Assessing the Antioxidant Properties, In Vitro Cytotoxicity and Antitumoral Effects of Polyphenol-Rich Perilla leaves Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Extraction Processes
2.2. Total Flavonoids, Phenols, and Anthocyanins Quantification
2.2.1. Total Flavonoids Quantification
2.2.2. Total Phenol Quantification
2.2.3. Total Anthocyanin Quantification
2.2.4. UHPLC-PDA Quantification and Identification of Extracted Compounds
2.2.5. Chemicals and Materials
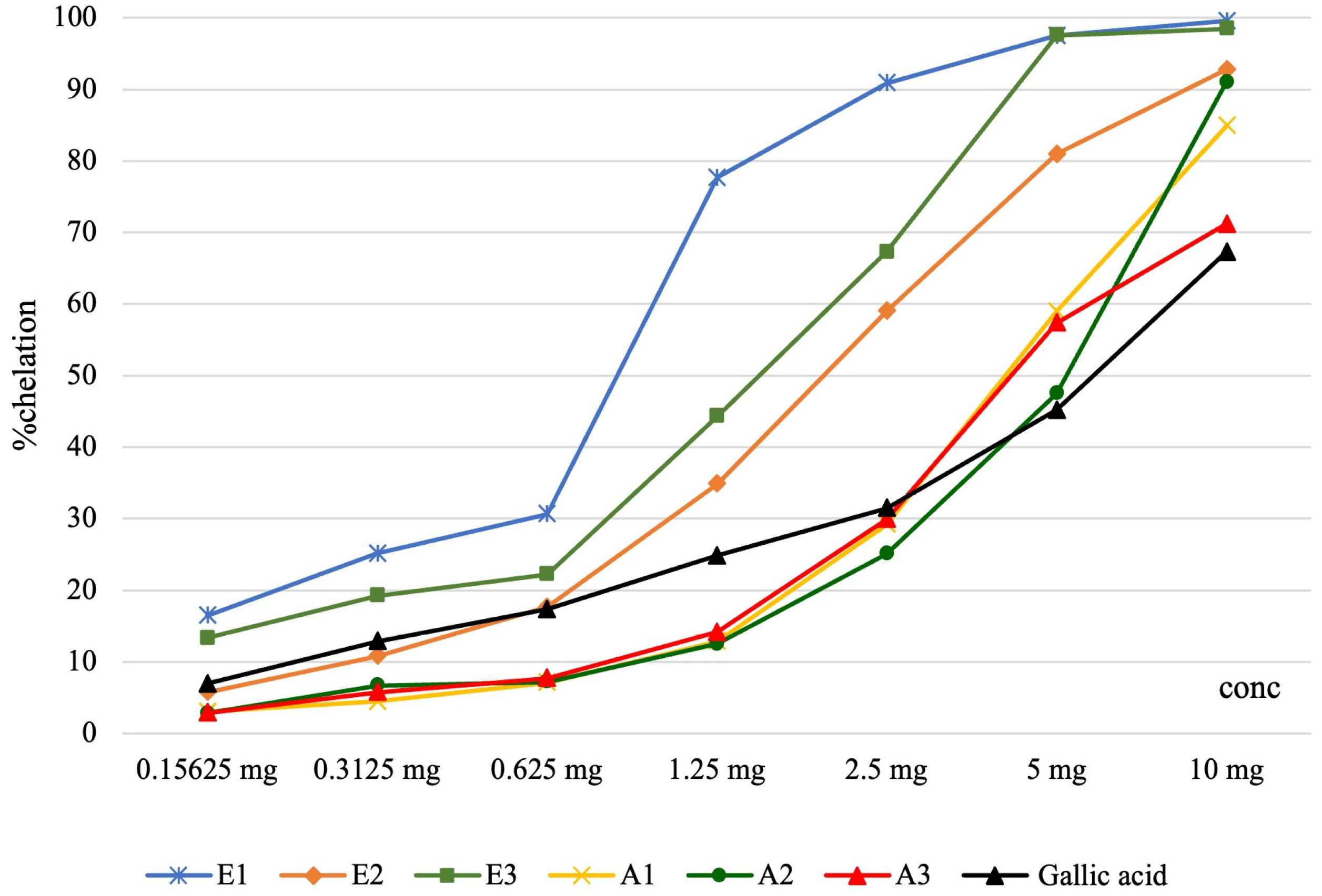
2.3. Iron Chelation Capacity Assessment
- -
- Dimethyl sulfoxide, DMSO (Merck, KgaA, Darmstadt, Germany);
- -
- 0.1 M Acetate Buffer, pH 5.25;
- -
- Iron (II) sulfate heptahydrate (Merck, KgaA, Darmstadt, Germany);
- -
- 2 mM Iron (II) sulfate in 0.2 M HCl;
- -
- Ferrozine (sodium salt of 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4′’ disulfonic acid) (Fluka, Sigma-Aldrich, Steinheim, Germany);
- -
- 5 mM Ferrozine Solution;
- -
- Hydrochloric Acid (Merck, Darmstadt, Germany);
- -
- UV-VIS Spectrophotometer ABL & E Jasco V-550 (Tokyo, Japan);
- -
- pH Meter Hanna Instruments pH210, Microprocessor pH-meter;
- -
- Vortex Mixer Velp Scientifica (Usmate Velate, Italy);
- -
- Ultra Clear TWF Water Purification System;
- -
- Test samples—solutions obtained by dissolving dried extracts in DMSO—hydroalcoholic extracts (E1, E2, E3), extracts in acetone: ethanol (7:3) acidified (A1, A2, A3); solution concentration for analysis: 0.078125–10 mg/mL.
- We mixed 0.2 mL of the test sample solution in ultrapure water, 0.74 mL of 0.1 M acetate buffer (pH 5.25), and 0.02 mL of 2 mM iron sulfate in 0.2 M hydrochloric acid. After 10–15 s of agitation, we added 0.04 mL of 5 mM ferrozine solution.
- After 10 min of incubation in the dark, we measured the absorbance of the solution at 562 nm against a control prepared under the same conditions as the sample (ultrapure water was used instead of iron sulfate solution).
- Simultaneously, we prepared the control solution and its control: the control contained 0.2 mL of ultrapure water, 0.74 mL of 0.1 M acetate buffer (pH 5.25), 0.02 mL of 2 mM iron sulfate in 0.2 M hydrochloric acid, and 0.04 mL of 5 mM ferrozine solution.
- Gallic acid was used as a reference substance, and gallic acid solutions in DMSO were processed under the same conditions as the ethanolic extract.
- All determinations were carried out in triplicate, and results were expressed as the mean of three determinations ± standard deviation.
