Optimization and Bioactive Evaluation of Bifurcaria bifurcata Antioxidant-Rich Extracts for Functional Food and Pharmaceutical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Algae Collection and Preparation
2.3. Microwave-Assisted Extraction
2.4. Experimental Design, Modeling, and Optimization
2.5. Model Independent Variables
2.5.1. Yield
2.5.2. Total Polyphenol Content (TPC)
2.5.3. DPPH• Scavenging Activity
| Experimental Design | Responses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coded Values | Natural Values | ||||||||||
| X1 | X2 | X3 | X1: t | X2: P | X3:S | Y | TPC | DPPH• | ABTS | BCM | |
| min | Bar | % | mg/g dw | mg FE/g dw | nM R·/g dw | nM R·/g dw | µM βC/g dw | ||||
| 1 | −1 | −1 | −1 | 7.5 | 5.6 | 20.3 | 426.77 | 57.12 | 32.70 | 41.73 | 0.0902 |
| 2 | −1 | −1 | 1 | 7.5 | 5.6 | 79.7 | 330.76 | 46.50 | 32.12 | 159.51 | 0.1037 |
| 3 | −1 | 1 | −1 | 7.5 | 16.4 | 20.3 | 514.74 | 69.46 | 39.99 | 44.61 | 0.1060 |
| 4 | −1 | 1 | 1 | 7.5 | 16.4 | 79.7 | 362.82 | 52.28 | 34.79 | 280.31 | 0.0354 |
| 5 | 1 | −1 | −1 | 20.5 | 5.6 | 20.3 | 541.44 | 61.89 | 42.05 | 53.86 | 0.1279 |
| 6 | 1 | −1 | 1 | 20.5 | 5.6 | 79.7 | 370.05 | 52.19 | 29.83 | 56.22 | 0.0481 |
| 7 | 1 | 1 | −1 | 20.5 | 16.4 | 20.3 | 462.27 | 61.70 | 41.84 | 29.30 | 0.0913 |
| 8 | 1 | 1 | 1 | 20.5 | 16.4 | 79.7 | 326.94 | 48.42 | 26.19 | 184.17 | 0.0712 |
| 9 | 1.68 | 0 | 0 | 25 | 11 | 50 | 423.36 | 58.72 | 32.49 | 168.26 | 0.0806 |
| 10 | −1.68 | 0 | 0 | 3 | 11 | 50 | 339.81 | 64.29 | 44.94 | 165.00 | 0.0870 |
| 11 | 0 | −1.68 | 0 | 14 | 2 | 50 | 323.71 | 44.04 | 30.59 | 84.13 | 0.0950 |
| 12 | 0 | 1.68 | 0 | 14 | 20 | 50 | 457.90 | 63.23 | 38.15 | 85.67 | 0.0757 |
| 13 | 0 | 0 | −1.68 | 14 | 11 | 0 | 452.43 | 43.58 | 26.07 | 180.96 | 0.0959 |
| 14 | 0 | 0 | 1.68 | 14 | 11 | 100 | 100.33 | 19.23 | 13.25 | 22.51 | 0.0017 |
| 15 | −1.68 | −1.68 | −1.68 | 3 | 2 | 0 | 283.57 | 24.48 | 12.20 | 216.08 | 0.0677 |
| 16 | −1.68 | −1.68 | 1.68 | 3 | 2 | 100 | 59.64 | 6.07 | 2.22 | 4.46 | 0.0017 |
| 17 | −1.68 | 1.68 | −1.68 | 3 | 20 | 0 | 393.25 | 33.17 | 17.52 | 18.40 | 0.0781 |
| 18 | −1.68 | 1.68 | 1.68 | 3 | 20 | 100 | 115.62 | 16.39 | 11.76 | 19.92 | 0.0046 |
| 19 | 1.68 | −1.68 | −1.68 | 25 | 2 | 0 | 345.64 | 31.39 | 16.46 | 181.43 | 0.1029 |
| 20 | 1.68 | −1.68 | 1.68 | 25 | 2 | 100 | 55.48 | 2.93 | 1.46 | 2.70 | 0.0017 |
| 21 | 1.68 | 1.68 | −1.68 | 25 | 20 | 0 | 365.07 | 32.35 | 17.77 | 36.29 | 0.0391 |
| 22 | 1.68 | 1.68 | 1.68 | 25 | 20 | 100 | 112.57 | 11.38 | 14.17 | 36.33 | 0.0096 |
| 23 | 0 | 0 | 0 | 14 | 11 | 50 | 385.65 | 62.49 | 33.79 | 169.41 | 0.0946 |
| 24 | 0 | 0 | 0 | 14 | 11 | 50 | 371.11 | 69.10 | 32.96 | 195.22 | 0.0950 |
| 25 | 0 | 0 | 0 | 14 | 11 | 50 | 411.94 | 62.68 | 27.71 | 199.82 | 0.0908 |
| 26 | 0 | 0 | 0 | 14 | 11 | 50 | 369.34 | 62.99 | 30.63 | 119.88 | 0.0926 |
| 27 | 0 | 0 | 0 | 14 | 11 | 50 | 400.93 | 59.00 | 23.27 | 180.36 | 0.1029 |
| 28 | 0 | 0 | 0 | 14 | 11 | 50 | 380.33 | 48.41 | 23.03 | 183.10 | 0.0959 |
2.5.4. Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid) ABTS•+ Scavenging Activity
2.5.5. β-Carotene Discoloration Method (BCM)
2.6. Mathematical Model
2.7. Numerical Methods, Statistical Analysis, and Figures
2.8. Biological Activities Assessment at the Optimal BB Extract
2.8.1. Antimicrobial Assay
2.8.2. ROS and RNS Scavenging Activity
2.8.3. Enzyme Inhibition Assays
2.8.4. Cell Line Studies
Anti-Inflammatory Activity
Cytotoxicity Activity Assay
3. Results and Discussion
3.1. Mathematical Modelling of the Optimization
