Different Oxidative Stress and Inflammation Patterns of Diseased Left Anterior Descending Coronary Artery versus Internal Thoracic Artery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Procedure
2.3. Blood Sampling
2.4. Oxidative Stress Assays
2.5. Cytokines Determination
2.6. Statistical Analysis
3. Results
3.1. Patients
3.2. Procedure Outcome
3.3. Laboratory Findings
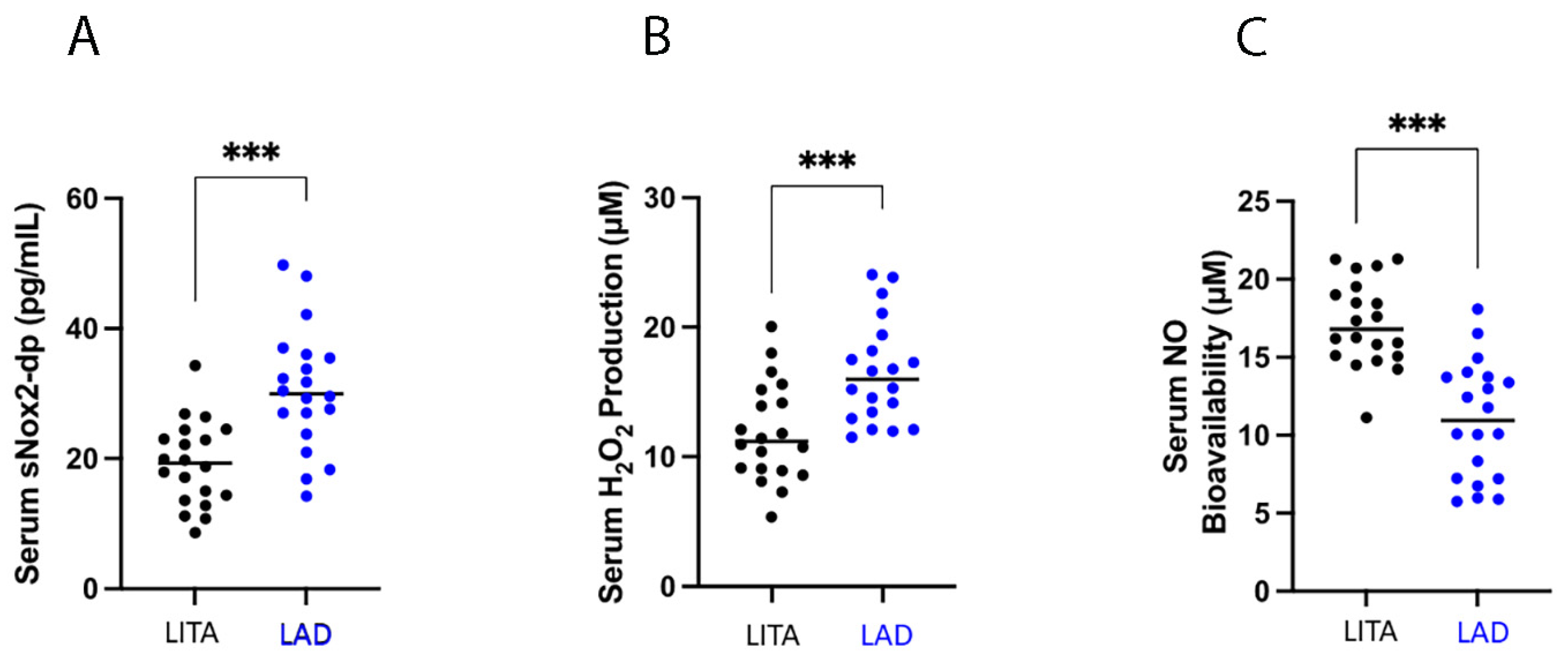
3.3.1. Oxidative Stress
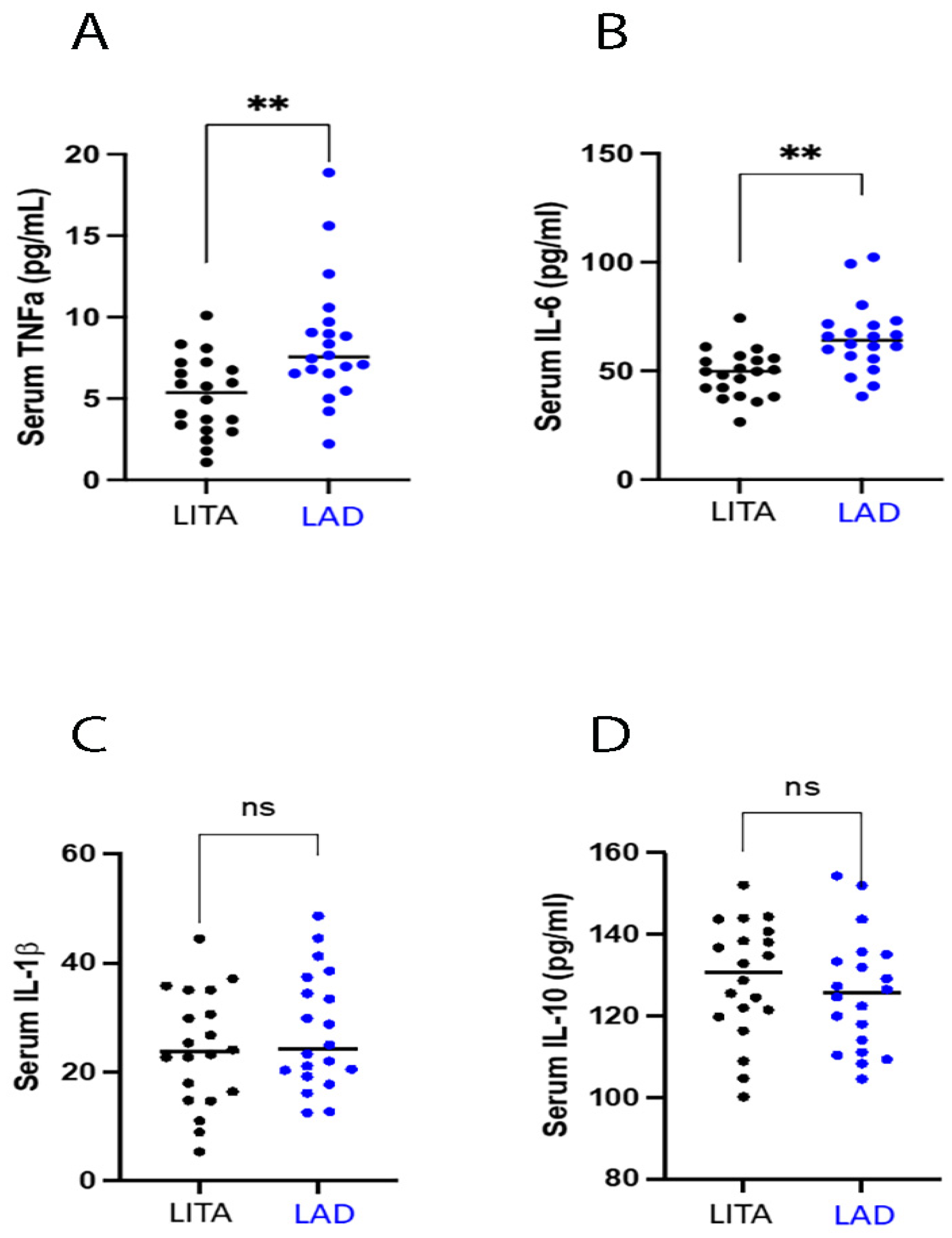
3.3.2. Inflammatory Status
4. Discussion
4.1. Possible Translational Implications in Coronary Surgery
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallino, A.; Aboyans, V.; Diehm, C.; Cosentino, F.; Stricker, H.; Falk, E.; Schouten, O.; Lekakis, J.; Amann-Vesti, B.; Siclari, F.; et al. Non-coronary atherosclerosis. Eur. Heart J. 2014, 35, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Aday, A.W.; Rose, L.M.; Ridker, P.M. Residual inflammatory risk on treatment with PCSK9 inhibition and statin therapy. Circulation 2018, 138, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., 3rd; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. American Heart Association. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Zacho, J.; Tybjaerg-Hansen, A.; Jensen, J.S.; Grande, P.; Sillesen, H.; Nordestgaard, B.G. Genetically elevated C-reactive protein and ischemic vascular disease. N. Engl. J. Med. 2008, 359, 1897–1908. [Google Scholar] [CrossRef]
- Ahn, J.H.; Tantry, U.S.; Kang, M.G.; Park, H.W.; Koh, J.S.; Bae, J.S.; Cho, S.Y.; Kim, K.H.; Jang, J.Y.; Park, J.R.; et al. Residual Inflammatory Risk and its Association with Events in East Asian Patients After Coronary Intervention. JACC Asia 2022, 2, 323–337. [Google Scholar] [CrossRef]
- Rymer, J.A.; Newby, L.K. Failure to launch: Targeting inflammation in acute coronary syndromes. J. Am. Coll. Cardiol. Basic Trans. Sci. 2017, 2, 484–497. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.C.; Lopes, N.; Soares, P.R.; Góis, A.F.; Stolf, N.A.; Oliveira, S.A.; Hueb, W.A.; Ramires, J.A. Five-year follow-up of angiographic disease progression after medicine, angioplasty, or surgery. J. Cardiothorac. Surg. 2010, 5, 91. [Google Scholar] [CrossRef]
- Zhang, M.; Guddeti, R.R.; Matsuzawa, Y.; Sara, J.D.; Kwon, T.G.; Liu, Z.; Sun, T.; Lee, S.J.; Lennon, R.J.; Bell, M.R.; et al. Left Internal Mammary Artery Versus Coronary Stents: Impact on Downstream Coronary Stenoses and Conduit Patency. J. Am. Heart Assoc. 2016, 5, e003568. [Google Scholar] [CrossRef]
