Targeting Cysteine Oxidation in Thrombotic Disorders
Abstract
:1. Introduction
2. The Biochemistry of Cysteines
2.1. Cysteine Reactivity: Not All Are Created Equal
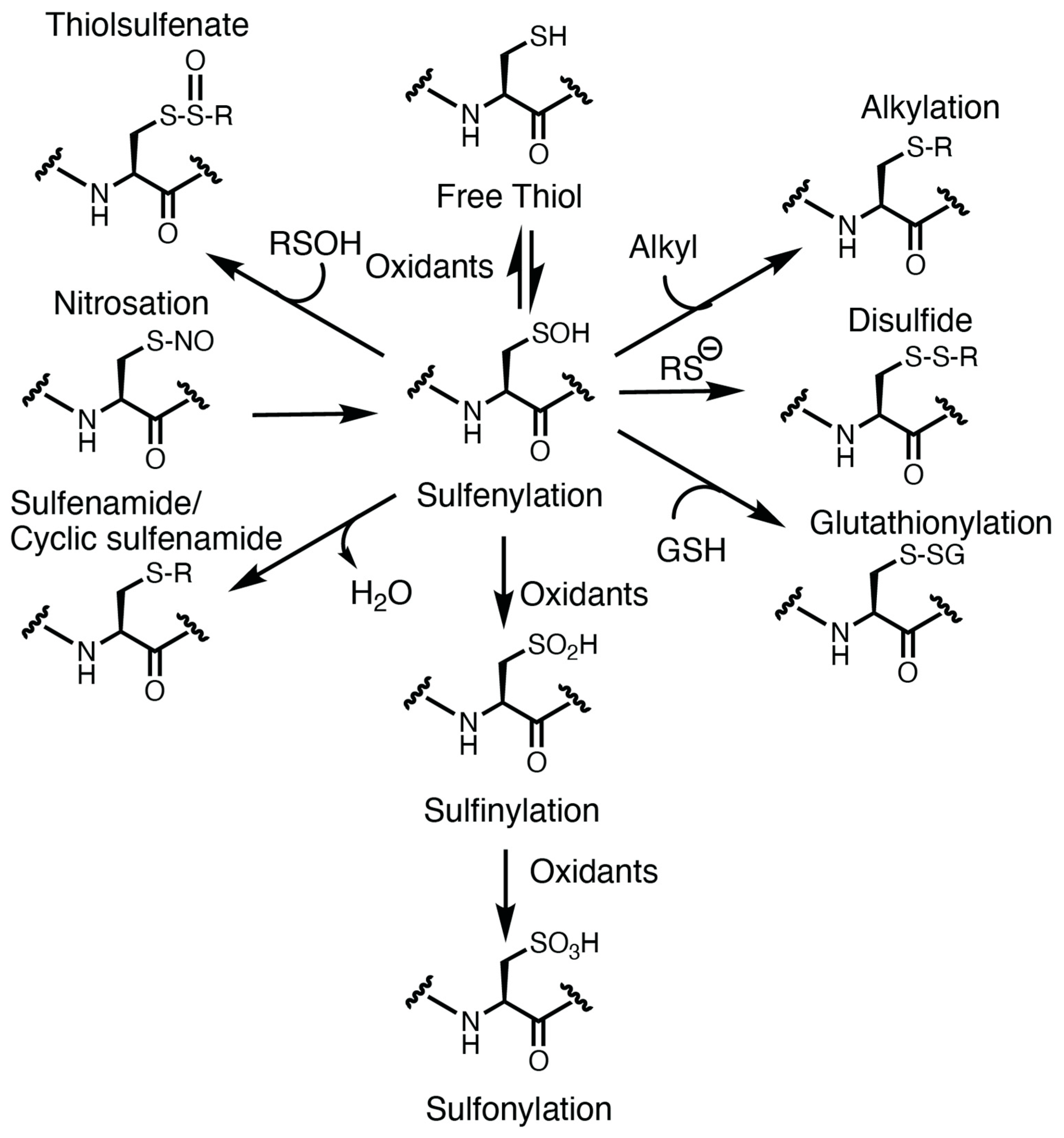
2.2. Cysteine Oxoforms
3. Oxidative Cysteine Modification in Oxidative Stress-Driven Thrombosis
3.1. Thrombosis Is a Multifaceted Process
3.2. CD36 Links Oxidative Stress to Prothrombotic Cysteine Oxidative Signaling
3.3. Thiol Isomerases Convert Redox Cues to a Thrombotic Response
4. Targeting Oxidative Cysteine Modification with Carbon Nucleophiles
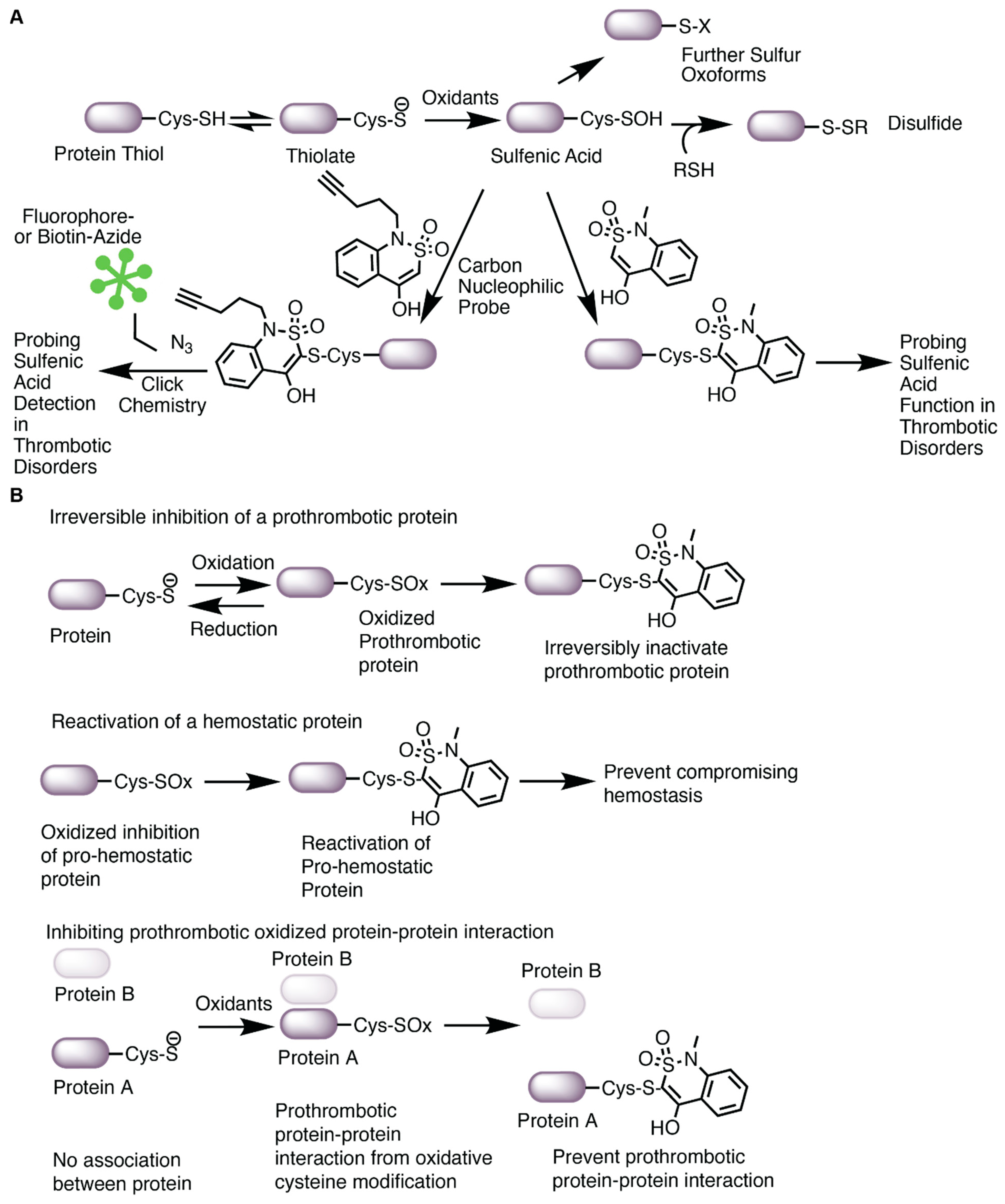
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Morotti, A.; Barale, C.; Melchionda, E.; Russo, I. Platelet Redox Imbalance in Hypercholesterolemia: A Big Problem for a Small Cell. Int. J. Mol. Sci. 2022, 23, 11446. [Google Scholar] [CrossRef]
- Vaidya, A.R.; Wolska, N.; Vara, D.; Mailer, R.K.; Schröder, K.; Pula, G. Diabetes and Thrombosis: A Central Role for Vascular Oxidative Stress. Antioxidants 2021, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Barale, C.; Russo, I. Influence of Cardiometabolic Risk Factors on Platelet Function. Int. J. Mol. Sci. 2020, 21, 623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zennadi, R. Oxidative Stress and Thrombosis during Aging: The Roles of Oxidative Stress in RBCs in Venous Thrombosis. Int. J. Mol. Sci. 2020, 21, 4259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, S.; Li, X.; Wen, X.; Liu, S.; Zu, R.; Ren, H.; Li, T.; Yang, C.; Luo, H. Research progress on the interaction between oxidative stress and platelets: Another avenue for cancer? Pharmacol. Res. 2023, 191, 106777. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Czajkowsky, D.M. SARS-CoV-2 infection and oxidative stress: Pathophysiological insight into thrombosis and therapeutic opportunities. Cytokine Growth Factor Rev. 2022, 63, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.D.; Sparkenbaugh, E.M. The invisible string of coagulation, complement, iron, and inflammation in sickle cell disease. Curr. Opin. Hematol. 2023, 30, 153–158. [Google Scholar] [CrossRef]
- Bettiol, A.; Galora, S.; Argento, F.R.; Fini, E.; Emmi, G.; Mattioli, I.; Bagni, G.; Fiorillo, C.; Becatti, M. Erythrocyte oxidative stress and thrombosis. Expert Rev. Mol. Med. 2022, 24, e31. [Google Scholar] [CrossRef]
- Nocella, C.; Bartimoccia, S.; Cammisotto, V.; D’amico, A.; Pastori, D.; Frati, G.; Sciarretta, S.; Rosa, P.; Felici, C.; Riggio, O.; et al. Oxidative Stress in the Pathogenesis of Antiphospholipid Syndrome: Implications for the Atherothrombotic Process. Antioxidants 2021, 10, 1790. [Google Scholar] [CrossRef]
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Demasi, M.; Augusto, O.; Bechara, E.J.; Bicev, R.N.; Cerqueira, F.M.; da Cunha, F.M.; Denicola, A.; Gomes, F.; Miyamoto, S.; Netto, L.E.; et al. Oxidative Modification of Proteins: From Damage to Catalysis, Signaling, and Beyond. Antioxid. Redox Signal. 2021, 35, 1016–1080. [Google Scholar] [CrossRef]
- Yang, M.; Smith, B.C. Cysteine and methionine oxidation in thrombotic disorders. Curr. Opin. Chem. Biol. 2023, 76, 102350. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Carroll, K.S. Sulfenic acid chemistry, detection and cellular lifetime. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 847–875. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Eom, G.H.; Kang, G. Nitrosative Stress and Human Disease: Therapeutic Potential of Denitrosylation. Int. J. Mol. Sci. 2021, 22, 9794. [Google Scholar] [CrossRef] [PubMed]
- Aussel, L.; Ezraty, B. Methionine Redox Homeostasis in Protein Quality Control. Front. Mol. Biosci. 2021, 8, 665492. [Google Scholar] [CrossRef]
- Sun, H.; Jia, H.; Kendall, O.; Dragelj, J.; Kubyshkin, V.; Baumann, T.; Mroginski, M.-A.; Schwille, P.; Budisa, N. Halogenation of tyrosine perturbs large-scale protein self-organization. Nat. Commun. 2022, 13, 4843. [Google Scholar] [CrossRef]
- Marino, S.M.; Gladyshev, V.N. Cysteine Function Governs Its Conservation and Degeneration and Restricts Its Utilization on Protein Surfaces. J. Mol. Biol. 2010, 404, 902–916. [Google Scholar] [CrossRef]