2.4. Determination of Hydroxyl Radical Scavenging Capacity
- -
- Dimethyl sulfoxide (Merck, KgaA, Darmstadt, Germany);
- -
- Ferrous sulfate heptahydrate (Merck, KgaA, Darmstadt, Germany);
- -
- 1.5 mM ferrous sulfate solution in distilled water;
- -
- 6 mM hydrogen peroxide solution in distilled water (Sigma-Aldrich, Steinheim, Germany);
- -
- 20 mM sodium salicylate solution in distilled water;
- -
- Hanna Instruments pH210 Microprocessor pH-meter (Padova, Italy);
- -
- Velp Scientifica Vortex agitator (Usmate Velate, Italy);
- -
- Ultra Clear TWF water purification apparatus (Günzburg, Germany);
- -
- ABL and E Jasco V-550 UV-VIS spectrophotometer (Tokyo, Japan);
- -
- Test samples—solutions obtained by dissolving dry extracts in DMSO—hydroalcoholic extracts (E1, E2, E3), extracts in acetone: ethanol 7:3 acidic mixture (A1, A2, A3); concentration of analyzed solutions: 0.078125–10 mg/mL.
2.5. Determining the Scavenging Capacity of the Superoxide Radical Anion
- -
- Dimethyl sulfoxide (Merck, KgaA, Darmstadt, Germany);
- -
- TRIS (Sigma-Aldrich, Steinheim, Germany);
- -
- TRIS buffer pH 8—0.4845 g of TRIS dissolved in 180 mL of distilled water, adjusted to pH 8 with 6M HCl solution, and completed with distilled water to 200 mL;
- -
- Reduced nicotinamide adenine dinucleotide sodium salt (NADHNa2) (Sigma-Aldrich, Steinheim, Germany);
- -
- 557 µM solution of reduced nicotinamide adenine dinucleotide sodium salt (NADHNa2) in TRIS buffer pH 8;
- -
- nitroblue tetrazolium (Fluka, Steinheim, Germany);
- -
- 108 µM solution of nitroblue tetrazolium in TRIS buffer pH 8;
- -
- Phenazine methosulfate (Fluka, Sigma-Aldrich, Steinheim, Germany);
- -
- 45 µM solution of phenazine methosulfate in TRIS buffer pH 8;
- -
- pH meter Hanna Instruments pH210, Microprocessor pH-meter (Padova, Italy);
- -
- Vortex mixer Velp Scientifica (Usmate Velate, Italy);
- -
- Ultra Clear TWF water purification apparatus (Günzburg, Germany);
- -
- UV-VIS spectrophotometer ABL & E Jasco V-550 (Tokyo, Japan);
- -
- Test samples—solutions obtained by dissolving dried extracts in DMSO—hydroalcoholic extracts (E1, E2, E3), extracts in a mixed solution of acetone: ethanol 7:3 (A1, A2, A3); concentration of the analyzed solutions: 0.078125–10 mg/mL.
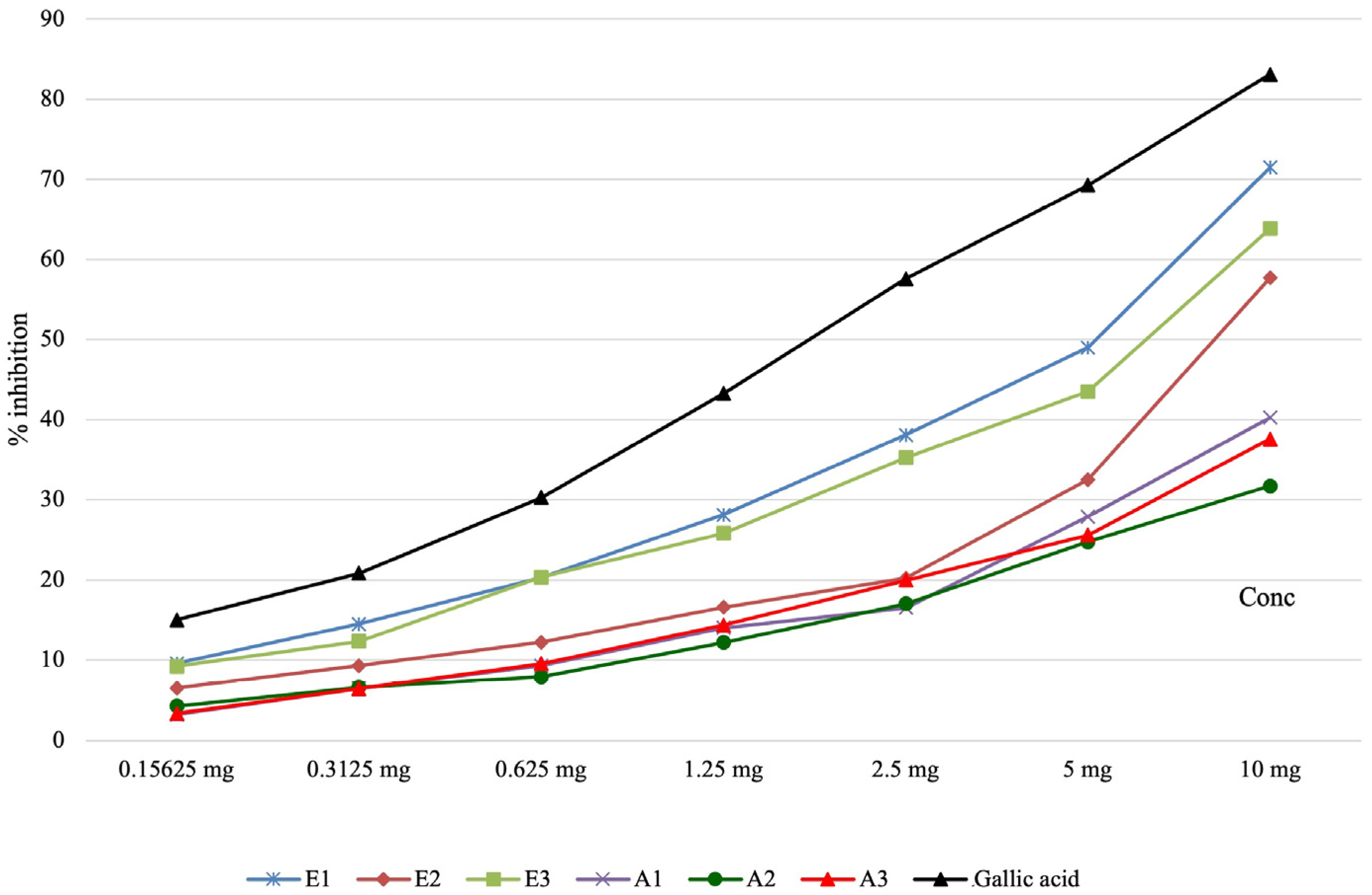
2.6. Determination of the Lipoxygenase Inhibition Capacity of Perilla frutescens Extracts
- -
- Dimethyl sulfoxide (DMSO) (Merck, KgaA, Darmstadt, Germany);
- -
- 0.1 M borate buffer pH 9—Dissolve 6.2 g of boric acid in 950 mL of distilled water, adjust to pH 9 with 1M NaOH, and make up to 1000 mL with distilled water;
- -
- Linoleic acid (Sigma-Aldrich, Steinheim, Germany) 0.16 mM in 0.1 M borate buffer pH 9;
- -
- Soybean lipoxygenase (Sigma-Aldrich, Steinheim, Germany) in 0.1 M borate buffer pH 9;
- -
- UV-VIS spectrophotometer ABL & E Jasco V-550 (Tokyo, Japan);
- -
- pH meter Hanna Instruments pH210, Microprocessor pH-meter (Padova, Italy);
- -
- Vortex mixer Velp Scientifica (Usmate Velate, Italy);
- -
- Ultra Clear TWF water purification system (Günzburg, Germany);
- -
- Test samples—solutions obtained by dissolving dried extracts in DMSO—hydroalcoholic extracts (E1, E2, E3), extracts in a mixture of acetone: ethanol 7:3 (A1, A2, A3); concentration of the analyzed solutions: 0.078125–10 mg/mL.