| Coefficients | Parametric Responses to the CCCD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Extract | Chemical | Antioxidant Activity | |||||||||
| Yield | TPC | DPPH• | ABTS•+ | BCM | |||||||
| (A) FITTING COEFFICIENTS OBTAINED | |||||||||||
| Intercept | b0 | 408.105 | ±17.983 | 61.645 | ±2.237 | 28.649 | ±2.473 | 170.106 | ±14.562 | 0.095 | ±0.006 |
| Linear effect | b1 | ns | −0.385 | ±0.131 | ns | −43.670 | ±23.646 | ns | |||
| b2 | 17.384 | ±10.519 | 2.597 | ±1.309 | 1.857 | ±1.006 | 61.489 | ±23.646 | −0.005 | ±0.003 | |
| b3 | −80.007 | ±10.519 | −6.451 | ±1.309 | −3.113 | ±1.006 | 121.825 | ±23.646 | −0.021 | ±0.003 | |
| Quadratic effect | b11 | ns | ns | 4.563 | ±1.636 | ns | ns | ||||
| b22 | ns | ns | 3.026 | ±1.636 | −33.331 | ±11.353 | ns | ||||
| b33 | −28.218 | ±16.798 | −8.872 | ±2.090 | −2.174 | ±1.636 | −28.079 | ±11.353 | −0.019 | ±0.003 | |
| Cubic effect | b111 | ns | ns | ns | 15.440 | ±9.306 | ns | ||||
| b22 | ns | ns | ns | −30.280 | ±9.306 | ns | |||||
| b333 | ns | ns | ns | −55.446 | ±9.306 | ns | |||||
| Interactive effect | b12 | ns | ns | ns | ns | ns | |||||
| b13 | ns | ns | ns | ns | ns | ||||||
| b23 | ns | ns | ns | 14.363 | ±5.008 | ns | |||||
| b123 | ns | ns | ns | ns | 0.003 | ±0.001 | |||||
| b1122 | ns | ns | ns | ns | ns | ||||||
| b1133 | ns | ns | ns | ns | ns | ||||||
| b2233 | ns | ns | ns | ns | ns | ||||||
| b112233 | −4.865 | ±2.022 | −0.734 | ±0.252 | −1.422 | ±0.332 | 2.760 | ±1.839 | ns | ||
| R2 | 0.8916 | 0.9290 | 0.8792 | 0.8871 | 0.8608 | ||||||
| (B) OPTIMAL CONDITIONS AND RESPONSE VALUES | |||||||||||
| Optimum conditions | X1: t (min) | 14.00 | ±1.87 | 3.00 | ±0.87 | 3.00 | ±0.87 | 7.57 | ±1.38 | 25.00 | ±2.50 |
| X2: P (bar) | 20.00 | ±2.24 | 20.00 | ±2.24 | 20.00 | ±2.24 | 14.25 | ±1.89 | 2.00 | ±0.71 | |
| X3: S (%) | 7.85 | ±1.40 | 43.49 | ±3.30 | 46.58 | ±3.41 | 71.94 | ±4.24 | 26.04 | ±2.55 | |
| Responses | mg/g dw | mg/g dw | nM R•/g dw | nM R•/g dw | µM βC/g dw | ||||||
| 494.05 | ±54.64 | 67.37 | ±18.47 | 53.42 | ±11.72 | 275.94 | ±14.48 | 0.11 | ±1.48 | ||
3.2. Influence of Extraction Conditions on Response Variables
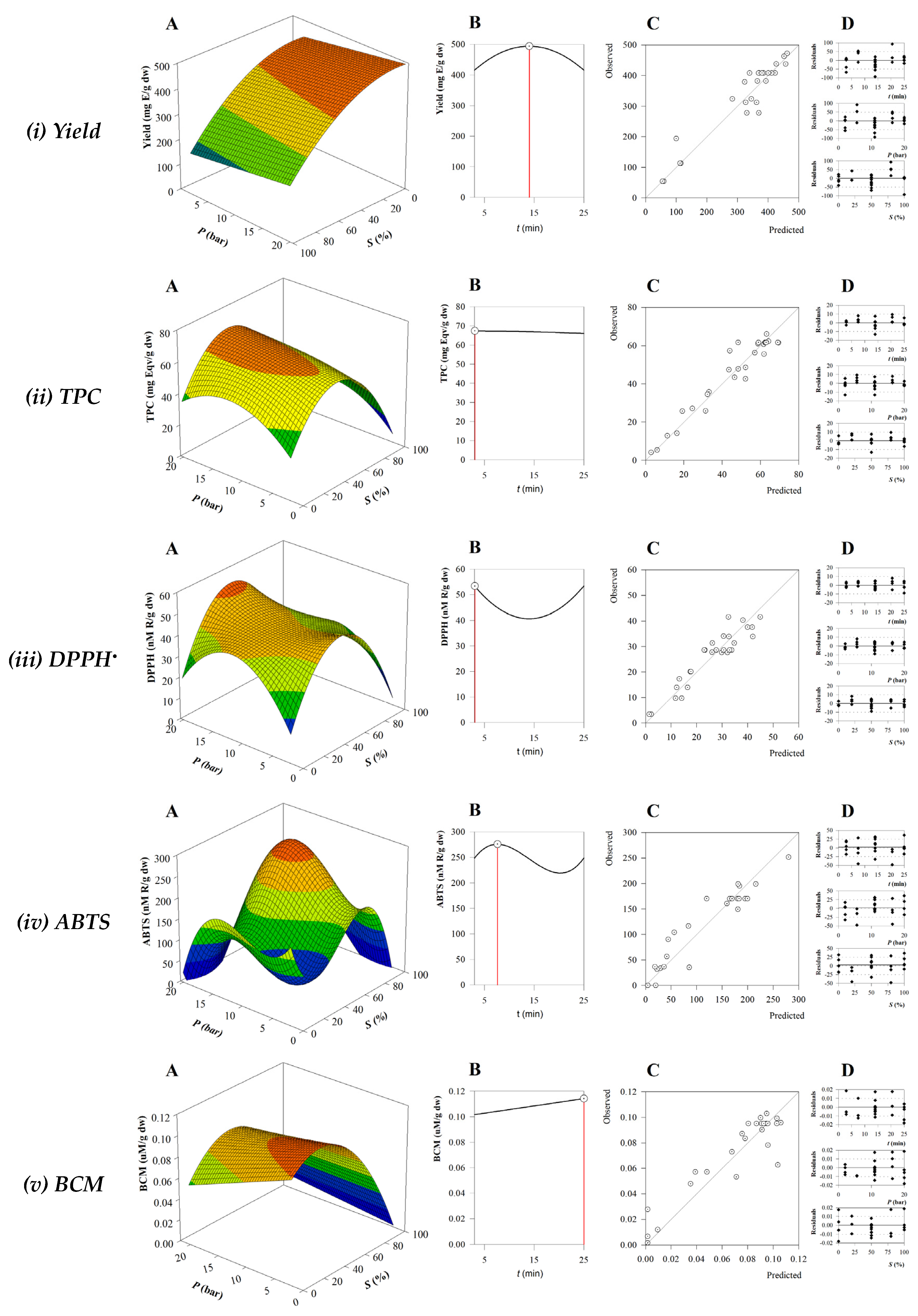
3.3. Biological Activity of the BB Optimal Extract
3.3.1. Antimicrobial Activity
| A: Antimicrobial activity | |||
| MIC (mg/mL) | Inhibition zone (mm) | ||
| Escherichia coli | NI | NI | Lactic acid 40% (18.96 ± 3.59) |
| Staphylococcus epidermidis | 8 | 15.86 ± 1.43 | Lactic acid 40% (17.43 ± 3.41) |
| Bacillus cereus | 1.2 | 5.45 ± 1.06 | Lactic acid 40% (17.10 ± 3.36) |
| Staphylococcus aureus | >8 | 12.06 ± 1.88 | Lactic acid 40% (19.08 ± 4.59) |
| Salmonella enteritidis | 1.2 | 9.77 ± 1.63 | Lactic acid 40% (18.06 ± 2.93) |
| Pseudomonas aeruginosa | >8 | 10.86 ± 0.23 | Lactic acid 40% (19.45 ± 1.45) |
| B:Antioxidant activity (ROS, RNS) | |||
| IC50 (µg/mL) | IC50 (µg/mL) | ||
| Hydroxyl radical (●OH) | 446.1 ± 22.3 | Ascorbic acid (183 µg/mL) | |
| Nitric oxide (●NO) | 55.35 ±2.76 | Ascorbic acid (446 µg/mL) | |
| Superoxide anion (O2●–) | 44.25 ± 2.21 | Ascorbic acid (160 µg/mL) | |
| Hydrogen peroxide (H2O2) | 108.4 ± 5.4 | Ascorbic acid (51 µg/mL) | |
| C:Enzymatic inhibition | |||
| IC50 (µg/mL) | IC50 (µg/mL) | ||
| Tyrosinase | 329.6 ± 16.4 | Kojic acid (1.82 µg/mL) | |
| Monoamine oxidase A (MAO-A) | 133.6 ± 6.68 | Clorgyline (25 ng/mL) | |
| Monoamine oxidase B (MAO-B) | 154.8 ± 7.74 | Selegiline (2.1 µg/mL) | |
| Acetylcholinesterase (AChE) | >2000 | Galantamine (0.92 µg/mL) | |