- Chiu, J.; Hogg, P.J. Allosteric disulfides: Sophisticated molecular structures enabling flexible protein regulation. J. Biol. Chem. 2019, 294, 2949–5908. [Google Scholar] [CrossRef]
- Awoonor-Williams, E.; Rowley, C.N. How Reactive are Druggable Cysteines in Protein Kinases? J. Chem. Inf. Model. 2018, 58, 1935–1946. [Google Scholar] [CrossRef]
- Liu, R.; Zhan, S.; Che, Y.; Shen, J. Reactivities of the Front Pocket N-Terminal Cap Cysteines in Human Kinases. J. Med. Chem. 2022, 65, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Pace, N.J.; Weerapana, E. Zinc-Binding Cysteines: Diverse Functions and Structural Motifs. Biomolecules 2014, 4, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Flaumenhaft, R. Oxidative Cysteine Modification of Thiol Isomerases in Thrombotic Disease: A Hypothesis. Antioxid. Redox Signal. 2021, 35, 1134–1155. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.S.; Gibbins, J.M. Thiol Isomerases Orchestrate Thrombosis and Hemostasis. Antioxid. Redox Signal. 2021, 35, 1116–1133. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Guzmán, C.A.; Ruiz-Pernía, J.J.; Zinovjev, K.; Tuñón, I. Unveiling the Mechanistic Singularities of Caspases: A Computational Analysis of the Reaction Mechanism in Human Caspase-1. ACS Catal. 2023, 13, 4348–4361. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Carroll, K.S. Cysteine-Mediated Redox Signaling: Chemistry, Biology, and Tools for Discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.M.; Gladyshev, V.N. Analysis and Functional Prediction of Reactive Cysteine Residues. J. Biol. Chem. 2012, 287, 4419–4425. [Google Scholar] [CrossRef]
- Roos, G.; Foloppe, N.; Messens, J. Understanding the pKa of Redox Cysteines: The Key Role of Hydrogen Bonding. Antioxid. Redox Signal. 2013, 18, 94–127. [Google Scholar] [CrossRef]
- Bak, D.W.; Bechtel, T.J.; Falco, J.A.; Weerapana, E. Cysteine reactivity across the subcellular universe. Curr. Opin. Chem. Biol. 2019, 48, 96–105. [Google Scholar] [CrossRef]
- Yang, R.; Zhu, T.; Xu, J.; Zhao, Y.; Kuang, Y.; Sun, M.; Chen, Y.; He, W.; Wang, Z.; Jiang, T.; et al. Organic Fluorescent Probes for Monitoring Micro-Environments in Living Cells and Tissues. Molecules 2023, 28, 3455. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, K.; Chen, T.; Sargsyan, K.; Lim, C. From quantum-derived principles underlying cysteine reactivity to combating the COVID-19 pandemic. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1607. [Google Scholar] [CrossRef] [PubMed]
- Røhr, K.; Hammerstad, M.; Andersson, K.K. Tuning of Thioredoxin Redox Properties by Intramolecular Hydrogen Bonds. PLoS ONE 2013, 8, e69411. [Google Scholar] [CrossRef] [PubMed]
- Karala, A.-R.; Lappi, A.-K.; Ruddock, L.W. Modulation of an Active-Site Cysteine pKa Allows PDI to Act as a Catalyst of both Disulfide Bond Formation and Isomerization. J. Mol. Biol. 2010, 396, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Kortemme, T.; Creighton, T.E. Ionisation of Cysteine Residues at the Termini of Model α-Helical Peptides. Relevance to Unusual Thiol pKaValues in Proteins of the Thioredoxin Family. J. Mol. Biol. 1995, 253, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Truong, T.H.; Garcia, F.J.; Homann, A.; Gupta, V.; Leonard, S.E.; Carroll, K.S. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 2011, 8, 57–64. [Google Scholar] [CrossRef]
- Truong, T.H.; Ung, P.M.-U.; Palde, P.B.; Paulsen, C.E.; Schlessinger, A.; Carroll, K.S. Molecular Basis for Redox Activation of Epidermal Growth Factor Receptor Kinase. Cell Chem. Biol. 2016, 23, 837–848. [Google Scholar] [CrossRef]
- Rosenkranz, A.A.; Slastnikova, T.A. Epidermal Growth Factor Receptor: Key to Selective Intracellular Delivery. Biochemistry 2020, 85, 967–1092. [Google Scholar] [CrossRef]
- Akter, S.; Fu, L.; Jung, Y.; Conte, M.L.; Lawson, J.R.; Lowther, W.T.; Sun, R.; Liu, K.; Yang, J.; Carroll, K.S. Chemical proteomics reveals new targets of cysteine sulfinic acid reductase. Nat. Chem. Biol. 2018, 14, 995–1004. [Google Scholar] [CrossRef]
