Effect of Curcumin Intake on Skeletal Muscle Oxygen Saturation Parameters in Older Participants
Abstract
1. Introduction
2. Methods
2.1. Participants
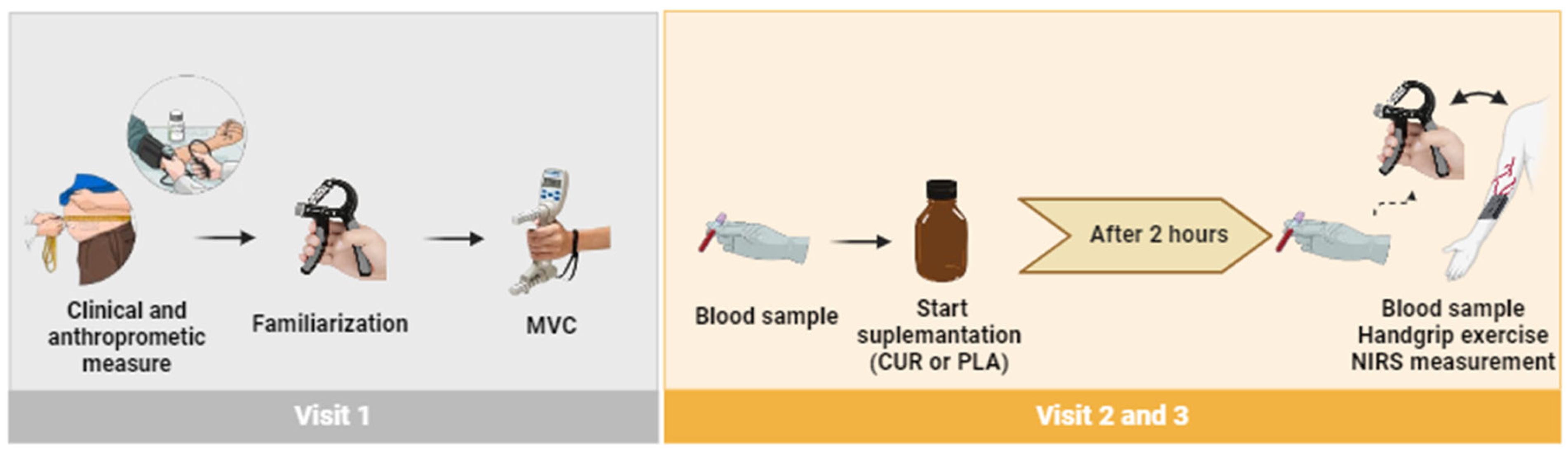
2.2. Experimental Design
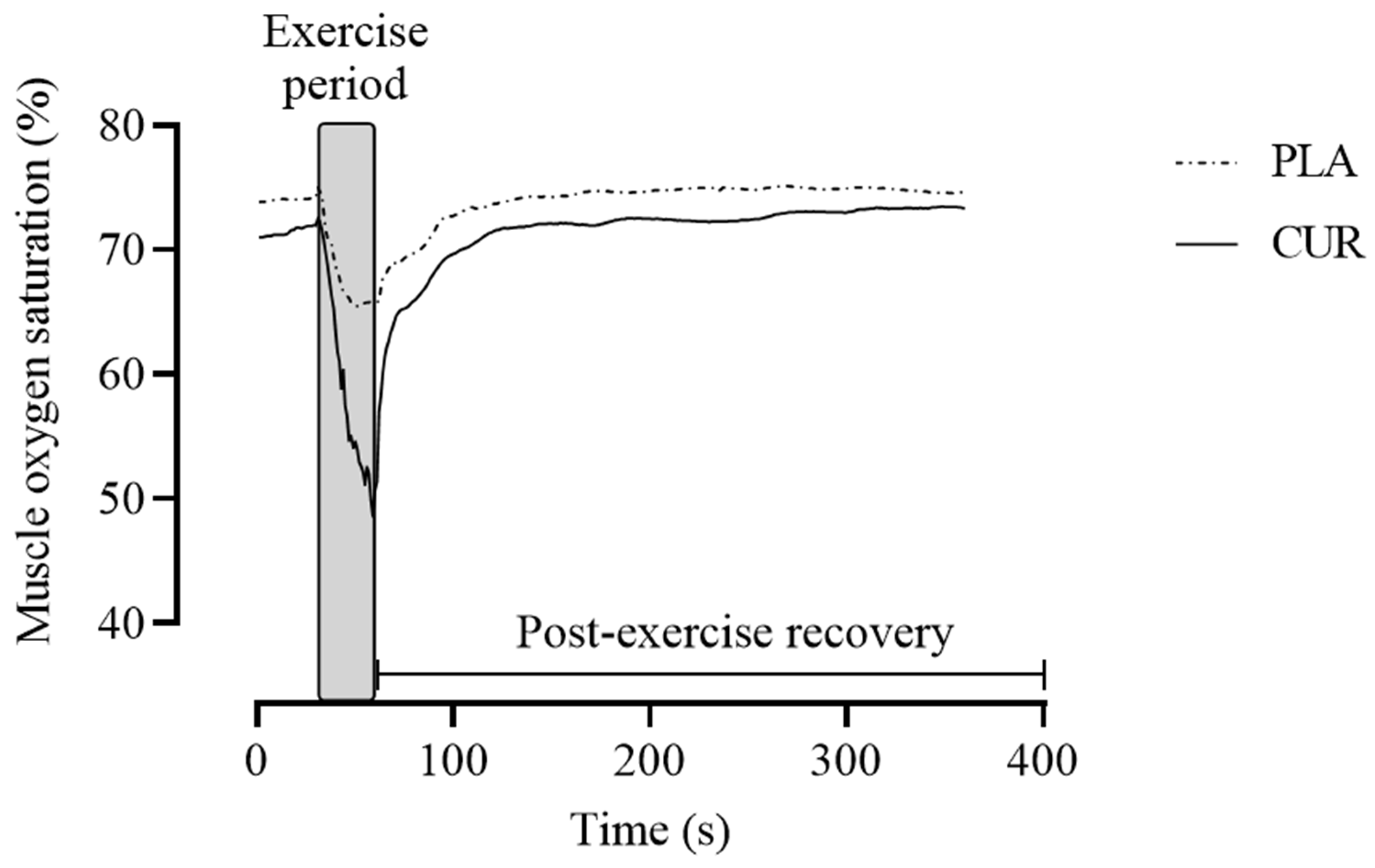
2.3. Muscle Oxygen Saturation Parameters
2.4. Circulating NO Metabolites Analysis
2.5. Statistical Analysis
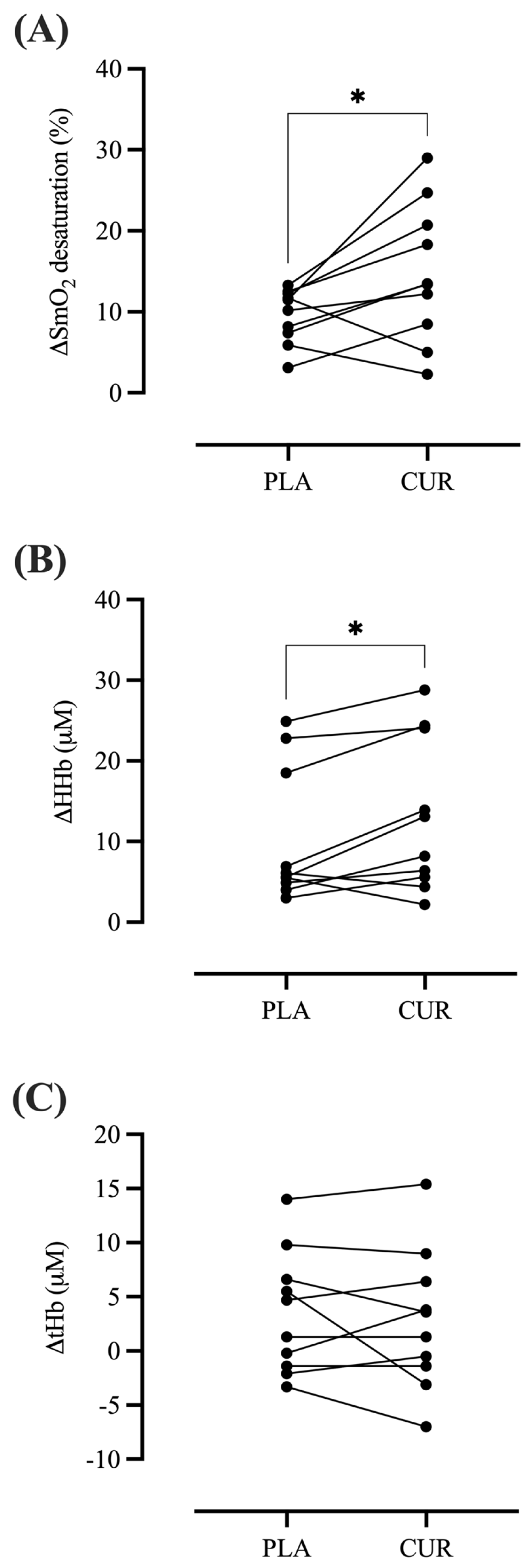
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donato, A.J.; Eskurza, I.; Silver, A.E.; Levy, A.S.; Pierce, G.L.; Gates, P.E.; Seals, D.R. Direct evidence of endothelial oxidative stress with aging in humans: Relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ. Res. 2007, 100, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.A.; Brosnan, M.J.; McIntyre, M.; Graham, D.; Dominiczak, A.F. Superoxide excess in hypertension and aging: A common cause of endothelial dysfunction. Hypertension 2001, 37 Pt 2, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Kojda, G.; Harrison, D. Interactions between NO and reactive oxygen species: Pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc. Res. 1999, 43, 562–571. [Google Scholar] [CrossRef]
- Price, D.T.; Vita, J.A.; Keaney, J.F. Redox control of vascular nitric oxide bioavailability. Antioxid. Redox Signal 2000, 2, 919–935. [Google Scholar] [CrossRef]
- Donato, A.J.; Morgan, R.G.; Walker, A.E.; Lesniewski, L.A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell Cardiol. 2015, 89 Pt B, 122–135. [Google Scholar] [CrossRef]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef]
- Landmesser, U.; Dikalov, S.; Price, S.R.; McCann, L.; Fukai, T.; Holland, S.M.; Mitch, W.E.; Harrison, D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Investig. 2003, 111, 1201–1209. [Google Scholar] [CrossRef]
- Stamler, J.S.; Meissner, G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001, 81, 209–237. [Google Scholar] [CrossRef]
- Kobayashi, J.; Ohtake, K.; Murata, I.; Sonoda, K. Nitric oxide bioavailability for red blood cell deformability in the microcirculation: A review of recent progress. Nitric Oxide Biol. Chem. 2022, 129, 25–29. [Google Scholar] [CrossRef]
