Protective Role of Sulforaphane against Physiological Toxicity of Triphenyltin in Common Carp (Cyprinus carpio haematopterus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Experimental Diets
2.2. Fish and Experimental Design
2.3. Sample Collection
2.4. Analytic Procedures
2.4.1. Hematological and Plasma Biochemical Parameters
2.4.2. Histological Examination
2.4.3. Analysis of Antioxidant Status
2.4.4. Related Gene Expression Analysis
2.4.5. Calculation Formula
2.5. Statistical Analysis
3. Results
3.1. Effects of TPT and SFN on the Growth Performance of Cyprinus carpio haematopterus
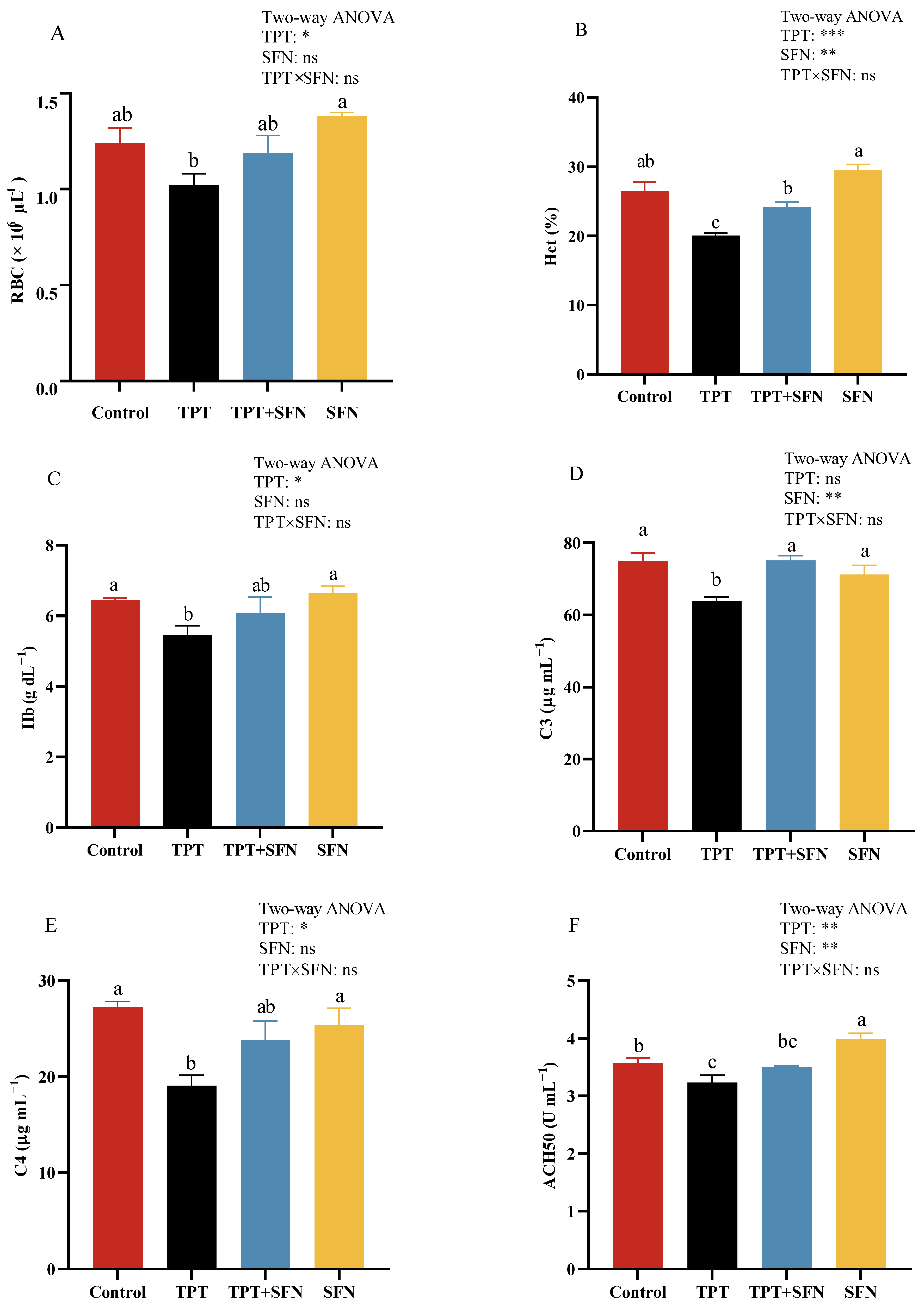
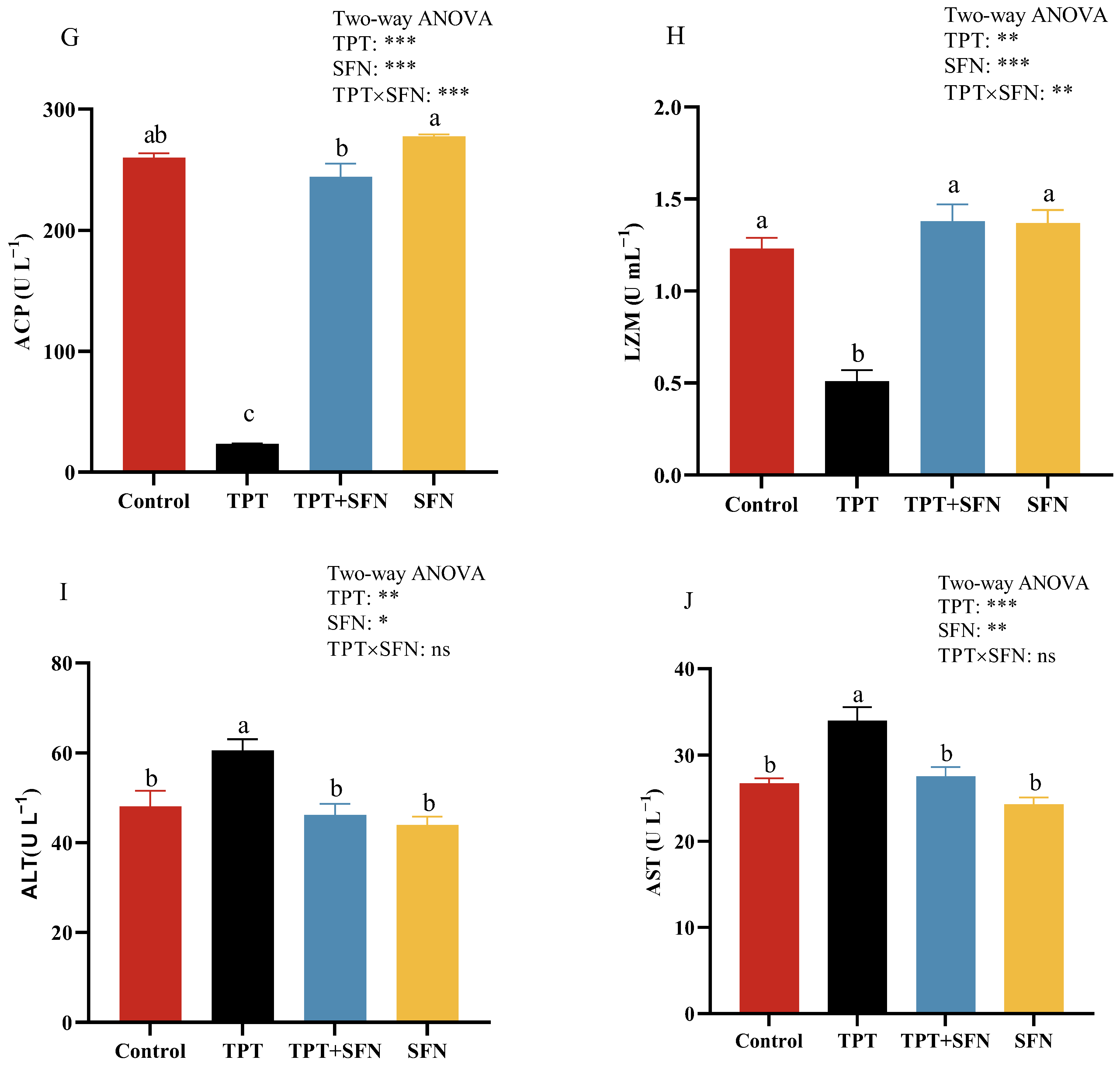
3.2. Effects of TPT and SFN on the Hematological and Plasma Immune Parameters of Cyprinus carpio haematopterus
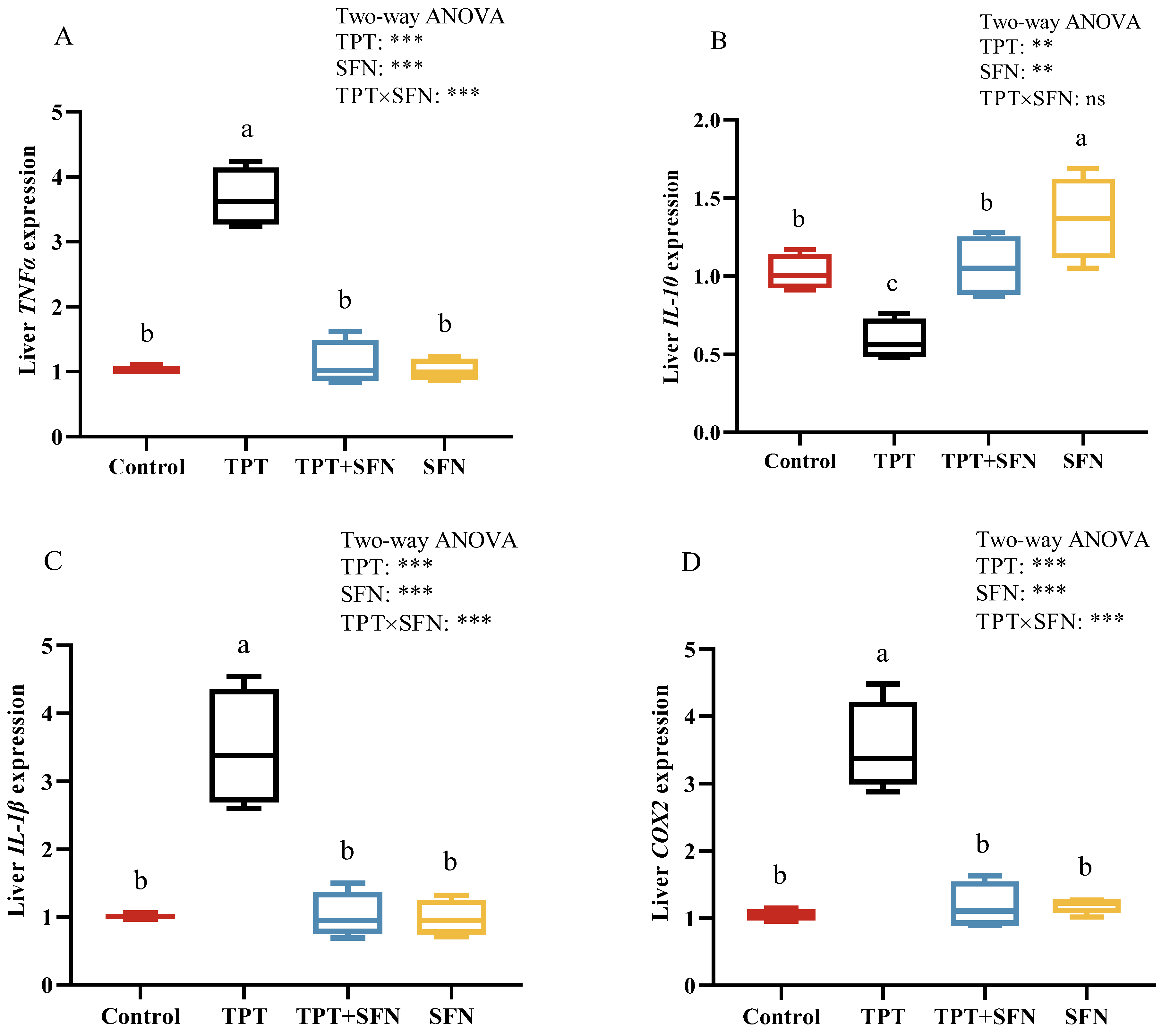
3.3. Effects of TPT and SFN on Liver Immune-Related Gene Expressions of Cyprinus carpio haematopterus
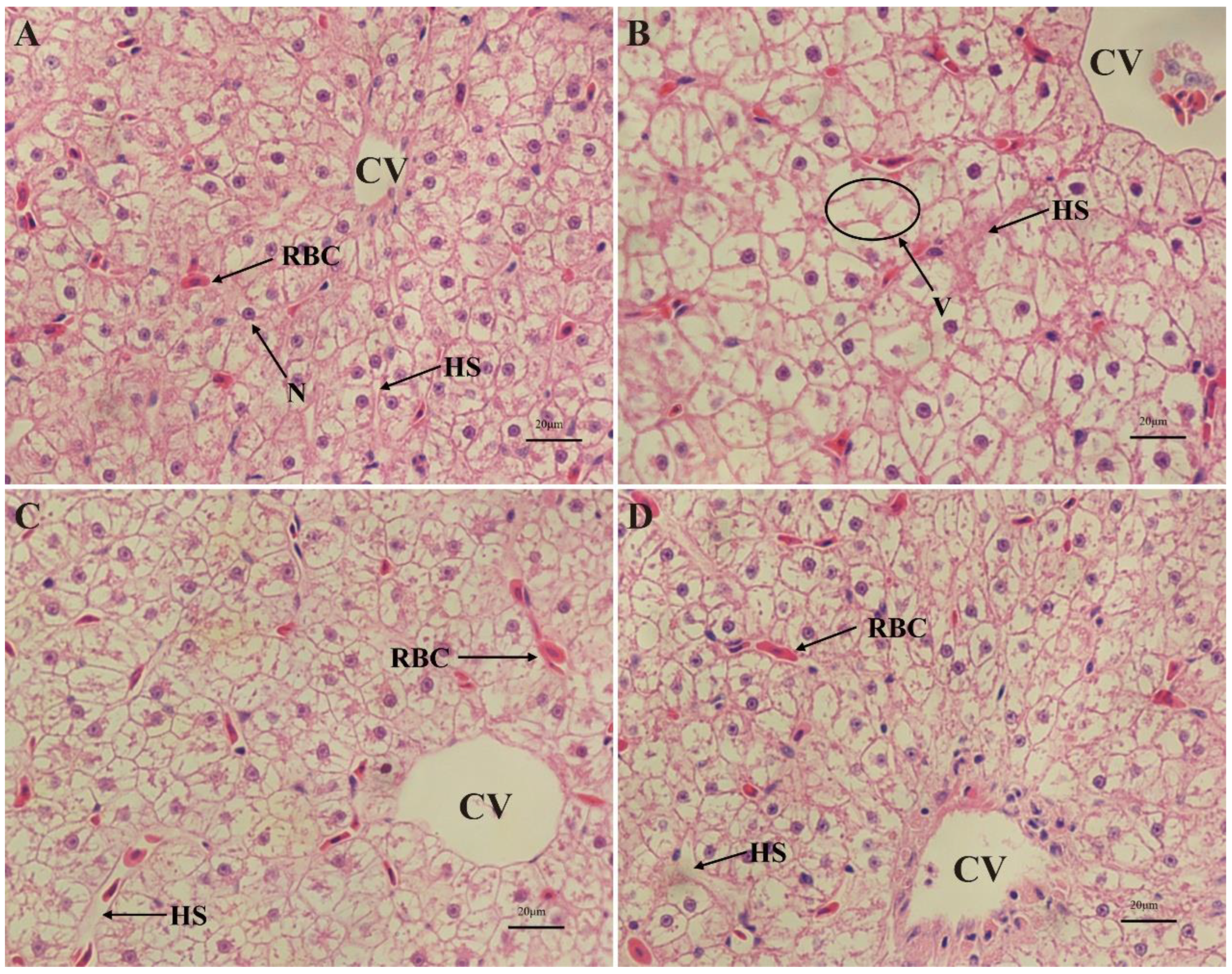
3.4. Liver Histological Examinations of Cyprinus carpio haematopterus
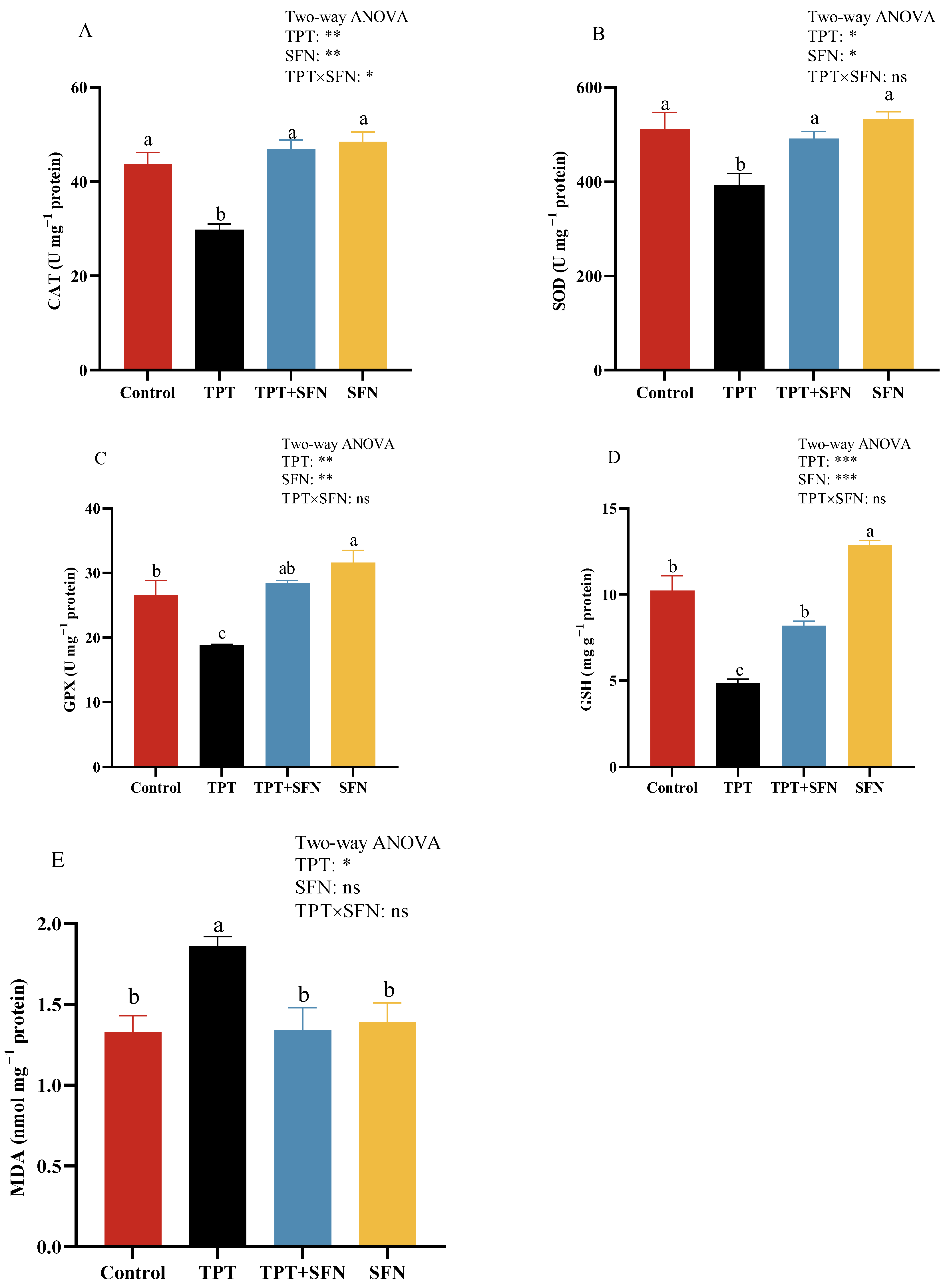
3.5. Effects of TPT and SFN on Liver Antioxidant Enzyme Activities of Cyprinus carpio haematopterus
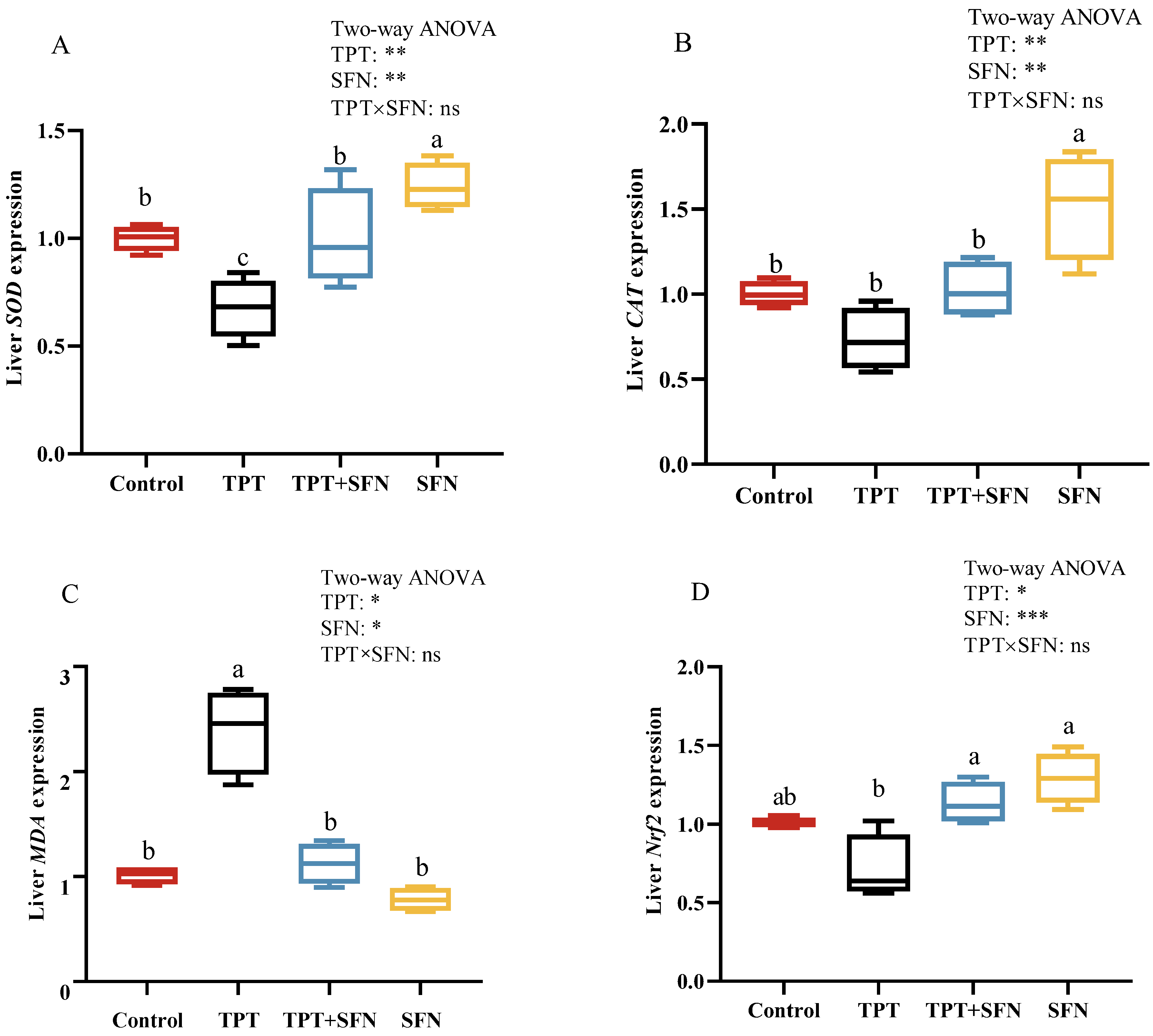
3.6. Effects of TPT and SFN on Liver Antioxidant Related Gene Expressions of Cyprinus carpio haematopterus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, S.W.; Li, P.; Li, Z.H. Review on endocrine disrupting toxicity of triphenyltin from the perspective of species evolution: Aquatic, amphibious and mammalian. Chemosphere 2021, 269, 128711. [Google Scholar] [CrossRef]
- Antizar-Ladislao, B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. A review. Environ. Int. 2008, 34, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Bettin, C.; Oehlmann, J.; Stroben, E. TBT-induced imposex in marine neogastropods is mediated by an increasing androgen level. Helgol. Meeresunters. 1996, 50, 299–317. [Google Scholar] [CrossRef]
- Sham, R.C.T.; Ho, K.K.Y.; Zhou, G.J.; Li, Y.; Wang, X.H.; Leung, K.M.Y. Occurrence and ecological risks of phenyltin compounds in the marine environment of Hong Kong. Mar. Pollut. Bull. 2020, 154, 111093. [Google Scholar] [CrossRef] [PubMed]