- Petroff, J.T.; Omlid, S.M.; Haloi, N.; Sith, L.; Johnson, S.; McCulla, R.D. Reactions of sulfenic acids with amines, thiols, and thiolates studied by quantum chemical calculations. Comput. Theor. Chem. 2020, 1189, 112979. [Google Scholar] [CrossRef]
- Rehder, D.S.; Borges, C.R. Cysteine sulfenic Acid as an Intermediate in Disulfide Bond Formation and Nonenzymatic Protein Folding. Biochemistry 2010, 49, 7748–7755. [Google Scholar] [CrossRef] [PubMed]
- Branchford, B.R.; Flood, V.H. 51—Bleeding and Thrombosis. In Nelson Pediatric Symptom-Based Diagnosis: Common Diseases and Their Mimics, 2nd ed.; Kliegman, R.M., Toth, H., Bordini, B.J., Basel, D., Eds.; Elsevier: Philadelphia, PA, USA, 2023; pp. 942–964.e1. [Google Scholar]
- Neubauer, K.; Zieger, B. Endothelial cells and coagulation. Cell Tissue Res. 2022, 387, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Braune, S.; Küpper, J.-H.; Jung, F. Effect of Prostanoids on Human Platelet Function: An Overview. Int. J. Mol. Sci. 2020, 21, 9020. [Google Scholar] [CrossRef] [PubMed]
- Gambaryan, S. The Role of NO/sGC/cGMP/PKG Signaling Pathway in Regulation of Platelet Function. Cells 2022, 11, 3704. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.; Smolenski, A. Cyclic nucleotide-dependent inhibitory signaling interweaves with activating pathways to determine platelet responses. Res. Pract. Thromb. Haemost. 2018, 2, 558–571. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.-J.; Yang, X.; Yang, B.; Zhang, X.; Zhang, J.; Zhang, Q.; Cheng, X.-D.; Wang, J.-H.; Yu, N.-W. Purinergic signaling: A potential therapeutic target for ischemic stroke. Purinergic Signal. 2023, 19, 173–183. [Google Scholar] [CrossRef]
- Scridon, A. Platelets and Their Role in Hemostasis and Thrombosis—From Physiology to Pathophysiology and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 12772. [Google Scholar] [CrossRef]
- Li, W.; Febbraio, M.; Reddy, S.P.; Yu, D.-Y.; Yamamoto, M.; Silverstein, R.L. CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. J. Clin. Investig. 2010, 120, 3996–4006. [Google Scholar] [CrossRef]
- Jin, R.C.; Mahoney, C.E.; Anderson, L.; Ottaviano, F.; Croce, K.; Leopold, J.A.; Zhang, Y.-Y.; Tang, S.-S.; Handy, D.E.; Loscalzo, J.; et al. Glutathione Peroxidase-3 Deficiency Promotes Platelet-Dependent Thrombosis In Vivo. Circulation 2011, 123, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Dayal, S.; Wilson, K.M.; Motto, D.G.; Miller, F.J., Jr.; Chauhan, A.K.; Lentz, S.R. Hydrogen Peroxide Promotes Aging-Related Platelet Hyperactivation and Thrombosis. Circulation 2013, 127, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Magwenzi, S.; Woodward, C.; Wraith, K.S.; Aburima, A.; Raslan, Z.; Jones, H.; McNeil, C.; Wheatcroft, S.; Yuldasheva, N.; Febbriao, M.; et al. Oxidized LDL activates blood platelets through CD36/NOX2–mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood 2015, 125, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cooley, B.C.; Li, W.; Chen, Y.; Vasquez-Vivar, J.; Scoggins, N.O.; Cameron, S.J.; Morrell, C.N.; Silverstein, R.L. Platelet CD36 promotes thrombosis by activating redox sensor ERK5 in hyperlipidemic conditions. Blood 2017, 129, 2917–2927. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Kakuta, S.; Iwakura, Y.; Takayama, N.; Ooehara, J.; Otsu, M.; Kamiya, A.; Petrich, B.G.; et al. In vivo imaging visualizes discoid platelet aggregations without endothelium disruption and implicates contribution of inflammatory cytokine and integrin signaling. Blood 2012, 119, e45–e56. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; McIntyre, T.M.; Silverstein, R.L. Ferric chloride-induced murine carotid arterial injury: A model of redox pathology. Redox Biol. 2013, 1, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Trostchansky, A.; Alarcon, M. An Overview of Two Old Friends Associated with Platelet Redox Signaling, the Protein Disulfide Isomerase and NADPH Oxidase. Biomolecules 2023, 13, 848. [Google Scholar] [CrossRef]