- Valooran, G.J.; Nair, S.K.; Chandrasekharan, K. Rare case of proximal coronary plaque regression after distal arterial grafting. Indian Heart J. 2016, 68 (Suppl. S2), S47–S50. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef]
- Lubos, E.; Handy, D.E.; Loscalzo, J. Role of oxidative stress and nitric oxide in atherothrombosis. Front. Biosci. 2008, 13, 5323–5344. [Google Scholar] [CrossRef] [PubMed]
- Cammisotto, V.; Nocella, C.; Bartimoccia, S.; Sanguigni, V.; Francomano, D.; Sciarretta, S.; Pastori, D.; Peruzzi, M.; Cavarretta, E.; D’Amico, A.; et al. The Role of Antioxidants Supplementation in Clinical Practice: Focus on Cardiovascular Risk Factors. Antioxidants 2021, 10, 146. [Google Scholar] [CrossRef]
- Antoniades, C.; Demosthenous, M.; Reilly, S.; Margaritis, M.; Zhang, M.H.; Antonopoulos, A.; Marinou, K.; Nahar, K.; Jayaram, R.; Tousoulis, D.; et al. Myocardial redox state predicts in-hospital clinical outcome after cardiac surgery effects of short-term pre-operative statin treatment. J. Am. Coll. Cardiol. 2012, 59, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kattach, H.; Ratnatunga, C.; Pillai, R.; Channon, K.M.; Casadei, B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J. Am. Coll. Cardiol. 2008, 51, 68–74. [Google Scholar] [CrossRef]
- Wu, J.H.; Marchioli, R.; Silletta, M.G.; Masson, S.; Sellke, F.W.; Libby, P.; Milne, G.L.; Brown, N.J.; Lombardi, F.; Damiano, R.J., Jr.; et al. Oxidative Stress Biomarkers and Incidence of Postoperative Atrial Fibrillation in the Omega-3 Fatty Acids for Prevention of Postoperative Atrial Fibrillation (OPERA) Trial. J. Am. Heart Assoc. 2015, 4, e001886. [Google Scholar] [CrossRef] [PubMed]
- Ulus, A.T.; Aksoyek, A.; Ozkan, M.; Katircioglu, S.F.; Basu, S. Cardiopulmonary bypass as a cause of free radical-induced oxidative stress and enhanced blood-borne isoprostanes in humans. Free. Radic. Biol. Med. 2003, 34, 911–917. [Google Scholar] [CrossRef]
- Nocella, C.; D’Amico, A.; Cammisotto, V.; Bartimoccia, S.; Castellani, V.; Loffredo, L.; Marini, L.; Ferrara, G.; Testa, M.; Motta, G.; et al. Structure, Activation, and Regulation of NOX2: At the Crossroad between the Innate Immunity and Oxidative Stress-Mediated Pathologies. Antioxidants 2023, 12, 429. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Kampoli, A.M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH oxidases in vascular pathology. Antioxid. Redox Signal. 2014, 20, 2794–2814. [Google Scholar] [CrossRef]
- Daiber, A.; Chlopicki, S. Revisiting pharmacology of oxidative stress and endothelial dysfunction in cardiovascular disease: Evidence for redox-based therapies. Free Radic. Biol. Med. 2020, 157, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Yudkin, J.S.; Kumari, M.; Humphries, S.E.; Mohamed-Ali, V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis 2000, 148, 209–214. [Google Scholar] [CrossRef]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The Role of Cytokines in the Development of Atherosclerosis. Biochemistry 2016, 81, 1358–1370. [Google Scholar] [CrossRef]
- Tokgozoglu, L.; Ergene, O.; Kinay, O.; Nazli, C.; Hascelik, G.; Hoscan, Y. Plasma interleukin-6 levels are increased in coronary artery ectasia. Acta Cardiol. 2004, 59, 515–519. [Google Scholar] [CrossRef]
- Aydin, M.; Tekin, I.O.; Dogan, S.M.; Yildirim, N.; Arasli, M.; Sayin, M.R.; Aktop, Z. The levels of tumor necrosis factor-alpha and interleukin-6 in patients with isolated coronary artery ectasia. Mediat. Inflamm. 2009, 2009, 106145. [Google Scholar] [CrossRef]
- Uzui, H.; Harpf, A.; Liu, M.; Doherty, T.M.; Shukla, A.; Chai, N.N.; Tripathi, P.V.; Jovinge, S.; Wilkin, D.J.; Asotra, K.; et al. Increased expression of membrane type 3-matrix metalloproteinase in human atherosclerotic plaque: Role of activated macrophages and inflammatory cytokines. Circulation 2002, 106, 3024–3030. [Google Scholar] [CrossRef]
- Tuomisto, K.; Jousilahti, P.; Sundvall, J.; Pajunen, P.; Salomaa, V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality. A population-based, prospective study. Thromb. Haemost. 2006, 95, 511–518. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Libby, P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: Further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur. Heart J. 2020, 41, 2153–2163. [Google Scholar] [CrossRef]
- Loffredo, L.; Ben, M.D.; Bartimoccia, S.; Castellani, V.; Mancinella, M.; Ciacci, P.; Orlando, F.; Paraninfi, A.; Angelico, F.; Ferro, D.; et al. Chocolate enriched by extra virgin olive oil improves endothelial function and oxidative stress in patients with diabetes. Nutrition 2021, 90, 111270. [Google Scholar] [CrossRef]
- Carnevale, R.; Nocella, C.; Schiavon, S.; Cammisotto, V.; Cotugno, M.; Forte, M.; Valenti, V.; Marchitti, S.; Vecchio, D.; Biondi Zoccai, G.; et al. Beneficial effects of a combination of natural product activators of autophagy on endothelial cells and platelets. Br. J. Pharmacol. 2021, 178, 2146–2159. [Google Scholar] [CrossRef]