- Sham, R.C.T.; Tao, L.S.R.; Mak, Y.K.Y.; Yau, J.K.C.; Wai, T.C.; Ho, K.K.Y.; Zhou, G.J.; Li, Y.Y.; Wang, X.H.; Leung, K.M.Y. Occurrence and trophic magnification profile of triphenyltin compounds in marine mammals and their corresponding food webs. Environ. Int. 2020, 137, 105567. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Watanabe, H.; Takanobu, H.; Shigemoto, Y.; Yamagishi, T.; Iguchi, T.; Tatarazako, N. Effects of triphenyltin on reproduction in Japanese medaka (Oryzias latipes) across two generations. Aquat. Toxicol. 2017, 192, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Cai, X.W.; Liang, Z.F.; Duan, D.D.; Diao, X.P.; Zhang, J.L. An integrative investigation of developmental toxicities induced by triphenyltin in a larval coral reef fish, Amphiprion ocellaris. Sci. Total Environ. 2023, 867, 161487. [Google Scholar] [CrossRef]
- Yao, F.; Li, Y.F.; Ru, H.J.; Wu, L.Y.; Xiao, Z.G.; Ni, Z.H.; Chen, D.Q.; Zhong, L.Q. Thyroid disruption and developmental toxicity caused by triphenyltin (TPT) in zebrafish embryos/larvae. Toxicol. Appl. Pharmacol. 2020, 394, 114957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.N.; Wang, J.H.; Qi, Q.; Yang, L.; Sun, P.; Yuan, X.Y. Modulatory effect of fructooligosaccharide against triphenyltin-induced oxidative stress and immune suppression in goldfish (Carassius auratus). Ecotoxicol. Environ. Saf. 2021, 212, 111966. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhang, C.N.; Li, E.C.; Jin, M.M.; Huang, M.X.; Cui, W.; Lin, Y.Y.; Shi, Y.J. Triphenyltin exposure affects mating behaviors and attractiveness to females during mating in male guppies (Poecilia reticulata). Ecotoxicol. Environ. Saf. 2019, 169, 76–84. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Li, P.; He, S.W.; Xing, S.Y.; Cao, Z.H.; Zhao, X.L.; Sun, C.C.; Li, Z.H. Combined effect of microplastic and triphenyltin: Insights from the gut-brain axis. Environ. Sci. Ecotechnol. 2023, 16, 100266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Li, P.; He, S.W.; Xing, S.Y.; Cao, Z.H.; Zhao, X.L.; Sun, C.C.; Li, Z.H. Assessing the ecotoxicity of combined exposure to triphenyltin and norfloxacin at environmental levels: A case study of immunotoxicity and metabolic regulation in carp (Cyprinus carpio). Chemosphere 2023, 313, 137381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Zuo, Z.H.; He, C.Y.; Wu, D.; Chen, Y.X.; Wang, C.G. Inhibition of thyroidal status related to depression of testicular development in Sebastiscus marmoratus exposed to tributyltin. Aquat. Toxicol. 