Signaling Paradigms of H2S-Induced Vasodilation: A Comprehensive Review
Abstract
:1. Introduction
2. Methodology for Systematic Literature Review
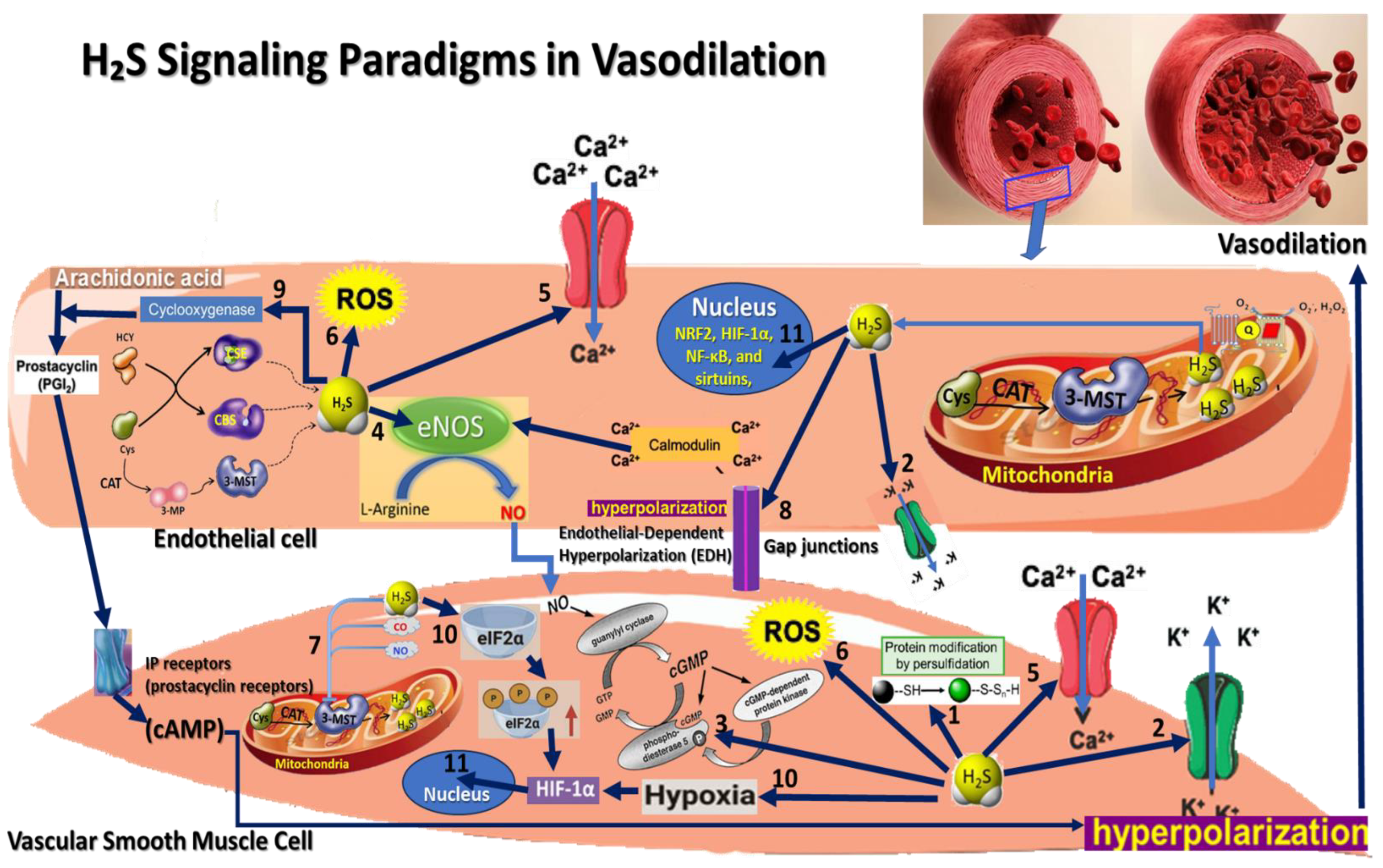
3. Hydrogen Sulfide Signaling Pathways Paradigms in Vasodilation
3.1. Persulfidation Signaling
3.2. K_ATP Channel Activation Paradigm
3.3. cGMP Pathway Activation Paradigm
3.4. Endothelial Nitric Oxide Synthase (eNOS) Activation Paradigm
3.5. Calcium Signaling Modulation Paradigm
3.6. Redox Signaling and Antioxidant Effects Paradigm
3.7. Interaction with Other Gasotransmitters Paradigm
3.8. Endothelial-Dependent Hyperpolarization (EDH) Paradigm
3.9. Interaction with Prostacyclin Signaling Paradigm
3.10. Hypoxia Response Paradigm
3.11. H2S Interaction with Transcription Factors
4. Therapeutic Mechanisms of H2S Resulted from Inducing Vasodilation
5. Preclinical and Clinical Evidence
5.1. In Vitro Evidence
5.2. Preclinical Evidence
5.3. Clinical Evidence
6. Discussion and Future Directions and Challenges
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Munteanu, C.; Dumitrascu, M. Therapeutic muds. Balneo Res. J. 2011, 2, 12–16. [Google Scholar] [CrossRef]
- Hoteteu, M.; Munteanu, C.; Ionescu, E.V.; Almășan, R.E. Bioactive substances of the Techirghiol therapeutic mud. Balneo Res. J. 2018, 9, 5–10. [Google Scholar] [CrossRef]
- Carbajo, J.M.; Maraver, F. Sulphurous mineral waters: New applications for health. Evid.-Based Complement. Altern. Med. 2017, 2017, 8034084. [Google Scholar] [CrossRef]
- Smith, H.M.; Pluth, M.D. Advances and Opportunities in H2S Measurement in Chemical Biology. JACS Au 2023, 3, 2677–2691. [Google Scholar] [CrossRef]
- Cheleschi, S.; Tenti, S.; Seccafico, I.; Gálvez, I.; Fioravanti, A.; Ortega, E. Balneotherapy year in review 2021: Focus on the mechanisms of action of balneotherapy in rheumatic diseases. Environ. Sci. Pollut. Res. 2022, 29, 8054–8073. [Google Scholar] [CrossRef]
- Carretero, M.I. Clays in pelotherapy. A review. Part I: Mineralogy, chemistry, physical and physicochemical properties. Appl. Clay Sci. 2020, 189, 105526. [Google Scholar] [CrossRef]
- Carretero, M.I. Clays in pelotherapy. A review. Part II: Organic compounds, microbiology and medical applications. Appl. Clay Sci. 2020, 189, 105531. [Google Scholar] [CrossRef]
- Gomes, C.; Carretero, M.I.; Pozo, M.; Maraver, F.; Cantista, P.; Armijo, F.; Legido, J.L.; Teixeira, F.; Rautureau, M.; Delgado, R. Peloids and pelotherapy: Historical evolution, classification and glossary. Appl. Clay Sci. 2013, 75–76, 28–38. [Google Scholar] [CrossRef]
- Munteanu, C.; Popescu, C.; Munteanu, D.; Hoteteu, M.; Iliescu, M.G.; Ionescu, E.V.; Stanciu, L.; Oprea, D.; Minea, M.; Oprea, C.; et al. Biological Evaluation of Balneotherapeutic Mud and Sulfurous Mineral Waters: Insights from In Vivo and In Vitro Studies. Balneo PRM Res. J. 2024, 15, 702. [Google Scholar] [CrossRef]
- Munteanu, C.; Hoteteu, M.; Munteanu, D.; Onose, G. The effects of Mineral Waters from Slănic Moldova’s Spring 1 and Spring 1 bis on Fibroblast activity: An In Vitro Study. Balneo PRM Res. J. 2023, 14, 1. [Google Scholar] [CrossRef]
- Munteanu, C.; Hoteteu, M.; Munteanu, D.; Onose, G. Mineral waters from Spring 1 and Spring 1 bis from Slănic Moldova—Molecular mechanisms responsible for triggering the prophylactic and therapeutic effects. Balneo PRM Res. J. 2023, 14, 592. [Google Scholar] [CrossRef]
- Albertini, M.C.; Dacha, M.; Teodori, L.; Conti, M.E. Drinking mineral waters: Biochemical effects and health implications the state-of-the-art. Int. J. Environ. Health 2007, 1, 153–169. [Google Scholar] [CrossRef]
- Baricz, A.; Levei, E.A.; Șenilă, M.; Pînzaru, S.C.; Aluaş, M.; Vulpoi, A.; Filip, C.; Tripon, C.; Dădârlat, D.; Buda, D.M.; et al. Comprehensive mineralogical and physicochemical characterization of recent sapropels from Romanian saline lakes for potential use in pelotherapy. Sci. Rep. 2021, 11, 18633. [Google Scholar] [CrossRef]
- Ma, T.; Song, X.; Ma, Y.; Hu, H.; Bai, H.; Li, Y.; Gao, L. The effect of thermal mineral waters on pain relief, physical function and quality of life in patients with osteoarthritis A systematic review and meta-analysis. Medicine 2021, 100, e24488. [Google Scholar] [CrossRef]
- Protano, C.; Vitali, M.; De Giorgi, A.; Marotta, D.; Crucianelli, S.; Fontana, M. Balneotherapy using thermal mineral water baths and dermatological diseases: A systematic review. Int. J. Biometeorol. 2024, 68, 1005–1013. [Google Scholar] [CrossRef]