- Salvatore, S.S.; Zelenski, K.N.; Perkins, R.K. Age-Related Changes in Skeletal Muscle Oxygen Utilization. J. Funct. Morphol. Kinesiol. 2022, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Dipla, K.; Triantafyllou, A.; Koletsos, N.; Papadopoulos, S.; Sachpekidis, V.; Vrabas, I.S.; Gkaliagkousi, E.; Zafeiridis, A.; Douma, S. Impaired Muscle Oxygenation and Elevated Exercise Blood Pressure in Hypertensive Patients: Links with Vascular Stiffness. Hypertension 2017, 70, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, N.; Choi, Y.; Miyaki, A.; Tanabe, Y.; Sugawara, J.; Ajisaka, R.; Maeda, S. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr. Res. 2012, 32, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.M.; Stoner, L.; Rowlands, D.S.; Caldwell, A.R.; Sanders, E.; Kreutzer, A.; Mitchell, J.B.; Purpura, M.; Jäger, R. Novel Form of Curcumin Improves Endothelial Function in Young, Healthy Individuals: A Double-Blind Placebo Controlled Study. J. Nutr. Metab. 2016, 2016, 1089653. [Google Scholar] [CrossRef]
- Santos-Parker, J.R.; Strahler, T.R.; Bassett, C.J.; Bispham, N.Z.; Chonchol, M.B.; Seals, D.R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging 2017, 9, 187–208. [Google Scholar] [CrossRef]
- Barber-Chamoux, N.; Milenkovic, D.; Verny, M.A.; Habauzit, V.; Pereira, B.; Lambert, C.; Richard, D.; Boby, C.; Mazur, A.; Lusson, J.R.; et al. Substantial Variability Across Individuals in the Vascular and Nutrigenomic Response to an Acute Intake of Curcumin: A Randomized Controlled Trial. Mol. Nutr. Food Res. 2018, 62, 1700418. [Google Scholar] [CrossRef]
- Choi, Y.; Tanabe, Y.; Akazawa, N.; Zempo-Miyaki, A.; Maeda, S. Curcumin supplementation attenuates the decrease in endothelial function following eccentric exercise. J. Exerc. Nutr. Biochem. 2019, 23, 7–12. [Google Scholar] [CrossRef]
- de Oliveira, G.; Alvares, T.S. Effect of curcumin on endothelial function in humans and their proposed physiological mechanism: Insights in formulating curcumin products supplementation. PharmaNutrition 2022, 22, 100313. [Google Scholar] [CrossRef]
- Rezende, C.; Oliveira, G.V.; Volino-Souza, M.; Castro, P.; Murias, J.M.; Alvares, T.S. Turmeric root extract supplementation improves pre-frontal cortex oxygenation and blood volume in older males and females: A randomised cross-over, placebo-controlled study. Int. J. Food Sci. Nutr. 2022, 73, 274–283. [Google Scholar] [CrossRef]