2009, 94, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Hamed, H.S. Antagonistic effects of dietary guava (Psidium guajava) leaves extract on growth, hemato-biochemical, and immunity response of cypermethrin-intoxicated Nile tilapia, Oreochromis niloticus, fingerlings. Aquaculture 2020, 529, 735668. [Google Scholar] [CrossRef]
- Yang, S.P.; Huang, Y.X.; Chen, B.K.; Liu, H.L.; Huang, Y.C.; Cai, S.H.; Jian, J.C. Protective effects of sulphoraphane on oxidative damage caused by ammonia in litopenaeus vannamei. Aquac. Res. 2022, 53, 1197–1204. [Google Scholar] [CrossRef]
- Mahn, A.; Castillo, A. Potential of sulforaphane as a natural immune system enhancer: A review. Molecules 2021, 26, 752. [Google Scholar] [CrossRef]
- Zhao, F.F.; Zhang, J.L.; Chang, N. Epigenetic modification of Nrf2 by sulforaphane increases the antioxidative and anti-inflammatory capacity in a cellular model of Alzheimer’s disease. Eur. J. Pharmacol. 2018, 824, 1–10. [Google Scholar] [CrossRef]
- Wang, B.K.; Zhang, C.N.; Zhang, Q.; Zhang, L.; Jiang, X.Y.; Feng, J.X.; Yang, X.L.; Wang, Y.H. Can sulforaphane alter growth performance, innate immunity and antioxidant capability of common carp (Cyprinus carpio Huanghe var)? Aquac. Rep. 2023, 31, 101666. [Google Scholar] [CrossRef]
- Blaxhall, P.C.; Daisley, K.W. Routine haematological methods for use with fish blood. J. Fish Biol. 1973, 5, 771–781. [Google Scholar] [CrossRef]
- Montero, D.; Tort, L.M.; Izquierdo, S.; Robaina, L.; Vergara, J.M. Depletion of serum alternative complement pathway activity in gilthead seabream caused by a-tocopherol and n-3 HUFA dietary deficiencies. Fish Physiol. Biochem. 1998, 18, 399–407. [Google Scholar] [CrossRef]
- Tang, H.G.; Wu, T.X.; Zhao, Z.Y.; Pan, X.D. Effects of fish protein hydrolysate on growth performance and humoral immune response in large yellow croaker (Pseudosciaena crocea R.). J. Zhejiang Univ. Sci. 2008, 9, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Tort, L.; Gómez, E.; Montero, D.; Sunyer, J.O. Serum haemolytic and agglutinating activity as indicators of fish immunocompetence: Their suitability in stress and dietary studies. Aquac. Int. 1996, 4, 31–41. [Google Scholar] [CrossRef]