- Ansari, S.A.; Keshava, S.; Pendurthi, U.R.; Rao, L.V.M. Oxidative Stress Product, 4-Hydroxy-2-Nonenal, Induces the Release of Tissue Factor-Positive Microvesicles from Perivascular Cells into Circulation. Arter. Thromb. Vasc. Biol. 2021, 41, 250–265. [Google Scholar] [CrossRef]
- Ansari, S.A.; Pendurthi, U.R.; Rao, L.V.M. The lipid peroxidation product 4-hydroxy-2-nonenal induces tissue factor decryption via ROS generation and the thioredoxin system. Blood Adv. 2017, 1, 2399–2413. [Google Scholar] [CrossRef]
- Jourdan, A.; Aguejouf, O.; Imbault, P.; Doutremepuich, F.; Inamo, J.; Doutremepuich, C. Experimental thrombosis model induced by free radicals. Application to aspirin and other different substances. Thromb. Res. 1995, 79, 109–123. [Google Scholar] [CrossRef]
- Berger, M.; Wraith, K.; Woodward, C.; Aburima, A.; Raslan, Z.; Hindle, M.S.; Moellmann, J.; Febbraio, M.; Naseem, K.M. Dyslipidemia-associated atherogenic oxidized lipids induce platelet hyperactivity through phospholipase Cγ2-dependent reactive oxygen species generation. Platelets 2018, 30, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Silverstein, R.L. CD36 signaling in vascular redox stress. Free. Radic. Biol. Med. 2019, 136, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Berberich, A.J.; Hegele, R.A. A Modern Approach to Dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. 1—Overview of Lipids and Atherosclerosis. In Clinical Lipidology, 3rd ed.; Ballantyne, C.M., Ed.; Elsevier: New Delhi, India, 2024; pp. 1–15.e12. [Google Scholar]
- Zhong, S.; Li, L.; Shen, X.; Li, Q.; Xu, W.; Wang, X.; Tao, Y.; Yin, H. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic. Biol. Med. 2019, 144, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Naseem, K.M. Oxidised Low-Density Lipoprotein-Induced Platelet Hyperactivity—Receptors and Signalling Mechanisms. Int. J. Mol. Sci. 2022, 23, 9199. [Google Scholar] [CrossRef] [PubMed]
- Podrez, E.A.; Byzova, T.V.; Febbraio, M.; Salomon, R.G.; Ma, Y.; Valiyaveettil, M.; Poliakov, E.; Sun, M.; Finton, P.J.; Curtis, B.R.; et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat. Med. 2007, 13, 1086–1095. [Google Scholar] [CrossRef]
- Podrez, E.A.; Poliakov, E.; Shen, Z.; Zhang, R.; Deng, Y.; Sun, M.; Finton, P.J.; Shan, L.; Gugiu, B.; Fox, P.L.; et al. Identification of a Novel Family of Oxidized Phospholipids That Serve as Ligands for the Macrophage Scavenger Receptor CD36. J. Biol. Chem. 2002, 277, 38503–38516. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Jamieson, G.A. Platelet glycocalicin. I. Orientation of glycoproteins of the human platelet surface. J. Biol. Chem. 1976, 251, 5944–5949. [Google Scholar] [CrossRef]
- Bendas, G.; Schlesinger, M. The Role of CD36/GPIV in Platelet Biology. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Berger, M.; Raslan, Z.; Aburima, A.; Magwenzi, S.; Wraith, K.S.; Spurgeon, B.E.J.; Hindle, M.S.; Law, R.; Febbraio, M.; Naseem, K.M. Atherogenic lipid stress induces platelet hyperactivity through CD36-mediated hyposensitivity to prostacyclin: The role of phosphodiesterase 3A. Haematologica 2020, 105, 808–819. [Google Scholar] [CrossRef]
- Chen, K.; Febbraio, M.; Li, W.; Silverstein, R.L. A Specific CD36-Dependent Signaling Pathway Is Required for Platelet Activation by Oxidized Low-Density Lipoprotein. Circ. Res. 2008, 102, 1512–1519. [Google Scholar] [CrossRef]
- Zhu, W.; Li, W.; Silverstein, R.L. Advanced glycation end products induce a prothrombotic phenotype in mice via interaction with platelet CD36. Blood 2012, 119, 6136–6144. [Google Scholar] [CrossRef]
- Banesh, S.; Ramakrishnan, V.; Trivedi, V. Mapping of phosphatidylserine recognition region on CD36 ectodomain. Arch. Biochem. Biophys. 2018, 660, 1–10. [Google Scholar] [CrossRef]
