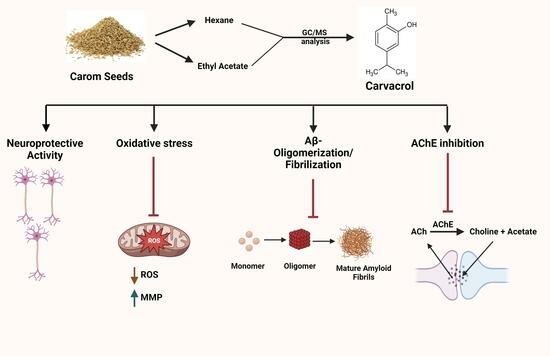
Trachyspermum ammi Bioactives Promote Neuroprotection by Inhibiting Acetylcholinesterase, Aβ-Oligomerization/Fibrilization, and Mitigating Oxidative Stress In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Extraction
2.3. Gas Chromatography–Mass Spectrometry (GC-MS) Method
2.4. Determination of Total Phenolic Content
2.5. Determination of Total Flavonoids Content
2.6. Determination of Antioxidant Capacity
2.6.1. 2,2′-Azino-bis (3-Ethylbenzothiazoline-6-Sulfonic Acid) [ABTS] Radical Scavenging Assay
2.6.2. Free Radical Scavenging using a 2,2-Diphenyl-1-picrylhydrazylhydrate (DPPH) Radical Assay
2.6.3. Ferric-Reducing Antioxidant Potential (FRAP) Assay
2.7. Anti-Acetylcholinesterase Activity
2.8. Anti-Advanced Glycation End-Product (AGE) Activity
2.9. Anti-Aβ1–42-Fibrilization Activity
2.10. Anti-Aβ1–42-Oligomerization Activity
2.11. Cell Culture
2.11.1. Cell Viability Assay
2.11.2. Neuroprotective Activity Assay
2.11.3. Measurement of Intracellular Reactive Oxygen Species
2.11.4. Mitochondrial Membrane Potential (ΔΨm) Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Evaluation and Antioxidant Ability of Carom Extract
3.2. GC–MS Analysis of Carom Extract
3.3. In Vitro Anti-Acetylcholinesterase Activity
3.4. In Vitro Anti-Glycation Potential of Carom
3.5. Effect of Carom on Aβ-Fibrilization and Oligomerization
3.6. Non-Toxic Effect of Carom in SH-SY5Y Cells
3.7. Neuroprotective Effect of Carom against H2O2-Induced Oxidative Stress in SH-SY5Y Cells
3.8. Alleviation of ROS Levels in SH-SY5Y Cells via Carom
3.9. Protective Effect of Carom Extract on Mitochondrial Membrane Potential of SH-SY5Y Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimer’s Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement 2023, 19, 1598–1695. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid.-Based Complement. Altern. Med. 2020, 2020, 6565396. [Google Scholar] [CrossRef] [PubMed]
- Zarshenas, M.M.; Moein, M.; Samani, S.M.; Petramfar, P. An overview on ajwain (Trachyspermum ammi) pharmacological effects; modern and traditional. J. Nat. Remedies 2013, 14, 98–105. [Google Scholar]
- Preedy, V.R.; Watson, R.R. Nuts and Seeds in Health and Disease Prevention, 2nd ed.; Academic Press: Cambridge, MA, USA, 2020; p. 598. ISBN 9780128185537. [Google Scholar]
- Singh, H.; Meghwal, M. Ajwain a potential source of phytochemical for better health. Pharma Innov 2019, 8, 599–603. [Google Scholar]
- Timalsina, B.; Haque, M.N.; Choi, H.J.; Dash, R.; Moon, I.S. Thymol in Trachyspermum ammi seed extract exhibits neuroprotection, learning, and memory enhancement in scopolamine-induced Alzheimer’s disease mouse model. Phytother. Res. 2023, 37, 2811–2826. [Google Scholar] [CrossRef]
- Sachan, N.; Saraswat, N.; Chandra, P.; Khalid, M.; Kabra, A. Isolation of Thymol from Trachyspermum ammi Fruits for Treatment of Diabetes and Diabetic Neuropathy in STZ-Induced Rats. Biomed. Res. Int. 2022, 2022, 8263999. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.H.; Choi, S.; Choi, B.Y.; Suh, S.W. Carvacrol Inhibits Expression of Transient Receptor Potential Melastatin 7 Channels and Alleviates Zinc Neurotoxicity Induced by Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 13840. [Google Scholar] [CrossRef]
