A Single-Cell Atlas of the Substantia Nigra Reveals Therapeutic Effects of Icaritin in a Rat Model of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Drugs
2.2. Animals Care
2.3. Rotenone Rat Model of PD
2.4. Behavioral Assessments
2.4.1. Rearing Behavior Test
2.4.2. Wire Grip Test
2.5. Measurement of Dopamine, Serotonin, and Their Metabolites in Striatum
2.6. Western Blotting Analysis
2.7. Isolation of the Substantia Nigra in Rats with PD and Cell Dissociation
2.8. Single-Cell RNA-Seq Library Preparation and Sequencing
2.9. GSVA and SCENIC Analysis
2.10. Analysis of Cell–Cell Interactions
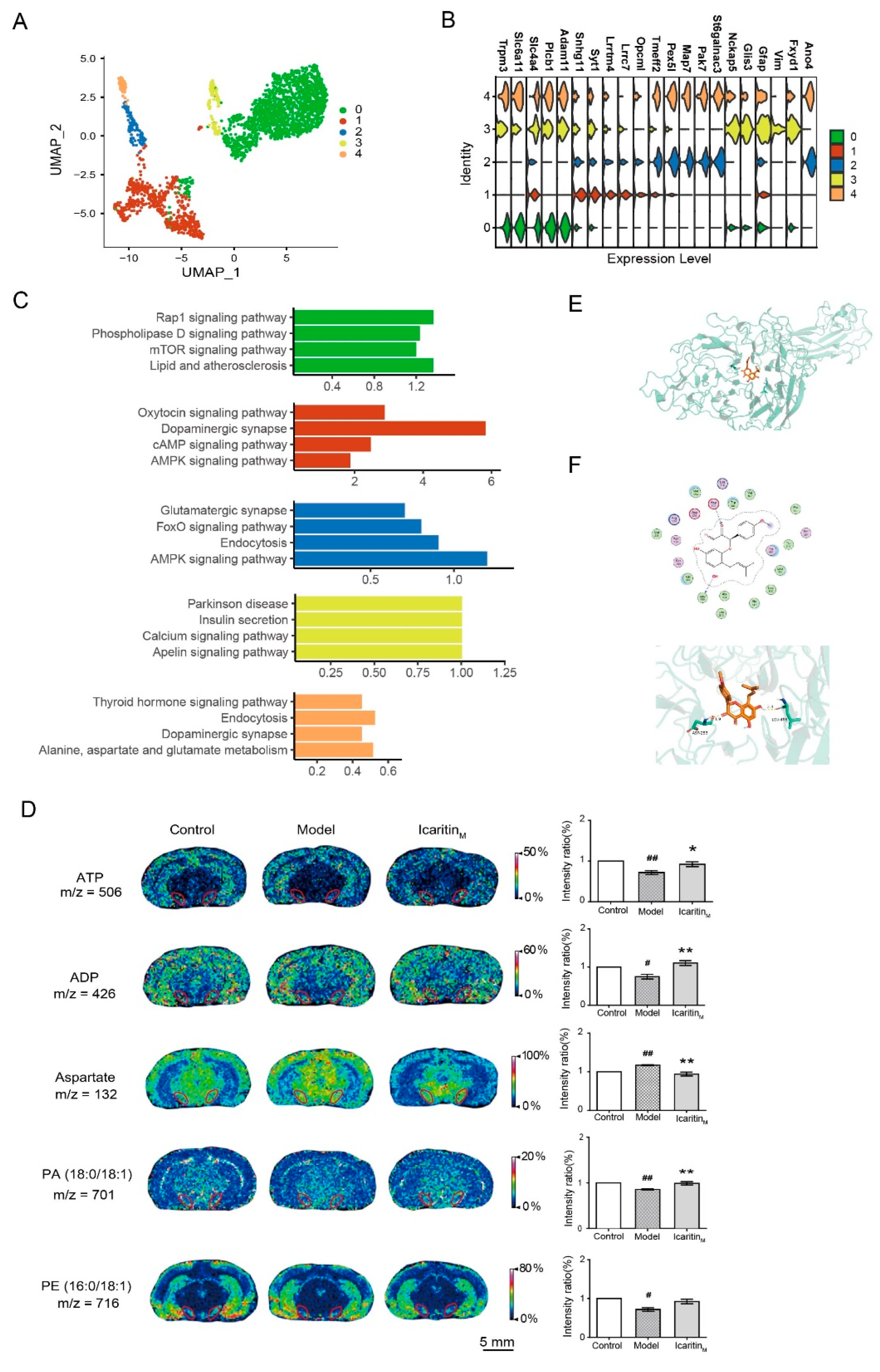
2.11. Molecular Docking
2.12. Matrix-Assisted Laser Desorption/Ionisation–Mass Spectrometry Imaging (MALDI-MSI)
2.13. Statistical Analysis
3. Results
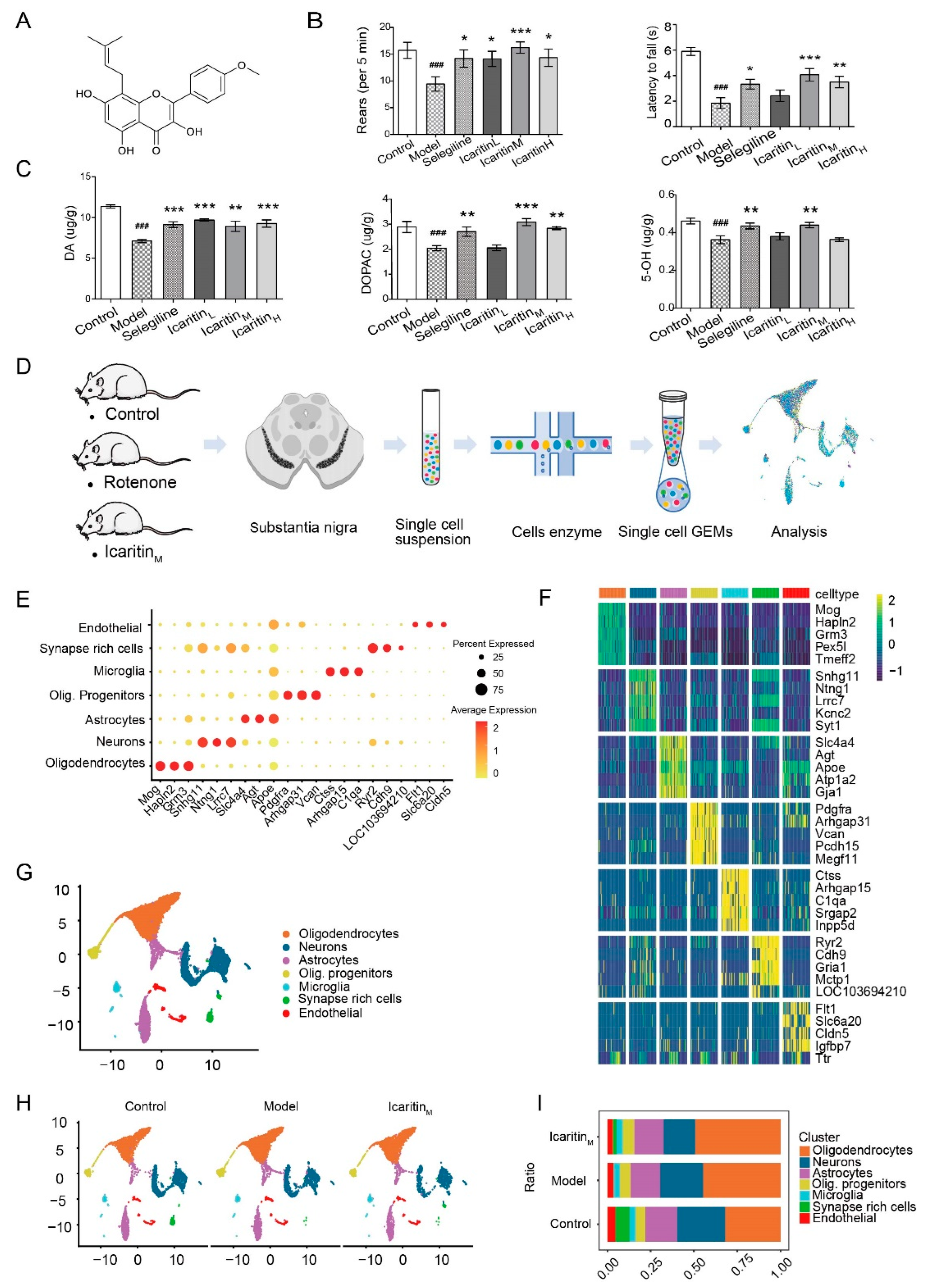
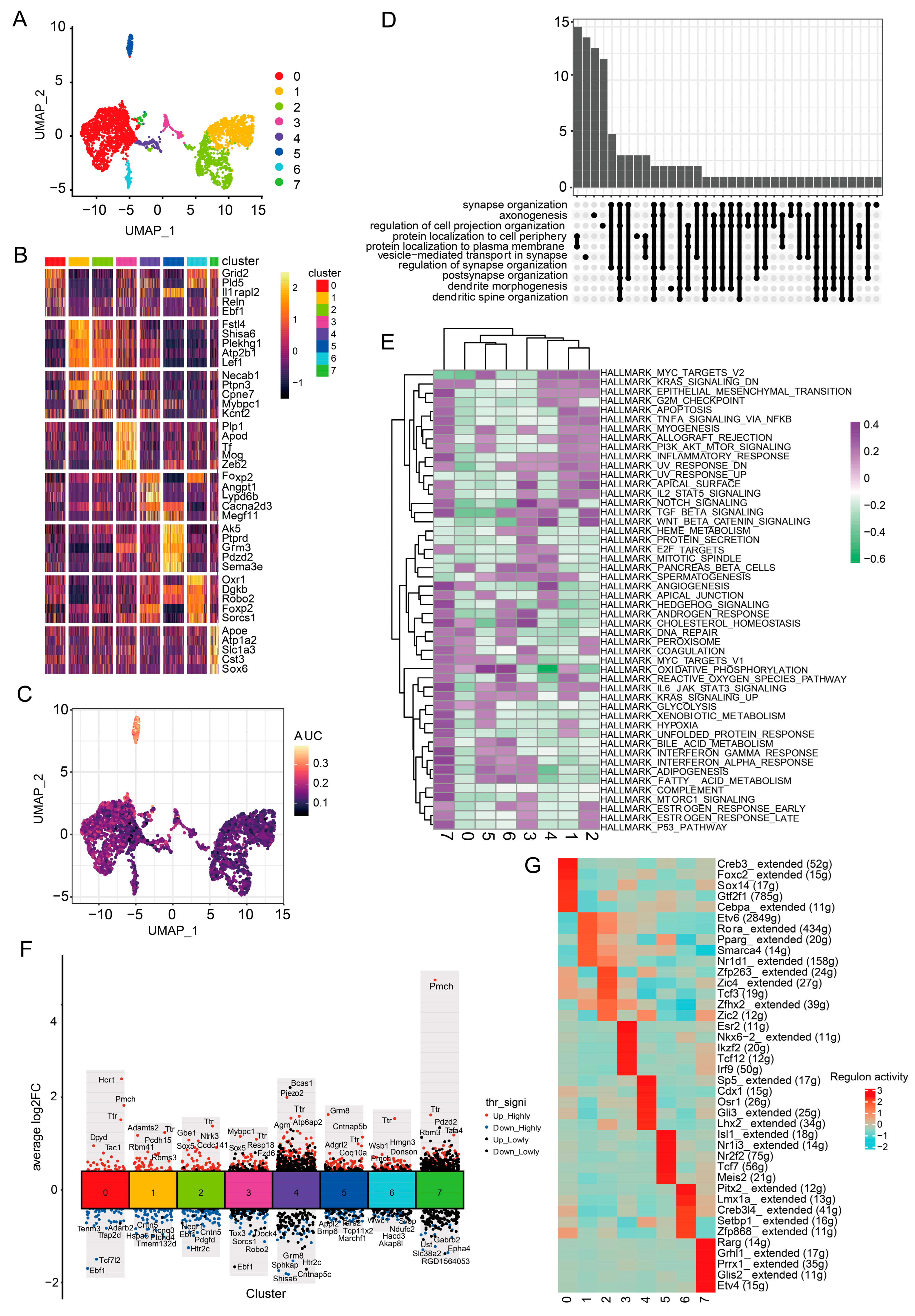
3.1. Establishment of the Rat Model of PD and Single-Cell Transcriptome Atlas of Cells in the Substantia Nigra
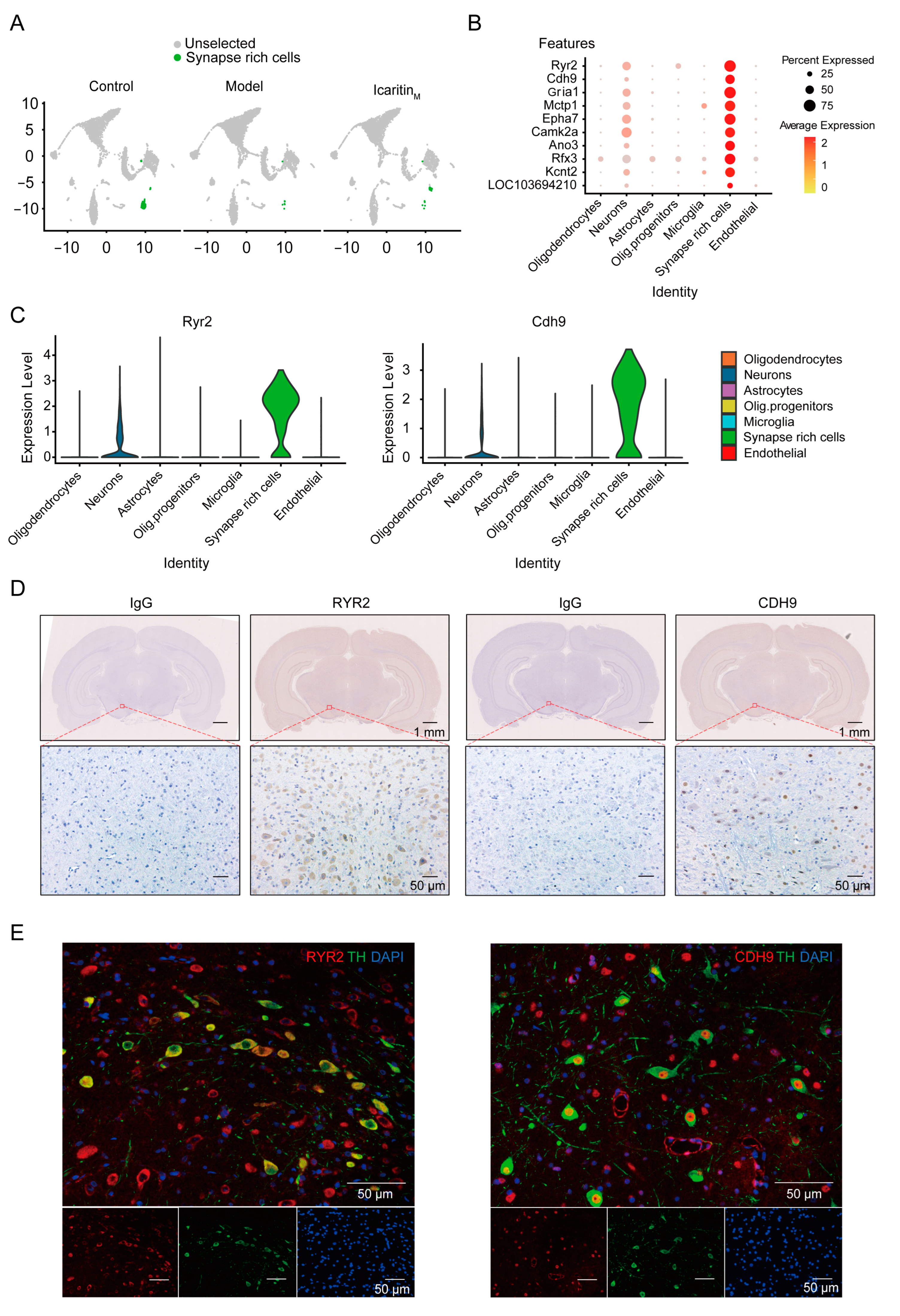
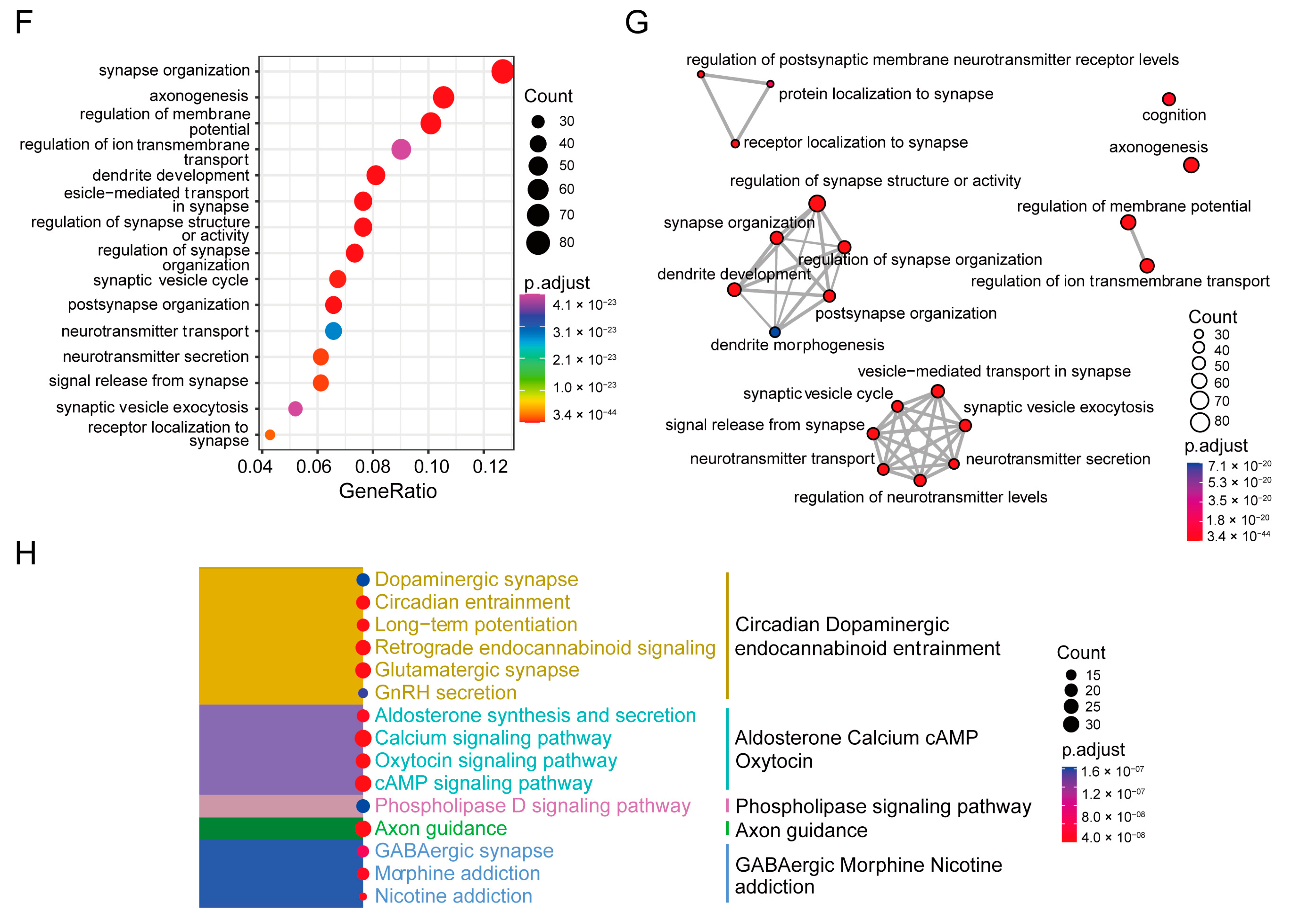
3.2. Cells Characterized by Specific Synapse-Related Genes Are Significantly Reduced in the Substantia Nigra of Rats with PD and Recovered by Icaritin Treatment
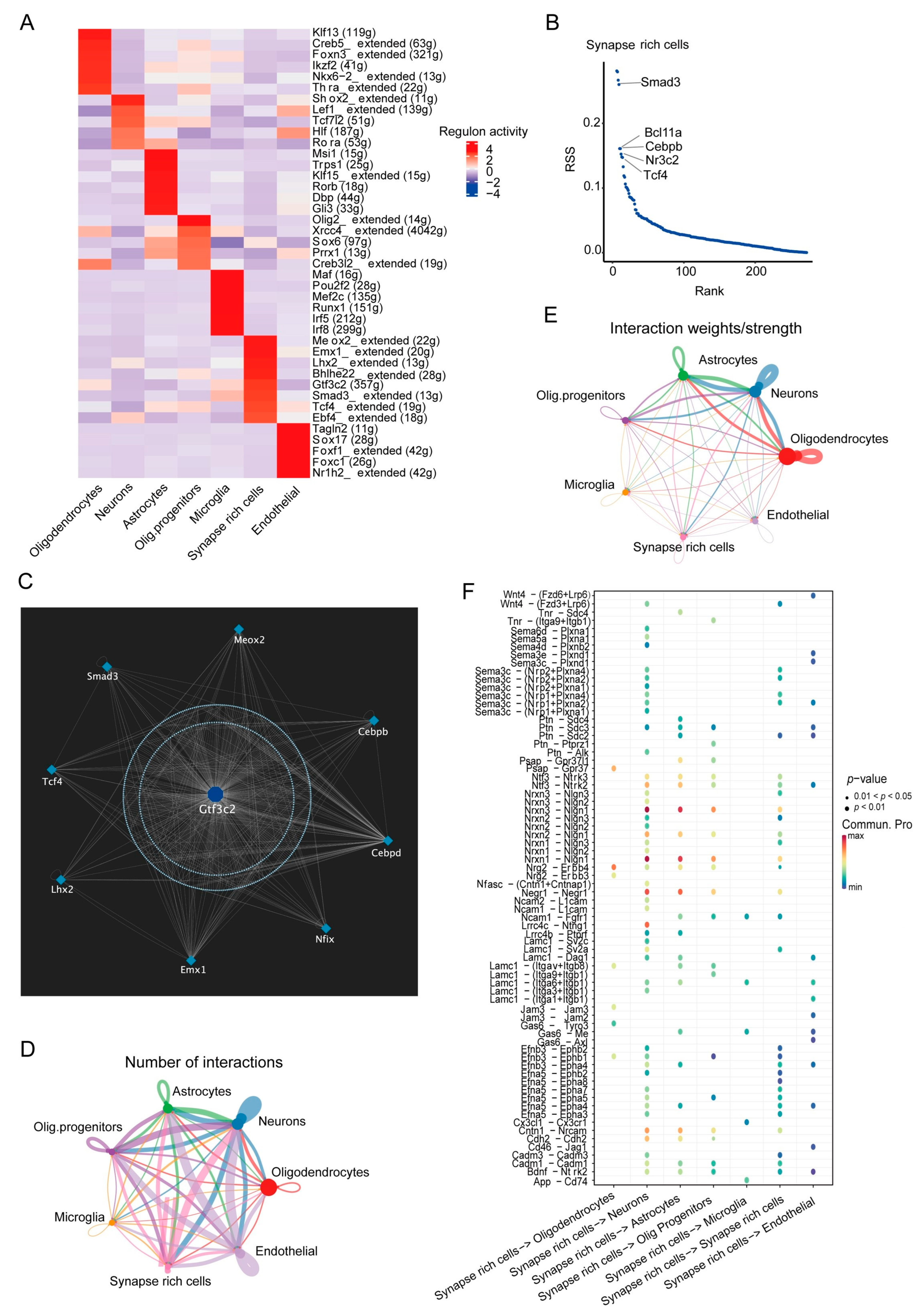
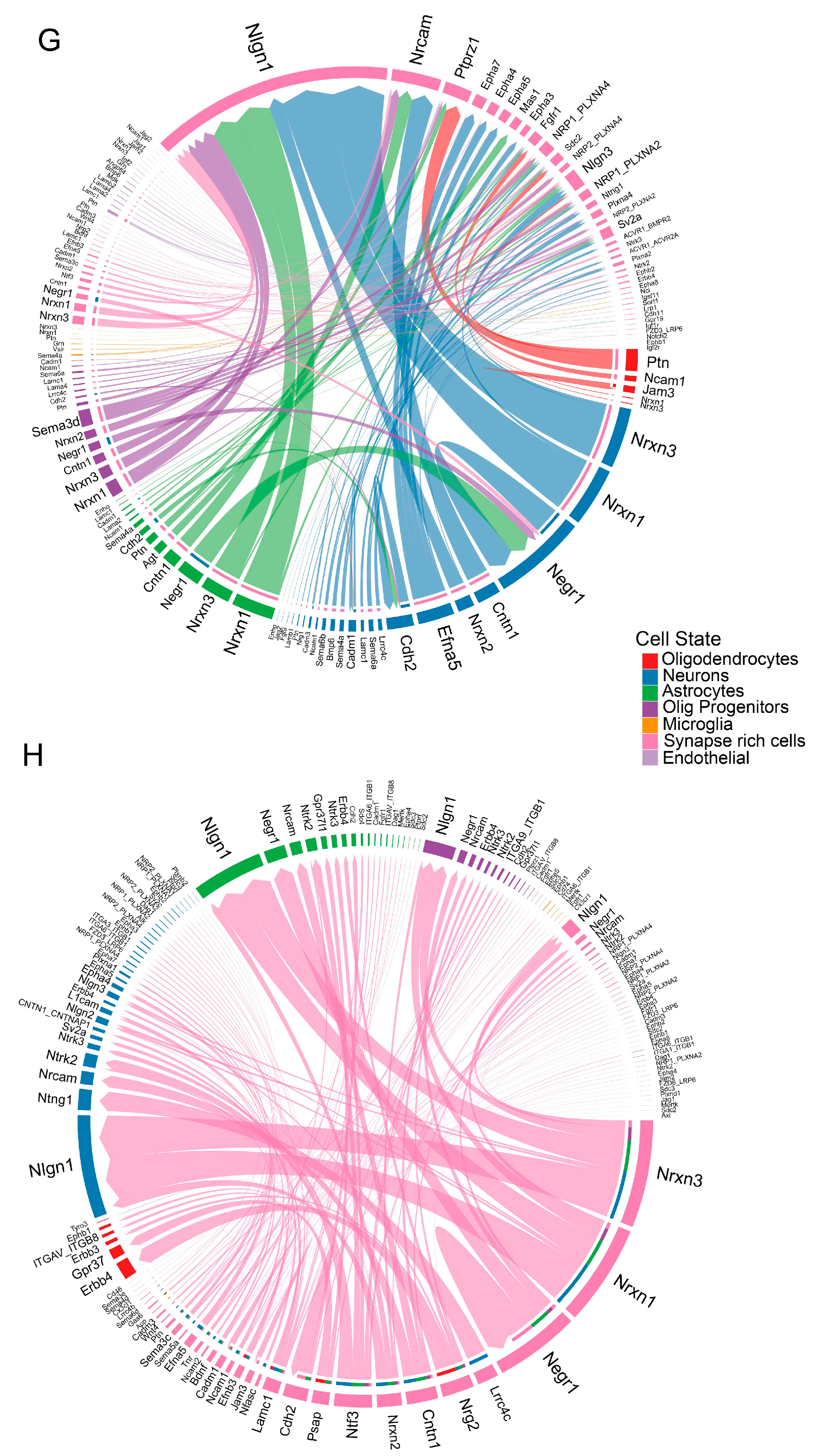
3.3. SRCs Interact Tightly with Other Cell Populations in the Substantia Nigra of Rats with PD
3.4. Icaritin Treatment Improves Neuroinflammation, Oxidative Stress, and Synaptic Vesicle-Mediated Transport-Related Pathways in Neurons of the Substantia Nigra from Rats with PD
3.5. Amelioration of Icaritin on the Energy Metabolism, Phospholipid Metabolism, and Amino Acid Metabolism-Related Pathways in Astrocyte of the Substantia Nigra from Rats with PD
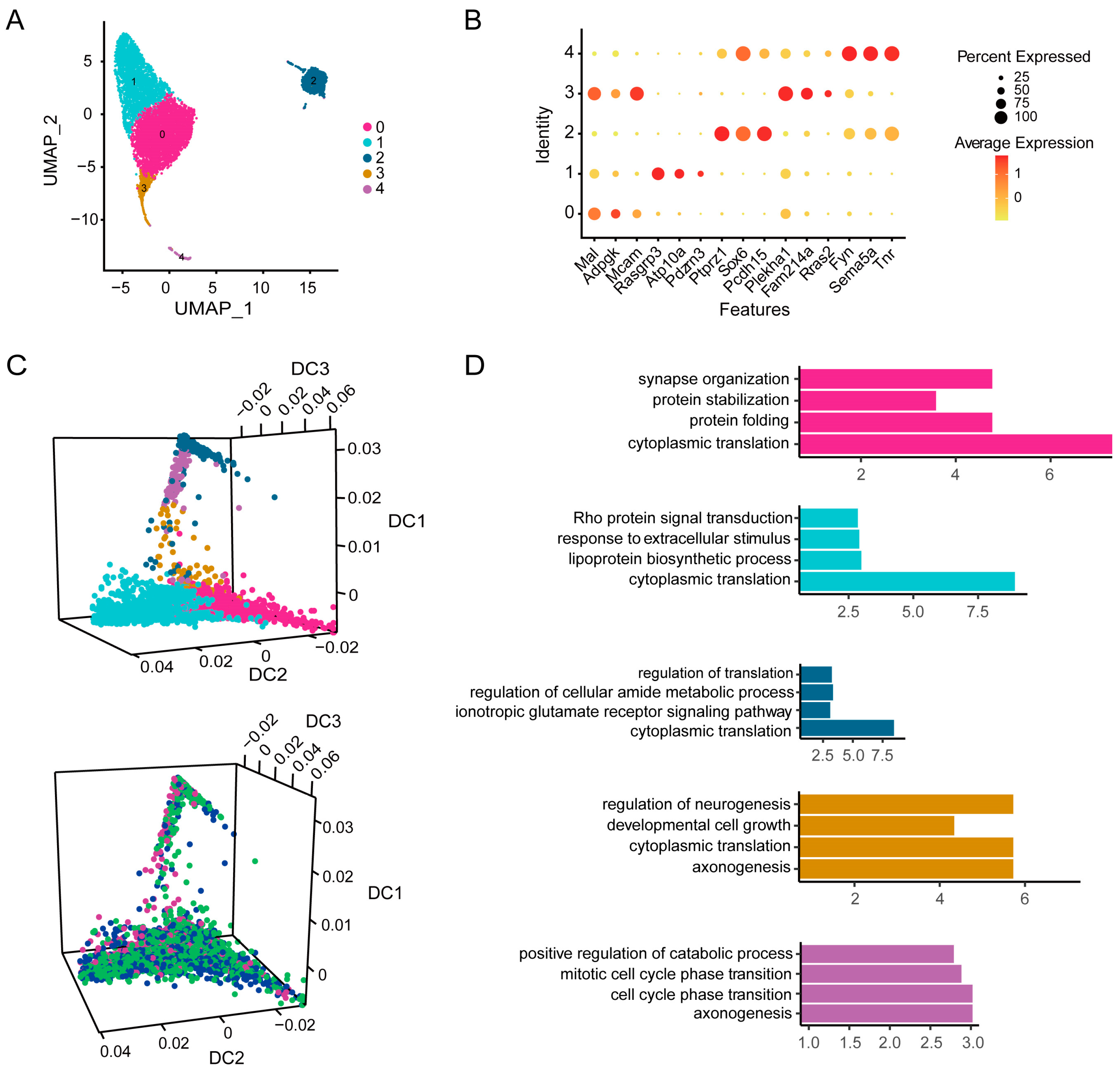
3.6. Icaritin Treatment Mainly Affects Cytoplasmic Translation and Protein Synthesis Related Pathways in Oligodendrocytes and Oligodendrocyte Progenitor Cells of the Substantia Nigra from Rats with PD
3.7. Icaritin Treatment Modulates Energy Metabolism and Oxidative Stress of Microglia and Endothelial Cells in the Substantia Nigra of Rats with PD
4. Discussion