- Ghosh, A.; Li, W.; Febbraio, M.; Espinola, R.G.; McCrae, K.R.; Cockrell, E.; Silverstein, R.L. Platelet CD36 mediates interactions with endothelial cell–derived microparticles and contributes to thrombosis in mice. J. Clin. Investig. 2008, 118, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, D.P.; Hajj-Ali, R.A.; Chen, Y.; Silverstein, R.L. Extracellular Vesicles Activate a CD36-Dependent Signaling Pathway to Inhibit Microvascular Endothelial Cell Migration and Tube Formation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.; Magwenzi, S.; Aburima, A.; Naseem, K.M. Thrombospondin-1 induces platelet activation through CD36-dependent inhibition of the cAMP/protein kinase A signaling cascade. Blood 2010, 116, 4297–4306. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.-Y.; Ramakrishnan, D.P.; Silverstein, R.L. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood 2013, 122, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Coburn, C.T.; Knapp, F.F., Jr.; Febbraio, M.; Beets, A.L.; Silverstein, R.L.; Abumrad, N.A. Defective Uptake and Utilization of Long Chain Fatty Acids in Muscle and Adipose Tissues of CD36 Knockout Mice. J. Biol. Chem. 2000, 275, 32523–32529. [Google Scholar] [CrossRef]
- Bachmann, A.; Metwally, N.G.; Allweier, J.; Cronshagen, J.; Tauler, M.d.P.M.; Murk, A.; Roth, L.K.; Torabi, H.; Wu, Y.; Gutsmann, T.; et al. CD36—A Host Receptor Necessary for Malaria Parasites to Establish and Maintain Infection. Microorganisms 2022, 10, 2356. [Google Scholar] [CrossRef] [PubMed]
- Stuart, L.M.; Deng, J.; Silver, J.M.; Takahashi, K.; Tseng, A.A.; Hennessy, E.J.; Ezekowitz, R.A.B.; Moore, K.J. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J. Cell Biol. 2005, 170, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Olonisakin, T.F.; Li, H.; Xiong, Z.; Kochman, E.J.K.; Yu, M.; Qu, Y.; Hulver, M.; Kolls, J.K.; Croix, C.S.; Doi, Y.; et al. CD36 Provides Host Protection against Klebsiella pneumoniae Intrapulmonary Infection by Enhancing Lipopolysaccharide Responsiveness and Macrophage Phagocytosis. J. Infect. Dis. 2016, 214, 1865–1875. [Google Scholar] [CrossRef]
- Dobri, A.-M.; Dudău, M.; Enciu, A.-M.; Hinescu, M.E. CD36 in Alzheimer’s Disease: An Overview of Molecular Mechanisms and Therapeutic Targeting. Neuroscience 2021, 453, 301–311. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, C.; Gao, H.; Bilodeau, M.L.; Zhang, Z.; Croce, K.; Liu, S.; Morooka, T.; Sakuma, M.; Nakajima, K.; et al. Platelet-derived S100 family member myeloid-related protein-14 regulates thrombosis. J. Clin. Investig. 2014, 124, 2160–2171. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, W.; Major, J.; Rahaman, S.O.; Febbraio, M.; Silverstein, R.L. Vav guanine nucleotide exchange factors link hyperlipidemia and a prothrombotic state. Blood 2011, 117, 5744–5750. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Lee, T.; Lee, N.; Yang, E.G.; Lee, C.; Lee, J.; Moon, E.-Y.; Ha, J.; Park, H. Src activates HIF-1α not through direct phosphorylation of HIF-1α-specific prolyl-4 hydroxylase 2 but through activation of the NADPH oxidase/Rac pathway. Carcinogenesis 2011, 32, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Biological chemistry of superoxide radicals. ChemTexts 2020, 6, 7. [Google Scholar] [CrossRef]
- Heppner, D.E. Structural insights into redox-active cysteine residues of the Src family kinases. Redox Biol. 2021, 41, 101934. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, W.; Harberg, C.; Chen, W.; Yue, H.; Ferreira, R.B.; Wynia-Smith, S.L.; Carroll, K.S.; Zielonka, J.; Flaumenhaft, R.; et al. Cysteine sulfenylation by CD36 signaling promotes arterial thrombosis in dyslipidemia. Blood Adv. 2020, 4, 4494–4507. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Kholmukhamedov, A.; Schulte, M.L.; Cooley, B.C.; Scoggins, N.O.; Wood, J.P.; Cameron, S.J.; Morrell, C.N.; Jobe, S.M.; Silverstein, R.L. Platelet CD36 signaling through ERK5 promotes caspase-dependent procoagulant activity and fibrin deposition in vivo. Blood Adv. 2018, 2, 2848–2861. [Google Scholar] [CrossRef]
- Le, N.-T. The significance of ERK5 catalytic-independent functions in disease pathways. Front. Cell Dev. Biol. 2023, 11, 1235217. [Google Scholar] [CrossRef]
- Postiglione, A.E.; Adams, L.L.; Ekhator, E.S.; Odelade, A.E.; Patwardhan, S.; Chaudhari, M.; Pardue, A.S.; Kumari, A.; LeFever, W.A.; Tornow, O.P.; et al. Hydrogen peroxide-dependent oxidation of ERK2 within its D-recruitment site alters its substrate selection. iScience 2023, 26, 107817. [Google Scholar] [CrossRef]
