intern. Med. 2022, 37, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Zamboli, P.; Fiorini, F.; D’Amelio, A.; Fatuzzo, P.; Granata, A. Color Doppler ultrasound and arteriovenous fistulas for hemodialysis. J. Ultrasound 2014, 17, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Tabbara, M.; Duque, J.C.; Selman, G.; Falcon, N.S.; Paez, A.; Griswold, A.J.; Ramos-Echazabal, G.; Hernandez, D.R.; Velazquez, O.C.; et al. Transcriptomics of Human Arteriovenous Fistula Failure: Genes Associated With Nonmaturation. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2019, 74, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ene-Iordache, B.; Remuzzi, A. Disturbed flow in radial-cephalic arteriovenous fistulae for haemodialysis: Low and oscillating shear stress locates the sites of stenosis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2012, 27, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Isaji, T.; Hu, H.; Yamamoto, K.; Bai, H.; Santana, J.M.; Kuo, A.; Kuwahara, G.; Foster, T.R.; Hanisch, J.J.; et al. Stimulation of Caveolin-1 Signaling Improves Arteriovenous Fistula Patency. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Yang, B.; Kilari, S.; Li, Y.; Zhao, C.; Sharma, A.; Misra, S. Evaluation of Venous Stenosis Angioplasty in a Murine Arteriovenous Fistula Model. J. Vasc. Interv. Radiol. 2019, 30, 1512–1521.e3. [Google Scholar] [CrossRef]
- Konner, K.; Nonnast-Daniel, B.; Ritz, E. The arteriovenous fistula. J. Am. Soc. Nephrol. 2003, 14, 1669–1680. [Google Scholar] [CrossRef]
- McNally, J.S.; Davis, M.E.; Giddens, D.P.; Saha, A.; Hwang, J.; Dikalov, S.; Jo, H.; Harrison, D.G. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2290–H2297. [Google Scholar] [CrossRef]
- Fang, S.Y.; Roan, J.N.; Lin, Y.; Hsu, C.H.; Chang, S.W.; Huang, C.C.; Tsai, Y.C.; Lam, C.F. Rosuvastatin suppresses the oxidative response in the venous limb of an arteriovenous fistula and enhances the fistula blood flow in diabetic rats. J. Vasc. Res. 2014, 51, 81–89. [Google Scholar] [CrossRef]
- Lee, H.S.; Yun, S.J.; Ha, J.M.; Jin, S.Y.; Ha, H.K.; Song, S.H.; Kim, C.D.; Bae, S.S. Prostaglandin D(2) stimulates phenotypic changes in vascular smooth muscle cells. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, K.; Guo, Y.; Li, Y.; Zhou, S.; Lu, C.; Cai, C.; Yang, H.; Li, Y.; Wang, W. Identification and Validation of PTGS2 Gene as an Oxidative Stress-Related Biomarker for Arteriovenous Fistula Failure. Antioxidants 2024, 13, 5. https://doi.org/10.3390/antiox13010005
Hu K, Guo Y, Li Y, Zhou S, Lu C, Cai C, Yang H, Li Y, Wang W. Identification and Validation of PTGS2 Gene as an Oxidative Stress-Related Biomarker for Arteriovenous Fistula Failure. Antioxidants. 2024; 13(1):5. https://doi.org/10.3390/antiox13010005
Chicago/Turabian StyleHu, Ke, Yi Guo, Yuxuan Li, Shunchang Zhou, Chanjun Lu, Chuanqi Cai, Hongjun Yang, Yiqing Li, and Weici Wang. 2024. "Identification and Validation of PTGS2 Gene as an Oxidative Stress-Related Biomarker for Arteriovenous Fistula Failure" Antioxidants 13, no. 1: 5. https://doi.org/10.3390/antiox13010005
APA StyleHu, K., Guo, Y., Li, Y., Zhou, S., Lu, C., Cai, C., Yang, H., Li, Y., & Wang, W. (2024). Identification and Validation of PTGS2 Gene as an Oxidative Stress-Related Biomarker for Arteriovenous Fistula Failure. Antioxidants, 13(1), 5. https://doi.org/10.3390/antiox13010005