- Obach, A.; Quentel, C.; Laurencin, F.B. Effects of alpha-tocopherol and dietary oxidized fish oil on the immune response of sea bass Dicentrarchus labrax. Dis. Aquat. Org. 1993, 15, 175–185. [Google Scholar] [CrossRef]
- Krajnović-Ozretić, M.; Ozretić, B. Estimation of the enzymes LDH, GOT and GPT in plasma of grey mullet Mugil auratus and their significance in liver intoxication. Dis. Aquat. Org. 1987, 3, 187–193. [Google Scholar] [CrossRef]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Lygren, B.; Hamre, K.; Waagbø, R. Effects of dietary pro- and antioxidants on some protective mechanisms and health parameters in Atlantic salmon. J. Aquat. Anim. Health 1999, 11, 211–221. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rehage, J.S.; Lynn, S.G.; Hammond, J.I.; Palmer, B.D.; Sih, A. Effects of larval exposure to triphenyltin on the survival, growth, and behavior of larval and juvenile Ambystoma barbouri salamanders. Environ. Toxicol. Chem. 2002, 21, 807–815. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Khattab, Y.A.E.; Ahmad, M.H.; Shalaby, A.M.E. Compensatory growth, feed utilization, whole-body composition, and hematological changes in starved Juvenile Nile tilapia, Oreochromis niloticus (L.). J. Appl. Aquac. 2006, 18, 17–36. [Google Scholar] [CrossRef]
- Jahazi, M.A.; Hoseinifar, S.H.; Jafari, V.; Hajimoradloo, A.; Doan, H.V.; Paolucci, M. Dietary supplementation of polyphenols positively affects the innate immune response, oxidative status, and growth performance of common carp, Cyprinus carpio L. Aquaculture 2020, 517, 734709. [Google Scholar] [CrossRef]
- Wang, B.K.; Wang, Y.H.; Jia, T.; Feng, J.X.; Qu, C.Y.; Wu, X.J.; Yang, X.L.; Zhang, Q. Changes in physiological responses and immunity of blunt snout bream Megalobrama amblycephala from transport stress. Fish Physiol. Biochem. 2022, 48, 1183–1192. [Google Scholar] [CrossRef]
- Nakayama, A.; Kurokawa, Y.; Harino, H.; Kawahara, E.; Miyadai, T.; Seikai, T.; Kawai, S. Effects of tributyltin on the immune system of Japanese flounder (Paralichthys olivaceus). Aquat. Toxicol. 2007, 83, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.N.; Jiang, D.X.; Wang, J.H.; Qi, Q. The effects of TPT and dietary quercetin on growth, hepatic oxidative damage and apoptosis in zebrafish. Ecotoxicol. Environ. Saf. 2021, 224, 112697. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Huang, W.B.; Wang, Q.W.; Wu, M.L.; Liu, J.; Wang, K.J. Effects of tributyltin and benzo[a]pyrene on the immune-associated activities of hemocytes and recovery responses in the gastropod abalone, Haliotis diversicolor. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Wu, L.Y.; Lee, C.H.; Chen, Y.L.; Hsueh, S.C.; Lu, H.F.; Liao, N.C.; Chung, J.G. Sulforaphane promotes immune responses in a WEHI-3–induced leukemia mouse model through enhanced phagocytosis of macrophages and natural killer cell activities in vivo. Mol. Med. Rep. 2016, 13, 4023–4029. [Google Scholar] [CrossRef]
- He, S.W.; Yu, D.D.; Li, P.; Zhang, M.; Xing, S.Y.; Sun, C.C.; Li, Z.H. Triphenyltin exposure causes changes in health-associated gut microbiome and metabolites in marine medaka. Environ. Pollut. 2021, 288, 117751. [Google Scholar] [CrossRef]
- Herseth, J.I.; Refsnes, M.; Låg, M.; Schwarze, P.E. Role of IL-1β and COX2 in silica-induced IL-6 release and loss of pneumocytes in co-cultures. Toxicol. Vitr. 2009, 23, 1342–1353. [Google Scholar] [CrossRef]
- Thejass, P.; Kuttan, G. Immunomodulatory activity of sulforaphane, a naturally occurring isothiocyanate from broccoli (brassica oleracea). Phytomedicine 2007, 14, 538–545. [Google Scholar] [CrossRef]
- Yousefi, M.; Shabunin, S.V.; Vatnikov, Y.A.; Kulikov, E.V.; Adineh, H.; Hamidi, M.K.; Hoseini, S.M. Effects of lavender (Lavandula angustifolia) extract inclusion in diet on growth performance, innate immunity, immune-related gene expression, and stress response of common carp, Cyprinus carpio. Aquaculture 2020, 515, 734588. [Google Scholar] [CrossRef]
- Zemheri-Navruz, F.; Acar, Ü.; Yılmaz, S. Dietary supplementation of olive leaf extract increases haematological, serum biochemical parameters and immune related genes expression level in common carp (Cyprinus carpio) juveniles. Fish Shellfish Immunol. 2019, 89, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Sharma, P.; Dhiman, S.K.; Chadha, P.; Saini, H.S. Biochemical, genotoxic, histological and ultrastructural effects on liver and gills of fresh water fish Channa punctatus exposed to textile industry intermediate 2 ABS. Chemosphere 2022, 287, 132103. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.; Serpeloni, J.M.; Batista, B.L.; Souza, S.S.; Barbosa, F. Identification and distribution of mercury species in rat tissues following administration of thimerosal or methylmercury. Arch. Toxicol. 2010, 84, 891–896. [Google Scholar] [CrossRef]