| Butyrylcholinesterase (BuChE) | 712.9 ± 35 | Galantamine (4.92 µg/mL) | |
| D: Anti-inflammatory activity | |||
| IC50 (µg/mL) | IC50 (µg/mL) | ||
| RAW264.7 | 320.5 ± 10.6 | Dexamethasone 7.23 ± 0.85 | |
| E: Cytotoxic effects | |||
| IC50 (µg/mL) | IC50 (µg/mL) | ||
| A549 (lung adenocarcinoma) | 87.57 ± 2.04 | Ellipticine (<1 mg/mL) | |
| HEPG2 (hepatocellular carcinoma) | 52.85 ± 2.71 | Ellipticine (0.85 ± 0.046) | |
| AGS (gastric adenocarcinoma) | 38.75 ± 2.18 | Ellipticine (<1 mg/mL) | |
| Vero | 85.85 ± 2.67 | Ellipticine (<1 mg/mL) | |
3.3.2. Reactive Oxygen and Nitrogen Scavenging Activity
3.3.3. In Vitro Neuroprotective Properties
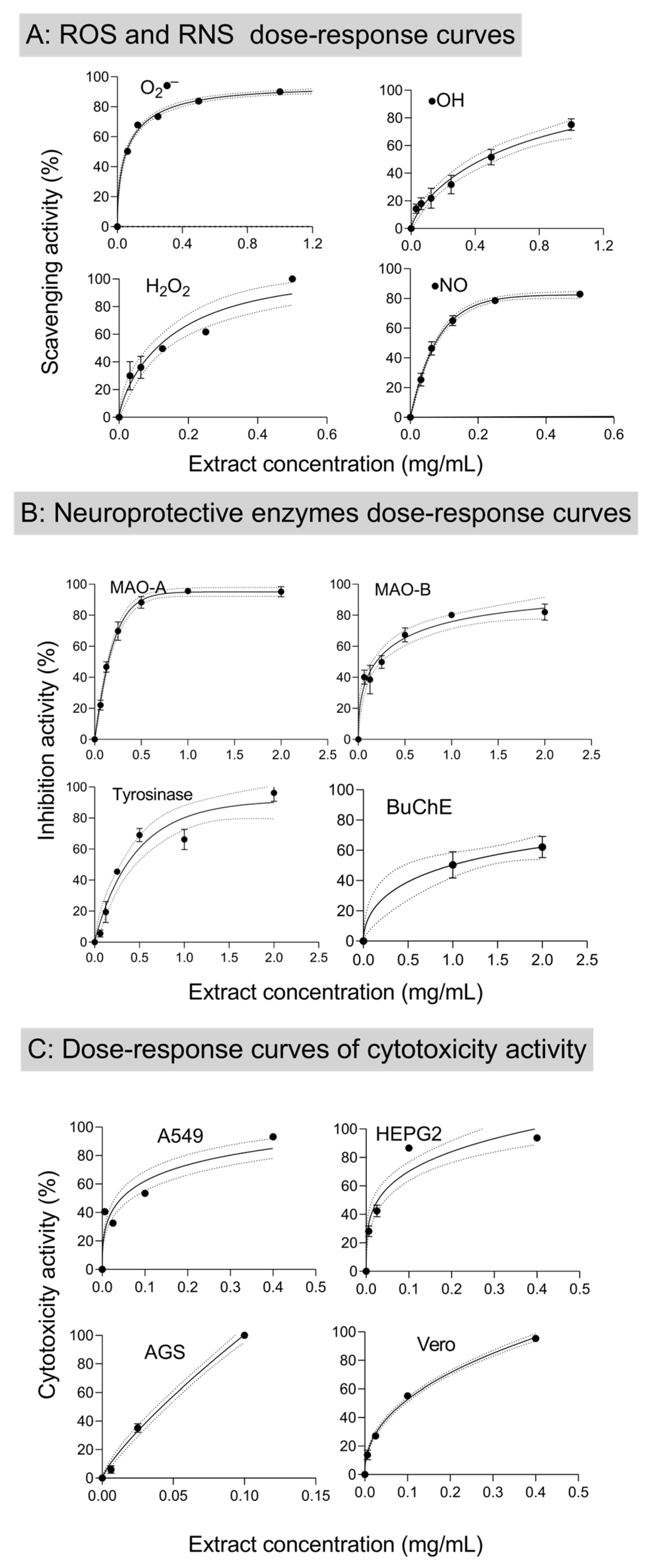
3.3.4. Anti-Inflammatory and Cytotoxic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, S.M.; Carvalho, L.G.; Silva, P.J.; Rodrigues, M.S.; Pereira, O.R. Bioproducts from Seaweeds: A Review with Special Focus on the Iberian Peninsula. Curr. Org. Chem. 2014, 18, 896–917. [Google Scholar] [CrossRef]
- Parsaeimehr, A.; Lutzu, G.A. Algae as a Novel Source of Antimicrobial Compounds: Current and Future Perspectives. In Antibiotic Resistance; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128036686. [Google Scholar]
- Spavieri, J.; Allmendinger, A.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Guiry, M.D.; Blunden, G.; Tasdemir, D. Antimycobacterial, Antiprotozoal and Cytotoxic Potential of Twenty-One Brown Algae (Phaeophyceae) from British and Irish Waters. Phyther. Res. 2010, 24, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave Assisted Extraction of Phenolic Compounds from Four Economic Brown Macroalgae Species and Evaluation of Their Antioxidant Activities and Inhibitory Effects on α-Amylase, α-Glucosidase, Pancreatic Lipase and Tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Le Lann, K.; Rumin, J.; Cérantola, S.; Culioli, G.; Stiger-Pouvreau, V. Spatiotemporal Variations of Diterpene Production in the Brown Macroalga Bifurcaria Bifurcata from the Western Coasts of Brittany (France). J. Appl. Phycol. 2014, 26, 1207–1214. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary Fibre and Physicochemical Properties of Several Edible Seaweeds from the Northwestern Spanish Coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Silva, A.; Cassani, L.; Grosso, C.; Garcia-Oliveira, P.; Morais, S.L.; Echave, J.; Carpena, M.; Xiao, J.; Barroso, M.F.; Simal-Gandara, J.; et al. Recent Advances in Biological Properties of Brown Algae-Derived Compounds for Nutraceutical Applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 1283–1311. [Google Scholar] [CrossRef]
- Pais, A.C.S.; Saraiva, J.A.; Rocha, S.M.; Silvestre, A.J.D.; Santos, S.A.O. Current Research on the Bioprospection of Linear Diterpenes from Bifurcaria Bifurcata: From Extraction Methodologies to Possible Applications. Mar. Drugs 2019, 17, 556. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Freitas, R.; Martins, A.; Pinteus, S.; Ribeiro, J.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Antioxidant and Neuroprotective Potential of the Brown Seaweed Bifurcaria Bifurcata in an in Vitro Parkinson’s Disease Model. Mar. Drugs 2019, 17, 85. [Google Scholar] [CrossRef]