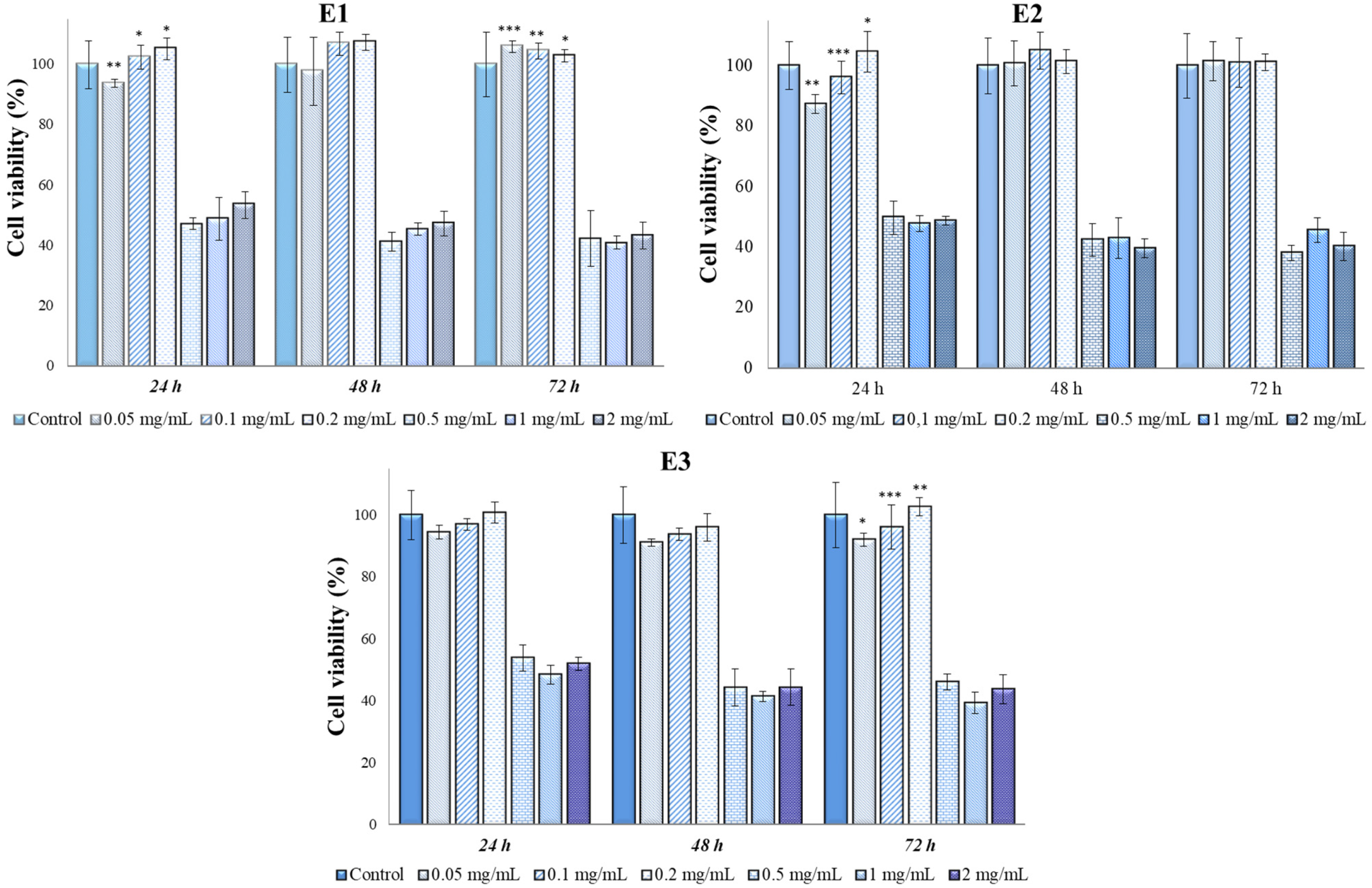
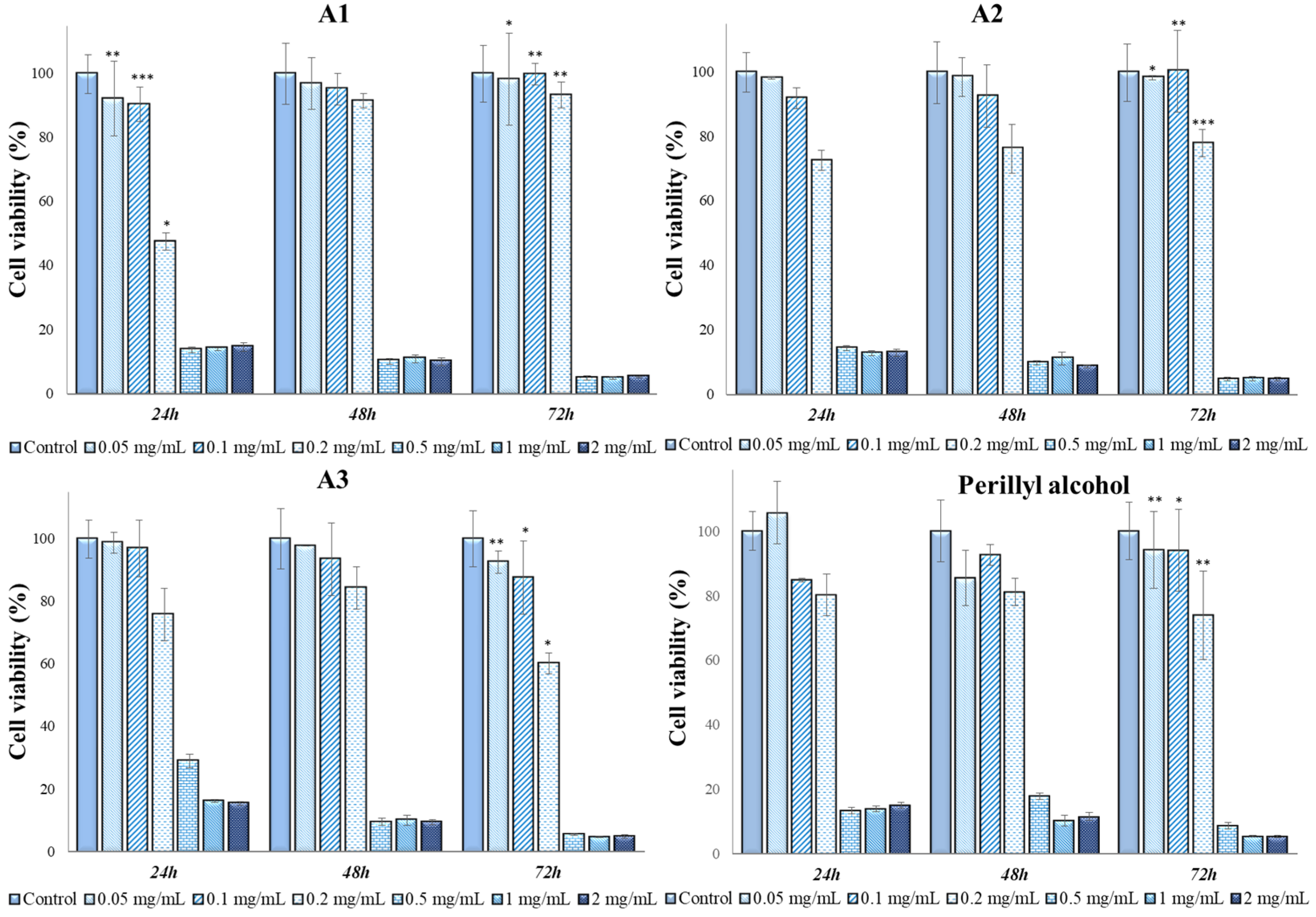
2.7. In Vitro Cytotoxicity Tests and Antitumor Action of Perilla Leaves Extracts
2.8. Statistical Analyses
3. Results
3.1. Quantification of the Total Phenols, Flavonoids and Anthocyanins from Ethanolic and Acetone: Ethanol Extracts of Perilla frutescens
3.2. Determination of the In Vitro Antioxidant Action of Perilla frutescens Extracts
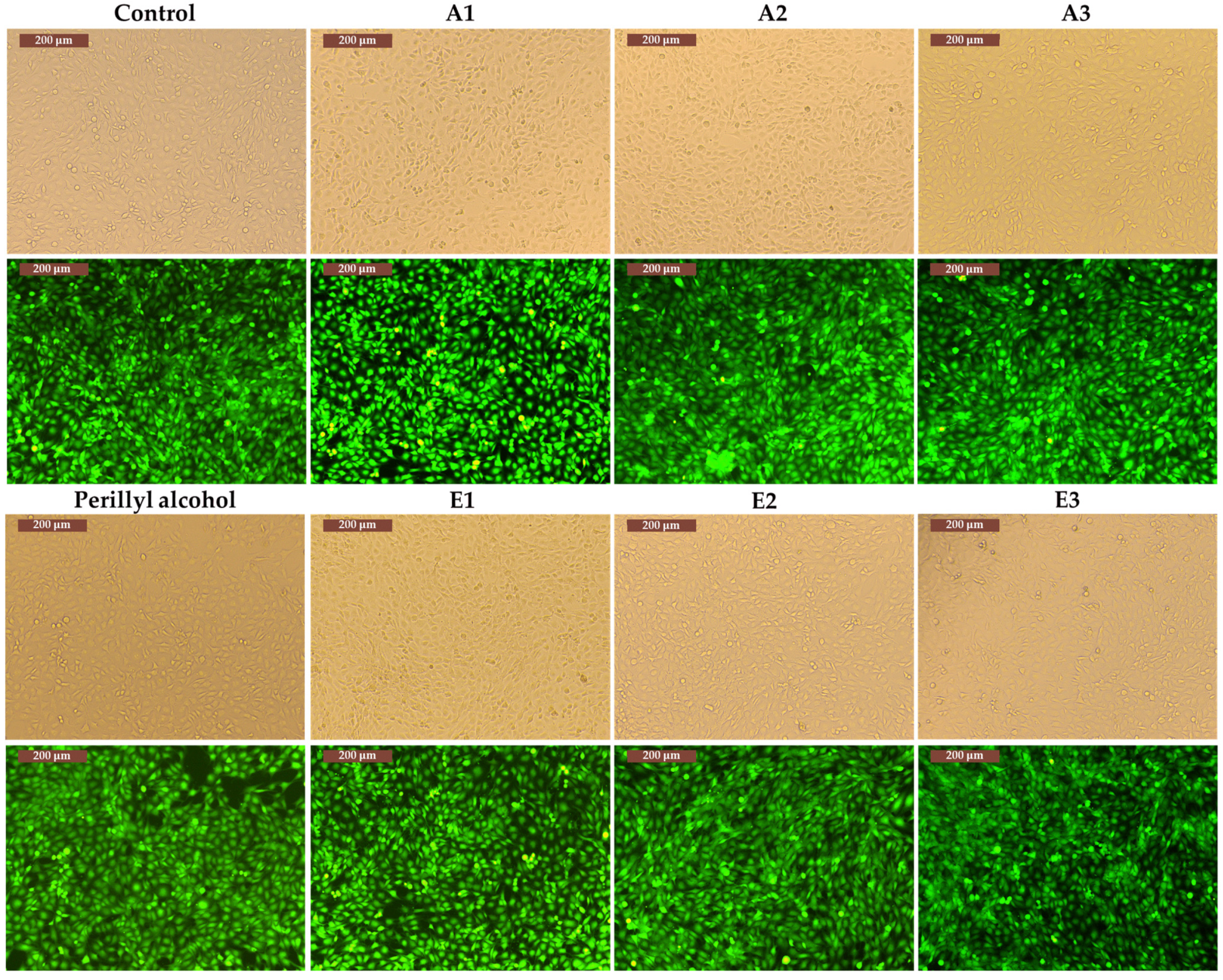
3.3. Determination of In Vitro Cytotoxicity and Antitumor Effects of Perilla frutescens Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adam, G.; Robu, S.; Flutur, M.M.; Cioanca, O.; Vasilache, I.A.; Adam, A.M.; Mircea, C.; Nechita, A.; Harabor, V.; Harabor, A.; et al. Applications of Perilla frutescens Extracts in Clinical Practice. Antioxidants 2023, 12, 727. [Google Scholar] [CrossRef] [PubMed]
- Adam, G.; Adam, A.M.; Robu, S.; Harabor, V.; Harabor, A.; Nechita, A.; Marin, D.B.; Morariu, I.D.; Cioanca, O.; Vasilache, I.A.; et al. The Effects of Perilla frutescens Extracts on IgA Nephropathy: A Systematic Review and Meta-Analysis. Pharmaceuticals 2023, 16, 988. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Nan, Y.; Chen, G.; Ning, N.; Du, Y.; Lu, D.; Yang, Y.; Meng, F.; Yuan, L. The Role and Mechanism of Perilla frutescens in Cancer Treatment. Molecules 2023, 28, 5883. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.L.; Shin, Y.S.; Choi, S.H.; Oh, S.; Kim, K.; Jeong, H.S.; Mo, J.S. Extracts of Perilla frutescens var. Acuta (Odash.) Kudo Leaves Have Antitumor Effects on Breast Cancer Cells by Suppressing YAP Activity. Evid. Based Complement. Altern. Med. 2021, 2021, 5619761. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Garcia, P.J.B.; Huang, S.K.; De Castro-Cruz, K.A.; Leron, R.B.; Tsai, P.W. An In Vitro Evaluation and Network Pharmacology Analysis of Prospective Anti-Prostate Cancer Activity from Perilla frutescens. Plants 2023, 12, 3006. [Google Scholar] [CrossRef]
- Meng, L.; Lozano, Y.F.; Gaydou, E.M.; Li, B. Antioxidant activities of polyphenols extracted from Perilla frutescens varieties. Molecules 2008, 14, 133–140. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, N.; Yeo, J.Y.; Seo, D.G.; Kim, S.; Lee, J.S.; Hwang, K.W.; Park, S.Y. Anti-Amyloidogenic Effects of Asarone Derivatives from Perilla frutescens Leaves against Beta-Amyloid Aggregation and Nitric Oxide Production. Molecules 2019, 24, 4297. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, B.; Kim, S.; Kim, M.S.; Kim, H.; Hwang, S.R.; Kim, K.; Lee, J.H. Characterization of metabolite profiles from the leaves of green perilla (Perilla frutescens) by ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry and screening for their antioxidant properties. J. Food Drug Anal. 2017, 25, 776–788. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar] [PubMed]
- Batir Marin, D.; Cioanca, O.; Apostu, M.; Tuchilus, C.; Mircea, C.; Robu, S.; Tutunaru, D.; Corciova, A.; Hancianu, M. The Comparative Study of Equisetum pratense, E. sylvaticum, E. telmateia: Accumulation of Silicon, Antioxidant and Antimicrobial Screening. Rev. De Chim. 2019, 70, 2519–2523. [Google Scholar] [CrossRef]