- Nithianandarajah-Jones, G.N.; Wilm, B.; Goldring, C.E.; Müller, J.; Cross, M.J. ERK5: Structure, regulation and function. Cell. Signal. 2012, 24, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Febbraio, M.; Silverstein, R.L. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Investig. 2009, 119, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Netto, L.E.S.; Machado, L.E.S.F. Preferential redox regulation of cysteine-based protein tyrosine phosphatases: Structural and biochemical diversity. FEBS J. 2022, 289, 5480–5504. [Google Scholar] [CrossRef] [PubMed]
- Sonkar, V.K.; Kumar, R.; Jensen, M.; Wagner, B.A.; Sharathkumar, A.A.; Miller, F.J., Jr.; Fasano, M.; Lentz, S.R.; Buettner, G.R.; Dayal, S. Nox2 NADPH oxidase is dispensable for platelet activation or arterial thrombosis in mice. Blood Adv. 2019, 3, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Xu, Y.; Tan, Y.; Shi, H.; Jin, P.; Li, Y.; Teng, J.; Liu, H.; Pan, H.; Hu, Q.; et al. CD36 mediates SARS-CoV-2-envelope-protein-induced platelet activation and thrombosis. Nat. Commun. 2023, 14, 5077. [Google Scholar] [CrossRef] [PubMed]
- Pokrovskaya, I.D.; Yadav, S.; Rao, A.; McBride, E.; Kamykowski, J.A.; Zhang, G.; Aronova, M.A.; Leapman, R.D.; Storrie, B. 3D ultrastructural analysis of α-granule, dense granule, mitochondria, and canalicular system arrangement in resting human platelets. Res. Pract. Thromb. Haemost. 2020, 4, 72–85. [Google Scholar] [CrossRef]
- Cole, L.W. The Evolution of Per-cell Organelle Number. Front. Cell Dev. Biol. 2016, 4, 85. [Google Scholar] [CrossRef]
- Fuentes, E.; Araya-Maturana, R.; Urra, F.A. Regulation of mitochondrial function as a promising target in platelet activation-related diseases. Free. Radic. Biol. Med. 2019, 136, 172–182. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, M.; Huang, W.; Chen, W.; Zhao, Y.; Schulte, M.L.; Volberding, P.; Gerbec, Z.; Zimmermann, M.T.; Zeighami, A.; et al. Mitochondrial Metabolic Reprogramming by CD36 Signaling Drives Macrophage Inflammatory Responses. Circ. Res. 2019, 125, 1087–1102. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Bierl, C.; Voetsch, B.; Jin, R.C.; Handy, D.E.; Loscalzo, J. Determinants of Human Plasma Glutathione Peroxidase (GPx-3) Expression. J. Biol. Chem. 2004, 279, 26839–26845. [Google Scholar] [CrossRef] [PubMed]
- Voetsch, B.; Jin, R.C.; Bierl, C.; Deus-Silva, L.; Camargo, E.C.; Annichino-Bizacchi, J.M.; Handy, D.E.; Loscalzo, J. Role of Promoter Polymorphisms in the Plasma Glutathione Peroxidase (GPx-3) Gene as a Risk Factor for Cerebral Venous Thrombosis. Stroke 2008, 39, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Voetsch, B.; Jin, R.C.; Bierl, C.; Benke, K.S.; Kenet, G.; Simioni, P.; Ottaviano, F.; Damasceno, B.P.; Annichino-Bizacchi, J.M.; Handy, D.E.; et al. Promoter polymorphisms in the plasma glutathione peroxidase (GPx-3) gene: A novel risk factor for arterial ischemic stroke among young adults and children. Stroke 2007, 38, 41–49. [Google Scholar] [CrossRef]
- Dayal, S.; Gu, S.X.; Hutchins, R.D.; Wilson, K.M.; Wang, Y.; Fu, X.; Lentz, S.R. Deficiency of Superoxide Dismutase Impairs Protein C Activation and Enhances Susceptibility to Experimental Thrombosis. Arter. Thromb. Vasc. Biol. 2015, 35, 1798–1804. [Google Scholar] [CrossRef]
- Sonkar, V.K.; Eustes, A.S.; Ahmed, A.; Jensen, M.; Solanki, M.V.; Swamy, J.; Kumar, R.; Fidler, T.P.; Houtman, J.C.; Allen, B.G.; et al. Endogenous SOD2 (Superoxide Dismutase) Regulates Platelet-Dependent Thrombin Generation and Thrombosis During Aging. Arter. Thromb. Vasc. Biol. 2023, 43, 79–91. [Google Scholar] [CrossRef]
- Yang, M.; Chiu, J.; Scartelli, C.; Ponzar, N.; Patel, S.; Patel, A.; Ferreira, R.B.; Keyes, R.F.; Carroll, K.S.; Pozzi, N.; et al. Sulfenylation links oxidative stress to protein disulfide isomerase oxidase activity and thrombus formation. J. Thromb. Haemost. 2023, 21, 2137–2150. [Google Scholar] [CrossRef]
- Badrnya, S.; Schrottmaier, W.C.; Kral, J.B.; Yaiw, K.-C.; Volf, I.; Schabbauer, G.; Söderberg-Nauclér, C.; Assinger, A. Platelets Mediate Oxidized Low-Density Lipoprotein–Induced Monocyte Extravasation and Foam Cell Formation. Arter. Thromb. Vasc. Biol. 2014, 34, 571–580. [Google Scholar] [CrossRef]