- Munteanu, C.; Munteanu, D.; Onose, G. Hydrogen sulfide (H2S)—Therapeutic relevance in rehabilitation and balneotherapy Systematic literature review and meta-analysis based on the PRISMA paradig. Balneo PRM Res. J. 2021, 12, 176–195. [Google Scholar] [CrossRef]
- Botzer, A.; Finkelstein, Y.; Unger, R. Blood Pressure Regulation Evolved from Basic Homeostatic Components. Biomedicines 2021, 9, 469. [Google Scholar] [CrossRef]
- Munteanu, C. Hydrogen Sulfide and Oxygen Homeostasis in Atherosclerosis: A Systematic Review from Molecular Biology to Therapeutic Perspectives. Int. J. Mol. Sci. 2023, 24, 8376. [Google Scholar] [CrossRef]
- Lv, B.; Chen, S.; Tang, C.; Jin, H.; Du, J.; Huang, Y. Hydrogen sulfide and vascular regulation—An update. J. Adv. Res. 2021, 27, 85–97. [Google Scholar] [CrossRef]
- Pandey, T.; Pandey, V. Hydrogen sulfide (H2S) metabolism: Unraveling cellular regulation, disease implications, and therapeutic prospects for precision medicine. Nitric Oxide 2024, 144, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Shang, Q.; Yao, J.; Ji, Y. Hydrogen sulfide: A gaseous signaling molecule modulates tissue homeostasis: Implications in ophthalmic diseases. Cell Death Dis. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Tang, G.; Wu, L.; Liang, W.; Wang, R. Direct stimulation of KATP channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol. Pharmacol. 2005, 68, 1757–1764. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Q.; Zhu, D. An update on hydrogen sulfide and nitric oxide interactions in the cardiovascular system. Oxidative Med. Cell. Longev. 2018, 2018, 4579140. [Google Scholar] [CrossRef]
- Munaron, L.; Avanzato, D.; Moccia, F.; Mancardi, D. Hydrogen sulfide as a regulator of calcium channels. Cell Calcium 2013, 53, 77–84. [Google Scholar] [CrossRef]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef]
- Cirino, G.; Szabo, C.; Papapetropoulos, A. Physiological roles of hydrogen sulfide in mammalian cells, tissues, and organs. Physiol. Rev. 2023, 103, 31–276. [Google Scholar] [CrossRef]
- Tinker, A.; Aziz, Q.; Thomas, A. The role of ATP-sensitive potassium channels in cellular function and protection in the cardiovascular system. Br. J. Pharmacol. 2014, 171, 12–23. [Google Scholar] [CrossRef]
- Lynch, M.J.; Crane, B.R. Design, Validation, and Application of an Enzyme-Coupled Hydrogen Sulfide Detection Assay. Biochemistry 2018, 58, 474–483. [Google Scholar] [CrossRef]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell 2012, 45, 13–24. [Google Scholar] [CrossRef]
- Wu, B.; Teng, H.; Yang, G.; Wu, L.; Wang, R. Hydrogen sulfide inhibits the translational expression of hypoxia-inducible factor-1α. Br. J. Pharmacol. 2012, 167, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pan, L.; Zhuo, Y.; Gong, Q.; Rose, P.; Zhu, Y. Hypoxia-inducible factor-1α is involved in the pro-angiogenic effect of hydrogen sulfide under hypoxic stress. Biol. Pharm. Bull. 2010, 33, 1550–1554. [Google Scholar] [CrossRef]
- Munteanu, C.; Turnea, M.A.; Rotariu, M. Hydrogen Sulfide: An Emerging Regulator of Oxidative Stress and Cellular Homeostasis—A Comprehensive One-Year Review. Antioxidants 2023, 12, 1737. [Google Scholar] [CrossRef]
- Bhatia, M.; Gaddam, R.R. Hydrogen Sulfide in Inflammation: A Novel Mediator and Therapeutic Target. Antioxid. Redox Signal. 2021, 34, 1368–1377. [Google Scholar] [CrossRef]
- Bolić, B.; Mijušković, A.; Popović-Bijelić, A.; Nikolić-Kokić, A.; Spasić, S.; Blagojević, D.; Spasić, M.B.; Spasojević, I. Reactions of superoxide dismutases with HS−/H2S and superoxide radical anion: An in vitro EPR study. Nitric Oxide 2015, 51, 19–23. [Google Scholar] [CrossRef]
- Wang, M.; Tang, J.; Zhang, S.; Pang, K.; Zhao, Y.; Liu, N.; Huang, J.; Kang, J.; Dong, S.; Li, H.; et al. Exogenous H2S initiating Nrf2/GPx4/GSH pathway through promoting Syvn1-Keap1 interaction in diabetic hearts. Cell Death Discov. 2023, 9, 1–14. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shackelford, R.E.; Shen, X.; Dominic, P.; Kevil, C.G. Sulfide regulation of cardiovascular function in health and disease. Nat. Rev. Cardiol. 2022, 20, 109–125. [Google Scholar] [CrossRef]
- Kabil, O.; Motl, N.; Banerjee, R. H2S and its role in redox signaling. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2014, 1844, 1355–1366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, C.-T.; Devarie-Baez, N.O.; Hamsath, A.; Fu, X.-D.; Xian, M. S-Persulfidation: Chemistry, Chemical Biology, and Significance in Health and Disease. Antioxid. Redox Signal. 2020, 33, 1092–1114. [Google Scholar] [CrossRef]