- Horta, A.; Pinteus, S.; Alves, C.; Fino, N.; Silva, J.; Fernandez, S.; Rodrigues, A.; Pedrosa, R. Antioxidant and Antimicrobial Potential of the Bifurcaria Bifurcata Epiphytic Bacteria. Mar. Drugs 2014, 12, 1676–1689. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Pinteus, S.; Simões, T.; Horta, A.; Silva, J.; Tecelão, C.; Pedrosa, R. Bifurcaria Bifurcata: A Key Macro-Alga as a Source of Bioactive Compounds and Functional Ingredients. Int. J. Food Sci. Technol. 2016, 51, 1638–1646. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins Are Polyphenolic Metabolites of Brown Algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Delazar, A.; Nahar, L.; Hamedeyazdan, S.; Sarker, S.D. Microwave-Assisted Extraction in Natural Products Isolation. Nat. Prod. Isol. 2012, 864, 89–115. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Rittà, M.; Donalisio, M.; Mariatti, F.; You, S.G.; Lembo, D.; Cravotto, G. Effect of Different Non-Conventional Extraction Methods on the Antibacterial and Antiviral Activity of Fucoidans Extracted from Nizamuddinia Zanardinii. Int. J. Biol. Macromol. 2019, 124, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Cikos, A.-M.M.; Jokic, S.; Subaric, D.; Jerkovic, I.; Ciko, A.M.; Jokić, S.; Šubarić, D.; Jerković, I.; Cikos, A.-M.M.; Jokic, S.; et al. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef] [PubMed]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Kovačević, D.B.; Garofulić, I.E.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Microwave-Hydrothermal Extraction and Degradation of Fucoidan from Supercritical Carbon Dioxide Deoiled Undaria pinnatifida. Ind. Eng. Chem. Res. 2013, 52, 7940–7946. [Google Scholar] [CrossRef]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-Assisted Extractions of Active Ingredients from Plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Hunter, J.S. Multi-Factor Experimental Designs for Exploring Response Surfaces. Ann. Math. Stat. 1957, 28, 195–241. [Google Scholar] [CrossRef]
- Toan, T.Q.; Phong, T.D.; Tien, D.D.; Linh, N.M.; Mai Anh, N.T.; Hong Minh, P.T.; Duy, L.X.; Nghi, D.H.; Pham Thi, H.H.; Nhut, P.T.; et al. Optimization of Microwave-Assisted Extraction of Phlorotannin From Sargassum Swartzii (Turn.) C. Ag. with Ethanol/Water. Nat. Prod. Commun. 2021, 16, 1934578X2199618. [Google Scholar] [CrossRef]
- Cassani, L.; Silva, A.; Carpena, M.; Pellegrini, M.C.; García-Pérez, P.; Grosso, C.; Barroso, M.F.; Simal-Gandara, J.; Gómez-Zavaglia, A.; Prieto, M.A. Phytochemical Compounds with Promising Biological Activities from Ascophyllum Nodosum Extracts Using Microwave-Assisted Extraction. Food Chem. 2024, 438, 138037. [Google Scholar] [CrossRef] [PubMed]
- Huis in’t Veld, J.H.J. Microbial and Biochemical Spoilage of Foods: An Overview. Int. J. Food Microbiol. 1996, 33, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus Epidermidis Infections. Ann. Intern. Med. 1983, 99, 834. [Google Scholar] [CrossRef]
- Nagatsu, T.; Nakashima, A.; Watanabe, H.; Ito, S.; Wakamatsu, K. Neuromelanin in Parkinson’s Disease: Tyrosine Hydroxylase and Tyrosinase. Int. J. Mol. Sci. 2022, 23, 4176. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.; Vieira, M.; Delerue-Matos, C.; Grosso, C.; Soares, C. Biological Potential, Gastrointestinal Digestion, Absorption, and Bioavailability of Algae-Derived Compounds with Neuroprotective Activity: A Comprehensive Review. Mar. Drugs 2022, 20, 362. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Thomas-Guyon, H.; Jacquot, C.; Jugé, M.; Culioli, G.; Ortalo-Magné, A.; Piovetti, L.; Roussakis, C. An Extract from the Brown Alga Bifurcaria Bifurcata Induces Irreversible Arrest of Cell Proliferation in a Non-Small-Cell Bronchopulmonary Carcinoma Line. J. Appl. Phycol. 2006, 18, 87–93. [Google Scholar] [CrossRef]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Paz, M.; Gúllon, P.; Barroso, M.F.; Carvalho, A.P.; Domingues, V.F.; Gomes, A.M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C. Brazilian Fruit Pulps as Functional Foods and Additives: Evaluation of Bioactive Compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Okawa, M.; Kinjo, J.; Nohara, T.; Ono, M. DPPH (1,1-Diphenyl-2-Picrylhydrazyl) Radical Scavenging Activity of Flavonoids Obtained from Some Medicinal Plants. Biol. Pharm. Bull. 2001, 24, 1202–1205. [Google Scholar] [CrossRef]
