Abstract
With the development of society and the improvement of people’s health consciousness, the demand for antioxidants is increasing. As a natural antioxidant with no toxic side effects, antioxidant peptides are widely used in food, cosmetics, medicine, and other fields because of their strong antioxidant capacity and easy absorption by the human body. Plant-derived antioxidant peptides have attracted more attention than animal-derived antioxidant peptides because plants are more diverse than animals and produce a large number of protein-rich by-products during the processing of their products, which are the main source of antioxidant peptides. In this review, we summarize the source, structure and activity, other biological functions, mechanism of action, and comprehensive applications of plant antioxidant peptides, and look forward to their future development trends, which will provide a reference for further research and development of plant antioxidant peptides.
1. Introduction
Reactive oxygen species (ROS) are small molecules derived from oxygen, which are by-products of the normal metabolism of human cells, mainly including hydroxyl free radicals (•OH), superoxide anion free radicals (O2•−), singlet oxygen, and hydrogen peroxide (H2O2). It has important functions in the organism, such as promoting cell signaling and proliferation and other life processes [1,2,3]. Studies have shown that excess ROS can unbalance the redox state of cells in vivo, leading to oxidative stress in the organism when it exceeds the effective antioxidant response capacity of cells [4]. Oxidative stress can affect cell ingredients, such as lead to impaired protein synthesis, DNA mutations, RNA damage, and lipid peroxidation [5,6], which in turn leads to a range of diseases such as diabetes, cardiovascular disease, cancer, and Parkinson’s disease [7,8,9,10].
In order to avoid the effects caused by oxidative stress, natural or synthetic antioxidants have been widely used in food, medicine, and cosmetics. However, it has been found that some synthetic antioxidants can cause certain damage to the human body [11,12], so the search for safe and effective natural antioxidants has become a hot topic of concern.
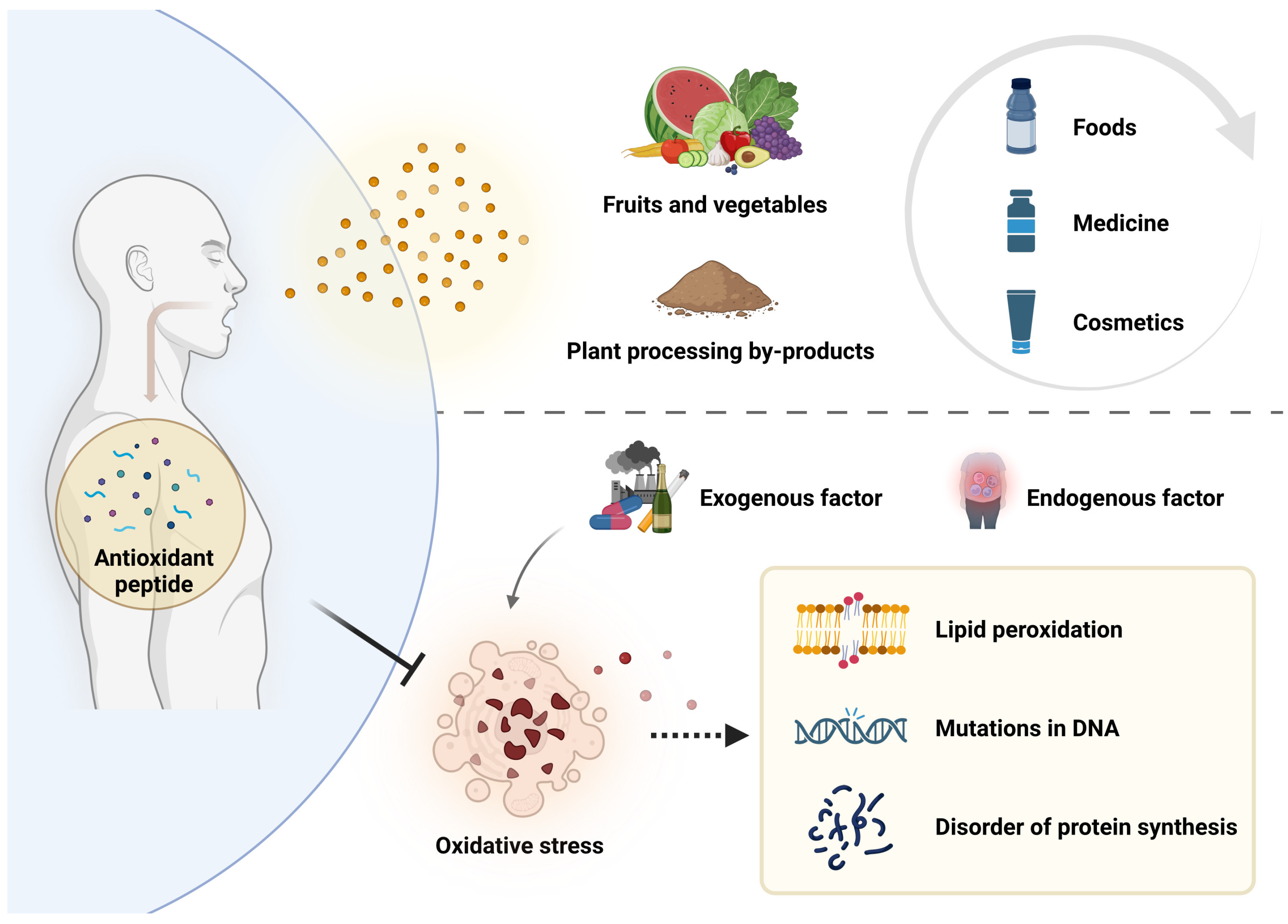
Plant antioxidant peptide is a kind of plant bioactive peptide with antioxidant function, most of which is composed of 2–20 amino acids. Its molecular weight is lower than 0.3 kDa [13] and it can effectively scavenge excess reactive oxygen radicals in the body, protect the normal structure and function of cells and mitochondria, prevent the occurrence of lipid peroxidation, and then play the role of delaying aging and preventing diseases [14,15]. In recent years, antioxidant peptides have attracted wide attention due to their significant antioxidant activity and easy absorption by the human body. Since many antioxidant peptides are derived from plants, it has become a hotspot to extract antioxidant peptides from plants and apply them in the fields of food, medicine, and cosmetics (Figure 1).
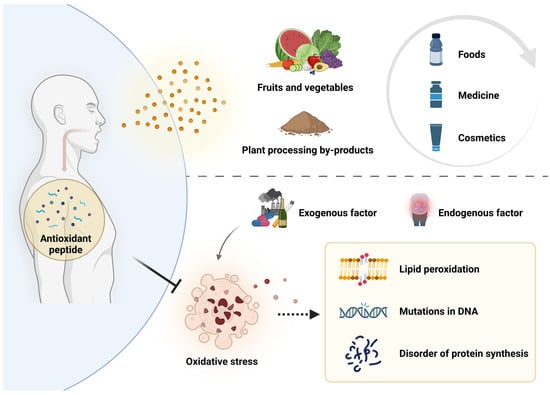
Figure 1.
Causes and effects of oxidative stress, and inhibition of oxidative stress by plant antioxidant peptides.
This paper summarizes the sources, structures and activities, other biological functions, mechanisms of action, and comprehensive applications of plant antioxidant peptides, discusses the current constraints on the development of the plant antioxidant peptide industry, and looks forward to its future development trends to provide a reference for its further research and development.
2. Sources of Plant Antioxidant Peptides
Plant antioxidant peptides have a wide range of sources and exist in all parts of plants. In addition, plant processing is prone to produce a large number of cheap industrial by-products, such as walnut meal and rice bran, which have a high protein content within them and are also regarded as one of the main sources of plant antioxidant peptides. Table 1 divides plant antioxidant peptides according to their sources and lists the amino acid sequences of peptides with antioxidant functions.

Table 1.
Sources of plant antioxidant peptides.
3. Structure and Activity of Plant Antioxidant Peptides
Studies have shown that the activity strength of antioxidant peptides is related to their structure, length, amino acid composition, and other factors [41,42]. The activity is positively correlated with the hydrophobicity, isoelectric point, and net charge of the peptide and negatively correlated with the molecular weight size [38]. Antioxidant peptides with aromatic or hydrophobic residues are able to exhibit stronger antioxidant properties. The experimental results of antioxidant properties of different antioxidant peptides are listed in Table 2 and their structural features are summarized.