- Hamidie, R.D.R.; Shibaguchi, T.; Yamada, T.; Koma, R.; Ishizawa, R.; Saito, Y.; Hosoi, T.; Masuda, K. Curcumin induces mitochondrial biogenesis by increasing cyclic AMP levels via phosphodiesterase 4A inhibition in skeletal muscle. Br. J. Nutr. 2021, 126, 1642–1650. [Google Scholar] [CrossRef]
- Soto-Urquieta, M.G.; López-Briones, S.; Pérez-Vázquez, V.; Saavedra-Molina, A.; González-Hernández, G.A.; Ramírez-Emiliano, J. Curcumin restores mitochondrial functions and decreases lipid peroxidation in liver and kidneys of diabetic db/db mice. Biol. Res. 2014, 47, 74. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Muthalib, M.; Quaresima, V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: Recent developments. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2011, 369, 4577–4590. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, T.; McCully, K.K.; Niwayama, M.; Chance, B. The use of muscle near-infrared spectroscopy in sport, health and medical sciences: Recent developments. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2011, 369, 4591–4604. [Google Scholar] [CrossRef]
- Grassi, B.; Quaresima, V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: A review from an exercise physiology perspective. J. Biomed. Opt. 2016, 21, 091313. [Google Scholar] [CrossRef]
- Alvares, T.S.; Oliveira, G.V.; Soares, R.; Murias, J.M. Near-infrared spectroscopy-derived total haemoglobin as an indicator of changes in muscle blood flow during exercise-induced hyperaemia. J. Sports Sci. 2020, 38, 751–758. [Google Scholar] [CrossRef]
- Volino-Souza, M.; de Oliveira, G.; Barros-Santos, E.; Conte-Junior, C.; Alvares, T. The impact of beetroot juice intake on muscle oxygenation and performance during rhythmic handgrip exercise. PharmaNutrition 2020, 14, 100215. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Yang, Y.; Lu, Y. The Determination of Nitrate and Nitrite in Human Urine and Blood by High-Performance Liquid Chromatography and Cloud-Point Extraction. J. Chromatogr. Sci. 2015, 53, 1169–1177. [Google Scholar] [CrossRef]