- Aron, N.; Bogdan-Goroftei, R.-E.; Boev, M.; Marin, D.; Ramos-Villarroel, A.; Iancu, A.-V. Innovative Fermented Soy Drink with the Sea Buckthorn Syrup and the Probiotics Co-Culture of Lactobacillus Paracasei ssp. Paracasei (L. Casei® 431) and Bifidobacterium Animalis ssp. Lactis (Bb-12®). Fermentation 2023, 9, 806. [Google Scholar] [CrossRef]
- Russo, D. Flavonoids and the structure-antioxidant activity relationship. J. Pharmacogn. Nat. Prod. 2018, 4, 1–2. [Google Scholar] [CrossRef]
- Amić, D.; Davidović-Amić, D.; Bešlo, D.; Trinajstić, N. Structure-radical scavenging activity relationships of flavonoids. Croat. Chem. Acta 2003, 76, 55–61. [Google Scholar]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant activity and healthy benefits of natural pigments in fruits: A review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef]
- Mandal, S.; Hazra, B.; Sarkar, R.; Biswas, S.; Mandal, N. Assessment of the antioxidant and reactive oxygen species scavenging activity of methanolic extract of Caesalpinia crista leaf. Evid.-Based Complement. Altern. Med. 2011, 2011, 173768. [Google Scholar] [CrossRef]
- Humulescu, I.; Flutur, M.-M.; Cioanca, O.; Mircea, C.; Robu, S.; Marin-Batir, D.; Spac, A.; Corciova, A.; Hancianu, M. Comparative chemical and biological activity of selective herbal extracts. Farmacia 2021, 69, 861–866. [Google Scholar] [CrossRef]
- Lungu, I.I.; Marin-Batîr, D.; Panainte, A.; Mircea, C.; Tuchiluș, C.; Ștefanache, A.; Szasz, F.A.; Grigorie, D.; Robu, S.; Cioancă, O. Catechin-Zinc-Complex: Synthesis, Characterization and Biological Activity Assessment. Farmacia 2023, 71, 755–763. [Google Scholar] [CrossRef]
- Dinis, T.C.; Maderia, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Lim, D.W.; Choi, J. Assessment of Size-Dependent Antimicrobial and Cytotoxic Properties of Silver Nanoparticles. Adv. Mater. Sci. Eng. 2014, 2014, 763807. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, D. Antioxidant activities of different fractions of polysaccharide purified from Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2007, 68, 54–58. [Google Scholar] [CrossRef]
- Malterud, K.E.; Rydland, K.M. Inhibitors of 15-lipoxygenase from orange peel. J. Agric. Food Chem. 2000, 48, 5576–5580. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Zhang, M.; Hu, J.; Zhang, Y.; Yang, L.; Hou, X. Chemical Compositions, Extraction Optimizations, and In Vitro Bioactivities of Flavonoids from Perilla Leaves (Perillae folium) by Microwave-Assisted Natural Deep Eutectic Solvents. Antioxidants 2023, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Wu, C.H.; Hung, L.S.; Lin, B.F. Ethanol Extract of Perilla frutescens Suppresses Allergen-Specific Th2 Responses and Alleviates Airway Inflammation and Hyperreactivity in Ovalbumin-Sensitized Murine Model of Asthma. Evid. Based Complement. Altern. Med. 2015, 2015, 324265. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Zhang, Z.; Ren, Z.; Yang, F. Extraction, preparative monomer separation and antibacterial activity of total polyphenols from Perilla frutescens. Food Funct. 2022, 13, 880–890. [Google Scholar] [CrossRef]