Table 2.
Structural characteristics and activities of plant antioxidant peptides.
In summary, it was found that most of the plant antioxidant peptides contained high levels of aromatic amino acids and hydrophobic amino acids, which suggests that they have a non-negligible role in the antioxidant function exerted by plant-derived peptides. Yu et al. [37] used the hydrolysate of corn gluten meal to determine its antioxidant activity and found that the peptides with a molecular weight of less than 1 kDa had high antioxidant activity, and three new antioxidant peptides were isolated from them (IEFL, SAADL, and RYLL), which contained the proportion of hydrophobic amino acids of 75%, 60%, and 50%, and the IEFL and RYLL also contained aromatic amino acids. Therefore, it was hypothesized that these high contents of amino acids might be one of the key reasons for the antioxidant function of peptides with molecular weights less than 1 kDa. In addition to the content of the two amino acids mentioned above, other factors such as the content of antioxidant amino acids, the presence of repetitive amino acid sequences, and the position of some amino acids in the peptide chain also have an effect on the level of antioxidant peptide activity in plants. Zhang et al. [22] isolated four peptides, VVFVDRL, VIYVVDLR, IYVVDLR, and IYVFVR, from soybean hydrolyzed protein. The results showed that they were highly active in scavenging free radicals, which might be related to the high content of hydrophobic amino acids and antioxidant amino acids. In addition, the repetitive amino acid sequence “VV” in VVFVDRL, VIYVVDLR, and IYVVDLR may be another reason for the enhanced antioxidant properties.
4. Other Biological Functions of Plant Antioxidant Peptides
It has been found that some antioxidant peptides have biological functions such as anticancer, anti-hypertension, and anti-inflammation while exerting antioxidant activities. By synthesizing the literature, other biological functions possessed by some antioxidant peptides were summarized and are presented in Table 3, together with their amino acid sequences and sources.
In conclusion, it was found that the above plant antioxidant peptides were able to exert biological functions other than antioxidant functions by regulating the expression levels of different disease-related factors. To investigate whether four bioactive peptides (corn, wheat, egg white, and soybean) could effectively lower blood pressure, Zou et al. [43] made a solution and injected it into spontaneously hypertensive rats. The results showed that wheat peptide could significantly lower the systolic blood pressure and inhibit the expression levels of angiotensin 2 and tumor necrosis factor-α (TNF-α) in spontaneously hypertensive rats, thus exerting anti-hypertensive function. In addition to anti-hypertension, wheat peptide can significantly reduce the content of MDA in rats by enhancing the activity of antioxidant enzymes, as well as activate the glutathione (GSH) synthesis pathway or provide raw materials for GSH synthesis, and increase the content of GSH in rats, so as to play an antioxidant function. Wei et al. [44] also investigated other biological functions of walnut antioxidant peptides and found that they significantly improved cognitive and memory deficits in bisphenol AF-treated zebrafish and ethanol-treated rats. This result may be related to the ability of walnut antioxidant peptides to promote the expression of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GDNF) as well as inhibit oxidative stress.

Table 3.
Other biological functions of plant antioxidant peptides.
Table 3.
Other biological functions of plant antioxidant peptides.
| Amino Acid Sequence | Source | Other Biological Functions | Reference |
|---|---|---|---|
| —— | Cicer arietinum seed | Hypoglycemic | [45] |
| —— | Amaranthus hypochondriacus seed | Anti-thrombotic | [46] |
| —— | Dioscorea polystachya stem | Anti-hypertensive | [47] |
| —— | Oryza sativa by-product | Anti-hypertensive | [48,49] |
| —— | Triticum aestivum by-product | Anti-hypertensive | [50] |
| IF | Solanum tuberosum stem | Anti-hypertensive | [19,51] |
| PWLNFK FSIAWPR GSHWPFGGK | Chenopodium quinoa seed | Anti-hypertensive | [52] |
| PADVTPEEKPEV | Helianthus annuus seed | Anti-inflammatory | [32] |
| —— | Glycine max seed | Anti-aging Anti-hypertensive Anti-inflammatory | [50,53] |
| LY GHS RALP | Brassica rapa seed | Anti-hypertensive Anti-inflammatory | [28] |
| QGRPWG PSRADIY AYNIPVNIAR CTLEW VQTL LGYEN GGW VYY LLPF | Juglans regia by-product | Anticancer Anti-hypertensive Improvement in cognitive and memory disorders | [33,44,54,55] |
5. Mechanisms of Action of Plant Antioxidant Peptides
It has been demonstrated that antioxidant peptides exert antioxidant activity mainly through three mechanisms, including direct scavenging of free radicals, chelation of pro-oxidant metal ions, and enhancement of antioxidant defenses, thereby inhibiting oxidative stress in organisms [19,56,57].
5.1. Direct Scavenging of Free Radicals
Antioxidant peptides can directly scavenge free radicals by providing hydrogen atoms or electrons, while the hydrogen- or electron-donating effect depends on their amino acid residues [58]. It has been shown that Tyr, Phenylalanine (Phe), and Tryptophan (Trp) can provide electrons to free radicals to transform them into stable molecules while maintaining their structural stability through resonance structures [41,59]. Hydrophobic amino acids such as Leu, Val, Isoleucine (Ile), and Proline (Pro) transfer electrons to free radicals and thus exhibit excellent free radical scavenging activity [60]. The Glutamate (Glu) side chain produced after partial deamidation of Glutamine (Gln) residues can act as a reducing agent to provide electrons to free radicals for scavenging [61]. In addition, the sulfhydryl group on Cys residues not only has the ability to provide electrons but also transfers hydrogen protons to free radicals [62]. This suggests that some amino acid residues can act as both electron and hydrogen donors.
5.2. Chelation of Pro-Oxidant Metal Ions
It has been shown that antioxidant peptides can chelate with pro-oxidant metal ions, change the chemical reactivity of the metal, form insoluble metal complexes, or spatially impede metal–lipid interactions, thereby preventing the formation of ROS for antioxidant effects [58]. Positively charged transition metal ions such as Cu2+ and Fe2+ can form ionic bonds with the carboxyl groups on antioxidant peptides and form coordination bonds with amine functional groups on them [61]. In addition, some groups on the side chains of amino acid residues, such as sulfhydryl groups on Cys, imidazolyl groups on His, indolyl groups on Trp, and hydroxyl groups on Threonine (Thr), can also bind to metal ions and thus exert antioxidant activity [63].
Torres-Fuentes et al. [64] purified metal ion chelating peptides from chickpea protein hydrolysate and investigated their properties. The results showed that these chelating peptides, when combined with pro-oxidant metal ions, contributed to inhibiting ROS production and thus exerted antioxidant effects. In addition, this study also found that the metal chelating activity of chickpea protein hydrolysate was directly proportional to the content of His. Wang et al. [35] purified and identified the antioxidant peptides from cottonseed protein and found that cottonseed protein hydrolysate had good Fe2+ chelating activity, and its free radical activity was positively correlated with Fe2+ chelating activity. In addition, amino acid composition analysis showed that cottonseed protein hydrolysate was rich in Glu and Aspartic acid (Asp), which could act as an effective metal ion chelator to prevent the formation of free radicals, thus helping the antioxidant peptides to play a role.
5.3. Enhancement of the Antioxidant Defence System
Studies have shown that the body’s antioxidant defense system is divided into a non-enzymatic antioxidant system and an enzymatic antioxidant system. The non-enzymatic antioxidant system mainly includes GSH, carotene, melatonin, etc., while the enzymatic antioxidant system mainly includes SOD, CAT, and GPH-Px [58,65].