- Haddadi, H.; Rajaei, Z.; Alaei, H.; Shahidani, S. Chronic treatment with carvacrol improves passive avoidance memory in a rat model of Parkinson’s disease. Arq. De Neuro-Psiquiatr. 2018, 76, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Manouchehrabadi, M.; Farhadi, M.; Azizi, Z.; Torkaman-Boutorabi, A. Carvacrol protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro models of Parkinson’s disease. Neurotox. Res. 2020, 37, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Azizi, Z.; Majlessi, N.; Choopani, S.; Naghdi, N. Neuroprotective effects of carvacrol against Alzheimer’s disease and other neurodegenerative diseases: A review. Avicenna J. Phytomedicine 2022, 12, 371–387. [Google Scholar]
- Azizi, Z.; Ebrahimi, S.; Saadatfar, E.; Kamalinejad, M.; Majlessi, N. Cognitive-enhancing activity of thymol and carvacrol in two rat models of dementia. Behav. Pharmacol. 2012, 23, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Packer, L., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar] [CrossRef]
- Ribarova, F.; Atanassova, M.; Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. JU Chem. Met. 2005, 40, 255–260. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.; Groot, A.d.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. Int. J. Plant Chem. Biochem. Technol. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Singh, P.; Jayaramaiah, R.H.; Agawane, S.B.; Vannuruswamy, G.; Korwar, A.M.; Anand, A.; Dhaygude, V.S.; Shaikh, M.L.; Joshi, R.S.; Boppana, R.; et al. Potential Dual Role of Eugenol in Inhibiting Advanced Glycation End Products in Diabetes: Proteomic and Mechanistic Insights. Sci. Rep. 2016, 6, 18798. [Google Scholar] [CrossRef]
- Tan, M.A.; Tan, B.L.U.; Nonato, M.G.; An, S.S.A. Neuroprotective effects on amyloid-beta induced cytotoxicity of Pandanus clementis Merr. 3 Biotech 2021, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Kim, D.Y.; Shim, K.H.; Sharma, N.; An, S.S.A. Multi-Targeting Neuroprotective Effects of Syzygium aromaticum Bud Extracts and Their Key Phytocompounds against Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 8148. [Google Scholar] [CrossRef] [PubMed]
- Alvariño, R.; Alonso, E.; Lacret, R.; Oves-Costales, D.; Genilloud, O.; Reyes, F.; Alfonso, A.; Botana, L.M. Caniferolide A, a macrolide from Streptomyces caniferus, attenuates neuroinflammation, oxidative stress, amyloid-beta, and tau pathology in vitro. Mol. Pharm. 2019, 16, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D. Girija. Evaluation of in vitro antioxidant and antimicrobial activities of various spices of Indian origin. Int. J. Pharm. Pharm. Sci. 2015, 8, 137–141. [Google Scholar]
- Goswami, N.; Chatterjee, S. Assessment of free radical scavenging potential and oxidative DNA damage preventive activity of Trachyspermum ammi L. (carom) and Foeniculum vulgare Mill. (fennel) seed extracts. Biomed. Res. Int. 2014, 2014, 582767. [Google Scholar] [CrossRef] [PubMed]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules 2011, 16, 7672–7690. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A. Therapeutic application of carvacrol: A comprehensive review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef]
- Güneş Bayır, A.; Kızıltan, H.Ş.; Koçyiğit, A. Dietary Interventions in Gastrointestinal Diseases: Foods, Nutrients, and Dietary Supplements, 1st ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; p. 358. ISBN 9780128144688. [Google Scholar]