- Proctor, D.N.; Shen, P.H.; Dietz, N.M.; Eickhoff, T.J.; Lawler, L.A.; Ebersold, E.J.; Loeffler, D.L.; Joyner, M.J. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J. Appl. Physiol. 1998, 85, 68–75. [Google Scholar] [CrossRef]
- Kutsuzawa, T.; Shioya, S.; Kurita, D.; Haida, M.; Yamabayashi, H. Effects of age on muscle energy metabolism and oxygenation in the forearm muscles. Med. Sci. Sports Exerc. 2021, 33, 901–906. [Google Scholar] [CrossRef]
- Ichimura, S.; Murase, N.; Osada, T.; Kime, R.; Homma, T.; Ueda, C.; Nagasawa, T.; Motobe, M.; Hamaoka, T.; Katsumura, T. Age and activity status affect muscle reoxygenation time after maximal cycling exercise. Med. Sci. Sports Exerc. 2006, 38, 1277–1281. [Google Scholar] [CrossRef]
- de Oliveira, G.V.; Soares, R.N.; Volino-Souza, M.; Leitão, R.; Murias, J.M.; Alvares, T.S. The effects of aging and cardiovascular risk factors on microvascular function assessed by near-infrared spectroscopy. Microvasc. Res. 2019, 126, 103911. [Google Scholar] [CrossRef] [PubMed]
- Shannon, O.M.; Clifford, T.; Seals, D.R.; Craighead, D.H.; Rossman, M.J. Nitric oxide, aging and aerobic exercise: Sedentary individuals to Master’s athletes. Nitric Oxide Biol. Chem. 2022, 125–126, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nisoli, E.; Falcone, S.; Tonello, C.; Cozzi, V.; Palomba, L.; Fiorani, M.; Pisconti, A.; Brunelli, S.; Cardile, A.; Francolini, M.; et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc. Natl. Acad. Sci. USA 2004, 101, 16507–16512. [Google Scholar] [CrossRef]
- Boonla, O.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P.; Prachaney, P.; Greenwald, S.E. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide Biol. Chem. 2014, 42, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.J.; Chen, C.W.; Tsai, S.Y.; Wang, P.Y.; Owaga, E.; Hsieh, R.H. Curcumin supplementation ameliorated vascular dysfunction and improved antioxidant status in rats fed a high-sucrose, high-fat diet. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2018, 43, 669–676. [Google Scholar] [CrossRef]
- Lauer, T.; Preik, M.; Rassaf, T.; Strauer, B.E.; Deussen, A.; Feelisch, M.; Kelm, M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc. Natl. Acad. Sci. USA 2001, 98, 12814–12819. [Google Scholar] [CrossRef]