Since antioxidant peptides can scavenge excess ROS present in the body, they can act as exogenous antioxidants to offset the depletion of endogenous antioxidants in the body. Studies have shown that gamma-glutamyltransferase (GTT) can degrade exogenous and endogenous GSH when stimulated by external conditions such as ethanol, UV light, and toxins [66]. Chen et al. [67] investigated an antioxidant peptide (NDAEYGICGF) purified from microalgae protein hydrolysate and found that it could exhibit antioxidant effects on ethanol-induced oxidative stress in human hepatocellular carcinoma cells (HepG2) by inhibiting the expression level of GTT protein. In addition, the docking model of NDAEYGICGF with GGT suggests that the peptide may be mediated by hydrogen bonds and thus interact with the GGT active site, causing inhibition of its enzymatic activity.
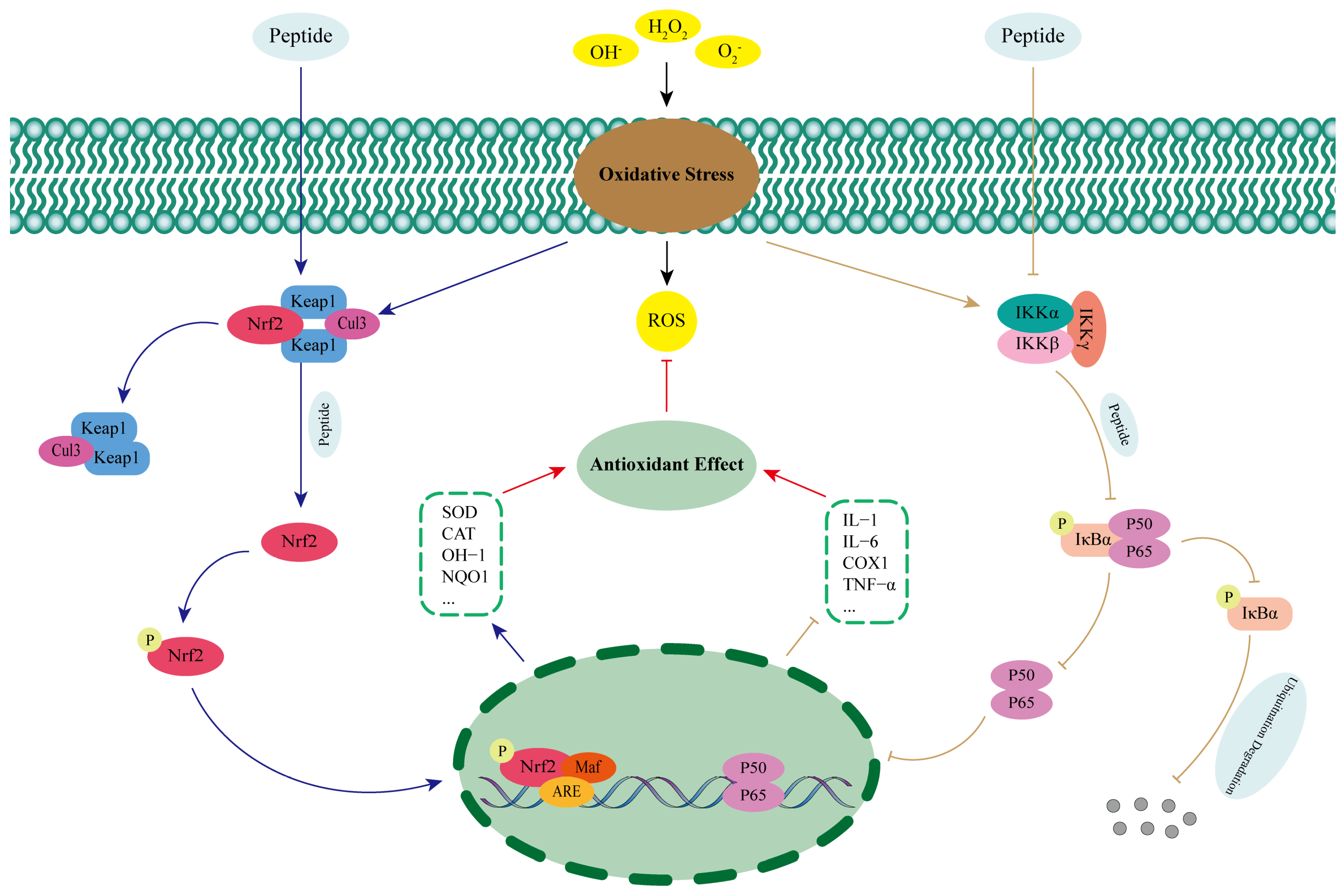
The nuclear factor-erythroid 2-related factor 2 (Nrf2) is a key factor in the regulation of oxidative stress in humans [68]. Normally, Nrf2 excess in the cytoplasm is mediated by Kelch-like ECH-associated protein 1 (Keap1) binding to cullin-RING E3 ligases 3 (Cul3), which is then degraded [69]. When under oxidative stress, excess ROS promotes the release of Nrf2 from the Nrf2-Keap1-Cul3 complex and into the nucleus, where it forms a heterodimer with V-Maf musculoaponeurotic fibrosarcoma (Maf) and recognizes the antioxidant reaction element (ARE) and binds to it, which in turn promotes the expression of antioxidant enzymes and phase II detoxification enzymes and exerts antioxidant effects (Figure 2) [70,71]. Studies have shown that antioxidant peptides can activate the Keap1-Nrf2-ARE signaling pathway by competitively binding to the active site of Keap1, interfering with the interaction between Keap1 and Nrf2, and promoting the release of Nrf2 and its entry into the nucleus [72]. Wu et al. [73] successfully identified two kinds of antioxidant peptides (IAY and TIL) that could significantly reduce oxidative damage in human umbilical vein endothelial cells (HUVECs) from Bangia fusco-purpurea and explored their antioxidant mechanisms in depth. The results showed that the two peptides were able to significantly increase the expression levels of Nrf2 protein and mRNA, promote Nrf2 nuclear translocation, and activate the expression of antioxidant enzymes and cytoprotective proteins under H2O2 stimulation. When Nrf2 was silenced, the antioxidant and anti-apoptotic effects of IAY and TIL on H2O2-induced HUVECs were inhibited. Therefore, it was hypothesized that the two antioxidant peptides could enter the cytoplasm of H2O2-induced HUVECs and exert antioxidant effects by occupying the binding site between Nrf2 and Keap1. Antioxidant peptides isolated from broken rice and rice bran proteins have also been shown to occupy the binding site between Keap1 and Nrf2 by techniques such as molecular docking and Western Blotting, promoting Nrf2 nuclear translocation, recognizing the ARE, and increasing the expression level of antioxidant enzymes, such as SOD and CAT, thus exerting antioxidant effects and reducing the level of oxidative damage to the cells [68,74].
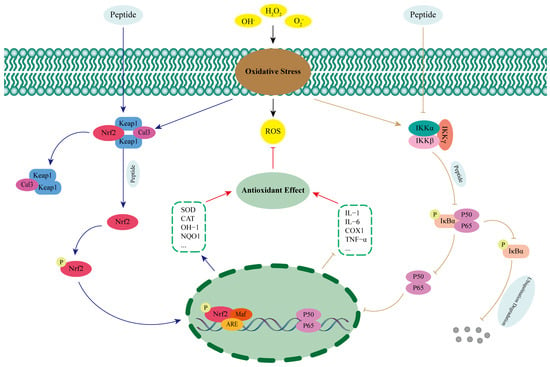
Figure 2.
Regulation of antioxidant peptides on the Keap1-Nrf2-ARE and NF-κB signaling pathways.