- Javed, M.; Ahmad, I.; Usmani, N.; Ahmad, M. Studies on biomarkers of oxidative stress and associated genotoxicity and histopathology in Channa punctatus from heavy metal polluted canal. Chemosphere 2016, 151, 210–219. [Google Scholar] [CrossRef]
- Macêdo, A.K.S.; dos Santos, K.P.E.; Brighenti, L.S.; Windmöller, C.C.; Barbosa, F.A.R.; de Azambuja Ribeiro, R.I.M.; dos Santos, H.B.; Thomé, R.G. Histological and molecular changes in gill and liver of fish (Astyanax lacustris Lütken, 1875) exposed to water from the Doce basin after the rupture of a mining tailings dam in Mariana, MG, Brazil. Sci. Total Environ. 2020, 735, 139505. [Google Scholar] [CrossRef]
- Trivedi, S.P.; Ratn, A.; Awasthi, Y.; Kumar, M.; Trivedi, A. In vivo assessment of dichlorvos induced histological and biochemical impairments coupled with expression of p53 responsive apoptotic genes in the liver and kidney of fish, Channa punctatus (Bloch, 1793). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 245, 109032. [Google Scholar] [CrossRef]
- Yildirim, Z.; Kilic, N. Effects of taurine and age on cerebellum antioxidant status and oxidative stress. Int. J. Gerontol. 2011, 5, 166–170. [Google Scholar] [CrossRef]
- Barbosa, M.L.; Menese, A.P.M.; Aguiar, R.P.S.; Sousa, J.M.C.; Cavalcante, A.A.C.; Maluf, S.W. Oxidative stress, antioxidant defense and depressive disorders: A systematic review of biochemical and molecular markers. Neurol. Psychiatry Brain Res. 2020, 36, 65–72. [Google Scholar] [CrossRef]
- Ma, J.S.; Wang, B.K.; Pu, C.C.; Chang, K.; Cheng, Y.F.; Sun, R.Y.; Qi, Q.; Xu, R.Y.; Chen, J.L.; Zhang, C.N. Protective effects of sulforaphane on inflammation, oxidative stress and intestinal dysbacteriosis induced by triphenyltin in Cyprinus carpio haematopterus. Fish Shellfish Immunol. 2023, 142, 109135. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, C.E.; Calderón-Oliver, M.; Pedraza-Chaverri, J.; Chirino, Y.I. Protective effect of sulforaphane against oxidative stress: Recent advances. Exp. Toxicol. Pathol. 2012, 64, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, M.; D’Argenio, G.; Lembo, V.; Palumbo, A.; Castellano, I. Antifibrotic Effect of Marine Ovothiol in an In Vivo Model of Liver Fibrosis. Oxid. Med. Cell. Longev. 2018, 2018, 5045734. [Google Scholar] [CrossRef]