- Liu, Y.; Wei, J.; Ma, K.-T.; Li, C.-L.; Mai, Y.-P.; Qiu, X.-X.; Wei, H.; Hou, N.; Luo, J.-D. Carvacrol protects against diabetes-induced hypercontractility in the aorta through activation of the PI3K/Akt pathway. Biomed. Pharmacother. 2020, 125, 109825. [Google Scholar] [CrossRef]
- Spalletta, S.; Flati, V.; Toniato, E.; Di Gregorio, J.; Marino, A.; Pierdomenico, L.; Marchisio, M.; D’Orazi, G.; Cacciatore, I.; Robuffo, I. Carvacrol reduces adipogenic differentiation by modulating autophagy and ChREBP expression. PLoS ONE 2018, 13, e0206894. [Google Scholar] [CrossRef] [PubMed]
- Rolim, M.d.O.P.; de Almeida, A.R.; da Rocha Pitta, M.G.; de Melo Rêgo, M.J.B.; Quintans-Júnior, L.J.; Quintans, J.d.S.S.; Heimfarth, L.; Scotti, L.; Scotti, M.T.; da Cruz, R.M.D. Design, synthesis and pharmacological evaluation of CVIB, a codrug of carvacrol and ibuprofen as a novel anti-inflammatory agent. Int. Immunopharmacol. 2019, 76, 105856. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Maio, A.; Nostro, A. Poly (lactic acid)/carvacrol-based materials: Preparation, physicochemical properties, and antimicrobial activity. Appl. Microbiol. Biotechnol. 2020, 104, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Ezz-Eldin, Y.M.; Aboseif, A.A.; Khalaf, M.M. Potential anti-inflammatory and immunomodulatory effects of carvacrol against ovalbumin-induced asthma in rats. Life Sci. 2020, 242, 117222. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.H.; Mohamed, H.H.; Elsabagh, D.A.; Mohamed, S.A.; Noreldin, A.E.; Al Jaouni, S.K.; Alsenosy, A.A. Eugenol and carvacrol attenuate brain d-galactose-induced aging-related oxidative alterations in rats. Environ. Sci. Pollut. Res. 2022, 29, 47436–47447. [Google Scholar] [CrossRef] [PubMed]
- Sisti, F.M.; Dos Santos, N.A.G.; do Amaral, L.; Dos Santos, A.C. The neurotrophic-like effect of carvacrol: Perspective for axonal and synaptic regeneration. Neurotox. Res. 2021, 39, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bhardwaj, M.; Shukla, S.; Min, S.H.; Choi, D.K.; Bajpai, V.K.; Huh, Y.S.; Kang, S.C. Carvacrol inhibits cytochrome P450 and protects against binge alcohol-induced liver toxicity. Food Chem. Toxicol. 2019, 131, 110582. [Google Scholar] [CrossRef]
- A Food Additive Database. Center for Food Safety and Applied Nutrition, Office of Premarket Approva. 2004. Available online: https://www.fda.gov/food/food-additives-petitions/substances-added-food-formerly-eafus (accessed on 5 September 2023).
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- FDA. Substances Added to Food. 2023. Available online: https://www.accessdata.fda.gov/scripts/fdcc/?set=FoodSubstances&sort=Sortterm&order=ASC&startrow=1&type=basic&search=thymol (accessed on 8 September 2023).
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Hung, N.H.; Quan, P.M.; Satyal, P.; Dai, D.N.; Hoa, V.V.; Huy, N.G.; Giang, L.D.; Ha, N.T.; Huong, L.T.; Hien, V.T. Acetylcholinesterase Inhibitory Activities of Essential Oils from Vietnamese Traditional Medicinal Plants. Molecules 2022, 27, 7092. [Google Scholar] [CrossRef]