Nuclear transcription factor-kappa B (NF-κB) is a key intracellular regulatory molecule involved in a variety of physiological processes such as inflammation, innate immunity, and apoptosis, and its most predominantly activated form is a heterodimer consisting of p65 and p50 [75,76]. Under normal physiological conditions, NF−kappa−B inhibitor alpha (IκBα) can bind to NF-κB, mask nuclear localization signals, and inhibit its nuclear translocation. When stimulated by oxidative stress, inhibitor of kappa B kinase (IKK), consisting of three subunits, inhibitor of kappa B kinase alpha (IKKα), inhibitor of kappa B kinase betta (IKKβ), and inhibitor of kappa B kinase gamma (IKKγ), is activated, leading to phosphorylation of IκBα as well as ubiquitination degradation, which in turn promotes NF-κB release. Free NF-κB rapidly translocates to the nucleus and specifically induces the transcription and expression of pro-inflammatory and apoptotic genes, leading to cellular damage (Figure 2) [76,77,78]. Zhang et al. [79] investigated the mechanism of action of antioxidant peptide (YWDHNNPQIR) extracted from rapeseed to alleviate renal fibrosis in diabetic nephropathy and found that it was able to significantly inhibit the expression of p65 in diabetic mice, and at the same time, it had a significant inhibitory effect on the high glucose-induced phosphorylation of p65 and transforming growth factor beta 1 (TGF-β1) in the glomerular thylakoid membrane cells of rats. This suggests that the antioxidant peptide can exert antioxidant effects by inhibiting the NF-κB signaling pathway. Tong et al. [80] investigated the antioxidant mechanism of rice-derived peptide AAGALPS and found that it significantly improved inflammation and oxidative damage in vascular endothelial cells induced by Tumor Necrosis Factor Alpha (TNF-α). Meanwhile, protein immunoblotting experiments revealed that compared with TNF-α alone, the expression level of IKKα was significantly inhibited by the combined treatment of AAGALPS and TNF-α, and the intracellular levels of IκBα were increased. This demonstrated that the antioxidant peptide was able to inhibit the NF-κB pathway and exert antioxidant effects by hindering IKK activation and slowing down IκBα degradation.
6. Integrated Application of Plant Antioxidant Peptides (Figure 3)
6.1. Food Field
6.1.1. Used as an Antioxidant
It has been found that lipids and other nutrients within food products are susceptible to damage by oxidation reactions during transportation and storage, resulting in changes in color and flavor and even the production of harmful substances [81].
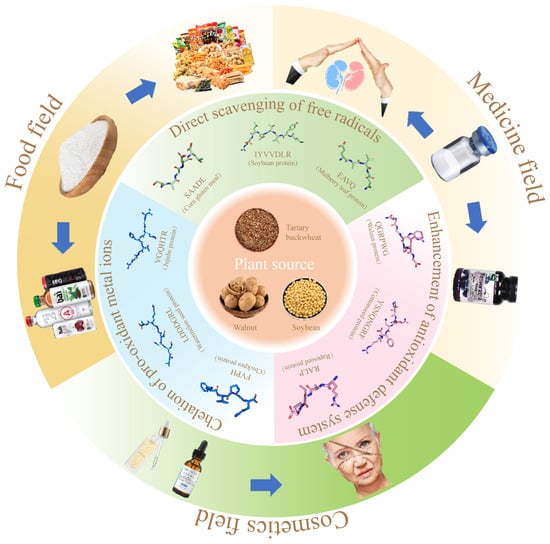
Figure 3.
The mechanism of antioxidant peptides from plants and their comprehensive applications in food, medicine, and cosmetics.
Figure 3.
The mechanism of antioxidant peptides from plants and their comprehensive applications in food, medicine, and cosmetics.
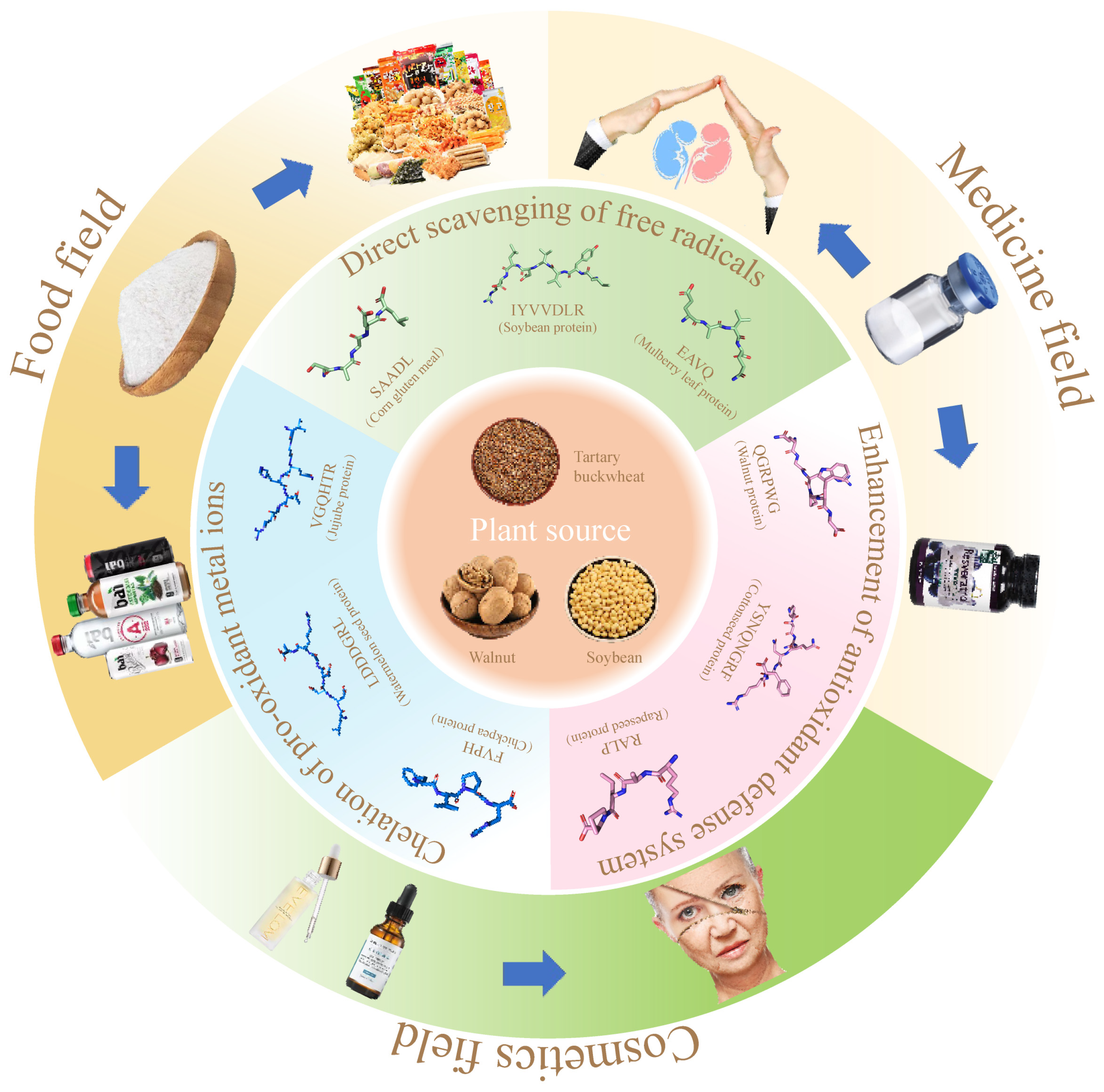
To solve this problem, many synthetic antioxidants such as butyl hydroxyisol, butyl hydroxytoluene, or tert-butylhydroquinone are used in foods. Although they are effective in inhibiting the oxidation process of nutrients such as lipids, their safety is still not guaranteed [82]. It has been reported that plant antioxidant peptides have a significant inhibitory effect on lipid peroxidation during food transport and storage, can effectively maintain the stability of food flavor and nutrients, and have higher antioxidant activity compared with synthetic antioxidants [83,84]. Therefore, the use of plant antioxidant peptides as antioxidants and their incorporation into foods can be helpful in alleviating concerns about food safety.
Hu et al. [85] added the hydrolysate of corn gluten meal to pork meal and determined the degree of inhibition of lipid oxidation of pork meal during the 16-day storage period. The results showed that the hydrolysate of corn gluten meal could effectively inhibit lipid peroxidation of pork meal, so it can be added into food as an antioxidant to improve the stability of the product in transport and storage and prolong the shelf life of food.
6.1.2. Development of Functional Products
It has been reported that plant antioxidant peptides are easily absorbed by the intestinal tract and can maintain a relatively intact structure to enter the circulatory system and give full play to their antioxidant functions, so they can be used as a strong candidate for the development of functional foods [86].