- Prieto, M.A.; Curran, T.P.; Gowen, A.; Vázquez, J.A. An Efficient Methodology for Quantification of Synergy and Antagonism in Single Electron Transfer Antioxidant Assays. Food Res. Int. 2015, 67, 284–298. [Google Scholar] [CrossRef]
- Murado, M.A.; Prieto, M.A. NOEC and LOEC as Merely Concessive Expedients: Two Unambiguous Alternatives and Some Criteria to Maximize the Efficiency of Dose–Response Experimental Designs. Sci. Total Environ. 2013, 461–462, 576–586. [Google Scholar] [CrossRef]
- Weibull, W.; Sweden, S. A Statistical Distribution Function of Wide Applicability. J. Appl. Mech. 1951, 18, 293–297. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard —Eleventh Edition; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2012; Volume 32, ISBN 1562387812. [Google Scholar]
- Soares, C.; Paíga, P.; Marques, M.; Neto, T.; Carvalho, A.P.; Paiva, A.; Simões, P.; Costa, L.; Bernardo, A.; Fernández, N.; et al. Multi-Step Subcritical Water Extracts of Fucus Vesiculosus L. and Codium Tomentosum Stackhouse: Composition, Health-Benefits and Safety. Processes 2021, 9, 893. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Valentão, P.; Pereira, J.A.; Silva, B.M.; Tavares, F.; Andrade, P.B. Ficus Carica L.: Metabolic and Biological Screening. Food Chem. Toxicol. 2009, 47, 2841–2846. [Google Scholar] [CrossRef]
- Gülçin, İ.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Screening of Antiradical and Antioxidant Activity of Monodesmosides and Crude Extract from Leontice smirnowii Tuber. Phytomedicine 2006, 13, 343–351. [Google Scholar] [CrossRef]
- Mancini, S.; Nardo, L.; Gregori, M.; Ribeiro, I.; Mantegazza, F.; Delerue-Matos, C.; Masserini, M.; Grosso, C. Functionalized Liposomes and Phytosomes Loading Annona Muricata L. Aqueous Extract: Potential Nanoshuttles for Brain-Delivery of Phenolic Compounds. Phytomedicine 2018, 42, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Geng, M. Free Radical Scavenging Activities of Pigment Extract from Hibiscus syriacus L. Petals in Vitro. Afr. J. Biotechnol. 2012, 11, 429–435. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for Tyrosinase Inhibitors among Extracts of Seashore Plants and Identification of Potent Ihibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Cassani, L.; Lourenço-Lopes, C.; Barral-Martinez, M.; Chamorro, F.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Thermochemical Characterization of Eight Seaweed Species and Evaluation of Their Potential Use as an Alternative for Biofuel Production and Source of Bioactive Compounds. Int. J. Mol. Sci. 2022, 23, 2355. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yan, J.; Liu, R.; Marcone, M.F.; Aisa, H.A.; Tsao, R. Optimization of Microwave-Assisted Extraction of Phenolics from Potato and Its Downstream Waste Using Orthogonal Array Design. Food Chem. 2012, 133, 1292–1298. [Google Scholar] [CrossRef]