- Kurt, B.Z.; Gazioglu, I.; Dag, A.; Salmas, R.E.; Kayık, G.; Durdagi, S.; Sonmez, F. Synthesis, anticholinesterase activity and molecular modeling study of novel carbamate-substituted thymol/carvacrol derivatives. Bioorganic Med. Chem. 2017, 25, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Nazzaro, F. Anti-Cholinesterase and Anti-α-Amylase Activities and Neuroprotective Effects of Carvacrol and p-Cymene and Their Effects on Hydrogen Peroxide Induced Stress in SH-SY5Y Cells. Int. J. Mol. Sci. 2023, 24, 6073. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Kartal, M.; Kan, Y.; Şener, B. Activity of essential oils and individual components against acetyland butyrylcholinesterase. Z. Fuer Naturforschung C 2008, 63, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Jukic, M.; Politeo, O.; Maksimovic, M.; Milos, M.; Milos, M. In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother. Res. 2007, 21, 259–261. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.M.; Andreolla, M.C.; Ribeiro, T.C.; Gonçalves, A.E.; Medeiros, A.R.; de Souza, A.S.; Ferreira, L.L.G.; Andricopulo, A.D.; Yunes, R.A.; de Oliveira, A.S. Structure-activity relationships of sulfonamides derived from carvacrol and their potential for the treatment of Alzheimer’s disease. RSC Med. Chem. 2020, 11, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.G.; da Costa, R.A.; de Oliveira, M.S.; da Cruz, J.N.; Figueiredo, P.L.B.; Brasil, D.d.S.B.; Nascimento, L.D.; Chaves Neto, A.M.d.J.; de Carvalho Junior, R.N.; Andrade, E.H.d.A. Chemical profile of Lippia thymoides, evaluation of the acetylcholinesterase inhibitory activity of its essential oil, and molecular docking and molecular dynamics simulations. PLoS ONE 2019, 14, e0213393. [Google Scholar] [CrossRef] [PubMed]
- Tupe, R.S.; Sankhe, N.M.; Shaikh, S.A.; Kemse, N.G.; Khaire, A.A.; Phatak, D.V.; Parikh, J.U. Nutraceutical properties of dietary plants extracts: Prevention of diabetic nephropathy through inhibition of glycation and toxicity to erythrocytes and HEK293 cells. Pharm. Biol. 2015, 53, 40–50. [Google Scholar] [CrossRef]
- Ahmed, H. Cumin Seeds: The Functional Food with an Ability to Inhibit the Deleterious Phenomenon of Glycation. Zia. J. Pharm. 2021, 2, 62–66. [Google Scholar]
- Morimitsu, Y.; Yoshida, K.; Esaki, S.; Hirota, A. Protein glycation inhibitors from thyme (Thymus vulgaris). Biosci. Biotechnol. Biochem. 1995, 59, 2018–2021. [Google Scholar] [CrossRef]
- Abbasi, S.; Gharaghani, S.; Benvidi, A.; Rezaeinasab, M. New insights into the efficiency of thymol synergistic effect with p-cymene in inhibiting advanced glycation end products: A multi-way analysis based on spectroscopic and electrochemical methods in combination with molecular docking study. J. Pharm. Biomed. Anal. 2018, 150, 436–451. [Google Scholar] [CrossRef]
- Voropai, E.; Samtsov, M.; Kaplevskii, K.; Maskevich, A.; Stepuro, V.; Povarova, O.; Kuznetsova, I.; Turoverov, K.; Fink, A.; Uverskii, V. Spectral properties of thioflavin T and its complexes with amyloid fibrils. J. Appl. Spectrosc. 2003, 70, 868–874. [Google Scholar] [CrossRef]
- Gour, N.; Koshti, B.; Kshtriya, V.S. A Chemical Perspective to the Anti-Amyloid Action of Compounds and a Nanoparticle Based Assay for Screening Amyloid Inhibitors. ChemRxiv 2019. [Google Scholar]