- Zheng, T.J.; Kohs, T.C.; Mueller, P.A.; Pang, J.; Reitsma, S.E.; Parra-Izquierdo, I.; Melrose, A.; Yang, L.; Choi, J.; Zientek, K.D.; et al. Effect of antiplatelet agents and tyrosine kinase inhibitors on oxLDL-mediated procoagulant platelet activity. Blood Adv. 2022, 7, 1366–1378. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, S.; Raghavamenon, A.; Garelnabi, M.O.; Santanam, N. Oxidized low-density lipoprotein. Methods Mol. Biol. 2010, 610, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Quan, S.; Schneider, I.; Pan, J.; Von Hacht, A.; Bardwell, J.C.A. The CXXC Motif Is More than a Redox Rheostat. J. Biol. Chem. 2007, 282, 28823–28833. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Furie, B.C.; Coughlin, S.R.; Furie, B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J. Clin. Investig. 2008, 118, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, C.; von Brühl, M.-L.; Manukyan, D.; Grahl, L.; Lorenz, M.; Altmann, B.; Dlugai, S.; Hess, S.; Konrad, I.; Orschiedt, L.; et al. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J. Clin. Investig. 2008, 118, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Jasuja, R.; Passam, F.H.; Kennedy, D.R.; Kim, S.H.; van Hessem, L.; Lin, L.; Bowley, S.R.; Joshi, S.S.; Dilks, J.R.; Furie, B.; et al. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J. Clin. Investig. 2012, 122, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.I.; Schlechter, B.L.; Stopa, J.D.; Liebman, H.A.; Aggarwal, A.; Puligandla, M.; Caughey, T.; Bauer, K.A.; Kuemmerle, N.; Wong, E.; et al. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight 2019, 4, e125851. [Google Scholar] [CrossRef]
- Jasuja, R.; Furie, B.; Furie, B.C. Endothelium-derived but not platelet-derived protein disulfide isomerase is required for thrombus formation in vivo. Blood 2010, 116, 4665–4674. [Google Scholar] [CrossRef]
- Kim, K.; Hahm, E.; Li, J.; Holbrook, L.-M.; Sasikumar, P.; Stanley, R.G.; Ushio-Fukai, M.; Gibbins, J.M.; Cho, J. Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood 2013, 122, 1052–1061. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Y.; Wang, L.; Rauova, L.; Hayes, V.M.; Poncz, M.; Essex, D.W. The C-terminal CGHC motif of protein disulfide isomerase supports thrombosis. J. Clin. Investig. 2015, 125, 4391–4406. [Google Scholar] [CrossRef]
- Xiao, R.; Lundström-Ljung, J.; Holmgren, A.; Gilbert, H.F. Catalysis of thiol/disulfide exchange. Glutaredoxin 1 and protein-disulfide isomerase use different mechanisms to enhance oxidase and reductase activities. J. Biol. Chem. 2005, 280, 21099–21106. [Google Scholar] [CrossRef]
- Saurin, A.T.; Neubert, H.; Brennan, J.P.; Eaton, P. Widespread sulfenic acid formation in tissues in response to hydrogen peroxide. Proc. Natl. Acad. Sci. USA 2004, 101, 17982–17987. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991, 266, 4244–4250. [Google Scholar] [CrossRef]
- Gaspar, R.S.; Laurindo, F.R.M. Sulfenylation: An emerging element of the protein disulfide isomerase code for thrombosis. J. Thromb. Haemost. 2023, 21, 2054–2057. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Yang, J.; Liebler, D.C.; Carroll, K.S. Diverse Redoxome Reactivity Profiles of Carbon Nucleophiles. J. Am. Chem. Soc. 2017, 139, 5588–5595. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P. Kinetics and Mechanisms of Thiol–Disulfide Exchange Covering Direct Substitution and Thiol Oxidation-Mediated Pathways. Antioxid. Redox Signal. 2013, 18, 1623–1641. [Google Scholar] [CrossRef]
- Kaetzel, C.S.; Rao, C.K.; Lamm, M.E. Protein disulphide-isomerase from human placenta and rat liver. Purification and immunological characterization with monoclonal antibodies. Biochem. J. 1987, 241, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Mandel, R.; Ryser, H.J.; Ghani, F.; Wu, M.; Peak, D. Inhibition of a reductive function of the plasma membrane by bacitracin and antibodies against protein disulfide-isomerase. Proc. Natl. Acad. Sci. USA 1993, 90, 4112–4116. [Google Scholar] [CrossRef]