- Baylis, C.; Vallance, P. Measurement of nitrite and nitrate levels in plasma and urine--what does this measure tell us about the activity of the endogenous nitric oxide system? Curr. Opin. Nephrol. Hypertens. 1998, 7, 59–62. [Google Scholar] [CrossRef]
- Coggan, A.R.; Abduljalil, A.M.; Swanson, S.C.; Earle, M.S.; Farris, J.W.; Mendenhall, L.A.; Robitaille, P.M. Muscle metabolism during exercise in young and older untrained and endurance-trained men. J. Appl. Physiol. 1993, 75, 2125–2133. [Google Scholar] [CrossRef]
- McCully, K.K.; Fielding, R.A.; Evans, W.J.; Leigh, J.S., Jr.; Posner, J.D. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J. Appl. Physiol. 1993, 75, 813–819. [Google Scholar] [CrossRef]
- Chung, S.; Rosenberry, R.; Ryan, T.E.; Munson, M.; Dombrowsky, T.; Park, S.; Nasirian, A.; Haykowsky, M.J.; Nelson, M.D. Near-infrared spectroscopy detects age-related differences in skeletal muscle oxidative function: Promising implications for geroscience. Physiol. Rep. 2018, 6, e13588. [Google Scholar] [CrossRef]
- Murias, J.M.; Spencer, M.D.; Keir, D.A.; Paterson, D.H. Systemic and vastus lateralis muscle blood flow and O2 extraction during ramp incremental cycle exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R720–R725. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Sahebkar, A.; Amiri, M.; Davoudi, S.M.; Beiraghdar, F.; Hoseininejad, S.L.; Kolivand, M. Improvement of sulphur mustard-induced chronic pruritus, quality of life and antioxidant status by curcumin: Results of a randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2012, 108, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Suzuki, K.; Kim, H.K.; Otsuka, Y.; Imaizumi, A.; Miyashita, M.; Sakamoto, S. Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int. J. Sports Med. 2014, 35, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Golab, F.; Morvaridzadeh, M.; Potter, E.; Akbari-Fakhrabadi, M.; Farsi, F.; Tanbakooei, S.; Shidfar, F. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: A randomized placebo-controlled clinical trial. Diabetes Metab. Syndr. 2020, 14, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Deane, C.S.; Din, U.S.U.; Sian, T.S.; Smith, K.; Gates, A.; Lund, J.N.; Williams, J.P.; Rueda, R.; Pereira, S.L.; Atherton, P.J.; et al. Curcumin Enhances Fed-State Muscle Microvascular Perfusion but Not Leg Glucose Uptake in Older Adults. Nutrients 2022, 14, 1313. [Google Scholar] [CrossRef]