- Sharoar, M.G.; Thapa, A.; Shahnawaz, M.; Ramasamy, V.S.; Woo, E.-R.; Shin, S.Y.; Park, I.-S. Keampferol-3-O-rhamnoside abrogates amyloid beta toxicity by modulating monomers and remodeling oligomers and fibrils to non-toxic aggregates. J. Biomed. Sci. 2012, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Gazit, E. Mechanisms of amyloid fibril self-assembly and inhibition: Model short peptides as a key research tool. FEBS J. 2005, 272, 5971–5978. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, Y.; Nishimura, S.; Murasugi, T.; Kaneko, I.; Meguro, M.; Marumoto, S.; Kogen, H.; Koyama, K.; Oda, T. A novel β-sheet breaker, RS-0406, reverses amyloid β-induced cytotoxicity and impairment of long-term potentiation in vitro. Br. J. Pharmacol. 2002, 137, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Amtul, Z.; Westaway, D.; Cechetto, D.F.; Rozmahel, R.F. Oleic acid ameliorates amyloidosis in cellular and mouse models of Alzheimer’s disease. Brain Pathol. 2011, 21, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.P.; Kumar, N.; Chauhan, B.S.; Garg, P. Carbamate as a potential anti-Alzheimer’s pharmacophore: A review. Drug Dev. Res. 2023, 84, 1624–1651. [Google Scholar] [CrossRef]
- Song, J.; Kim, Y.-S.; Lee, D.H.; Lee, S.H.; Park, H.J.; Lee, D.; Kim, H. Neuroprotective effects of oleic acid in rodent models of cerebral ischaemia. Sci. Rep. 2019, 9, 10732. [Google Scholar] [CrossRef]
- Im, D.; Kim, S.; Yoon, G.; Hyun, D.G.; Eom, Y.-G.; Lee, Y.E.; Sohn, C.H.; Choi, J.-M.; Kim, H.I. Decoding the Roles of Amyloid-β (1–42)’s Key Oligomerization Domains toward Designing Epitope-Specific Aggregation Inhibitors. JACS Au 2023, 3, 1065–1075. [Google Scholar] [CrossRef]
- Kobayashi, H.; Murata, M.; Kawanishi, S.; Oikawa, S. Polyphenols with Anti-Amyloid β Aggregation Show Potential Risk of Toxicity Via Pro-Oxidant Properties. Int. J. Mol. Sci. 2020, 21, 3561. [Google Scholar] [CrossRef]
- Necula, M.; Kayed, R.; Milton, S.; Glabe, C.G. Small Molecule Inhibitors of Aggregation Indicate That Amyloid β Oligomerization and Fibrillization Pathways Are Independent and Distinct. J. Biol. Chem. 2007, 282, 10311–10324. [Google Scholar] [CrossRef] [PubMed]
- Orlando, R.A.; Gonzales, A.M.; Royer, R.E.; Deck, L.M.; Vander Jagt, D.L. A chemical analog of curcumin as an improved inhibitor of amyloid Abeta oligomerization. PLoS ONE 2012, 7, e31869. [Google Scholar] [CrossRef] [PubMed]
- Azizi, Z.; Salimi, M.; Amanzadeh, A.; Majelssi, N.; Naghdi, N. Carvacrol and thymol attenuate cytotoxicity induced by amyloid β25-35 via activating protein kinase C and inhibiting oxidative stress in PC12 cells. Iran. Biomed. J. 2020, 24, 243. [Google Scholar] [CrossRef]
- Aydin, E.; Turkez, H.; Keles, M.S. The effect of carvacrol on healthy neurons and N2a cancer cells: Some biochemical, anticancerogenicity and genotoxicity studies. Cytotechnology 2014, 66, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.-w.; Xie, Z.-x.; Wang, B.-f.; Zhong, Z.-h.; Chen, X.-y.; Sun, Y.-h.; Sun, Q.-f.; Yang, G.-y.; Bian, L.-g. Carvacrol protects neuroblastoma SH-SY5Y cells against Fe2+-induced apoptosis by suppressing activation of MAPK/JNK-NF-κB signaling pathway. Acta Pharmacol. Sin. 2015, 36, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.A.; Targowska-Duda, K.; Klimek, K.; Ginalska, G.; Jóźwiak, K.; Waksmundzka-Hajnos, M.; Cieśla, Ł. Volatile terpenoids as potential drug leads in Alzheimer’s disease. Open Chem. 2017, 15, 332–343. [Google Scholar] [CrossRef]
- Smolecule. Vinyltriphenylphosphonium Bromide. 2023. Available online: https://www.smolecule.com/products/s714471 (accessed on 10 September 2023).