Chen et al. [87] isolated three antioxidant peptides, HGEPGQQQR, VAPFPEVFGK, and HNVADPQR, from walnut hydrolyzed protein, which inhibited oxidative stress by acting at key sites in the Keap1-Nrf2 pathway and could significantly improve the survival of ethanol-induced injury in HepG2 cells. Overall, these three antioxidant peptides may serve as potential hepatoprotective agents and be used in the development of functional foods. Samaei et al. [88] explored the functional properties of faba bean protein hydrolysate and investigated its effect on apple juice quality. It was found that the solubility of the broad bean protein hydrolysate was significantly increased, and its foamy ability was also improved to varying degrees. In addition, its hydrolysis products contained a variety of antioxidant peptides, which exerted their antioxidant effects through direct scavenging of free radicals or chelation with metal ions. The apple juice quality effect experiment found that the antioxidant peptide concentration below 3% had no significant effect on the flavor of apple juice except for some bitter taste. It has been found that orange juice can be used as a potential carrier of bioactive peptides [89], which can play a role in the development of functional beverages. Therefore, similar to orange juice, faba bean protein hydrolysate can also be added to apple juice to act as an antioxidant peptide carrier, and thus develop functional drinks with antioxidant effects.
In addition to the above plant protein hydrolysates, other plant protein hydrolysates can be used as functional ingredients and to develop food products with specific functions. For example, cardamom protein hydrolysate not only has excellent antioxidant properties but also has anti-fatigue activity, which can prolong weight-bearing swimming time in mice [89]; moreover, wheat protein and soybean protein hydrolysate can reduce blood pressure in spontaneously hypertensive rats while exerting antioxidant function [50].
6.2. Medicine Field
It has been found that oxidative stress caused by excessive ROS leads to many chronic diseases, such as neurological diseases and inflammatory diseases [90]. Antioxidant peptides can effectively remove excess ROS from the body and protect the normal structure and function of cells, so they have become a research hotspot in the medical field.
Diabetic nephropathy (DN) is a severe complication of diabetes mellitus and a major cause of end-stage renal disease [91]. Previous studies have proved that the development of DN is associated with excessive accumulation of extracellular matrix components and oxidative stress [92]. Zhang et al. [79] utilized an antioxidant peptide from rapeseed (YWDHNNPQIR) [93], which has been validated to have antioxidative stress properties, to investigate its pharmacological effects on DN renal fibrosis as well as high-glucose-induced glomerular mesangial dysfunction. The results showed that YWDHNNPQIR could inhibit DN renal fibrosis by inhibiting the MAPK and NF-κB pathways, and it is expected to be designed as a natural drug for treating DN renal fibrosis.
In addition to rapeseed antioxidant peptides, other plant antioxidant peptides have also been shown to have disease-delaying and therapeutic effects. For example, the antioxidant peptide (RQSHFANAQP) extracted from chickpeas can effectively inhibit the proliferation of breast cancer cells by increasing the p53 content [94]. Three peptide fractions (>10 kDa, 5–10 kDa, and <5 kDa) obtained from germinated soybeans have good antioxidant activity and can effectively inhibit the proliferation of breast and cervical cancer cell lines [95]. In addition, bioactive peptides (PELF and IALLIPF) isolated from foxtail millet can also inhibit the production of intracellular ROS and MDA, thus showing antioxidant activity, and it can alleviate inflammation by inhibiting the production of nitric oxide and pro-inflammatory cytokines [96].
Zhao et al. [97] identified five kinds of antioxidant peptides by ABTS after hydrolyzing wheat protein and treating them in an environment simulating the gastrointestinal enzyme system. It found that three peptides, CGFPGHC, RNF, and WF, lost their antioxidant properties. Therefore, preventing antioxidant peptides from being degraded is an issue that must be emphasized during drug preparation. Arturo et al. [98] used ionic gel and spray freeze-drying technology to embed antioxidant peptides to prepare gel nanoparticles. They found that antioxidant peptide activity could be well maintained and had little effect on cell viability. This suggests that nanoparticles can be used to embed antioxidant peptides and design an antioxidant peptide nanoparticle for delivery to the colon, which helps to maintain the bioavailability and pharmacological activity of antioxidant peptides.
6.3. Cosmetics Field
Modern studies have shown that skin aging is a process of degenerative changes in the appearance of the skin and the structure and function of the skin layers caused by intrinsic and extrinsic factors [99]. With the aging of the population in today’s society and the continuous improvement of living standards, the demand for anti-skin aging products is increasing, and how to prevent and delay skin aging has become a hot spot of concern.
Zhu et al. [100] isolated seven antioxidant peptides, including YLSF, LPSYVN, and SPHWNVN, from apricot using papain and the results of antioxidant activity assay showed that the scavenging rate of the above antioxidant peptides on ABTS free radicals was higher than that of GSH and ascorbic acid. In addition, the study also found that the above antioxidant peptides were able to significantly inhibit the extent of UV damage in mice. And based on the 4D label-free quantitative proteomics results, it was speculated that the apricot antioxidant peptides might inhibit UV-induced oxidative damage through three key pathways, including the base excision repair pathway. Mo et al. [101] used Lactobacillus plantarum to forage rice, extracted and purified a short peptide mixture (RFP) with less than 11 amino acids from the fermentation broth, and evaluated the antioxidant ability of RFP and its ability to remove ROS and MDA in human skin fibroblasts under oxidative stress induced by UVA. The results showed that RFP inhibited and delayed skin aging by promoting Nrf2 nuclear translocation, scavenging lipid oxidation products and excessive ROS, and enhancing the expression of antioxidant enzyme genes downstream of the Keap1-Nrf2-ARE pathway. Therefore, plant antioxidant peptides can be used as one of the new sources of raw materials for anti-aging cosmetics.
7. Conclusions
In recent years, as people have become increasingly aware of the important role of redox state balance in health status and the maintenance of health status, the production of antioxidant products that are safe, non-toxic, and have excellent antioxidant properties has become one of the demands of people. Plant-derived antioxidant peptides have attracted much attention in recent years, and have been used for product development in the food, medicine, and cosmetic fields because of their safety, easy absorption by the human body, excellent antioxidant properties, and wide range of sources. This paper summarizes the sources, structures, activities, and other biological functions of plant antioxidant peptides, and also elaborates on the mechanism of plant antioxidant peptides, detailing the antioxidant function of plant antioxidant peptides through Keap1-Nrf2-ARE, NF-κB, and other pathways. In addition, the application of plant antioxidant peptides in food, medicine, and cosmetics is also introduced.
Although the sources of plant antioxidant peptides are very wide, their extraction sources are mainly from various organs of plants, such as flowers, fruits, and seeds. It is less common to extract antioxidant peptides from the by-products of plant processing products. Numerous studies have shown that the by-products of processed plant products are high in protein content and yield, but are often discarded directly or used as low-value waste or feed, which seriously hampers the economic development of this plant industry [37,102]. In addition, the industrial-scale production of plant antioxidant peptides is also a major problem that needs to be solved urgently. The current method of producing plant antioxidant peptides is still relatively small-scale, time-consuming, and expensive, and the development of the plant antioxidant peptide industry is severely limited due to the high cost of large-scale production and subsequent purification of plant antioxidant peptides.
Therefore, in order to further develop plant antioxidant peptides in the food, medicine, and cosmetic fields, research on the by-products produced during the processing of plant products is essential. The extraction and purification techniques of plant antioxidant peptides also need to be improved, which is indispensable for their industrial-scale production. It is believed that with the development of molecular biology, cell biology, and bioinformatics, as well as the cross-integration of various disciplines, these problems will be overcome one by one, and the development and application of plant antioxidant peptides will take a new step forward.
Author Contributions
Conceptualization, Z.Z., Z.X. and L.M.; methodology, Z.Z., Z.X., Y.L. and Y.F.; investigation, Y.L., Y.F., Y.Z. and K.S.; writing—original draft preparation, Z.Z. and Z.X.; writing—review and editing, Y.L., Y.F. and L.M.; supervision, L.M.; funding acquisition, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Undergraduate Training Program for Innovation and Entrepreneurship of China (S202310541065X), the First-Class Discipline of Pharmaceutical Science of Hunan, the Project supported by Provincial Natural Science Foundation of Hunan (2024JJ8169), and the Research Fund of Hunan University of Chinese Medicine (2021XJJJ017).