- Aroca, A.; Gotor, C.; Bassham, D.C.; Romero, L.C. Hydrogen sulfide: From a toxic molecule to a key molecule of cell life. Antioxidants 2020, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [PubMed]
- Hill-Eubanks, D.C.; Werner, M.E.; Heppner, T.J.; Nelson, M.T. Calcium signaling in smooth muscle. Cold Spring Harb. Perspect. Biol. 2011, 3, a004549. [Google Scholar] [CrossRef]
- Cesarini, V.; Guida, E.; Campolo, F.; Crescioli, C.; Di Baldassarre, A.; Pisano, C.; Balistreri, C.R.; Ruvolo, G.; Jannini, E.A.; Dolci, S. Type 5 phosphodiesterase (PDE5) and the vascular tree: From embryogenesis to aging and disease. Mech. Ageing Dev. 2020, 190, 111311. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Kida, M.; Sugiyama, T.; Yoshimoto, T.; Ogawa, Y. Hydrogen sulfide increases nitric oxide production with calcium-dependent activation of endothelial nitric oxide synthase in endothelial cells. Eur. J. Pharm. Sci. 2013, 48, 211–215. [Google Scholar] [CrossRef]
- Jin, Y.; Yuan, H.; Liu, Y.; Zhu, Y.; Wang, Y.; Liang, X.; Gao, W.; Ren, Z.; Ji, X.; Wu, D. Role of hydrogen sulfide in health and disease. Medcomm 2024, 5, e661. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Qian, L.-L.; Wang, R.-X. Hydrogen Sulfide-Induced Vasodilation: The Involvement of Vascular Potassium Channels. Front. Pharmacol. 2022, 13, 911704. [Google Scholar] [CrossRef]
- Bootman, M.D.; Bultynck, G. Fundamentals of Cellular Calcium Signaling: A Primer. Cold Spring Harb. Perspect. Biol. 2019, 12, a038802. [Google Scholar] [CrossRef]
- Liang, G.H.; Xi, Q.; Leffler, C.W.; Jaggar, J.H. Hydrogen sulfide activates Ca2+ sparks to induce cerebral arteriole dilatation. J. Physiol. 2012, 590, 2709–2720. [Google Scholar] [CrossRef]
- Sandoo, A.; van Zanten, J.J.V.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The Endothelium and Its Role in Regulating Vascular Tone. Open Cardiovasc. Med. J. 2010, 4, 302. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Chen, T.; Tian, M.; Han, Y. Hydrogen sulfide: A multi-tasking signal molecule in the regulation of oxidative stress responses. J. Exp. Bot. 2020, 71, 2862–2869. [Google Scholar] [CrossRef]
- Corsello, T.; Komaravelli, N.; Casola, A. Role of hydrogen sulfide in NRF2- and sirtuin-dependent maintenance of cellular redox balance. Antioxidants 2018, 7, 129. [Google Scholar] [CrossRef]
- Olas, B. Hydrogen sulfide in signaling pathways. Clin. Chim. Acta 2015, 439, 212–218. [Google Scholar] [CrossRef]
- Hosoki, R.; Matsuki, N.; Kimura, H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Li, L.; Kostetski, I.; Chu, S.H.; Siau, J.L.; Bhatia, M.; Moore, P.K. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem. Biophys. Res. Commun. 2006, 343, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.; Dux, M.; Namer, B.; Miljkovic, J.; Cordasic, N.; Will, C.; Kichko, T.I.; de la Roche, J.; Fischer, M.; Suárez, S.A.; et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO–TRPA1–CGRP signalling pathway. Nat. Commun. 2014, 5, 4381. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Fernandez, B.O.; Santos, J.L.; Mergia, E.; Grman, M.; Nagy, P.; Kelm, M.; Butler, A.; Feelisch, M. Nitrosopersulfide (SSNO−) accounts for sustained NO bioactivity of S-nitrosothiols following reaction with sulfide. Redox Biol. 2014, 2, 234–244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyamoto, R.; Koike, S.; Takano, Y.; Shibuya, N.; Kimura, Y.; Hanaoka, K.; Urano, Y.; Ogasawara, Y.; Kimura, H. Polysulfides (H2Sn) produced from the interaction of hydrogen sulfide (H2S) and nitric oxide (NO) activate TRPA1 channels. Sci. Rep. 2017, 7, srep45995. [Google Scholar] [CrossRef]
- Sun, H.-J.; Wu, Z.-Y.; Nie, X.-W.; Bian, J.-S. Role of endothelial dysfunction in cardiovascular diseases: The link between inflammation and hydrogen sulfide. Front. Pharmacol. 2020, 10, 1568. [Google Scholar] [CrossRef]
- Gheibi, S.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Regulation of vascular tone homeostasis by NO and H2S: Implications in hypertension. Biochem. Pharmacol. 2018, 149, 42–59. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide 2013, 35, 5–20. [Google Scholar] [CrossRef]
- Rose, P.; Moore, P.K.; Zhu, Y.Z. H2S biosynthesis and catabolism: New insights from molecular studies. Cell. Mol. Life Sci. 2016, 74, 1391–1412. [Google Scholar] [CrossRef]