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Chen, H.Y. Why the Reactive Oxygen Species of the Fenton Reaction Switches from Oxoiron(IV) Species to Hydroxyl Radical in Phosphate Buffer Solutions? A Computational Rationale. ACS Omega 2019, 4, 14105–14113. [Google Scholar] [CrossRef]
- Pradeep, K.; Raj Mohan, C.V.; Gobianand, K.; Karthikeyan, S. Protective effect of Cassia fistula Linn. on diethylnitrosamine induced hepatocellular damage and oxidative stress in ethanol pretreated rats. Biol. Res. 2010, 43, 113–125. [Google Scholar] [CrossRef]
- Banik, S.; Rahman, M.M.; Sikder, M.T.; Saito, T.; Kurasaki, M. Protective effects of ajwain (Trachyspermum ammi L.) extract against cadmium-induced cytotoxicity and apoptosis in PC12 cells. J. Herb. Med. 2021, 26, 100423. [Google Scholar] [CrossRef]
- Wei, H.K.; Xue, H.X.; Zhou, Z.X.; Peng, J. A carvacrol–thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal 2017, 11, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Chenet, A.L.; Duarte, A.R.; de Almeida, F.J.S.; Andrade, C.M.B.; de Oliveira, M.R. Carvacrol Depends on Heme Oxygenase-1 (HO-1) to Exert Antioxidant, Anti-inflammatory, and Mitochondria-Related Protection in the Human Neuroblastoma SH-SY5Y Cells Line Exposed to Hydrogen Peroxide. Neurochem. Res. 2019, 44, 884–896. [Google Scholar] [CrossRef]
- Banik, S.; Akter, M.; Corpus Bondad, S.E.; Saito, T.; Hosokawa, T.; Kurasaki, M. Carvacrol inhibits cadmium toxicity through combating against caspase dependent/independent apoptosis in PC12 cells. Food Chem. Toxicol. 2019, 134, 110835. [Google Scholar] [CrossRef]
- Tiefensee Ribeiro, C.; Gasparotto, J.; Petiz, L.L.; Brum, P.O.; Peixoto, D.O.; Kunzler, A.; da Rosa Silva, H.T.; Bortolin, R.C.; Almeida, R.F.; Quintans-Junior, L.J.; et al. Oral administration of carvacrol/β-cyclodextrin complex protects against 6-hydroxydopamine-induced dopaminergic denervation. Neurochem. Int. 2019, 126, 27–35. [Google Scholar] [CrossRef]
- Meeran, M.N.; Jagadeesh, G.; Selvaraj, P. Thymol, a dietary monoterpene phenol abrogates mitochondrial dysfunction in β-adrenergic agonist induced myocardial infarcted rats by inhibiting oxidative stress. Chem.-Biol. Interact. 2016, 244, 159–168. [Google Scholar] [CrossRef]
- Shettigar, N.B.; Das, S.; Rao, N.B.; Rao, S.B. Thymol, a monoterpene phenolic derivative of cymene, abrogates mercury-induced oxidative stress resultant cytotoxicity and genotoxicity in hepatocarcinoma cells. Environ. Toxicol. 2015, 30, 968–980. [Google Scholar] [CrossRef]









| Vmax (μmol/min/mg) | Km (mM) | Inhibition | |
|---|---|---|---|
| No Inhibitor | 1.096 | 5.02 | |
| Carom-H (50 μg/mL) | 1.110 | 7.08 | |
| Carom-H (100 μg/mL) | 1.021 | 7.96 | Competitive |
| Carom-EA (50 μg/mL) | 1.196 | 7.72 | |
| Carom-EA (100 μg/mL) | 1.214 | 8.64 | Competitive |
| Carvacrol (50 μg/mL) | 1.114 | 14.76 | |
| Carvacrol (100 μg/mL) | 1.174 | 20.15 | Mixed |
| Thymol (50 μg/mL) | 1.117 | 6.37 | |
| Thymol (100 μg/mL) | 1.198 | 7.33 | Competitive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, H.; Yang, H.; Sharma, N.; An, S.S.A. Trachyspermum ammi Bioactives Promote Neuroprotection by Inhibiting Acetylcholinesterase, Aβ-Oligomerization/Fibrilization, and Mitigating Oxidative Stress In Vitro. Antioxidants 2024, 13, 9. https://doi.org/10.3390/antiox13010009
Sharma H, Yang H, Sharma N, An SSA. Trachyspermum ammi Bioactives Promote Neuroprotection by Inhibiting Acetylcholinesterase, Aβ-Oligomerization/Fibrilization, and Mitigating Oxidative Stress In Vitro. Antioxidants. 2024; 13(1):9. https://doi.org/10.3390/antiox13010009
Chicago/Turabian StyleSharma, Himadri, Hyewon Yang, Niti Sharma, and Seong Soo A An. 2024. "Trachyspermum ammi Bioactives Promote Neuroprotection by Inhibiting Acetylcholinesterase, Aβ-Oligomerization/Fibrilization, and Mitigating Oxidative Stress In Vitro" Antioxidants 13, no. 1: 9. https://doi.org/10.3390/antiox13010009
APA StyleSharma, H., Yang, H., Sharma, N., & An, S. S. A. (2024). Trachyspermum ammi Bioactives Promote Neuroprotection by Inhibiting Acetylcholinesterase, Aβ-Oligomerization/Fibrilization, and Mitigating Oxidative Stress In Vitro. Antioxidants, 13(1), 9. https://doi.org/10.3390/antiox13010009