- Milito, A.; Brancaccio, M.; D’Argenio, G.; Castellano, I. Natural Sulfur-Containing Compounds: An Alternative Therapeutic Strategy against Liver Fibrosis. Cells 2019, 8, 1356. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, W.L.; Xie, D.Q.; Jia, W. Sulforaphane alleviates hepatic ischemia–reperfusion injury through promoting the activation of Nrf-2/HO-1 signaling. Transpl. Immunol. 2021, 68, 101439. [Google Scholar] [CrossRef]
- Jiang, W.D.; Zhou, X.Q.; Zhang, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Vitamin A deficiency impairs intestinal physical barrier function of fish. Fish Shellfish Immunol. 2019, 87, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, S.H. Sulforaphane induces antioxidative and antiproliferative responses by generating reactive oxygen species in human bronchial epithelial BEAS-2B cells. J. Korean Med. Sci. 2011, 26, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.N.; Ma, J.S.; Wang, B.K.; Pu, C.C.; Chang, K.; Zhu, J.X.; Zhang, B.Y.; Li, J.J.; Qi, Q.; Xu, R.Y. Sulforaphane modulates some stress parameters in TPT-exposed Cyprinus carpio in relation to liver metabolome. Ecotoxicol. Environ. Saf. 2024, 284, 116882. [Google Scholar] [CrossRef]
- Cho, H.Y.; Miller-DeGraff, L.; Blankenship-Paris, T.; Wang, X.T.; Bell, D.A.; Lih, F.; Deterding, L.; Panduri, V.; Morgan, D.L.; Yamamoto, M.; et al. Sulforaphane enriched transcriptome of lung mitochondrial energy metabolism and provided pulmonary injury protection via Nrf2 in mice. Toxicol. Appl. Pharm. 2019, 364, 29–44. [Google Scholar] [CrossRef]
| Ingredients (%) | |
|---|---|
| Fish meal | 5.00 |
| Soybean meal | 32.00 |
| Rapeseed meal | 15.00 |
| Cottonseed meal | 15.00 |
| Fish oil | 1.00 |
| Soybean oil | 2.00 |
| Wheat flour | 21.00 |
| Wheat bran | 6.00 |
| Ca(H2PO4)2 | 1.80 |
| Premix 1 | 1.00 |
| Salt | 0.20 |
| Proximate analyses (% air-dry basis) | |
| Dry matter | 89.73 |
| Crude lipid | 4.04 |
| Crude protein | 35.82 |
| Crude ash | 9.45 |
| Gene | Sequences of Primers (5′-3′) | Accession Numbers |
|---|---|---|
| β-actin | F: TTGACTTCCCTGACAGCTGT | M24113.1 |
| R: CGAATCCGGCTTTGCACATA | ||
| CAT | F: ACAATGCAATCTCCATCGGC | XM_019068441.1 |
| R: CGTCTTGAGGTGCATTCTGG | ||