| Demographics | |
| n (male/female) | 10 (6/4) |
| Age (years) | 67 ± 4 |
| Body mass (kg) | 80 ± 12 |
| Height (m) | 1.65 ± 0.13 |
| Body mass index (kg/m2) | 30 ± 4 |
| Clinical | |
| Total cholesterol (mg/dL) | 163 ± 35 |
| HDL-cholesterol (mg/dL) | 40 ± 13 |
| LDL-cholesterol (mg/dL) | 104 ± 37 |
| Triglycerides (mg/dL) | 108 ± 37 |
| Fasting glucose (mg/dL) | 138 ± 57 |
| Systolic blood pressure (mmHg) | 131 ± 8 |
| Diastolic blood pressure (mmHg) | 81 ± 7 |
| Heart rate (bpm) | 66 ± 9 |
| Variable | PLA | CUR | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Nitrate (µM) | 44.25 ± 10.94 | 44.03 ± 10.16 | 43.09 ± 10.66 | 47.24 ±1 1.29 |
| Nitrate (%) | 0.71 ± 12.11 | 10.5 ± 17.11 | ||
| Nitrite (µM) | 0.263 ± 0.233 | 0.264 ± 0.268 | 0.240 ± 0.255 | 0.257 ± 0.273 |
| Nitrite (%) | −4.94 ± 17.28 | 6.82 ± 11.68 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Junior, O.J.F.; Pinheiro, V.d.S.; Souza, T.G.d.S.d.; Alvares, T.S. Effect of Curcumin Intake on Skeletal Muscle Oxygen Saturation Parameters in Older Participants. Antioxidants 2024, 13, 1175. https://doi.org/10.3390/antiox13101175
Ramos-Junior OJF, Pinheiro VdS, Souza TGdSd, Alvares TS. Effect of Curcumin Intake on Skeletal Muscle Oxygen Saturation Parameters in Older Participants. Antioxidants. 2024; 13(10):1175. https://doi.org/10.3390/antiox13101175
Chicago/Turabian StyleRamos-Junior, Olavo João Frederico, Vivian dos Santos Pinheiro, Tatiane Gomes dos Santos de Souza, and Thiago Silveira Alvares. 2024. "Effect of Curcumin Intake on Skeletal Muscle Oxygen Saturation Parameters in Older Participants" Antioxidants 13, no. 10: 1175. https://doi.org/10.3390/antiox13101175
APA StyleRamos-Junior, O. J. F., Pinheiro, V. d. S., Souza, T. G. d. S. d., & Alvares, T. S. (2024). Effect of Curcumin Intake on Skeletal Muscle Oxygen Saturation Parameters in Older Participants. Antioxidants, 13(10), 1175. https://doi.org/10.3390/antiox13101175