- Garland, C.J.; Dora, K.A. EDH: Endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol. 2017, 219, 152–161. [Google Scholar] [CrossRef]
- Goto, K.; Ohtsubo, T.; Kitazono, T. Endothelium-dependent hyperpolarization (EDH) in hypertension: The role of endothelial ion channels. Int. J. Mol. Sci. 2018, 19, 315. [Google Scholar] [CrossRef]
- Goto, K.; Kitazono, T. Endothelium-dependent hyperpolarization (EDH) in diabetes: Mechanistic insights and therapeutic implications. Int. J. Mol. Sci. 2019, 20, 3737. [Google Scholar] [CrossRef]
- de Wit, C.; Boettcher, M.; Schmidt, V.J. Signaling across myoendothelial gap junctions—Fact or fiction? Cell Commun. Adhes. 2008, 15, 231–245. [Google Scholar] [CrossRef]
- Ellinsworth, D.C.; Earley, S.; Murphy, T.V.; Sandow, S.L. Endothelial control of vasodilation: Integration of myoendothelial microdomain signalling and modulation by epoxyeicosatrienoic acids. Pflügers Arch. Eur. J. Physiol. 2013, 466, 389–405. [Google Scholar] [CrossRef]
- Gorini, F.; Del Turco, S.; Sabatino, L.; Gaggini, M.; Vassalle, C. H2S as a bridge linking inflammation, oxidative stress and endothelial biology: A possible defense in the fight against SARS-CoV-2 infection? Biomedicines 2021, 9, 1107. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.E.; Cleland, L.G.; Penglis, P.S.; Gamble, J.R.; James, M.J. Roles of Cyclooxygenase (COX)-1 and COX-2 in Prostanoid Production by Human Endothelial Cells: Selective Up-Regulation of Prostacyclin Synthesis by COX-2. J. Immunol. 2001, 167, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Biringer, R.G. A Review of Prostanoid Receptors: Expression, Characterization, Regulation, and Mechanism of Action. J. Cell Commun. Signal. 2020, 15, 155–184. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Vitvitsky, V.; Banerjee, R. Sulfur as a signaling nutrient through hydrogen sulfide. Annu. Rev. Nutr. 2014, 34, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef]
- Islam, K.N.; Nguyen, I.D.; Islam, R.; Pirzadah, H.; Malik, H. Roles of Hydrogen Sulfide (H2S) as a Potential Therapeutic Agent in Cardiovascular Diseases: A Narrative Review. Cureus 2024, 16, e64913. [Google Scholar] [CrossRef]
- Michiels, C. Physiological and Pathological Responses to Hypoxia. Am. J. Pathol. 2004, 164, 1875–1882. [Google Scholar] [CrossRef]
- Hajiaqaei, M.; Ranjbaran, M.; Kadkhodaee, M.; Shafie, A.; Abdi, A.; Lorian, K.; Kianian, F.; Seifi, B. Hydrogen sulfide upregulates hypoxia inducible factors and erythropoietin production in chronic kidney disease induced by 5/6 nephrectomized rats. Mol. Biol. Rep. 2024, 51, 916. [Google Scholar] [CrossRef]
- Lohninger, L.; Tomasova, L.; Praschberger, M.; Hintersteininger, M.; Erker, T.; Gmeiner, B.M.; Laggner, H. Hydrogen sulphide induces HIF-1α and Nrf2 in THP-1 macrophages. Biochimie 2015, 112, 187–195. [Google Scholar] [CrossRef]
- Kulandavelu, S.; Balkan, W.; Hare, J.M. Regulation of oxygen delivery to the body via hypoxic vasodilation. Proc. Natl. Acad. Sci. USA 2015, 112, 6254–6255. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; de la Lastra, J.M.P.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Sulfide—Pathological and Physiological Functions in Mammalian Cells. Cells 2023, 12, 2684. [Google Scholar] [CrossRef]
- Dogaru, B.G.; Munteanu, C. The Role of Hydrogen Sulfide (H2S) in Epigenetic Regulation of Neurodegenerative Diseases: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 12555. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed]
- Alique, M.; Sánchez-López, E.; Bodega, G.; Giannarelli, C.; Carracedo, J.; Ramírez, R. Hypoxia-Inducible Factor-1α: The Master Regulator of Endothelial Cell Senescence in Vascular Aging. Cells 2020, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Hashmp, S.F.; Sattar, M.Z.A.; Rathore, H.A.; Ahmadi, A.; Johns, E.J. A critical review on pharmacological significance of hydrogen sulfide (H₂S) on NF-κB concentration and icam-1 expression in renal ischemia reperfusion injury. Acta Pol. Pharm. 2017, 74, 747–752. [Google Scholar] [PubMed]
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 1–74. [Google Scholar] [CrossRef]
- Billman, G.E. Homeostasis: The Underappreciated and Far Too Often Ignored Central Organizing Principle of Physiology. Front. Physiol. 2020, 11, 200. [Google Scholar] [CrossRef]
- Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- Chun, Q.Y.; Wen, C.; Ling, L.X.; Wei, W.Y.; Hua, X.X. H2S protecting against lung injury following limb ischemia-reperfusion by alleviating inflammation and water transport abnormality in rats. Biomed. Environ. Sci. 2014, 27, 410–418. [Google Scholar] [CrossRef]
- Gao, X.; Jin, B.; Zhou, X.; Bai, J.; Zhong, H.; Zhao, K.; Huang, Z.; Wang, C.; Zhu, J.; Qin, Q. Recent advances in the application of gasotransmitters in spinal cord injury. J. Nanobiotechnol. 2024, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pushchina, E.V.; Marinina, K.S.; Myasoyedov, S.D. Hydrogen Sulfide and Pathophysiology of the CNS. Neurophysiology 2020, 52, 308–321. [Google Scholar] [CrossRef]
- Gluba-Brzózka, A.; Franczyk, B.; Rysz-Górzyńska, M.; Ławiński, J.; Rysz, J. Emerging anti-atherosclerotic therapies. Int. J. Mol. Sci. 2021, 22, 12109. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Wu, J.; Guo, W.; Zhu, Y.-Z. Atherosclerosis and the Hydrogen Sulfide Signaling Pathway—Therapeutic Approaches to Disease Prevention. Cell. Physiol. Biochem. 2017, 42, 859–875. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Rotariu, M.; Turnea, M.-A.; Anghelescu, A.; Albadi, I.; Dogaru, G.; Silișteanu, S.C.; Ionescu, E.V.; Firan, F.C.; Ionescu, A.M.; et al. Topical Reappraisal of Molecular Pharmacological Approaches to Endothelial Dysfunction in Diabetes Mellitus Angiopathy. Curr. Issues Mol. Biol. 2022, 44, 3378–3397. [Google Scholar] [CrossRef]
- Onose, G.; Anghelescu, A.; Blendea, D.; Ciobanu, V.; Daia, C.; Firan, F.C.; Oprea, M.; Spinu, A.; Popescu, C.; Ionescu, A.; et al. Cellular and Molecular Targets for Non-Invasive, Non-Pharmacological Therapeutic/Rehabilitative Interventions in Acute Ischemic Stroke. Int. J. Mol. Sci. 2022, 23, 907. [Google Scholar] [CrossRef]
- Chen, G.; Dou, Y.; Wang, Z. The role of hydrogen sulfide in stroke. Med Gas Res. 2016, 6, 79–84. [Google Scholar] [CrossRef]
- Onose, G.; Anghelescu, A.; Blendea, C.D.; Ciobanu, V.; Daia, C.O.; Firan, F.C.; Munteanu, C.; Oprea, M.; Spinu, A.; Popescu, C. Non-invasive, non-pharmacological/bio-technological interventions towards neurorestoration upshot after ischemic stroke, in adults—Systematic, synthetic, literature review. Front. Biosci. 2021, 26, 1204–1239. [Google Scholar] [CrossRef]
- Merz, T.; McCook, O.; Brucker, C.; Waller, C.; Calzia, E.; Radermacher, P.; Datzmann, T. H2S in Critical Illness—A New Horizon for Sodium Thiosulfate? Biomolecules 2022, 12, 543. [Google Scholar] [CrossRef]
- Munteanu, C.; Iordan, D.A.; Hoteteu, M.; Popescu, C.; Postoiu, R.; Onu, I.; Onose, G. Mechanistic Intimate Insights into the Role of Hydrogen Sulfide in Alzheimer’s Disease: A Recent Systematic Review. Int. J. Mol. Sci. 2023, 24, 15481. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, Z.; Lv, S.; Xu, S.; Liu, A.; Li, H.; Li, P.; Han, H.; Liu, Y. Elucidating VSMC phenotypic transition mechanisms to bridge insights into cardiovascular disease implications. Front. Cardiovasc. Med. 2024, 11, 1400780. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ouyang, C.; Zhang, H.; Gu, Y.; Deng, Y.; Du, C.; Cui, C.; Li, S.; Wang, W.; Kong, W.; et al. Vascular smooth muscle cell-derived hydrogen sulfide promotes atherosclerotic plaque stability via TFEB (transcription factor EB)-mediated autophagy. Autophagy 2022, 18, 2270–2287. [Google Scholar] [CrossRef]
- Ng, P.C.; Hendry-Hofer, T.B.; Witeof, A.E.; Brenner, M.; Mahon, S.B.; Boss, G.R.; Haouzi, P.; Bebarta, V.S. Hydrogen Sulfide Toxicity: Mechanism of Action, Clinical Presentation, and Countermeasure Development. J. Med. Toxicol. 2019, 15, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Sen, N. Functional and Molecular Insights of Hydrogen Sulfide Signaling and Protein Sulfhydration. J. Mol. Biol. 2017, 429, 543–561. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Altaany, Z.; Moccia, F.; Munaron, L.; Mancardi, D.; Wang, R. Hydrogen Sulfide and Endothelial Dysfunction: Relationship with Nitric Oxide. Curr. Med. Chem. 2014, 21, 3646–3661. [Google Scholar] [CrossRef]
- Coletta, C.; Papapetropoulos, A.; Erdelyi, K.; Olah, G.; Modis, K.; Panopoulos, P.; Asimakopoulou, A.; Gero, D.; Sharina, I.; Martin, E.; et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA 2012, 109, 9161–9166. [Google Scholar] [CrossRef] [PubMed]
- Stitham, J.; Midgett, C.; Martin, K.A.; Hwa, J. Prostacyclin: An Inflammatory Paradox. Front. Pharmacol. 2011, 2, 24. [Google Scholar] [CrossRef]