- Wu, Y.; Ahmad, S.S.; Zhou, J.; Wang, L.; Cully, M.P.; Essex, D.W. The disulfide isomerase ERp57 mediates platelet aggregation, hemostasis, and thrombosis. Blood 2012, 119, 1737–1746. [Google Scholar] [CrossRef]
- Shi, Y.; Carroll, K.S. Activity-Based Sensing for Site-Specific Proteomic Analysis of Cysteine Oxidation. Acc Chem. Res. 2020, 53, 20–31. [Google Scholar] [CrossRef]
- Gupta, V.; Carroll, K.S. Profiling the reactivity of cyclic C-nucleophiles towards electrophilic sulfur in cysteine sulfenic acid. Chem. Sci. 2016, 7, 400–415. [Google Scholar] [CrossRef]
- Boike, L.; Henning, N.J.; Nomura, D.K. Advances in covalent drug discovery. Nat. Rev. Drug Discov. 2022, 21, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Benitez, L.V.; Allison, W.S. The inactivation of the acyl phosphatase activity catalyzed by the sulfenic acid form of glycer-aldehyde 3-phosphate dehydrogenase by dimedone and olefins. J. Biol. Chem. 1974, 249, 6234–6243. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Jung, Y.; Tian, C.; Ferreira, R.B.; Cheng, R.; He, F.; Yang, J.; Carroll, K.S. Nucleophilic covalent ligand discovery for the cysteine redoxome. Nat. Chem. Biol. 2023, 19, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Backus, K.M.; Correia, B.E.; Lum, K.M.; Forli, S.; Horning, B.D.; González-Páez, G.E.; Chatterjee, S.; Lanning, B.R.; Teijaro, J.R.; Olson, A.J.; et al. Proteome-wide covalent ligand discovery in native biological systems. Nature 2016, 534, 570–574. [Google Scholar] [CrossRef]
- Bak, D.W. Flipping the polarity switch. Nat. Chem. Biol. 2023, 19, 1292–1293. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Zabetakis, I.; Lordan, R. Platelet aggregometry assay for evaluating the effects of platelet agonists and antiplatelet compounds on platelet function in vitro. MethodsX 2019, 6, 63–70. [Google Scholar] [CrossRef]
- Kholmukhamedov, A.; Jobe, S. Mitochondria and Platelet Cell Death. Thromb. Haemost. 2017, 117, 2207–2208. [Google Scholar] [CrossRef]
- Huang, W.; Li, R.; Zhang, J.; Cheng, Y.; Ramakrishnan, D.P.; Silverstein, R.L. A CD36 transmembrane domain peptide interrupts CD36 interactions with membrane partners on macrophages and inhibits atherogenic functions. Transl. Res. 2023, 254, 68–76. [Google Scholar] [CrossRef]
- Chen, Y.; Kennedy, D.J.; Ramakrishnan, D.P.; Yang, M.; Huang, W.; Li, Z.; Xie, Z.; Chadwick, A.C.; Sahoo, D.; Silverstein, R.L. Oxidized LDL–bound CD36 recruits an Na+/K+-ATPase–Lyn complex in macrophages that promotes atherosclerosis. Sci. Signal. 2015, 8, ra91. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; Yang, M.; Xin, G.; Cui, W.; Xie, Z.; Silverstein, R.L. Cardiotonic Steroids Stimulate Macrophage Inflammatory Responses Through a Pathway Involving CD36, TLR4, and Na/K-ATPase. Arter. Thromb. Vasc. Biol. 2017, 37, 1462–1469. [Google Scholar] [CrossRef]
- Miao, W.-M.; Vasile, E.; Lane, W.S.; Lawler, J. CD36 associates with CD9 and integrins on human blood platelets. Blood 2001, 97, 1689–1696. [Google Scholar] [CrossRef]
- Julio, A.R.; Shikwana, F.; Truong, C.; Burton, N.R.; Dominguez, E.; Turmon, A.; Cao, J.; Backus, K. Pervasive aggregation and depletion of host and viral proteins in response to cysteine-reactive electrophilic compounds. bioRxiv 2023. [Google Scholar] [CrossRef]
- Sonego, G.; Le, T.M.; Crettaz, D.; Abonnenc, M.; Tissot, J.; Prudent, M. Sulfenylome analysis of pathogen-inactivated platelets reveals the presence of cysteine oxidation in integrin signaling pathway and cytoskeleton regulation. J. Thromb. Haemost. 2021, 19, 233–247. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Silverstein, R.L. Targeting Cysteine Oxidation in Thrombotic Disorders. Antioxidants 2024, 13, 83. https://doi.org/10.3390/antiox13010083
Yang M, Silverstein RL. Targeting Cysteine Oxidation in Thrombotic Disorders. Antioxidants. 2024; 13(1):83. https://doi.org/10.3390/antiox13010083
Chicago/Turabian StyleYang, Moua, and Roy L. Silverstein. 2024. "Targeting Cysteine Oxidation in Thrombotic Disorders" Antioxidants 13, no. 1: 83. https://doi.org/10.3390/antiox13010083