| SOD | F: CACTGGCCTTACTCCTGGAA | JX977106.1 |
| R: GGTCCACCGTGAGTTTGATT | ||
| MDA | F: GCTGCCAGTTGGGTGTTCTTTTA | DQ983598.1 |
| R: CCATCCGTGACTCCAGTTATCAT | ||
| TNF-α | F: GCACCGAAACGACGGAAGA | AJ311800.2 |
| R: ACGACCATGTTTCCCCCAC | ||
| IL-10 | F: GGAGGGCTTTCCAGTGAGAC | MG759384.1 |
| R: TATCACACTTTGCCACCGCT | ||
| IL-1β | F: CACCCGCTGGATTTGTCAGA | AB757756.1 |
| R: ATGGTTGAGGTGGTCAGTATGG | ||
| COX2 | F: TCCGGTATGTGCAAAGCCGG | JX807772.1 |
| R: CCGCAGATTTCAGAGCATTGTC | ||
| Nrf2 | F: CTCCCGAGACGAACAGAGAG | MG759384.1 |
| R: GGTGCTTGGACATCATCTCG |
| Control | TPT | TPT + SFN | SFN | Two-Way ANOVA | |||
|---|---|---|---|---|---|---|---|
| TPT | SFN | Interaction | |||||
| Wi (g) | 56.97 ± 0.83 | 56.64 ± 0.87 | 57.48 ± 0.81 | 56.53 ± 1.00 | |||
| Wf (g) | 110.47 ± 2.24 a | 98.04 ± 2.56 b | 112.26 ± 2.21 a | 111.28 ± 1.08 a | * | ** | * |
| WGR (%) | 93.93 ± 1.16 a | 73.23 ± 5.35 b | 95.30 ± 2.30 a | 96.88 ± 2.07 a | ** | ** | * |
| SGR (% day−1) | 1.18 ± 0.04 a | 0.98 ± 0.05 b | 1.19 ± 0.02 a | 1.21 ± 0.02 a | * | ** | * |
| FCR | 1.29 ± 0.07 b | 1.62 ± 0.08 a | 1.35 ± 0.02 b | 1.23 ± 0.07 b | ** | * | ns |
| CF (%) | 2.31 ± 0.02 a | 1.95 ± 0.17 b | 2.32 ± 0.07 a | 2.47 ± 0.08 a | * | * | ns |
| HSI (%) | 1.49 ± 0.03 b | 1.91 ± 0.08 a | 1.67 ± 0.09 ab | 1.56 ± 0.04 b | ** | ns | * |
| VSI (%) | 5.54 ± 0.24 | 5.79 ± 0.23 | 5.60 ± 0.26 | 5.44 ±0.15 | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhang, C.; Ma, J.; Wang, Y.; Zhang, L.; Yang, X.; Jia, T.; Zhang, K.; Zhang, Q. Protective Role of Sulforaphane against Physiological Toxicity of Triphenyltin in Common Carp (Cyprinus carpio haematopterus). Antioxidants 2024, 13, 1173. https://doi.org/10.3390/antiox13101173
Wang B, Zhang C, Ma J, Wang Y, Zhang L, Yang X, Jia T, Zhang K, Zhang Q. Protective Role of Sulforaphane against Physiological Toxicity of Triphenyltin in Common Carp (Cyprinus carpio haematopterus). Antioxidants. 2024; 13(10):1173. https://doi.org/10.3390/antiox13101173
Chicago/Turabian StyleWang, Bingke, Chunnuan Zhang, Jianshuang Ma, Yanhui Wang, Ling Zhang, Xingli Yang, Tao Jia, Kaisong Zhang, and Qin Zhang. 2024. "Protective Role of Sulforaphane against Physiological Toxicity of Triphenyltin in Common Carp (Cyprinus carpio haematopterus)" Antioxidants 13, no. 10: 1173. https://doi.org/10.3390/antiox13101173