- Wenceslau, C.F.; McCarthy, C.G.; Earley, S.; England, S.K.; Filosa, J.A.; Goulopoulou, S.; Gutterman, D.D.; Isakson, B.E.; Kanagy, N.L.; Martinez-Lemus, L.A.; et al. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am. J. Physiol. Circ. Physiol. 2021, 321, H77–H111. [Google Scholar] [CrossRef]
- Schubert, R.; Gaynullina, D.; Shvetsova, A.; Tarasova, O.S. Myography of isolated blood vessels: Considerations for experimental design and combination with supplementary techniques. Front. Physiol. 2023, 14, 1176748. [Google Scholar] [CrossRef]
- Liu, L.; Yao, Y.; Liu, Y.; Hong, B.; Li, Z.; Chen, X.; Zhang, Y.; Fu, H.; Yang, D.; Yang, C. Targeted H2S-mediated gas therapy with pH-sensitive release property for myocardial ischemia–reperfusion injury by platelet membrane. Biomater. Res. 2024, 28, 0061. [Google Scholar] [CrossRef] [PubMed]
- Jama, H.A.; Muralitharan, R.R.; Xu, C.; O’DOnnell, J.A.; Bertagnolli, M.; Broughton, B.R.S.; Head, G.A.; Marques, F.Z. Rodent models of hypertension. Br. J. Pharmacol. 2021, 179, 918–937. [Google Scholar] [CrossRef] [PubMed]
- Lerman, L.O.; Kurtz, T.W.; Touyz, R.M.; Ellison, D.H.; Chade, A.R.; Crowley, S.D.; Mattson, D.L.; Mullins, J.J.; Osborn, J.; Eirin, A.; et al. Animal Models of Hypertension: A Scientific Statement From the American Heart Association. Hypertension 2019, 73, E87–E120. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Roles of Hydrogen Sulfide in Hypertension Development and Its Complications: What, So What, Now What. Hypertension 2023, 80, 936–944. [Google Scholar] [CrossRef]
- Al-Magableh, M.R.; Kemp-Harper, B.K.; Hart, J.L. Hydrogen sulfide treatment reduces blood pressure and oxidative stress in angiotensin II-induced hypertensive mice. Hypertens. Res. 2014, 38, 13–20. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Meng, H.; Duan, H.; Liu, X.; Wu, J.; Gao, F.; Wang, S.; Tan, R.; Yuan, J. Ischemia-reperfusion injury: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 1–39. [Google Scholar] [CrossRef]
- Kura, B.; Slezak, J. The Protective Role of Molecular Hydrogen in Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2024, 25, 7884. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, S.; Mao, C.; Qu, Y.; Xu, Z.; Xie, Y.; Jiang, D.; Song, Y. Therapeutic Potential of Hydrogen Sulfide in Ischemia and Reperfusion Injury. Biomolecules 2024, 14, 740. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Juriasingani, S.; Zhang, M.Y.; Sener, A. H2S donor molecules against cold ischemia-reperfusion injury in preclinical models of solid organ transplantation. Pharmacol. Res. 2021, 172, 105842. [Google Scholar] [CrossRef]
- Engelen, S.E.; Robinson, A.J.B.; Zurke, Y.-X.; Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: How to proceed? Nat. Rev. Cardiol. 2022, 19, 522–542. [Google Scholar] [CrossRef]
- Gisterå, A.; Ketelhuth, D.F.; Malin, S.G.; Hansson, G.K. Animal Models of Atherosclerosis–Supportive Notes and Tricks of the Trade. Circ. Res. 2022, 130, 1869–1887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fatima, M.; Hou, S.; Bai, L.; Zhao, S.; Liu, E. Research methods for animal models of atherosclerosis (Review). Mol. Med. Rep. 2021, 24, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Bennett, M.R. Vascular smooth muscle cells in atherosclerosis: Time for a re-assessment. Cardiovasc. Res. 2021, 117, 2326–2339. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.S.H.; Massam, B.D.; Kulkarni, S.S.; Lang, C.C. Pulmonary Arterial Hypertension: Pathophysiology and Treatment. Diseases 2018, 6, 38. [Google Scholar] [CrossRef]
- Turhan, K.; Alan, E.; Yetik-Anacak, G.; Sevin, G. H2S releasing sodium sulfide protects against pulmonary hypertension by improving vascular responses in monocrotaline-induced pulmonary hypertension. Eur. J. Pharmacol. 2022, 931, 175182. [Google Scholar] [CrossRef]
- Maron, B.A.; Abman, S.H.; Elliott, C.G.; Frantz, R.P.; Hopper, R.K.; Horn, E.M.; Nicolls, M.R.; Shlobin, O.A.; Shah, S.J.; Kovacs, G.; et al. Pulmonary arterial hypertension: Diagnosis, treatment, and novel advances. Am. J. Respir. Crit. Care Med. 2021, 203, 1472–1487. [Google Scholar] [CrossRef]