Acknowledgments
The authors are thankful to Bohou Xia and Haomei Tian of Hunan University of Chinese Medicine for their guidance on this paper and the financial support provided by Hunan University of Chinese Medicine.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Meghea, A. Nanoparticles Induce Oxidative and Endoplasmic Reticulum Stresses: Antioxidant Therapeutic Defenses. MRS Bull. 2020, 45, 868. [Google Scholar] [CrossRef]
- Rahman, M.S.; Choi, Y.H.; Choi, Y.S.; Alam, M.B.; Lee, S.H.; Yoo, J.C. A novel antioxidant peptide, purified from Bacillus amyloliquefaciens, showed strong antioxidant potential via Nrf-2 mediated heme oxygenase-1 expression. Food Chem. 2018, 239, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and anti-freezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Jiang, B.; Miao, M.; Mu, W. The effects of an antioxidative pentapeptide derived from chickpea protein hydrolysates on oxidative stress in Caco-2 and HT-29 cell lines. J. Funct. Foods 2014, 7, 719–726. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- García-Nebot, M.J.; Recio, I.; Hernández-Ledesma, B. Antioxidant activity and protective effects of peptide lunasin against oxidative stress in intestinal Caco-2 cells. Food Chem. Toxicol. 2014, 65, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Wang, W.; Kang, P.M. Oxidative Stress and Antioxidant Treatments in Cardiovascular Diseases. Antioxidants 2020, 9, 1292. [Google Scholar] [CrossRef]
- Gurer-Orhan, H.; Ince, E.; Konyar, D.; Saso, L.; Suzen, S. The Role of Oxidative Stress Modulators in Breast Cancer. Curr. Med. Chem. 2018, 25, 4084–4101. [Google Scholar] [CrossRef]
- Puspita, L.; Chung, S.Y.; Shim, J.W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain 2017, 10, 53. [Google Scholar] [CrossRef]
- Sun, Z.; Tang, Z.; Yang, X.; Liu, Q.S.; Zhang, J.; Zhou, Q.; Jiang, G. 3-tert-Butyl-4-hydroxyanisole Impairs Hepatic Lipid Metabolism in Male Mice Fed with a High-Fat Diet. Environ. Sci. Technol. 2022, 56, 3204–3213. [Google Scholar] [CrossRef]
- Gerhart, A.K.; Janz, D.M. Toxicity of Aqueous L-Selenomethionine and Tert-Butyl Hydroperoxide Exposure to Zebrafish (Danio rerio) Embryos Following Tert-Butyl Hydroquinone Treatment. Toxics 2019, 7, 44. [Google Scholar] [CrossRef]
- Apone, F.; Barbulova, A.; Colucci, M.G. Plant and Microalgae Derived Peptides Are Advantageously Employed as Bioactive Compounds in Cosmetics. Front. Plant Sci. 2019, 10, 756. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Jahandideh, F.; Wu, J. Food-derived bioactive peptides on inflammation and oxidative stress. BioMed Res. Int. 2014, 2014, 608979. [Google Scholar] [CrossRef]
- Fang, L.; Ren, D.; Cui, L.; Liu, C.; Wang, J.; Liu, W.; Min, W.; Liu, J. Antifatigue, Antioxidant and Immunoregulatory Effects of Peptides Hydrolyzed from Manchurian Walnut (Juglans mandshurica Maxim.) on Mice. Grain Oil Sci. Technol. 2019, 1, 44–52. [Google Scholar] [CrossRef]
- Long, Y.; Tao, H.; Wang, S.; Xing, B.; Wang, Z.; Liu, K.; Shao, Q.; Gao, F. Identification and Functional Validation of Two Novel Antioxidant Peptides in Saffron. Antioxidants 2024, 13, 378. [Google Scholar] [CrossRef]
- Memarpoor-Yazdi, M.; Mahaki, H.; Zare-Zardini, H. Antioxidant activity of protein hydrolysates and purified peptides from Zizyphus jujuba fruits. J. Funct. Foods 2013, 5, 62–70. [Google Scholar] [CrossRef]
- Sompinit, K.; Lersiripong, S.; Reamtong, O.; Pattarayingsakul, W.; Patikarnmonthon, N.; Panbangred, W. In vitro study on novel bioactive peptides with antioxidant and antihypertensive properties from edible rhizomes. LWT-Food Sci. Technol. 2020, 134, 110227. [Google Scholar] [CrossRef]
- Tsai, B.C.; Hsieh, D.J.; Lin, W.T.; Tamilselvi, S.; Day, C.H.; Ho, T.J.; Chang, R.L.; Viswanadha, V.P.; Kuo, C.H.; Huang, C.Y. Functional potato bioactive peptide intensifies Nrf2-dependent antioxidant defense against renal damage in hypertensive rats. Food Res. Int. 2020, 129, 108862. [Google Scholar] [CrossRef]
- Sun, C.; Tang, X.; Ren, Y.; Wang, E.; Shi, L.; Wu, X.; Wu, H. Novel Antioxidant Peptides Purified from Mulberry (Morus atropurpurea Roxb.) Leaf Protein Hydrolysates with Hemolysis Inhibition Ability and Cellular Antioxidant Activity. J. Agric. Food Chem. 2019, 67, 7650–7659. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Chen, Y.; Sun, H.; Liu, Y. Preparation and characterization of lipophilic antioxidative peptides derived from mung bean protein. Food Chem. 2022, 395, 133535. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Li, Y.; Wang, H.; Wang, Z.; Qi, B.; Sui, X.; Jiang, L. Purification and Characterization of Antioxidant Peptides from Alcalase-Hydrolyzed Soybean (Glycine max L.) Hydrolysate and Their Cytoprotective Effects in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2019, 67, 5772–5781. [Google Scholar] [CrossRef]
- Gao, J.; Li, T.; Chen, D.; Gu, H.; Mao, X. Identification and molecular docking of antioxidant peptides from hemp seed protein hydrolysates. LWT-Food Sci. Technol. 2021, 147, 111453. [Google Scholar] [CrossRef]
- Zhou, Y.; She, X.; Chen, Z.; Wei, Y.; Xiao, Y.; Zhou, X. Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn) protein-derived antioxidant peptides: Mechanisms of action and structure-activity relationship in Caco-2 cell models. Food Sci. Hum. Wellness 2022, 11, 1580–1590. [Google Scholar] [CrossRef]
- Liang, L.-L.; Cai, S.-Y.; Gao, M.; Chu, X.-M.; Pan, X.-Y.; Gong, K.-K.; Xiao, C.-W.; Chen, Y.; Zhao, Y.-Q.; Wang, B.; et al. Purification of antioxidant peptides of Moringa oleifera seeds and their protective effects on H2O2 oxidative damaged Chang liver cells. J. Funct. Foods 2020, 64, 103698. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Purification and identification of novel antioxidant peptides from watermelon seed protein hydrolysates and their cytoprotective effects on H2O2-induced oxidative stress. Food Chem. 2020, 327, 127059. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Contreras, M.D.M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef]
- He, R.; Wang, Y.; Yang, Y.; Wang, Z.; Ju, X.; Yuan, J. Rapeseed protein-derived ACE inhibitory peptides LY, RALP and GHS show antioxidant and anti-inflammatory effects on spontaneously hypertensive rats. J. Funct. Foods 2019, 55, 211–219. [Google Scholar] [CrossRef]
- Martineau-Côté, D.; Achouri, A.; Karboune, S.; L’Hocine, L. Antioxidant and Angiotensin-Converting Enzyme Inhibitory Activity of Faba Bean-Derived Peptides after In Vitro Gastrointestinal Digestion: Insight into Their Mechanism of Action. J. Agric. Food Chem. 2024, 72, 6432–6443. [Google Scholar] [CrossRef]
- Chai, T.T.; Xiao, J.; Mohana Dass, S.; Teoh, J.Y.; Ee, K.Y.; Ng, W.J.; Wong, F.C. Identification of antioxidant peptides derived from tropical jackfruit seed and investigation of the stability profiles. Food Chem. 2021, 340, 127876. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Sun, Q.; Song, G.; Huang, J. Extraction, identification and structure-activity relationship of antioxidant peptides from sesame (Sesamum indicum L.) protein hydrolysate. Food Res. Int. 2019, 116, 707–716. [Google Scholar] [CrossRef]