- Amarante, S.J.; Catarino, M.D.; Marçal, C.; Silva, A.M.S.; Ferreira, R.; Cardoso, S.M. Microwave-Assisted Extraction of Phlorotannins from Fucus Vesiculosus. Mar. Drugs 2020, 18, 559. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Alves, C.; Susano, P.; Martins, A.; Pinteus, S.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Exploring Marine Resources against Neurological Disorders—The Neuroprotective and Anti-Inflammatory Potential of the Brown Seaweed Bifurcaria Bifurcata. J. Appl. Phycol. 2022, 34, 2671–2688. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-Azino-Bis-3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2‘-Diphenyl-1-Picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.-W.; Cai, L.; Xing, Y.; Yu, J.; Ding, Z.-T. Re-Evaluation of ABTS*+ Assay for Total Antioxidant Capacity of Natural Products. Nat. Prod. Commun. 2015, 10, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Grina, F.; Ullah, Z.; Kaplaner, E.; Moujahid, A.; Eddoha, R.; Nasser, B.; Terzioğlu, P.; Yilmaz, M.A.; Ertaş, A.; Öztürk, M.; et al. In Vitro Enzyme Inhibitory Properties, Antioxidant Activities, and Phytochemical Fingerprints of Five Moroccan Seaweeds. S. Afr. J. Bot. 2020, 128, 152–160. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In Vitro Antioxidant Properties of Crude Extracts and Compounds from Brown Algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef] [PubMed]
- Agregán, R.; Munekata, P.E.; Domínguez, R.; Carballo, J.; Franco, D.; Lorenzo, J.M. Proximate Composition, Phenolic Content and in Vitro Antioxidant Activity of Aqueous Extracts of the Seaweeds Ascophyllum Nodosum, Bifurcaria Bifurcata and Fucus Vesiculosus. Effect of Addition of the Extracts on the Oxidative Stability of Canola Oil Unde. Food Res. Int. 2017, 99, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Zubia, M.; Fabre, M.S.; Kerjean, V.; Lann, K.L.; Stiger-Pouvreau, V.; Fauchon, M.; Deslandes, E. Antioxidant and Antitumoural Activities of Some Phaeophyta from Brittany Coasts. Food Chem. 2009, 116, 693–701. [Google Scholar] [CrossRef]
- Sarno, E.; Pezzutto, D.; Rossi, M.; Liebana, E.; Rizzi, V. A Review of Significant European Foodborne Outbreaks in the Last Decade. J. Food Prot. 2021, 84, 2059–2070. [Google Scholar] [CrossRef] [PubMed]
- Bubonja-Šonje, M.; Knežević, S.; Abram, M. Challenges to Antimicrobial Susceptibility Testing of Plant-Derived Polyphenolic Compounds. Arch. Ind. Hyg. Toxicol. 2020, 71, 300–311. [Google Scholar] [CrossRef]
- Santos, S.; Trindade, S.; Oliveira, C.; Parreira, P.; Rosa, D.; Duarte, M.; Ferreira, I.; Cruz, M.; Rego, A.; Abreu, M.; et al. Lipophilic Fraction of Cultivated Bifurcaria Bifurcata R. Ross: Detailed Composition and In Vitro Prospection of Current Challenging Bioactive Properties. Mar. Drugs 2017, 15, 340. [Google Scholar] [CrossRef]
- Rubiño, S.; Peteiro, C.; Aymerich, T.; Hortós, M. Brown Macroalgae (Phaeophyceae): A Valuable Reservoir of Antimicrobial Compounds on Northern Coast of Spain. Mar. Drugs 2022, 20, 775. [Google Scholar] [CrossRef] [PubMed]
- Biard, J.F.; Verbist, J.F.; Letourneux, Y.; Floch, R. Diterpene Ketols with Antimicrobial Activity from Bifurcaria Bifurcata (Author’s Transl). Planta Med. 1980, 11, 288–294. [Google Scholar] [CrossRef]
- Hellio, C.; Thomas-Guyon, H.; Culioli, G.; Piovettt, L.; Bourgougnon, N.; le Gal, Y. Marine Antifoulants from Bifurcaria Bifurcata (Phaeophyceae, Cystoseiraceae) and Other Brown Macroalgae. Biofouling 2001, 17, 189–201. [Google Scholar] [CrossRef]
- Ray, G.; Husain, S.A. Oxidants, Antioxidants and Carcinogenesis. Indian J. Exp. Biol. 2002, 40, 1213–1232. [Google Scholar] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Heo, S.; Park, E.; Lee, K.; Jeon, Y. Antioxidant Activities of Enzymatic Extracts from Brown Seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef]