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Goldberg, E.L.; Dixit, V.D. Drivers of age-related inflammation and strategies for healthspan extension. Immunol. Rev. 2015, 265, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Blandini, F.; Armentero, M.T. Animal models of Parkinson’s disease. FEBS J. 2012, 279, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Brück, D.; Wenning, G.K.; Stefanova, N.; Fellner, L. Glia and alpha-synuclein in neurodegeneration: A complex interaction. Neurobiol. Dis. 2016, 85, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Booth, H.D.E.; Hirst, W.D.; Wade-Martins, R. The Role of Astrocyte Dysfunction in Parkinson’s Disease Pathogenesis. Trends Neurosci. 2017, 40, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Hook, P.W.; McClymont, S.A.; Cannon, G.H.; Law, W.D.; Morton, A.J.; Goff, L.A.; McCallion, A.S. Single-Cell RNA-Seq of Mouse Dopaminergic Neurons Informs Candidate Gene Selection for Sporadic Parkinson Disease. Am. J. Hum. Genet. 2018, 102, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Kilfeather, P.; Khoo, J.H.; Wagner, K.; Liang, H.; Caiazza, M.C.; An, Y.; Zhang, X.; Chen, X.; Connor-Robson, N.; Shang, Z.; et al. Single-cell spatial transcriptomic and translatomic profiling of dopaminergic neurons in health, aging, and disease. Cell Rep. 2024, 43, 113784. [Google Scholar] [CrossRef]
- Yaghmaeian Salmani, B.; Lahti, L.; Gillberg, L.; Jacobsen, J.K.; Mantas, I.; Svenningsson, P.; Perlmann, T. Transcriptomic atlas of midbrain dopamine neurons uncovers differential vulnerability in a Parkinsonism lesion model. Elife 2024, 12, RP89482. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Robak, L.A.; Yu, M.; Cykowski, M.; Shulman, J.M. Genetics and Pathogenesis of Parkinson’s Syndrome. Annu. Rev. Pathol. 2023, 18, 95–121. [Google Scholar] [CrossRef]
- Theofanous, T.; Kourti, M. Abrogating Oxidative Stress as a Therapeutic Strategy Against Parkinson’s Disease: A Mini Review of the Recent Advances on Natural Therapeutic Antioxidant and Neuroprotective Agents. Med Chem. 2022, 18, 772–783. [Google Scholar] [CrossRef]
- Wu, H.; Liu, X.; Gao, Z.Y.; Lin, M.; Zhao, X.; Sun, Y.; Pu, X.P. Icaritin Provides Neuroprotection in Parkinson’s Disease by Attenuating Neuroinflammation, Oxidative Stress, and Energy Deficiency. Antioxidants 2021, 10, 529. [Google Scholar] [CrossRef]
- Lai, X.; Ye, Y.; Sun, C.; Huang, X.; Tang, X.; Zeng, X.; Yin, P.; Zeng, Y. Icaritin exhibits anti-inflammatory effects in the mouse peritoneal macrophages and peritonitis model. Int. Immunopharmacol. 2013, 16, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wahdan, S.A.; Tadros, M.G.; Khalifa, A.E. Antioxidant and antiapoptotic actions of selegiline protect against 3-NP-induced neurotoxicity in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Farombi, E.O.; Awogbindin, I.O.; Farombi, T.H.; Oladele, J.O.; Izomoh, E.R.; Aladelokun, O.B.; Ezekiel, I.O.; Adebambo, O.I.; Abah, V.O. Neuroprotective role of kolaviron in striatal redo-inflammation associated with rotenone model of Parkinson’s disease. Neurotoxicology 2019, 73, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.R.; Tapias, V.; Na, H.M.; Honick, A.S.; Drolet, R.E.; Greenamyre, J.T. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol. Dis. 2009, 34, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, W.; Zhang, J. Effects of novel anxiolytic 4-butyl-alpha-agarofuran on levels of monoamine neurotransmitters in rats. Eur. J. Pharmacol. 2004, 504, 39–44. [Google Scholar] [CrossRef]
- Yang, L.; Dai, R.; Wu, H.; Cai, Z.; Xie, N.; Zhang, X.; Shen, Y.; Gong, Z.; Jia, Y.; Yu, F.; et al. Unspliced XBP1 Counteracts β-Catenin to Inhibit Vascular Calcification. Circ. Res. 2022, 130, 213–229. [Google Scholar] [CrossRef]
- Herriges, M.J.; Swarr, D.T.; Morley, M.P.; Rathi, K.S.; Peng, T.; Stewart, K.M.; Morrisey, E.E. Long noncoding RNAs are spatially correlated with transcription factors and regulate lung development. Genes Dev. 2014, 28, 1363–1379. [Google Scholar] [CrossRef] [PubMed]
- Molecular Operating Environment (MOE) Software; Chemical Computing Group Inc.: Montreal, QC, Canada, 2019.
- George Paxinos, C.W. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Dieg, CA, USA, 2007. [Google Scholar]
- Liu, H.; Li, W.; He, Q.; Xue, J.; Wang, J.; Xiong, C.; Pu, X.; Nie, Z. Mass Spectrometry Imaging of Kidney Tissue Sections of Rat Subjected to Unilateral Ureteral Obstruction. Sci. Rep. 2017, 7, 41954. [Google Scholar] [CrossRef]
- Liu, H.; Chen, R.; Wang, J.; Chen, S.; Xiong, C.; Wang, J.; Hou, J.; He, Q.; Zhang, N.; Nie, Z.; et al. 1,5-Diaminonaphthalene hydrochloride assisted laser desorption/ionization mass spectrometry imaging of small molecules in tissues following focal cerebral ischemia. Anal. Chem. 2014, 86, 10114–10121. [Google Scholar] [CrossRef] [PubMed]
- Morabito, S.; Miyoshi, E.; Michael, N.; Shahin, S.; Martini, A.C.; Head, E.; Silva, J.; Leavy, K.; Perez-Rosendahl, M.; Swarup, V. Single-nucleus chromatin accessibility and transcriptomic characterization of Alzheimer’s disease. Nat. Genet. 2021, 53, 1143–1155. [Google Scholar] [CrossRef]
- Tiklová, K.; Nolbrant, S.; Fiorenzano, A.; Björklund, Å.K.; Sharma, Y.; Heuer, A.; Gillberg, L.; Hoban, D.B.; Cardoso, T.; Adler, A.F.; et al. Single cell transcriptomics identifies stem cell-derived graft composition in a model of Parkinson’s disease. Nat. Commun. 2020, 11, 2434. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xiao, X.; Zhang, Z.; Li, M. Genetic insights and neurobiological implications from NRXN1 in neuropsychiatric disorders. Mol. Psychiatry 2019, 24, 1400–1414. [Google Scholar] [CrossRef]
- Kuboyama, K.; Fujikawa, A.; Suzuki, R.; Tanga, N.; Noda, M. Role of Chondroitin Sulfate (CS) Modification in the Regulation of Protein-tyrosine Phosphatase Receptor Type Z (PTPRZ) Activity: PLEIOTROPHIN-PTPRZ-A SIGNALING IS INVOLVED IN OLIGODENDROCYTE DIFFERENTIATION. J. Biol. Chem. 2016, 291, 18117–18128. [Google Scholar] [CrossRef] [PubMed]
- Tanga, N.; Kuboyama, K.; Kishimoto, A.; Kiyonari, H.; Shiraishi, A.; Suzuki, R.; Watanabe, T.; Fujikawa, A.; Noda, M. The PTN-PTPRZ signal activates the AFAP1L2-dependent PI3K-AKT pathway for oligodendrocyte differentiation: Targeted inactivation of PTPRZ activity in mice. Glia 2019, 67, 967–984. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Masliah, E.; Reixach, N.; Buxbaum, J.N. Neuronal production of transthyretin in human and murine Alzheimer’s disease: Is it protective? J. Neurosci. 2011, 31, 12483–12490. [Google Scholar] [CrossRef] [PubMed]
- Mul, J.D.; O’Duibhir, E.; Shrestha, Y.B.; Koppen, A.; Vargoviç, P.; Toonen, P.W.; Zarebidaki, E.; Kvetnansky, R.; Kalkhoven, E.; Cuppen, E.; et al. Pmch-deficiency in rats is associated with normal adipocyte differentiation and lower sympathetic adipose drive. PLoS ONE 2013, 8, e60214. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.S.; Ufer, F.; Rothammer, N.; Di Liberto, G.; Binkle, L.; Haferkamp, U.; Sonner, J.K.; Engler, J.B.; Hornig, S.; Bauer, S.; et al. Neuronal metabotropic glutamate receptor 8 protects against neurodegeneration in CNS inflammation. J. Exp. Med. 2021, 218, e20201290. [Google Scholar] [CrossRef] [PubMed]
- Contrepas, A.; Walker, J.; Koulakoff, A.; Franek, K.J.; Qadri, F.; Giaume, C.; Corvol, P.; Schwartz, C.E.; Nguyen, G. A role of the (pro)renin receptor in neuronal cell differentiation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R250–R257. [Google Scholar] [CrossRef]
- Cruciat, C.M.; Ohkawara, B.; Acebron, S.P.; Karaulanov, E.; Reinhard, C.; Ingelfinger, D.; Boutros, M.; Niehrs, C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 2010, 327, 459–463. [Google Scholar] [CrossRef]
- McDermott, M.I.; Wang, Y.; Wakelam, M.J.O.; Bankaitis, V.A. Mammalian phospholipase D: Function, and therapeutics. Prog. Lipid Res. 2020, 78, 101018. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, C.; Jeong, J.W. AMP-activated protein kinase is activated in Parkinson’s disease models mediated by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Biochem. Biophys. Res. Commun. 2010, 391, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, J.A.; Lemus, M.B.; Stark, R.; Santos, V.V.; Thompson, A.; Rees, D.J.; Galic, S.; Elsworth, J.D.; Kemp, B.E.; Davies, J.S.; et al. Ghrelin-AMPK Signaling Mediates the Neuroprotective Effects of Calorie Restriction in Parkinson’s Disease. J. Neurosci. 2016, 36, 3049–3063. [Google Scholar] [CrossRef] [PubMed]
- Bartels, T.; De Schepper, S.; Hong, S. Microglia modulate neurodegeneration in Alzheimer’s and Parkinson’s diseases. Science 2020, 370, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kim, M.; Han, J. Icariin Metabolism by Human Intestinal Microflora. Molecules 2016, 21, 1158. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.F.; Chen, C.Y.; Chia, G.T.; Tan, B.B.J.; Chan, L.L.; Tan, E.K. The association between Parkinson’s disease and Sexual dysfunction: Clinical correlation and therapeutic implications. Ageing Res. Rev. 2022, 79, 101665. [Google Scholar] [CrossRef]
- Balobanova, N.; Odintsova, E.; Kozlova, Z.; Balobanova, S.; Kartashkina, N. The study of physical—Chemical and technological properties of Epimedium strelolist’s dry extract to create a solid dosage form for the treatment of erectile dysfunction. Georgian Med. News 2019, 137–140. [Google Scholar]
- Cai, R.; Zhang, Y.; Simmering, J.E.; Schultz, J.L.; Li, Y.; Fernandez-Carasa, I.; Consiglio, A.; Raya, A.; Polgreen, P.M.; Narayanan, N.S.; et al. Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases. J. Clin. Investig. 2019, 129, 4539–4549. [Google Scholar] [CrossRef] [PubMed]
- Elkouzi, A.; Vedam-Mai, V.; Eisinger, R.S.; Okun, M.S. Emerging therapies in Parkinson disease—Repurposed drugs and new approaches. Nat. Rev. Neurol. 2019, 15, 204–223. [Google Scholar] [CrossRef]
- Gileadi, T.E.; Swamy, A.K.; Hore, Z.; Horswell, S.; Ellegood, J.; Mohan, C.; Mizuno, K.; Lundebye, A.K.; Giese, K.P.; Stockinger, B.; et al. Effects of Low-Dose Gestational TCDD Exposure on Behavior and on Hippocampal Neuron Morphology and Gene Expression in Mice. Environ. Health Perspect. 2021, 129, 57002. [Google Scholar] [CrossRef]
- Yu, C.Y.; Gui, W.; He, H.Y.; Wang, X.S.; Zuo, J.; Huang, L.; Zhou, N.; Wang, K.; Wang, Y. Neuronal and astroglial TGFβ-Smad3 signaling pathways differentially regulate dendrite growth and synaptogenesis. Neuromolecular Med. 2014, 16, 457–472. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, X.; Wang, B.; Gao, F. Icariin alleviates transforming growth factor-β1-induced epithelial-mesenchymal transition by targeting Smad and MAPK signaling pathways. Am. J. Transl. Res. 2020, 12, 343–360. [Google Scholar] [PubMed]
- Yao, Y.; Kang, S.S.; Xia, Y.; Wang, Z.H.; Liu, X.; Muller, T.; Sun, Y.E.; Ye, K. A delta-secretase-truncated APP fragment activates CEBPB, mediating Alzheimer’s disease pathologies. Brain 2021, 144, 1833–1852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, N.; Wan, C.; Du, J.; Lin, J.; Wang, H. Icariin and Icariside II Reciprocally Stimulate Osteogenesis and Inhibit Adipogenesis of Multipotential Stromal Cells through ERK Signaling. Evid. Based Complement. Altern. Med. 2021, 2021, 8069930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fong, C.; Jia, Z.; Cui, L.; Yao, X.; Yang, M. Icariin Stimulates Differentiation and Suppresses Adipocytic Transdifferentiation of Primary Osteoblasts Through Estrogen Receptor-Mediated Pathway. Calcif. Tissue Int. 2016, 99, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.C.; Wheeler, M.A.; Lee, H.G.; Li, Z.; Sanmarco, L.M.; Thaploo, S.; Polonio, C.M.; Shin, S.W.; Scalisi, G.; Henry, A.R.; et al. Identification of astrocyte regulators by nucleic acid cytometry. Nature 2023, 614, 326–333. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, P.; Yang, J.; Huang, J.; Xu, P.; Hu, M.; Zhang, C.; Wang, B.; Peng, W. Integrated network pharmacology analysis and serum metabolomics to reveal the cognitive improvement effect of Bushen Tiansui formula on Alzheimer’s disease. J. Ethnopharmacol. 2020, 249, 112371. [Google Scholar] [CrossRef]
- Servián-Morilla, E.; Robles-Lanuza, E.; Sánchez-Hidalgo, A.C.; Camacho-Garcia, R.J.; Paez-Gomez, J.A.; Mavillard, F.; Saura, C.A.; Martinez-Mir, A.; Scholl, F.G. Proteolytic Processing of Neurexins by Presenilins Sustains Synaptic Vesicle Release. J. Neurosci. 2018, 38, 901–917. [Google Scholar] [CrossRef]
- Nguyen, M.; Wong, Y.C.; Ysselstein, D.; Severino, A.; Krainc, D. Synaptic, Mitochondrial, and Lysosomal Dysfunction in Parkinson’s Disease. Trends Neurosci. 2019, 42, 140–149. [Google Scholar] [CrossRef]
- Merino-Galán, L.; Jimenez-Urbieta, H.; Zamarbide, M.; Rodríguez-Chinchilla, T.; Belloso-Iguerategui, A.; Santamaria, E.; Fernández-Irigoyen, J.; Aiastui, A.; Doudnikoff, E.; Bézard, E.; et al. Striatal synaptic bioenergetic and autophagic decline in premotor experimental parkinsonism. Brain 2022, 145, 2092–2107. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, C.W.; Eom, J.H.; Jo, M.Y.; Hur, H.J.; Choi, S.K.; Lee, J.S.; Nam, S.T.; Jo, K.S.; Oh, Y.W.; et al. Preclinical and dose-ranging assessment of hESC-derived dopaminergic progenitors for a clinical trial on Parkinson’s disease. Cell Stem Cell 2023, 31, 25–38.e8. [Google Scholar] [CrossRef]
- Zu, S.; Li, Y.E.; Wang, K.; Armand, E.J.; Mamde, S.; Amaral, M.L.; Wang, Y.; Chu, A.; Xie, Y.; Miller, M.; et al. Single-cell analysis of chromatin accessibility in the adult mouse brain. Nature 2023, 624, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Wang, Y.F.; Shinoda, Y.; Kawahata, I.; Yamamoto, T.; Jia, W.B.; Yamamoto, H.; Mizobata, T.; Kawata, Y.; Fukunaga, K. Fatty acid-binding protein 7 triggers α-synuclein oligomerization in glial cells and oligodendrocytes associated with oxidative stress. Acta Pharmacol. Sin. 2022, 43, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Dehestani, M.; Kozareva, V.; Blauwendraat, C.; Fraenkel, E.; Gasser, T.; Bansal, V. Transcriptomic changes in oligodendrocytes and precursor cells predicts clinical outcomes of Parkinson’s disease. bioRxiv 2023. [Google Scholar] [CrossRef]
- Fang, Y.; Jiang, Q.; Li, S.; Zhu, H.; Xu, R.; Song, N.; Ding, X.; Liu, J.; Chen, M.; Song, M.; et al. Opposing functions of β-arrestin 1 and 2 in Parkinson’s disease via microglia inflammation and Nprl3. Cell Death Differ. 2021, 28, 1822–1836. [Google Scholar] [CrossRef] [PubMed]
- Pediaditakis, I.; Kodella, K.R.; Manatakis, D.V.; Le, C.Y.; Hinojosa, C.D.; Tien-Street, W.; Manolakos, E.S.; Vekrellis, K.; Hamilton, G.A.; Ewart, L.; et al. Modeling alpha-synuclein pathology in a human brain-chip to assess blood-brain barrier disruption. Nat. Commun. 2021, 12, 5907. [Google Scholar] [CrossRef]
- Brandebura, A.N.; Paumier, A.; Onur, T.S.; Allen, N.J. Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat. Rev. Neurosci. 2023, 24, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Vallée, J.N.; Lecarpentier, Y. Potential role of cannabidiol in Parkinson’s disease by targeting the WNT/β-catenin pathway, oxidative stress and inflammation. Aging 2021, 13, 10796–10813. [Google Scholar] [CrossRef] [PubMed]
| ID | Molecules | Full Name of Target | Source | Uniprot ID | Docking Score (Kcal/mol) |
|---|---|---|---|---|---|
| 1 | Icaritin | B cell CLL/lymphoma 11A (zinc finger protein) | Rat | D3ZSY3 | −8.56 |
| 2 | Icaritin | CCAAT/enhancer-binding protein beta | Rat | P21272 | −6.59 |
| 3 | Icaritin | Mineralocorticoid receptor | Rat | P22199 | −10.21 |
| 4 | Icaritin | Mothers against decapentaplegic homolog 3 | Rat | P84025 | −9.51 |
| 5 | Icaritin | Transcription factor 4 | Rat | Q62655 | −6.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Zhang, Z.-H.; Zhou, P.; Sui, X.; Liu, X.; Sun, Y.; Zhao, X.; Pu, X.-P. A Single-Cell Atlas of the Substantia Nigra Reveals Therapeutic Effects of Icaritin in a Rat Model of Parkinson’s Disease. Antioxidants 2024, 13, 1183. https://doi.org/10.3390/antiox13101183
Wu H, Zhang Z-H, Zhou P, Sui X, Liu X, Sun Y, Zhao X, Pu X-P. A Single-Cell Atlas of the Substantia Nigra Reveals Therapeutic Effects of Icaritin in a Rat Model of Parkinson’s Disease. Antioxidants. 2024; 13(10):1183. https://doi.org/10.3390/antiox13101183
Chicago/Turabian StyleWu, Hao, Zhen-Hua Zhang, Ping Zhou, Xin Sui, Xi Liu, Yi Sun, Xin Zhao, and Xiao-Ping Pu. 2024. "A Single-Cell Atlas of the Substantia Nigra Reveals Therapeutic Effects of Icaritin in a Rat Model of Parkinson’s Disease" Antioxidants 13, no. 10: 1183. https://doi.org/10.3390/antiox13101183
APA StyleWu, H., Zhang, Z.-H., Zhou, P., Sui, X., Liu, X., Sun, Y., Zhao, X., & Pu, X.-P. (2024). A Single-Cell Atlas of the Substantia Nigra Reveals Therapeutic Effects of Icaritin in a Rat Model of Parkinson’s Disease. Antioxidants, 13(10), 1183. https://doi.org/10.3390/antiox13101183