- Tonolo, F.; Coletta, S.; Fiorese, F.; Grinzato, A.; Albanesi, M.; Folda, A.; Ferro, S.; De Mario, A.; Piazza, I.; Mammucari, C.; et al. Sunflower seed-derived bioactive peptides show antioxidant and anti-inflammatory activity: From in silico simulation to the animal model. Food Chem. 2024, 439, 138124. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; John, A.; Jiang, Y.; Zhu, H.; Yang, B.; Wen, L. Structure identification of walnut peptides and evaluation of cellular antioxidant activity. Food Chem. 2022, 388, 132943. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Zhuang, Y.; Li, Y.; Tian, H.; Shi, P.; Li, G. Isolation of Novel ACE-Inhibitory and Antioxidant Peptides from Quinoa Bran Albumin Assisted with an In Silico Approach: Characterization, In Vivo Antihypertension, and Molecular Docking. Molecules 2019, 24, 4562. [Google Scholar] [CrossRef]
- Wang, L.; Ma, M.; Yu, Z.; Du, S.K. Preparation and identification of antioxidant peptides from cottonseed proteins. Food Chem. 2021, 352, 129399. [Google Scholar] [CrossRef]
- Wang, M.; Li, C.; Li, H.; Wu, Z.; Chen, B.; Lei, Y.; Shen, Y. In Vitro and In Silico Antioxidant Activity of Novel Peptides Prepared from Paeonia Ostii ‘Feng Dan’ Hydrolysate. Antioxidants 2019, 8, 433. [Google Scholar] [CrossRef]
- Yu, X.; Chen, Y.; Qi, Z.; Chen, Q.; Cao, Y.; Kong, Q. Preparation and identification of a novel peptide with high antioxidant activity from corn gluten meal. Food Chem. 2023, 424, 136389. [Google Scholar] [CrossRef]
- Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Anwar, F.; Abu Bakar, F.; Philip, R.; Saari, N. Identification and characterization of papain-generated antioxidant peptides from palm kernel cake proteins. Food Res. Int. 2014, 62, 726–734. [Google Scholar] [CrossRef]
- Chen, M.L.; Ning, P.; Jiao, Y.; Xu, Z.; Cheng, Y.H. Extraction of antioxidant peptides from rice dreg protein hydrolysate via an angling method. Food Chem. 2021, 337, 128069. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Z.; Zhao, W. Identification and action mechanism of novel antioxidative peptides from copra meal protein. LWT-Food Sci. Technol. 2023, 188, 115425. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Jung, W.K.; Je, J.Y.; Kim, S.K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, M.; Wang, Z.; Aluko, R.E.; He, R. Antihypertensive and antioxidant activities of enzymatic wheat bran protein hydrolysates. J. Food Biochem. 2020, 44, e13090. [Google Scholar] [CrossRef]
- Wei, W.; Wu, Q.; Wang, S.; Dong, C.; Shao, S.; Zhang, Z.; Zhang, X.; Zhang, X.; Kan, J.; Liu, F. Treatment with walnut peptide ameliorates memory impairment in zebrafish and rats: Promoting the expression of neurotrophic factors and suppressing oxidative stress. Food Funct. 2024, 15, 8043–8052. [Google Scholar] [CrossRef]
- Li, P.; Chen, G.; Liang, R.; Cai, K.; Chen, Z.; Yang, N.; Huang, W.; Xie, Z.; Chen, Y.; Liao, Q. Identification and Function Analysis of Novel Hypoglycemic and Antioxidant Peptides from Chickpea. Plant Foods Hum. Nutr. 2024. [Google Scholar] [CrossRef]
- Sabbione, A.C.; Ibañez, S.M.; Martínez, E.N.; Añón, M.C.; Scilingo, A.A. Antithrombotic and Antioxidant Activity of Amaranth Hydrolysate Obtained by Activation of an Endogenous Protease. Plant Foods Hum. Nutr. 2016, 71, 174–182. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, N.; Kai, N.; Tanoue, Y. Functional properties of autolysate and enzymatic hydrolysates from yam tsukuneimo (Dioscorea opposita Thunb.) tuber mucilage tororo: Antioxidative activity and antihypertensive activity. J. Food Sci. Technol. 2014, 51, 3838–3845. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT-Food Sci. Technol. 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F.; Xu, Y.X.; Tian, T.G.; Xu, Y.P. Antihypertensive and antioxidant effects of food-derived bioactive peptides in spontaneously hypertensive rats. Food Sci. Nutr. 2024, 00, 1–11. [Google Scholar] [CrossRef]
- Pihlanto, A.; Akkanen, S.; Korhonen, H.J. ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum). Food Chem. 2008, 109, 104–112. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Oliveira, L.; Martinez-Villaluenga, C.; Frias, J.; Elena Cartea, M.; Francisco, M.; Cristianini, M.; Peñas, E. High pressure-assisted enzymatic hydrolysis potentiates the production of quinoa protein hydrolysates with antioxidant and ACE-inhibitory activities. Food Chem. 2024, 447, 138887. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Xiang, J.; Li, B.; Zhao, M.; Hou, T. Selenium-containing soybean antioxidant peptides: Preparation and comprehensive comparison of different selenium supplements. Food Chem. 2021, 358, 129888. [Google Scholar] [CrossRef]
- Ma, S.; Huang, D.; Zhai, M.; Yang, L.; Peng, S.; Chen, C.; Feng, X.; Weng, Q.; Zhang, B.; Xu, M. Isolation of a novel bio-peptide from walnut residual protein inducing apoptosis and autophagy on cancer cells. BMC Complement. Altern. Med. 2015, 15, 413. [Google Scholar] [CrossRef]
- Chen, S.; Huan, P.; Ma, T.; Zhong, Y.; Ning, D.; Zhuang, Y. Walnut peptide relieves hypertension and associated kidney and heart injury by regulating the renin-angiotensin-aldosterone system and intestinal microbiota. J. Sci. Food Agric. 2024. [Google Scholar] [CrossRef]
- Power, O.; Jakeman, P.; FitzGerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2013, 44, 797–820. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Zhang, T.; Zhang, Z. Research progress on the mechanism of antioxidant peptide. J. Food Saf. Qual. 2022, 13, 3981–3988. [Google Scholar] [CrossRef]
- Moayedi, A.; Mora, L.; Aristoy, M.C.; Hashemi, M.; Safari, M.; Toldrá, F. ACE-Inhibitory and Antioxidant Activities of Peptide Fragments Obtained from Tomato Processing By-Products Fermented Using Bacillus subtilis: Effect of Amino Acid Composition and Peptides Molecular Mass Distribution. Appl. Biochem. Biotechnol. 2017, 181, 48–64. [Google Scholar] [CrossRef]
- Bamdad, F.; Chen, L. Antioxidant capacities of fractionated barley hordein hydrolysates in relation to peptide structures. Mol. Nutr. Food Res. 2013, 57, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Bamdad, F.; Ahmed, S.; Chen, L. Specifically designed peptide structures effectively suppressed oxidative reactions in chemical and cellular systems. J. Funct. Foods 2015, 18, 35–46. [Google Scholar] [CrossRef]
- Huo, J.; Luo, X.; Huang, M.; Wu, J.; Zhang, J.; Liu, X.; Li, H.; Sun, X. Identification and Antioxidant Activity of a Novel Peptide from Baijiu. Int. J. Pept. Res. Ther. 2020, 26, 1199–1210. [Google Scholar] [CrossRef]