- Jaumdally, R.; Varma, C.; Macfadyen, R.J.; Lip, G.Y. Coronary sinus blood sampling: An insight into local cardiac pathophysiology and treatment? Eur. Heart J. 2007, 28, 929–940. [Google Scholar] [CrossRef]
- Suárez-Cuenca, J.A.; Robledo-Nolasco, R.; Alcántara-Meléndez, M.A.; Díaz Hernández, L.J.; Vera-Gómez, E.; Hernández-Patricio, A.; Sánchez-Díaz, K.S.; Buendía-Gutiérrez, J.A.; Contreras-Ramos, A.; Ruíz-Hernández, A.S.; et al. Coronary circulating mononuclear progenitor cells and soluble biomarkers in the cardiovascular prognosis after coronary angioplasty. J. Cell Mol. Med. 2019, 23, 4844–4849. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, A.H.; Jang, C.; Jang, I.J.; Kim, K.B.; Cho, J.Y.; Hwang, H.Y. Comparison of the Plasma Metabolome Profiles between the Internal Thoracic Artery and Ascending Aorta in Patients Undergoing Coronary Artery Bypass Graft Surgery Using Gas Chromatography Time-of-Flight Mass Spectrometry. J. Korean Med. Sci. 2019, 34, e104. [Google Scholar] [CrossRef]
- Liberale, L.; Ministrini, S.; Carbone, F.; Camici, G.G.; Montecucco, F. Cytokines as therapeutic targets for cardio- and cerebrovascular diseases. Basic Res. Cardiol. 2021, 116, 23. [Google Scholar] [CrossRef]
- Ridker, P.M.; Devalaraja, M.; Baeres, F.M.M.; Engelmann, M.D.M.; Hovingh, G.K.; Ivkovic, M.; Lo, L.; Kling, D.; Pergola, P.; Raj, D.; et al. RESCUE Investigators. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2021, 397, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Jensen, C.; Meyer, A.S.P.; Zonoozi, A.A.M.; Honda, H. Efficacy and safety of interleukin-6 inhibition with ziltivekimab in patients at high risk of atherosclerotic events in Japan (RESCUE-2): A randomized, double-blind, placebo-controlled, phase 2 trial. J. Cardiol. 2023, 82, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Shiga, Y.; Miura, S.I.; Nishikawa, H.; Nakamura, A.; Arimura, T.; Mitsutake, R.; Iwata, A.; Saku, K. Regression of coronary plaque after coronary artery bypass graft. J. Cardiol. Cases 2012, 5, e92–e95. [Google Scholar] [CrossRef]
- Dodic, S.; Kovacevic, D.; Bjelobrk, M.; Petrovic, M.; Miljkovic, T.; Cankovic, M.; Vujin, B.; Cemerlic-Adjic, N.; Dodic, B. Spontaneous regression of proximal LAD subocclusive stenosis after left internal mammary artery bypass grafting. Herz 2015, 40, 79–81. [Google Scholar] [CrossRef]
- Carnevale, R.; Loffredo, L.; Nocella, C.; Bartimoccia, S.; Bucci, T.; De Falco, E.; Peruzzi, M.; Chimenti, I.; Biondi-Zoccai, G.; Pignatelli, P.; et al. Epicatechin and catechin modulate endothelial activation induced by platelets of patients with peripheral artery disease. Oxidative Med. Cell. Longev. 2014, 2014, 691015. [Google Scholar] [CrossRef]
| Patients Characteristics | |
|---|---|
| N° of patients | 20 |
| Age, y | 65 ± 10 |
| Range | 46–83 |
| Male sex | 18 (90) |
| BSA, m2 | 1.90 ± 0.16 |
| BMI, kg/m2 | 26.1 ± 2.9 |
| Familiar history of CAD | 6 (30) |
| Smoking, n (%) | |
| Never | 4 (20) |
| Former | 10 (50) |
| Active | 6 (30) |
| Hypertension | 17 (85) |
| Diabetes mellitus, n (%) | |
| Type 1 | 2 (10) |
| Type 2 | 6 (30) |
| Diabetes therapy, n (%) | |
| Oral | 2 (25) |
| Insulin | 2 (25) |
| Oral + insulin | 4 (50) |
| Dyslipidaemia | 15 (75) |
| COPD | 3 (15) |
| Paroxysmal AF | 1 (5) |
| History of MI, n (%) | |
| NSTEMI | 4 (20) |
| STEMI | 2 (10) |
| Previous PTCA | 5 (25) |
| Previous CVA | 1 (5) |
| CAD presentation, n (%) | |
| STEMI | 0 |
| NSTEMI | 2 (10) |
| Stable angina | 14 (70) |
| Asymptomatic | 4 (20) |
| Echocardiographic Characteristics | |
| LVEF, % | 56 ± 7 |
| LVEDD, mm | 46 ± 5 |
| LVESD, mm | 32 ± 5 |
| IVS, mm | 12 ± 2 |
| PW, mm | 10 ± 2 |
| TAPSE, mm | 26 ± 4 |
| sPAP, mmHg | 28 ± 9 |
| Coronary Angiography Characteristics | |
| N° of diseased vessels | 3.6 ± 1.0 |
| Syntax Score | 24 ± 5 |
| LAD stenosis, % | 85 ± 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salica, A.; Cammisotto, V.; Scaffa, R.; Folino, G.; De Paulis, R.; Carnevale, R.; Benedetto, U.; Saade, W.; Marullo, A.; Sciarretta, S.; et al. Different Oxidative Stress and Inflammation Patterns of Diseased Left Anterior Descending Coronary Artery versus Internal Thoracic Artery. Antioxidants 2024, 13, 1180. https://doi.org/10.3390/antiox13101180
Salica A, Cammisotto V, Scaffa R, Folino G, De Paulis R, Carnevale R, Benedetto U, Saade W, Marullo A, Sciarretta S, et al. Different Oxidative Stress and Inflammation Patterns of Diseased Left Anterior Descending Coronary Artery versus Internal Thoracic Artery. Antioxidants. 2024; 13(10):1180. https://doi.org/10.3390/antiox13101180
Chicago/Turabian StyleSalica, Andrea, Vittoria Cammisotto, Raffaele Scaffa, Giulio Folino, Ruggero De Paulis, Roberto Carnevale, Umberto Benedetto, Wael Saade, Antonino Marullo, Sebastiano Sciarretta, and et al. 2024. "Different Oxidative Stress and Inflammation Patterns of Diseased Left Anterior Descending Coronary Artery versus Internal Thoracic Artery" Antioxidants 13, no. 10: 1180. https://doi.org/10.3390/antiox13101180