- Khattak, S.; Zhang, Q.-Q.; Sarfraz, M.; Muhammad, P.; Ngowi, E.E.; Khan, N.H.; Rauf, S.; Wang, Y.-Z.; Qi, H.-W.; Wang, D.; et al. The role of hydrogen sulfide in respiratory diseases. Biomolecules 2021, 11, 682. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Xu, Q.; Zhou, K.; Shen, Y.; Guo, L.; Liu, H.; Ren, Z.; Jiang, Z. Hydrogen sulfide donors across time: From origins to cutting-edge applications. Nitric Oxide 2024, 144, 29–39. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Tain, Y.-L. Hydrogen sulfide in hypertension and kidney disease of developmental origins. Int. J. Mol. Sci. 2018, 19, 1438. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, F.; Yin, J.; Wu, S.; Zhou, X. Protective mechanisms of hydrogen sulfide in myocardial ischemia. J. Cell. Physiol. 2020, 235, 9059–9070. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Ma, Y.; Xie, L.; Ferro, A.; Ji, Y. Themed Section: Chinese Innovation in Cardiovascular Drug Discovery Emerging role of hydrogen sulfide in hypertension and related cardiovascular diseases Correspondence LINKED ARTICLES. Br. J. Pharmacol. 2015, 172, 5501. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, Z.; Luo, S.; Guo, W.; Zhu, Y.Z. The cardioprotective effects of hydrogen sulfide in heart diseases: From molecular mechanisms to therapeutic potential. Oxidative Med. Cell. Longev. 2015, 2015, 925167. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.J.; Calvert, J.W.; Butler, J.; Lefer, D.J. The Cardioprotective Actions of Hydrogen Sulfide in Acute Myocardial Infarction and Heart Failure. Scientifica 2014, 2014, 768607. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Iliodromitis, E.K.; Rassaf, T.; Schulz, R.; Papapetropoulos, A.; Ferdinandy, P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br. J. Pharmacol. 2014, 172, 1587–1606. [Google Scholar] [CrossRef]
- Lambert, J.P.; Nicholson, C.K.; Amin, H.; Amin, S.; Calvert, J.W. Hydrogen sulfide provides cardioprotection against myocardial/ischemia reperfusion injury in the diabetic state through the activation of the RISK pathway. Med. Gas Res. 2014, 4, 20. [Google Scholar] [CrossRef]
- Bechelli, C.; Macabrey, D.; Deglise, S.; Allagnat, F. Clinical Potential of Hydrogen Sulfide in Peripheral Arterial Disease. Int. J. Mol. Sci. 2023, 24, 9955. [Google Scholar] [CrossRef]
- Marinko, M.; Novaković, A. Hydrogen sulfide-releasing therapeutics: How far have we come in clinical studies? Arh. Za Farm. 2023, 73, 173–189. [Google Scholar] [CrossRef]
- Tinawi, M. New Trends in the Diagnosis and Management of Hypertension. Cureus 2022, 14, e22393. [Google Scholar] [CrossRef]
- Sitbon, O.; Gomberg-Maitland, M.; Granton, J.; Lewis, M.I.; Mathai, S.C.; Rainisio, M.; Stockbridge, N.L.; Wilkins, M.R.; Zamanian, R.T.; Rubin, L.J. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801908. [Google Scholar] [CrossRef]
- Johnsen, H.M.; Hiorth, M.; Klaveness, J. Molecular Hydrogen Therapy—A Review on Clinical Studies and Outcomes. Molecules 2023, 28, 7785. [Google Scholar] [CrossRef]
- Rodkin, S.V.; Nwosu, C.D. Role of Nitric Oxide and Hydrogen Sulfide in Neuronal and Glial Cell Death in Neurodegenerative Processes. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2023, 17, 223–242. [Google Scholar] [CrossRef]
- Eren, F.; Yilmaz, S.E. Neuroprotective approach in acute ischemic stroke: A systematic review of clinical and experimental studies. Brain Circ. 2022, 8, 172–179. [Google Scholar] [CrossRef]
- Gao, R.; Chen, G.; Zhang, J.-Y.; Ding, Y.-P.; Wang, Z.; Kong, Y. Hydrogen sulfide therapy in brain diseases: From bench to bedside. Med Gas Res. 2017, 7, 113–119. [Google Scholar] [CrossRef]
- Macabrey, D.; Longchamp, A.; Déglise, S.; Allagnat, F. Clinical Use of Hydrogen Sulfide to Protect Against Intimal Hyperplasia. Front. Cardiovasc. Med. 2022, 9, 876639. [Google Scholar] [CrossRef]
- Corvino, A.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Scognamiglio, A.; Caliendo, G.; Fiorino, F.; Severino, B. Trends in H2S-donors chemistry and their effects in cardiovascular diseases. Antioxidants 2021, 10, 429. [Google Scholar] [CrossRef]
- Norris, E.J.; Feilen, N.; Nguyen, N.H.; Culberson, C.R.; Shin, M.C.; Fish, M.; Clemens, M.G. Hydrogen sulfide modulates sinusoidal constriction and contributes to hepatic micorcirculatory dysfunction during endotoxemia. Am. J. Physiol. Liver Physiol. 2013, 304, G1070–G1078. [Google Scholar] [CrossRef]
- Maldonado, C.S.; Weir, A.; Rumbeiha, W.K. A comprehensive review of treatments for hydrogen sulfide poisoning: Past, present, and future. Toxicol. Mech. Methods 2022, 33, 183–196. [Google Scholar] [CrossRef]
- Shi, X.; Li, H.; Guo, F.; Li, D.; Xu, F. Novel ray of hope for diabetic wound healing: Hydrogen sulfide and its releasing agents. J. Adv. Res. 2024, 58, 105–115. [Google Scholar] [CrossRef]