- Díaz, M.; Dunn, C.M.; McClements, D.J.; Decker, E.A. Use of caseinophosphopeptides as natural antioxidants in oil-in-water emulsions. J. Agric. Food Chem. 2003, 51, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Alaiz, M.; Vioque, J. Affinity purification and characterisation of chelating peptides from chickpea protein hydrolysates. Food Chem. 2011, 129, 485–490. [Google Scholar] [CrossRef]
- Qi, J.H.; Dong, F.X. The relevant targets of anti-oxidative stress: A review. J. Drug Target. 2021, 29, 677–686. [Google Scholar] [CrossRef]
- Puchalska, P.; Marina, M.L.; García, M.C. Isolation and identification of antioxidant peptides from commercial soybean-based infant formulas. Food Chem. 2014, 148, 147–154. [Google Scholar] [CrossRef]
- Chen, M.-F.; Zhang, Y.Y.; He, M.D.; Li, C.Y.; Zhou, C.X.; Hong, P.Z.; Qian, Z.-J. Antioxidant Peptide Purified from Enzymatic Hydrolysates of Isochrysis Zhanjiangensis and Its Protective Effect against Ethanol Induced Oxidative Stress of HepG2 Cells. Biotechnol. Bioprocess Eng. 2019, 24, 308–317. [Google Scholar] [CrossRef]
- Ren, L.K.; Yang, Y.; Ma, C.M.; Fan, J.; Bian, X.; Liu, B.X.; Wang, D.F.; Zhu, P.Y.; Fu, Y.; Zhang, N. Identification and in silico analysis of novel antioxidant peptides in broken rice protein hydrolysate and its cytoprotective effect against H2O2-induced 2BS cell model. Food Res. Int. 2022, 162, 112108. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.; Cadenas, S. The Antioxidant Transcription Factor Nrf2 in Cardiac Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2021, 22, 11939. [Google Scholar] [CrossRef] [PubMed]
- Adelusi, T.I.; Du, L.; Hao, M.; Zhou, X.; Xuan, Q.; Apu, C.; Sun, Y.; Lu, Q.; Yin, X. Keap1/Nrf2/ARE signaling unfolds therapeutic targets for redox imbalanced-mediated diseases and diabetic nephropathy. Biomed. Pharmacother. 2020, 123, 109732. [Google Scholar] [CrossRef]
- Zborowski, V.A.; Heck, S.O.; Vencato, M.; Pinton, S.; Marques, L.S.; Nogueira, C.W. Keap1/Nrf2/HO-1 signaling pathway contributes to p-chlorodiphenyl diselenide antidepressant-like action in diabetic mice. Psychopharmacology 2020, 237, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Dong, Q.; Yu, C.; Chen, H.; Zhao, Y.; Zhang, B.; Yu, P.; Chen, M. Advances in Research on the Activity Evaluation, Mechanism and Structure-Activity Relationships of Natural Antioxidant Peptides. Antioxidants 2024, 13, 479. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yi, Z.; Chen, X.; Pan, N.; Su, X.; Shi, H.; Liu, Z. Screening and mechanistic study of antioxidant peptides from Bangia fusco-purpurea targeting the Keap1–Nrf2 pathway. Food Biosci. 2024, 59, 104122. [Google Scholar] [CrossRef]
- Ren, L.K.; Fan, J.; Yang, Y.; Liu, X.F.; Wang, B.; Bian, X.; Wang, D.F.; Xu, Y.; Liu, B.X.; Zhu, P.Y.; et al. Identification, in silico selection, and mechanism study of novel antioxidant peptides derived from the rice bran protein hydrolysates. Food Chem. 2023, 408, 135230. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D.; Wolenski, F.S. NF-κB: Where did it come from and why? Immunol. Rev. 2012, 246, 14–35. [Google Scholar] [CrossRef]
- Wu, S.; Liao, X.; Zhu, Z.; Huang, R.; Chen, M.; Huang, A.; Zhang, J.; Wu, Q.; Wang, J.; Ding, Y. Antioxidant and anti-inflammation effects of dietary phytochemicals: The Nrf2/NF-κB signalling pathway and upstream factors of Nrf2. Phytochemistry 2022, 204, 113429. [Google Scholar] [CrossRef]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Quan, Z.; Xiao, P.; Duan, J.A. New Insights into Antioxidant Peptides: An Overview of Efficient Screening, Evaluation Models, Molecular Mechanisms, and Applications. Antioxidants 2024, 13, 203. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, Z.; Bu, L.; An, C.; Wang, D.; Liu, X.; Zhang, J.; Yang, W.; Deng, B.; Xie, J.; et al. Rapeseed protein-derived antioxidant peptide RAP alleviates renal fibrosis through MAPK/NF-κB signaling pathways in diabetic nephropathy. Drug Des. Dev. Ther. 2018, 12, 1255–1268. [Google Scholar] [CrossRef]
- Tong, L.T.; Ju, Z.; Liu, L.; Wang, L.; Zhou, X.; Xiao, T.; Zhou, S. Rice-derived peptide AAGALPS inhibits TNF-α-induced inflammation and oxidative stress in vascular endothelial cells. Food Sci. Nutr. 2020, 8, 659–667. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Durand, E.; Beaubier, S.; Ilic, I.; Fine, F.; Kapel, R.; Villeneuve, P. Production and antioxidant capacity of bioactive peptides from plant biomass to counteract lipid oxidation. Curr. Res. Food Sci. 2021, 4, 365–397. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M.; Badakné, K.; Farmani, J.; Aluko, R.E. Antioxidant peptides: Overview of production, properties, and applications in food systems. Compr. Rev. Food Sci. Food Saf. 2023, 22, 46–106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lao, F.; Pan, X.; Wu, J. Food Protein-Derived Antioxidant Peptides: Molecular Mechanism, Stability and Bioavailability. Biomolecules 2022, 12, 1622. [Google Scholar] [CrossRef]
- Hu, R.; Chen, G.; Li, Y. Production and Characterization of Antioxidative Hydrolysates and Peptides from Corn Gluten Meal Using Papain, Ficin, and Bromelain. Molecules 2020, 25, 4091. [Google Scholar] [CrossRef]
- Fan, H.; Liu, H.; Zhang, Y.; Zhang, S.; Liu, T.; Wang, D. Review on plant-derived bioactive peptides: Biological activities, mechanism of action and utilizations in food development. J. Future Foods 2022, 2, 143–159. [Google Scholar] [CrossRef]
- Chen, P.; Huang, P.; Liang, Y.; Wang, Q.; Miao, J. The antioxidant peptides from walnut protein hydrolysates and their protective activity against alcoholic injury. Food Funct. 2024, 15, 5315–5328. [Google Scholar] [CrossRef]
- Samaei, S.P.; Ghorbani, M.; Tagliazucchi, D.; Martini, S.; Gotti, R.; Themelis, T.; Tesini, F.; Gianotti, A.; Gallina Toschi, T.; Babini, E. Functional, nutritional, antioxidant, sensory properties and comparative peptidomic profile of faba bean (Vicia faba, L.) seed protein hydrolysates and fortified apple juice. Food Chem. 2020, 330, 127120. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, W.; Lin, Y.; Du, C.; Huang, D.; Chen, S.; Yu, T.; Cong, X. Antioxidant and anti-fatigue activities of selenium-enriched peptides isolated from Cardamine violifolia protein hydrolysate. J. Funct. Foods 2021, 79, 104412. [Google Scholar] [CrossRef]
- Gong, X.; An, Q.; Le, L.; Geng, F.; Jiang, L.; Yan, J.; Xiang, D.; Peng, L.; Zou, L.; Zhao, G.; et al. Prospects of cereal protein-derived bioactive peptides: Sources, bioactivities diversity, and production. Crit. Rev. Food Sci. Nutr. 2022, 62, 2855–2871. [Google Scholar] [CrossRef]
- Choudhury, D.; Tuncel, M.; Levi, M. Diabetic nephropathy—A multifaceted target of new therapies. Discov. Med. 2010, 10, 406–415. [Google Scholar] [PubMed]
- He, X.; Kuang, G.; Zuo, Y.; Li, S.; Zhou, S.; Ou, C. The Role of Non-coding RNAs in Diabetic Nephropathy-Related Oxidative Stress. Front. Med. 2021, 8, 626423. [Google Scholar] [CrossRef]
- Xu, F.; Wang, L.; