Silibinin Suppresses Inflammatory Responses Induced by Exposure to Asian Sand Dust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physical and Chemical Characteristics of ASD
2.2. Procedure for Animal Experiment
2.3. Histological Examination
2.4. Western Blot Analysis
2.5. Cell Culture
2.6. Measurement of TNF-α in ASD-Stimulated RAW264.7 Cells
2.7. Measurement of p-p65 Expression in ASD-Stimulated RAW264.7 Cells
2.8. Statistical Analysis
3. Results
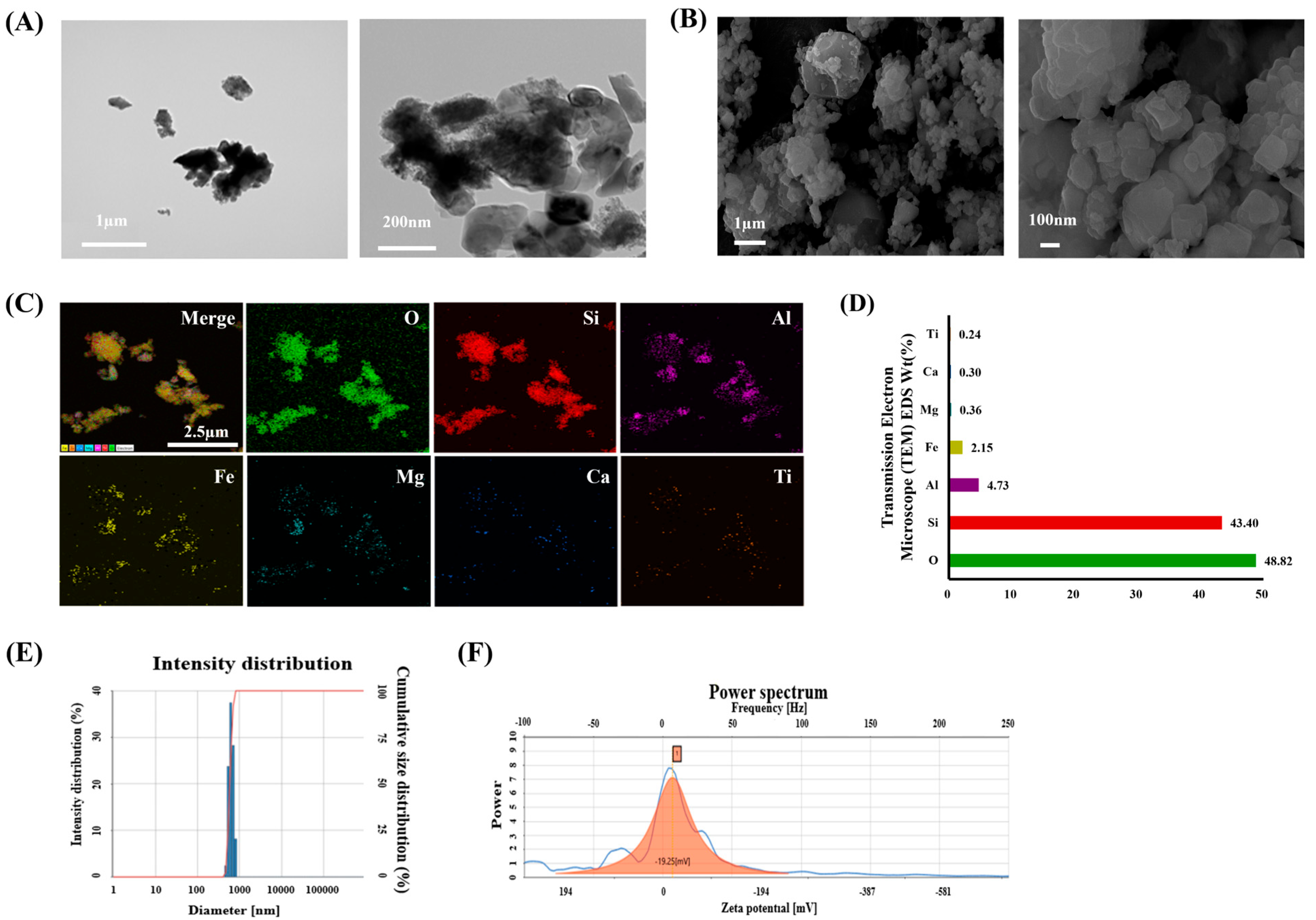
3.1. Characteristics of ASD
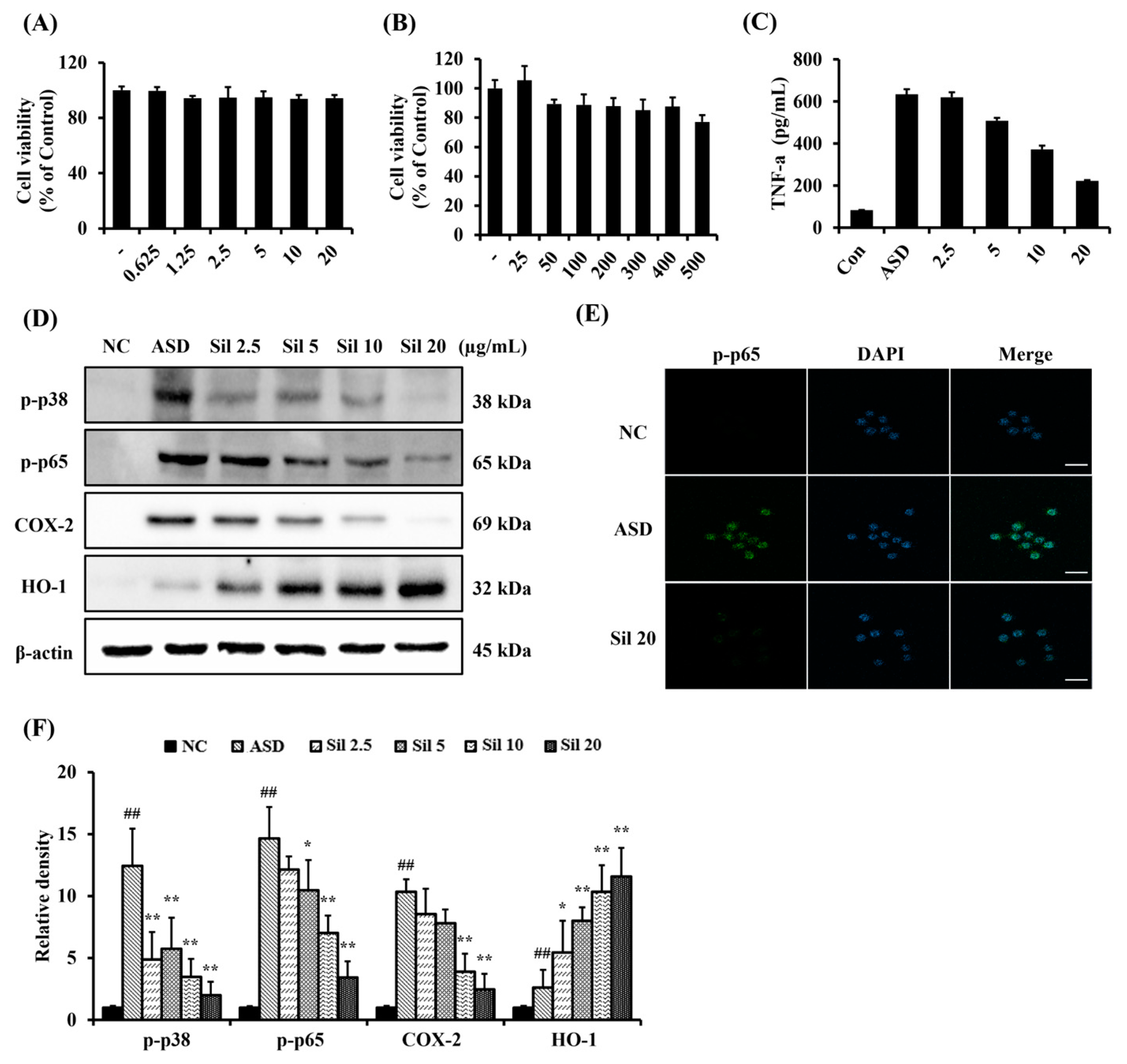
3.2. Silibinin Decreased Inflammatory Responses in ASD-Stimulated RAW264.7 Cells
3.3. Silibinin Reduced Inflammatory Mediators of BALF of ASD-Exposed Mice
3.4. Silibinin Decreased Inflammatory Response and Oxidative Stress in the Lung Tissue of ASD-Exposed Mice
3.5. Silibinin Suppressed Inflammatory Signaling in ASD-Exposed Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, J.M.; Kim, H.J.; Park, J.H.; Hwang, Y.J.; Lee, H.M. Asian Sand Dust Regulates IL-32 Production in Airway Epithelial Cells: Inhibitory Effect of Glucocorticoids. Am. J. Rhinol. Allergy 2019, 33, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.I.; Lim, J.O.; Pak, S.W.; Lee, S.J.; Shin, I.S.; Moon, C.; Heo, J.D.; Kim, J.C. Exposure to China dust exacerbates testicular toxicity induced by cyclophosphamide in mice. Toxicol. Res. 2023, 39, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, S.; Minami, H.; Abe, M.; Hasei, T.; Sera, N.; Yamamoto, S.; Funasaka, K.; Asakawa, D.; Watanabe, M.; Honda, N.; et al. Seasonal Fluctuations in Air Pollution in Dazaifu, Japan, and Effect of Long-Range Transport from Mainland East Asia. Biol. Pharm. Bull. 2015, 38, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Ye, M.K.; Lee, D.W.; Chae, M.H. Asian Sand Dust Particles Enhance the Development of Aspergillus fumigatus Biofilm on Nasal Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 3030. [Google Scholar] [CrossRef]
- Lei, Y.C.; Chan, C.C.; Wang, P.Y.; Lee, C.T.; Cheng, T.J. Effects of Asian dust event particles on inflammation markers in peripheral blood and bronchoalveolar lavage in pulmonary hypertensive rats. Environ. Res. 2004, 95, 71–76. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, X.; Wang, J.; Dandekar, A.; Kim, H.; Qiu, Y.; Xu, X.; Cui, Y.; Wang, A.; Chen, L.C.; et al. Exposure to fine airborne particulate matters induces hepatic fibrosis in murine models. J. Hepatol. 2015, 63, 1397–1404. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, J.; Yuan, L.; Ye, J.; Zhao, X.; Ren, G.; Chen, H. Silibinin alleviates intestinal inflammation via inhibiting JNK signaling in Drosophila. Front. Pharmacol. 2023, 14, 1246960. [Google Scholar] [CrossRef]
- Soleimani, V.; Delghandi, P.S.; Moallem, S.A.; Karimi, G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review. Phytother. Res. 2019, 33, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, D.L.; Xie, L.N.; Ma, Y.R.; Wu, P.P.; Li, C.; Liu, W.F.; Zhang, K.; Zhou, R.P.; Xu, X.T.; et al. Synergistic anti-inflammatory effects of silibinin and thymol combination on LPS-induced RAW264.7 cells by inhibition of NF-κB and MAPK activation. Phytomedicine 2020, 78, 153309. [Google Scholar] [CrossRef]
- Ma, Z.; Zang, W.; Wang, H.; Wei, X. Silibinin enhances anti-renal fibrosis effect of MK-521 via downregulation of TGF-β signaling pathway. Hum. Cell 2020, 33, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kadhim, M.M.; Turki Jalil, A.; Oudah, S.K.; Aminov, Z.; Alsaikhan, F.; Jawhar, Z.H.; Ramírez-Coronel, A.A.; Farhood, B. A systematic review of the protective effects of silymarin/silibinin against doxorubicin-induced cardiotoxicity. Cancer Cell Int. 2023, 23, 88. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Zheng, X.; Zhang, K.; Du, Z. Potent inhibitory effect of silibinin from milk thistle on skin inflammation stimuli by 12-O-tetradecanoylphorbol-13-acetate. Food Funct. 2015, 6, 3712–3719. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.W.; Zhang, C.; Hong, T.; Liu, D.H.; Wang, C.; Li, J.; He, X.K.; Xu, W.D. Silibinin alleviates inflammation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes and has a therapeutic effect on arthritis in rats. Sci. Rep. 2018, 8, 3241. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, H.; Jin, Z.; Lei, W.; Deng, C.; Yang, W.; Lu, C.; Hou, Y.; Zhang, Y.; Tang, R.; et al. Silibinin protects against sepsis and septic myocardial injury in an NR1H3-dependent pathway. Free Radic. Biol. Med. 2022, 187, 141–157. [Google Scholar] [CrossRef]
- Park, J.W.; Shin, N.R.; Shin, I.S.; Kwon, O.K.; Kim, J.S.; Oh, S.R.; Kim, J.H.; Ahn, K.S. Silibinin Inhibits Neutrophilic Inflammation and Mucus Secretion Induced by Cigarette Smoke via Suppression of ERK-SP1 Pathway. Phytother. Res. 2016, 30, 1926–1936. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jin, G.Y.; Guo, H.S.; Piao, H.M.; Li, L.; Li, G.Z.; Lin, Z.H.; Yan, G.H. Silibinin attenuates allergic airway inflammation in mice. Biochem. Biophys. Res. Commun. 2012, 427, 450–455. [Google Scholar] [CrossRef]
- Ko, J.W.; Shin, N.R.; Park, S.H.; Lee, I.C.; Ryu, J.M.; Kim, H.J.; Cho, Y.K.; Kim, J.C.; Shin, I.S. Silibinin inhibits the fibrotic responses induced by cigarette smoke via suppression of TGF-β1/Smad 2/3 signaling. Food Chem. Toxicol. 2017, 106, 424–429. [Google Scholar] [CrossRef]
- Im, H.; Kim, E.; Kwon, H.J.; Kim, H.; Ko, J.; Sung, Y.; Kim, S.H.; Lee, E.J.; Kwon, W.S.; Ryoo, Z.Y.; et al. Silibinin Mitigates Vanadium-induced Lung Injury via the TLR4/MAPK/NF-κB Pathway in Mice. In Vivo 2024, 38, 2179–2189. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.O.; Shin, N.R.; Seo, Y.S.; Nam, H.H.; Ko, J.W.; Jung, T.Y.; Lee, S.J.; Kim, H.J.; Cho, Y.K.; Kim, J.C.; et al. Silibinin Attenuates Silica Dioxide Nanoparticles-Induced Inflammation by Suppressing TXNIP/MAPKs/AP-1 Signaling. Cells 2020, 9, 678. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Jeung, Y.S.; Seo, M.H.; Yoon, J.Y.; Kim, D.Y.; Park, J.-W.; Han, J.H.; Jeong, S.H. Asian Dust and titanium dioxide particles–induced inflammation and oxidative DNA damage in C57BL/6 mice. Inhal. Toxicol. 2010, 22, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Kim, J.; Lee, M.; Park, J. Adverse impacts of Asian dust events on human health and the environment-A probabilistic risk assessment study on particulate matter-bound metals and bacteria in Seoul, South Korea. Sci. Total Environ. 2023, 875, 162637. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Song, J.Y.; Park, T.I.; Choi, W.S.; Kim, J.H.; Kwon, O.S.; Lee, J.Y. The effects of BRL-50481 on ovalbumin-induced asthmatic lung inflammation exacerbated by co-exposure to Asian sand dust in the murine model. Arch. Pharm. Res. 2022, 45, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Pak, S.W.; Lee, A.Y.; Kim, W.I.; Chae, S.W.; Cho, Y.K.; Ko, J.W.; Kim, T.W.; Kim, J.C.; Moon, B.C.; et al. Loranthus tanakae Franch. and Sav. Attenuates Respiratory Inflammation Caused by Asian Sand Dust. Antioxidants 2024, 13, 419. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.O.; Song, K.H.; Lee, I.S.; Lee, S.J.; Kim, W.I.; Pak, S.W.; Shin, I.S.; Kim, T. Cimicifugae Rhizoma Extract Attenuates Oxidative Stress and Airway Inflammation via the Upregulation of Nrf2/HO-1/NQO1 and Downregulation of NF-κB Phosphorylation in Ovalbumin-Induced Asthma. Antioxidants 2021, 10, 1626. [Google Scholar] [CrossRef]
- Chen, Y.S.; Sheen, P.C.; Chen, E.R.; Liu, Y.K.; Wu, T.N.; Yang, C.Y. Effects of Asian dust storm events on daily mortality in Taipei, Taiwan. Environ. Res. 2004, 95, 151–155. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Shimada, A.; Nemoto, M.; Morita, T.; Adilbish, A.; Bayasgalan, M.O. Adverse effects of inhaled sand dust particles on the respiratory organs of sheep and goats exposed to severe sand storms in Mongolia. Folia Histochem. Cytobiol. 2014, 52, 244–249. [Google Scholar] [CrossRef]
- Pak, S.W.; Lee, S.J.; Kim, W.I.; Yang, Y.G.; Cho, Y.K.; Kim, J.S.; Kim, T.W.; Ko, J.W.; Kim, J.C.; Kim, S.H.; et al. The effects of Pycnogenol, a pine bark extract on pulmonary inflammation by Asian sand dust in mice. Vet. Med. 2024, 69, 8–17. [Google Scholar] [CrossRef]
- Nakao, M.; Ishihara, Y.; Kim, C.H.; Hyun, I.G. The Impact of Air Pollution, Including Asian Sand Dust, on Respiratory Symptoms and Health-related Quality of Life in Outpatients With Chronic Respiratory Disease in Korea: A Panel Study. J. Prev. Med. Public Health 2018, 51, 130–139. [Google Scholar] [CrossRef]
- Sadakane, K.; Ichinose, T.; Maki, T.; Nishikawa, M. Co-exposure of peptidoglycan and heat-inactivated Asian sand dust exacerbates ovalbumin-induced allergic airway inflammation in mice. Inhal. Toxicol. 2022, 34, 231–243. [Google Scholar] [CrossRef]
- Thacker, E.L. Lung inflammatory responses. Vet. Res. 2006, 37, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.; Ross, R.; Frydas, I.; Kritas, S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVID-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 327–331. [Google Scholar] [PubMed]
- Yang, H.W.; Park, J.H.; Shin, J.M.; Lee, H.M.; Park, I.H. Asian Sand Dust Upregulates IL-6 and IL-8 via ROS, JNK, ERK, and CREB Signaling in Human Nasal Fibroblasts. Am. J. Rhinol. Allergy 2020, 34, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Rigon, C.; Marchiori, M.C.L.; da Silva Jardim, F.; Pegoraro, N.S.; Chaves, P.D.S.; Velho, M.C.; Beck, R.C.R.; Ourique, A.F.; Sari, M.H.M.; Oliveira, S.M.; et al. Hydrogel containing silibinin nanocapsules presents effective anti-inflammatory action in a model of irritant contact dermatitis in mice. Eur. J. Pharm. Sci. 2019, 137, 104969. [Google Scholar] [CrossRef]
- Xu, R.; Qiu, S.; Zhang, J.; Liu, X.; Zhang, L.; Xing, H.; You, M.; Wang, M.; Lu, Y.; Zhang, P.; et al. Silibinin Schiff Base Derivatives Counteract CCl4-Induced Acute Liver Injury by Enhancing Anti-Inflammatory and Antiapoptotic Bioactivities. Drug Des. Devel. Ther. 2022, 16, 1441–1456. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, R.; Han, Y.; Zeng, J.; Shi, L.; Mao, Y.; Sun, X.; Ji, Y.; Zhang, X.; Chen, Y.; et al. Silibinin Attenuates Experimental Periodontitis by Downregulation of Inflammation and Oxidative Stress. Oxidative Med. Cell. Longev. 2023, 2023, 5617800. [Google Scholar] [CrossRef]
- Son, Y.; Lee, H.J.; Rho, J.K.; Chung, S.Y.; Lee, C.G.; Yang, K.; Kim, S.H.; Lee, M.; Shin, I.S.; Kim, J.S. The ameliorative effect of silibinin against radiation-induced lung injury: Protection of normal tissue without decreasing therapeutic efficacy in lung cancer. BMC Pulm. Med. 2015, 15, 68. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, H.S.; Lim, Y.; Paik, J.H.; Kwon, O.K.; Kim, J.H.; Paryanto, I.; Yunianto, P.; Choi, S.; Oh, S.R.; et al. Rhododendron album Blume extract inhibits TNF-α/IFN-γ-induced chemokine production via blockade of NF-κB and JAK/STAT activation in human epidermal keratinocytes. Int. J. Mol. Med. 2018, 41, 3642–3652. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.W.; Shin, N.R.; Park, S.Y.; Kwon, O.K.; Park, H.A.; Lim, Y.; Ryu, H.W.; Yuk, H.J.; Kim, J.H.; et al. Picrasma quassiodes (D. Don) Benn. attenuates lipopolysaccharide (LPS)-induced acute lung injury. Int. J. Mol. Med. 2016, 38, 834–844. [Google Scholar] [CrossRef]
- Kim, A.H.; Chon, S.; Yoon, J.Y.; Kim, Y.J.; Kyung, S.Y.; Lee, S.P.; Park, J.W.; Jeong, S.H. The Effect of Particulate Matter 10 from Asian Dust on the Production of Reactive Oxygen Species, TGF-β, NF-κB, PDGF-α and Fibronectin in MRC-5 Fibroblast Cells. Tuberc. Respir. Dis. 2009, 67, 528–535. [Google Scholar] [CrossRef]
- Tian, L.; Li, W.; Wang, T. Therapeutic effects of silibinin on LPS-induced acute lung injury by inhibiting NLRP3 and NF-κB signaling pathways. Microb. Pathog. 2017, 108, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jiang, X.; Liu, Y.; Jiang, X.; Che, L.; Lin, Y.; Zhuo, Y.; Feng, B.; Fang, Z.; Hua, L.; et al. Silibinin Alleviates Lipopolysaccharide Induced Inflammation in Porcine Mammary Epithelial Cells via mTOR/NF-κB Signaling Pathway. Mol. Nutr. Food Res. 2023, 67, e2200715. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, C.; Shan, M. Vincamine alleviates brain injury by attenuating neuroinflammation and oxidative damage in a mouse model of Parkinson’s disease through the NF-κB and Nrf2/HO-1 signaling pathways. J. Biochem. Mol. Toxicol. 2024, 38, e23714. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.Y.; Lee, A.Y.; Song, J.H.; Lee, M.Y.; Lim, J.O.; Lee, S.J.; Ko, J.W.; Shin, N.R.; Kim, J.C.; Shin, I.S.; et al. Scrophularia koraiensis Nakai Attenuates Allergic Airway Inflammation via Suppression of NF-κB and Enhancement of Nrf2/HO-1 Signaling. Antioxidants 2020, 9, 99. [Google Scholar] [CrossRef]
- Wei, P.; Li, X.; Wang, S.; Dong, Y.; Yin, H.; Gu, Z.; Na, X.; Wei, X.; Yuan, J.; Cao, J.; et al. Silibinin Ameliorates Formaldehyde-Induced Cognitive Impairment by Inhibiting Oxidative Stress. Oxid. Med. Cell. Longev. 2022, 2022, 5981353. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-J.; Pak, S.-W.; Kim, W.-I.; Park, S.-H.; Cho, Y.-K.; Ko, J.-W.; Kim, T.-W.; Kim, J.-S.; Kim, J.-C.; Lim, J.-O.; et al. Silibinin Suppresses Inflammatory Responses Induced by Exposure to Asian Sand Dust. Antioxidants 2024, 13, 1187. https://doi.org/10.3390/antiox13101187
Lee S-J, Pak S-W, Kim W-I, Park S-H, Cho Y-K, Ko J-W, Kim T-W, Kim J-S, Kim J-C, Lim J-O, et al. Silibinin Suppresses Inflammatory Responses Induced by Exposure to Asian Sand Dust. Antioxidants. 2024; 13(10):1187. https://doi.org/10.3390/antiox13101187
Chicago/Turabian StyleLee, Se-Jin, So-Won Pak, Woong-Il Kim, Sin-Hyang Park, Young-Kwon Cho, Je-Won Ko, Tae-Won Kim, Joong-Sun Kim, Jong-Choon Kim, Je-Oh Lim, and et al. 2024. "Silibinin Suppresses Inflammatory Responses Induced by Exposure to Asian Sand Dust" Antioxidants 13, no. 10: 1187. https://doi.org/10.3390/antiox13101187