- Airanthi, M.K.W.A.; Hosokawa, M.; Miyashita, K. Comparative Antioxidant Activity of Edible Japanese Brown Seaweeds. J. Food Sci. 2011, 76, C104–C111. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Rodriguez, P.A.; Murillo-Álvarez, J.I.; Campa-Cordova, Á.I.; Angulo, C. Antioxidant Screening and Phenolic Content of Ethanol Extracts of Selected Baja California Peninsula Macroalgae. J. Food Sci. Technol. 2017, 54, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.-A.; Aedo, H.; Lopez-Torres, B.; Maximiliano, J.-E.; Martínez-Larrañaga, M.-R.; Anadón, A.; Martínez, M.; Peteiro, C.; Cueto, M.; Rubiño, S.; et al. Bifurcaria Bifurcata Extract Exerts Antioxidant Effects on Human Caco-2 Cells. Environ. Res. 2023, 231, 116141. [Google Scholar] [CrossRef]
- Kim, K.-N.; Heo, S.-J.; Yoon, W.-J.; Kang, S.-M.; Ahn, G.; Yi, T.-H.; Jeon, Y.-J. Fucoxanthin Inhibits the Inflammatory Response by Suppressing the Activation of NF-ΚB and MAPKs in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Ko, S.C.; Kang, S.M.; Kang, H.S.; Kim, J.P.; Kim, S.H.; Lee, K.W.; Cho, M.G.; Jeon, Y.J. Cytoprotective Effect of Fucoxanthin Isolated from Brown Algae Sargassum Siliquastrum against H2O2—Induced Cell Damage. Eur. Food Res. Technol. 2008, 228, 145–151. [Google Scholar] [CrossRef]
- Murakami, A.; Nakashima, M.; Koshiba, T.; Maoka, T.; Nishino, H.; Yano, M.; Sumida, T.; Kyung Kim, O.; Koshimizu, K.; Ohigashi, H. Modifying Effects of Carotenoids on Superoxide and Nitric Oxide Generation from Stimulated Leukocytes. Cancer Lett. 2000, 149, 115–123. [Google Scholar] [CrossRef]
- Baek, S.M.; Kwon, C.H.; Kim, J.H.; Woo, J.S.; Jung, J.S.; Kim, Y.K. Differential Roles of Hydrogen Peroxide and Hydroxyl Radical in Cisplatin-Induced Cell Death in Renal Proximal Tubular Epithelial Cells. J. Lab. Clin. Med. 2003, 142, 178–186. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a Stealthy Biological Oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef]
- Patil, P.O.; Bari, S.B.; Firke, S.D.; Deshmukh, P.K.; Donda, S.T.; Patil, D.A. A Comprehensive Review on Synthesis and Designing Aspects of Coumarin Derivatives as Monoamine Oxidase Inhibitors for Depression and Alzheimer’s Disease. Bioorg. Med. Chem. 2013, 21, 2434–2450. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Greig, N.H.; Lahiri, D.K.; Sambamurti, K. Butyrylcholinesterase: An Important New Target in Alzheimer’s Disease Therapy. Int. Psychogeriatr. 2002, 14, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Hira, S.; Saleem, U.; Anwar, F.; Sohail, M.F.; Raza, Z.; Ahmad, B. β-Carotene: A Natural Compound Improves Cognitive Impairment and Oxidative Stress in a Mouse Model of Streptozotocin-Induced Alzheimer’s Disease. Biomolecules 2019, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, L.; Yu, J.; Xiang, S.; Wang, J.; Zhang, J.; Yan, X.; Cui, W.; He, S.; Wang, Q. Fucoxanthin, a Marine Carotenoid, Reverses Scopolamine-Induced Cognitive Impairments in Mice and Inhibits Acetylcholinesterase in Vitro. Mar. Drugs 2016, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Kwon, O.I.; Hwang, H.J.; Shin, H.-C.; Yang, S. Therapeutic Effects of Phlorotannins in the Treatment of Neurodegenerative Disorders. Front. Mol. Neurosci. 2023, 16, 1193590. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Alves, C.; Pinteus, S.; Susano, P.; Simões, M.; Guedes, M.; Martins, A.; Rehfeldt, S.; Gaspar, H.; Goettert, M.; et al. Disclosing the Potential of Eleganolone for Parkinson’s Disease Therapeutics: Neuroprotective and Anti-Inflammatory Activities. Pharmacol. Res. 2021, 168, 105589. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, D.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Andronie-Cioara, F.L.; Toma, M.M.; Bungau, S.; Bumbu, A.G. Role of Monoamine Oxidase Activity in Alzheimer’s Disease: An Insight into the Therapeutic Potential of Inhibitors. Molecules 2021, 26, 3724. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Polyphenols from Brown Seaweeds (Ochrophyta, Phaeophyceae): Phlorotannins in the Pursuit of Natural Alternatives to Tackle Neurodegeneration. Mar. Drugs 2020, 18, 654. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Roy, A.; Jung, J.H.; Choi, J.S. Evaluation of the Inhibitory Effects of Eckol and Dieckol Isolated from Edible Brown Alga Eisenia Bicyclis on Human Monoamine Oxidases A and B. Arch. Pharm. Res. 2017, 40, 480–491. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, K.; Hwang, H.; Kim, S.-H.; Park, S.E.; Durai, P.; Park, K.; Kim, H.-S.; Jang, D.S.; Choi, J.S.; et al. New Monocyclic Terpenoid Lactones from a Brown Algae Sargassum Macrocarpum as Monoamine Oxidase Inhibitors. Plants 2022, 11, 1998. [Google Scholar] [CrossRef]
- Jung, H.A.; Roy, A.; Choi, J.S. In Vitro Monoamine Oxidase A and B Inhibitory Activity and Molecular Docking Simulations of Fucoxanthin. Fish. Sci. 2017, 83, 123–132. [Google Scholar] [CrossRef]
- Manandhar, B.; Wagle, A.; Seong, S.H.; Paudel, P.; Kim, H.-R.; Jung, H.A.; Choi, J.S. Phlorotannins with Potential Anti-Tyrosinase and Antioxidant Activity Isolated from the Marine Seaweed Ecklonia Stolonifera. Antioxidants 2019, 8, 240. [Google Scholar] [CrossRef]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Fino, N.; Rodrigues, A.I.; Mendes, S.; Pedrosa, R. Cytoprotective Effect of Seaweeds with High Antioxidant Activity from the Peniche Coast (Portugal). Food Chem. 2017, 218, 591–599. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.; Carpena, M.; Cassani, L.; Grosso, C.; Garcia-Oliveira, P.; Delerue-Matos, C.; Simal-Gandara, J.; Barroso, M.F.; Prieto, M.A. Optimization and Bioactive Evaluation of Bifurcaria bifurcata Antioxidant-Rich Extracts for Functional Food and Pharmaceutical Applications. Antioxidants 2024, 13, 1189. https://doi.org/10.3390/antiox13101189
Silva A, Carpena M, Cassani L, Grosso C, Garcia-Oliveira P, Delerue-Matos C, Simal-Gandara J, Barroso MF, Prieto MA. Optimization and Bioactive Evaluation of Bifurcaria bifurcata Antioxidant-Rich Extracts for Functional Food and Pharmaceutical Applications. Antioxidants. 2024; 13(10):1189. https://doi.org/10.3390/antiox13101189
Chicago/Turabian StyleSilva, Aurora, Maria Carpena, Lucia Cassani, Clara Grosso, Paula Garcia-Oliveira, Cristina Delerue-Matos, Jesus Simal-Gandara, Maria Fatima Barroso, and Miguel A. Prieto. 2024. "Optimization and Bioactive Evaluation of Bifurcaria bifurcata Antioxidant-Rich Extracts for Functional Food and Pharmaceutical Applications" Antioxidants 13, no. 10: 1189. https://doi.org/10.3390/antiox13101189
APA StyleSilva, A., Carpena, M., Cassani, L., Grosso, C., Garcia-Oliveira, P., Delerue-Matos, C., Simal-Gandara, J., Barroso, M. F., & Prieto, M. A. (2024). Optimization and Bioactive Evaluation of Bifurcaria bifurcata Antioxidant-Rich Extracts for Functional Food and Pharmaceutical Applications. Antioxidants, 13(10), 1189. https://doi.org/10.3390/antiox13101189
