- Yang, F.; Zhong, W.; Pan, S.; Wang, Y.; Xiao, Q.; Gao, X. Recent advances in the mechanism of hydrogen sulfide in wound healing in diabetes. Biochem. Biophys. Res. Commun. 2023, 692, 149343. [Google Scholar] [CrossRef]
- Munteanu, C.; Onose, G.; Turnea, M.-A.; Rotariu, M. Cellular and Molecular Homeostatic Microenvironmental imbalances in Osteoarthritis and Rheumatoid Arthritis. Balneo PRM Res. J. 2023, 14, 564. [Google Scholar] [CrossRef]
- Kloesch, B.; Liszt, M.; Krehan, D.; Broell, J.; Kiener, H.; Steiner, G. High concentrations of hydrogen sulphide elevate the expression of a series of pro-inflammatory genes in fibroblast-like synoviocytes derived from rheumatoid and osteoarthritis patients. Immunol. Lett. 2012, 141, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Nasi, S.; Ehirchiou, D.; Chatzianastasiou, A.; Nagahara, N.; Papapetropoulos, A.; Bertrand, J.; Cirino, G.; So, A.; Busso, N. The protective role of the 3-mercaptopyruvate sulfurtransferase (3-MST)-hydrogen sulfide (H2S) pathway against experimental osteoarthritis. Arthritis Res. Ther. 2020, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stummer, N.; Feichtinger, R.G.; Weghuber, D.; Kofler, B.; Schneider, A.M. Role of Hydrogen Sulfide in Inflammatory Bowel Disease. Antioxidants 2023, 12, 1570. [Google Scholar] [CrossRef]
- Mateus, I.; Prip-Buus, C. Hydrogen sulphide in liver glucose/lipid metabolism and non-alcoholic fatty liver disease. Eur. J. Clin. Investig. 2021, 52, e13680. [Google Scholar] [CrossRef]
- Wallace, J.L.; Wang, R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015, 14, 329–345. [Google Scholar] [CrossRef]
- Zaorska, E.; Tomasova, L.; Koszelewski, D.; Ostaszewski, R.; Ufnal, M. Hydrogen sulfide in pharmacotherapy, beyond the hydrogen sulfide-donors. Biomolecules 2020, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. A timeline of hydrogen sulfide (H2S) research: From environmental toxin to biological mediator. Biochem. Pharmacol. 2018, 149, 5–19. [Google Scholar] [CrossRef]
- Guidotti, T.L. Hydrogen sulfide: Advances in understanding human toxicity. Int. J. Toxicol. 2010, 29, 569–581. [Google Scholar] [CrossRef]
- Lu, Q.-B.; Ding, Y.; Fu, X.; Sun, H.-J.; Zhang, J.-R. Hydrogen sulfide in health and diseases: Cross talk with noncoding RNAs. Am. J. Physiol. Physiol. 2023, 324, C856–C877. [Google Scholar] [CrossRef]
- Rong, F.; Wang, T.; Zhou, Q.; Peng, H.; Yang, J.; Fan, Q.; Li, P. Intelligent polymeric hydrogen sulfide delivery systems for therapeutic applications. Bioact. Mater. 2023, 19, 198–216. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Anokhe, A.; Kalia, V. Biochemical Test for Detecting Hydrogen Sulphide (H2S) Producing Bacteria. Available online: https://www.researchgate.net/publication/356127970 (accessed on 21 September 2024).
- Hartle, M.D.; Pluth, M.D. A practical guide to working with H2S at the interface of chemistry and biology. Chem. Soc. Rev. 2016, 45, 6108–6117. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, L.; Jiang, J.; Wu, M.; Lin, P. Applications and challenges of CRISPR-Cas gene-editing to disease treatment in clinics. Precis. Clin. Med. 2021, 4, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Buret, A.G.; Allain, T.; Motta, J.-P.; Wallace, J.L. Effects of Hydrogen Sulfide on the Microbiome: From Toxicity to Therapy. Antioxid. Redox Signal. 2022, 36, 211–219. [Google Scholar] [CrossRef]
| Keywords | PubMed | Scopus | Web of Science | Total |
|---|---|---|---|---|
| “Hydrogen sulfide” [Title] AND “vasodilation” [Title] | 10 | 2 | 7 | 19 |
| “H2S” [Title] AND “vasodilation” [Title] | 4 | 0 | 4 | 8 |
| “Hydrogen sulfide” [Title] AND “vascular regulation” [Title] | 2 | 6 | 7 | 15 |
| “H2S” [Title] AND “vascular regulation” [Title] | 0 | 0 | 7 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, C.; Popescu, C.; Vlădulescu-Trandafir, A.-I.; Onose, G. Signaling Paradigms of H2S-Induced Vasodilation: A Comprehensive Review. Antioxidants 2024, 13, 1158. https://doi.org/10.3390/antiox13101158
Munteanu C, Popescu C, Vlădulescu-Trandafir A-I, Onose G. Signaling Paradigms of H2S-Induced Vasodilation: A Comprehensive Review. Antioxidants. 2024; 13(10):1158. https://doi.org/10.3390/antiox13101158
Chicago/Turabian StyleMunteanu, Constantin, Cristina Popescu, Andreea-Iulia Vlădulescu-Trandafir, and Gelu Onose. 2024. "Signaling Paradigms of H2S-Induced Vasodilation: A Comprehensive Review" Antioxidants 13, no. 10: 1158. https://doi.org/10.3390/antiox13101158