- Deguchi, Y.; Ito, M. Caffeic acid and rosmarinic acid contents in genus Perilla. J. Nat. Med. 2020, 74, 834–839. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kono, M.; Ito, A.; Ito, M. Anthocyanins in perilla plants and dried leaves. Phytochemistry 2018, 147, 158–166. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wang, Y.; Li, X.; Zhou, L.; Yang, J.; Guo, L. Metabolites and chemometric study of Perilla (Perilla frutescens) from different varieties and geographical origins. J. Food Sci. 2022, 87, 5240–5251. [Google Scholar] [CrossRef]
- Peng, Y.; Ye, J.; Kong, J. Determination of phenolic compounds in Perilla frutescens L. by capillary electrophoresis with electrochemical detection. J. Agric. Food Chem. 2005, 53, 8141–8147. [Google Scholar] [CrossRef] [PubMed]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Hydroxyl radical and hypochlorous acid scavenging activity of small centaury (Centaurium erythraea) infusion. A comparative study with green tea (Camellia sinensis). Phytomedicine 2003, 10, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D. The chemistry and biological effects of flavonoids and phenolic acids. Ann. N. Y. Acad. Sci. 1998, 854, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Parr, A.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Kwon, S.J.; Lee, J.H.; Moon, K.D.; Jeong, I.Y.; Ahn, D.U.; Lee, M.K.; Seo, K.I. Induction of apoptosis by isoegomaketone from Perilla frutescens L. in B16 melanoma cells is mediated through ROS generation and mitochondrial-dependent, -independent pathway. Food Chem. Toxicol. 2014, 65, 97–104. [Google Scholar] [CrossRef]
- Cho, B.O.; Jin, C.H.; Park, Y.D.; Ryu, H.W.; Byun, M.W.; Seo, K.I.; Jeong, I.Y. Isoegomaketone induces apoptosis through caspase-dependent and caspase-independent pathways in human DLD1 cells. Biosci. Biotechnol. Biochem. 2011, 75, 1306–1311. [Google Scholar] [CrossRef]
- Yeruva, L.; Pierre, K.J.; Elegbede, A.; Wang, R.C.; Carper, S.W. Perillyl alcohol and perillic acid induced cell cycle arrest and apoptosis in non small cell lung cancer cells. Cancer Lett. 2007, 257, 216–226. [Google Scholar] [CrossRef]
- Oturanel, C.E.; Kıran, İ.; Özşen, Ö.; Çiftçi, G.A.; Atlı, Ö. Cytotoxic, Antiproliferative and Apoptotic Effects of Perillyl Alcohol and Its Biotransformation Metabolite on A549 and HepG2 Cancer Cell Lines. Anticancer Agents Med. Chem. 2017, 17, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Elegbede, J.A.; Flores, R.; Wang, R.C. Perillyl alcohol and perillaldehyde induced cell cycle arrest and cell death in BroTo and A549 cells cultured in vitro. Life Sci. 2003, 73, 2831–2840. [Google Scholar] [CrossRef] [PubMed]





| Solvent | Perilla frutescens Species Variety | Extract Code | Extraction Yield (%/Mean and Standard Deviation) |
|---|---|---|---|
| 70% Ethanol | Perilla frutescens var. crispa f. purpurea | E1 | 58.22% (* 2.04: 1.18 ± 0.03) ab |
| 70% Ethanol | Perilla frutescens var. frutescens f. viridis | E2 | 58.79% (* 2.03: 1.19 ± 0.01) a |
| 70% Ethanol | Perilla frutescens var. frutescens f. crispidiscolor | E3 | 48.32% (* 2.02: 0.97 ± 0.01) c |
| Acetone: Ethanol (7:3) + Citric Acid | Perilla frutescens var. crispa f. purpurea | A1 | 38.51% (* 2.01: 0.77 ± 0.006) e |
| Acetone: Ethanol (7:3) + Citric Acid | Perilla frutescens var. frutescens f. viridis | A2 | 34.84% (* 2.04: 0.71 ± 0.02) f |
| Acetone: Ethanol (7:3) + Citric Acid | Perilla frutescens var. frutescens f. crispidiscolor | A3 | 44.47% (* 2.02: 0.90 ± 0.01) d |
| Sample | Total Phenols (mg Gallic Acid/mL Extract) | Total Flavonoids (mg Rutoside/mL Extract) | Anthocyanins (mg Cyanidol/mL Extract) |
|---|---|---|---|
| E1 | 2.15 ± 0.12 b | 0.17 ± 0.02 a | 0.10 ± 0.001 a |
| E2 | 2.36 ± 0.11 a | 0.09 ± 0.002 c | 0.007 ± 0.007 c |
| E3 | 2.13 ± 0.03 c | 0.11 ± 0.01 bc | 0.08 ± 0.001 b |
| A1 | 0.33 ± 0.02 d | 0.11 ± 0.009 bcd | 0.09 ± 0.001 d |
| A2 | 0.14 ± 0.03 f | 0.08 ± 0.002 cf | - |
| A3 | 0.28 ± 0.02 de | 0.10 ± 0.001 bcdef | 0.06 ± 0.001 e |
| Compound | Identified Compound (µg/mL Extract) | |||||
|---|---|---|---|---|---|---|
| E1 | E2 | E3 | A1 | A2 | A3 | |
| Gallic Acid | 0.249 a | 0.235 ab | 0.095 ac | - | 0.225 ad | 0.103 ae |
| Catechin | 1.526 c | 2.025 ac | 1.672 bc | 1.388 ec | 1.355 fc | 1.581 dc |
| Chlorogenic Acid | 1.15 bc | 0.388 cd | 4.448 a | 0.376 cedf | 0.633 ced | 0.331 cef |
| Caffeic Acid | 109.714 a | 55.12 c | 81.306 b | 8.582 e | 12.022 d | 7.108 f |
| Epi-Catechin | 1.014 abc | 0.870 abc | 0.912 abc | 0.769 abce | 0.653 bcef | 1.030 abcd |
| Syringic acid | 129.323 c | 244.033 a | 232.475 b | 20.57 e | 35.109 d | 19.603 f |
| P-Coumaric Acid | 11.163 a | 10.069 b | 7.097 c | 2.222 f | 2.25 fd | 3.788 d |
| Ferulic Acid | 2.423 abc | 2.429 abc | 2.551 abc | - | 0.538 e | 0.722 d |
| Ellagic Acid | 9.930 a | 5.865b | 4.807 c | 3.421 e | 3.677 d | 2.731 f |
| Cinnamic Acid | 70.465 a | - | 22.698 b | 16.544 d | 11.494 e | 25.218 c |
| Rosmarinic Acid | 67.665 a | 10.351 c | 14.871 b | 1.098 f | 3.177 e | 9.004 d |
| Quercitin | 19.663 a | 3.912 c | 16.828 b | - | - | 12.243 d |
| Kaemferol | 36.45 bc | 38.12 bc | 41.09 a | 22.05 de | 26.15 de | 19.47 ef |
| Isorhamnetin | 1.194 a | 0.805 bc | 0.745 bc | 0.138 ef | 0.101 def | 0.263 de |
| Apigenin | 3.400 c | 8.361 b | 10.656 a | 2.677 e | 4.061 d | 1.313 f |
| Pinostrobin | 31.827 a | 28.35 b | 24.081 cd | 23.601 e | - | 24.320 cd |
| Pinocembrin | 2.323 ab | 0.972 c | 2.302 ab | 0.387 fd | 0.447 def | 0.424 def |
| Crysin | 1.11 ce | 4.214 a | 3.239 b | 1.449 de | 1.354 cde | 0.56 f |
| Samples | Iron Chelation Capacity Assessment—IC50 (μg/mL Final Solution) |
|---|---|
| E1 | 166.36 ± 0.18 c |
| E2 | 386.69 ± 9.36 a |
| E3 | 297.01 ± 0.31 b |
| A1 | 811.88 ± 1.32 g |
| A2 | 1040.93 ± 0.17 e |
| A3 | 830.49 ± 0.82 f |
| Gallic acid | 1163.15 ± 2.63 d |
| Samples | Determination of Hydroxyl Radical Scavenging Capacity—IC50 (μg/mL final solution) |
| E1 | 122.62 ± 0.74 c |
| E2 | 154.58 ± 0.41 ab |
| E3 | 153.16 ± 1.11 ab |
| A1 | 156.96 ± 3.57 abe |
| A2 | - |
| A3 | - |
| Gallic acid | 177.29 ± 0.66 d |
| Samples | Determination of Superoxide Anion Scavenging Capacity—IC50 (μg/mL final solution) |
| E1 | 321.60 ± 9.12 c |
| E2 | 520.79 ± 16.90 ab |
| E3 | 470.08 ± 7.32 ab |
| A1 | 977.47 ± 39.37 e |
| A2 | - |
| A3 | 1741.66 ± 29.87 d |
| Gallic acid | 543.38 ± 15.43 af |
| Samples | Determination of the lipoxygenase inhibition capacity—IC50 (μg/mL final solution) |
| E1 | 85.99 ± 1.85 c |
| E2 | 134.77 ± 2.49 a |
| E3 | 104.10 ± 5.05 b |
| A1 | - |
| A2 | - |
| A3 | - |
| Gallic acid | 28.85 ± 0.76 d |
| Extracts | Time Frame | 2 mg/mL | 1 mg/mL | 0.5 mg/mL | Controls |
|---|---|---|---|---|---|
| E1 (mean and standard deviation) | 24 h | 53.50 ± 1.32 abc | 48.98 ± 3.95 abc | 47.03 ± 3.67 abc | 100.00 ± 7.19 d |
| E1 (mean and standard deviation) | 48 h | 47.33 ± 11.18 abc | 45.54 ± 3.82 abc | 41.33 ± 2.68 abc | 100 ± 9.16 d |
| E1 (mean and standard deviation) | 72 h | 43.36 ± 1.94 abc | 40.99 ± 2.53 abc | 42.20 ± 1.99 abc | 100 ± 20.64 d |
| E2 (mean and standard deviation) | 24 h | 48.74 ± 1.46 abc | 47.76 ± 2.60 abc | 49.84 ± 5.54 abc | 100.00 ± 7.19 d |
| E2 (mean and standard deviation) | 48 h | 39.70 ± 3.20 abc | 43.05 ± 6.69abc | 42.50 ± 5.33 abc | 100 ± 9.16 d |
| E2 (mean and standard deviation) | 72 h | 40.24 ± 4.64 abc | 45.68 ± 4.15 abc | 38.12 ± 2.56 abc | 100 ± 20.64 d |
| E3 (mean and standard deviation) | 24 h | 51.91 ± 2.11 a | 48.28 ± 3.12 a | 53.69 ± 4.21 a | 100.00 ± 7.19 d |
| E3 (mean and standard deviation) | 48 h | 44.21 ± 5.9 bc | 41.26 ± 1.63 bc | 44.21 ± 6 bc | 100 ± 9.16 d |
| E3 (mean and standard deviation) | 72 h | 43.64 ± 4.69 bc | 39.18 ± 3.43 bc | 45.99 ± 2.55 bc | 100 ± 20.64 d |
| A1 (mean and standard deviation) | 24 h | 51.21 ± 8.12 a | 55.34 ± 6.88 a | 49.84 ± 8.37 a | 100.00 ± 7.19 d |
| A1 (mean and standard deviation) | 48 h | 43.20 ± 3.86 bc | 42.35 ± 4.41 bc | 40.09 ± 2.30 bc | 100 ± 9.16 d |
| A1 (mean and standard deviation) | 72 h | 39.40 ± 2.02 bc | 39.71 ± 2.10 bc | 43.72 ± 3.86 bc | 100 ± 20.64 d |
| A2 (mean and standard deviation) | 24 h | 45.994 ± 2.95 abc | 47.46 ± 4.57 abc | 47.55 ± 3.21 abc | 100.00 ± 7.19 d |
| A2 (mean and standard deviation) | 48 h | 38.22 ± 1.76 abc | 44.60 ± 5.56 abc | 38.82 ± 4.53 abc | 100 ± 9.16 d |
| A2 (mean and standard deviation) | 72 h | 42.20 ± 3.18 abc | 41.30 ± 5.59 abc | 43.64 ± 6.53 abc | 100 ± 20.64 d |
| A3 (mean and standard deviation) | 24 h | 45.17 ± 6.06 abc | 43.15 ± 3.32 abc | 47.27 ± 6.18 abc | 100.00 ± 7.19 d |
| A3 (mean and standard deviation) | 48 h | 37.99 ± 4.90 abc | 36.97 ± 3.03 abc | 39.47 ± 2.56 abc | 100 ± 9.16 d |
| A3 (mean and standard deviation) | 72 h | 43.57 ± 2.45 abc | 40.69 ± 2.4 abc | 44.402 ± 1.66 abc | 100 ± 20.64 d |
| Perillyl alcohol (mean) | 24 h | 57.53 ± 5.82 a | 54.33 ± 9.36 a | 95.37 ± 7.03 a | 100.00 ± 7.19 d |
| Perillyl alcohol (mean) | 48 h | 46.16 ± 2.23 bc | 43.98± 4.62 bc | 81.69 ± 10.88 b | 100 ± 9.16 d |
| Perillyl alcohol (mean) | 72 h | 48.63 ± 2.43 bc | 45.68 ± 4.37 bc | 42.66 ± 2.15 c | 100 ± 20.64 d |
| Extracts | Time Frame | 2 mg/mL | 1 mg/mL | 0.5 mg/mL | Controls |
|---|---|---|---|---|---|
| E1 (mean and standard deviation) | 24 h | 13.84 ± 0.40 abc | 13.76 ± 0.91 abc | 14.40 ± 0.56 abc | 100 ± 6.08 d |
| E1 (mean and standard deviation) | 48 h | 10.29 ± 1.73 abc | 9.96 ± 0.89 abc | 11.29 ± 0.99 abc | 100 ± 9.58 d |
| E1 (mean and standard deviation) | 72 h | 5.63 ± 0.23 abc | 5.46 ± 0.65 abc | 5.02 ± 0.53 abc | 100 ± 8.91 d |
| E2 (mean and standard deviation) | 24 h | 14.57 ± 1.38 abc | 15.94 ± 0.63 abc | 16.54 ± 2.98 abc | 100 ± 6.08 d |
| E2 (mean and standard deviation) | 48 h | 10.66 ± 2.2 abc | 9.94 ± 1.15 abc | 10.17 ± 0.68 abc | 100 ± 9.58 d |
| E2 (mean and standard deviation) | 72 h | 4.73 ± 0.38 abc | 5.183 ± 0.44 abc | 5.13 ± 0.74 abc | 100 ± 8.91 d |
| E3 (mean and standard deviation) | 24 h | 15.65 ± 1.57 abc | 13.89 ± 1.46 abc | 15.17 ± 1.21 abc | 100 ± 6.08 d |
| E3 (mean and standard deviation) | 48 h | 9.92 ± 0.60 abc | 9.99 ± 0.65 abc | 10.15 ± 1.69 abc | 100 ± 9.58 d |
| E3 (mean and standard deviation) | 72 h | 5.21 ± 0.63 abc | 4.88 ± 0.52 abc | 5.66 ± 0.09 abc | 100 ± 8.91 d |
| A1 (mean and standard deviation) | 24 h | 14.63 ± 1.38 abc | 14.03 ± 0.43 abc | 13.60 ± 0.90 abc | 100 ± 6.08 d |
| A1 (mean and standard deviation) | 48 h | 9.98 ± 1.26 abc | 10.93 ± 1.23 abc | 10.17 ± 0.89 abc | 100 ± 9.58 d |
| A1 (mean and standard deviation) | 72 h | 5.24 ± 1.26 abc | 4.95 ± 1.23 abc | 4.89 ± 0.89 abc | 100 ± 8.91 d |
| A2 (mean and standard deviation) | 24 h | 12.95 ± 1.07 a | 12.83 ± 0.77 a | 14.40 ± 0.72 a | 100 ± 6.08 d |
| A2 (mean and standard deviation) | 48 h | 8.71 ± 0.47 b | 11.10 ± 1.98 b | 9.78 ± 0.64 b | 100 ± 9.58 d |
| A2 (mean and standard deviation) | 72 h | 4.81 ± 0.44 c | 4.911 ± 0.75 c | 4.73 ± 0.52 c | 100 ± 8.91 d |
| A3 (mean and standard deviation) | 24 h | 15.37 ± 0.56 a | 16.17 ± 0.43 a | 28.90 ± 2.15 a | 100 ± 6.08 d |
| A3 (mean and standard deviation) | 48 h | 9.46 ± 0.80 b | 10.05 ± 1.65 b | 9.52 ± 1.05 b | 100 ± 9.58 d |
| A3 (mean and standard deviation) | 72 h | 4.82 ± 0.46 c | 4.68 ± 0.29 c | 5.51 ± 0.41 c | 100 ± 8.91 d |
| Perillyl alcohol (mean and standard deviation) | 24 h | 15 ± 0.97 ab | 13.88 ± 0.82 ab | 13.43 ± 0.86 ab | 100 ± 6.08 d |
| Perillyl alcohol (mean and standard deviation) | 48 h | 11.50 ± 1.23 ab | 10.20 ± 1.64 ab | 17.78 ± 2.49 ab | 100 ± 9.58 d |
| Perillyl alcohol (mean and standard deviation) | 72 h | 5.39 ± 0.35 c | 5.42 ± 0.25 c | 8.76 ± 2.05 c | 100 ± 8.91 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam, G.; Cojocaru, F.D.; Verestiuc, L.; Cioanca, O.; Vasilache, I.-A.; Adam, A.-M.; Mircea, C.; Nechita, A.; Harabor, V.; Huzum, B.; et al. Assessing the Antioxidant Properties, In Vitro Cytotoxicity and Antitumoral Effects of Polyphenol-Rich Perilla leaves Extracts. Antioxidants 2024, 13, 58. https://doi.org/10.3390/antiox13010058
Adam G, Cojocaru FD, Verestiuc L, Cioanca O, Vasilache I-A, Adam A-M, Mircea C, Nechita A, Harabor V, Huzum B, et al. Assessing the Antioxidant Properties, In Vitro Cytotoxicity and Antitumoral Effects of Polyphenol-Rich Perilla leaves Extracts. Antioxidants. 2024; 13(1):58. https://doi.org/10.3390/antiox13010058
Chicago/Turabian StyleAdam, Gigi, Florina Daniela Cojocaru, Liliana Verestiuc, Oana Cioanca, Ingrid-Andrada Vasilache, Ana-Maria Adam, Cornelia Mircea, Aurel Nechita, Valeriu Harabor, Bogdan Huzum, and et al. 2024. "Assessing the Antioxidant Properties, In Vitro Cytotoxicity and Antitumoral Effects of Polyphenol-Rich Perilla leaves Extracts" Antioxidants 13, no. 1: 58. https://doi.org/10.3390/antiox13010058







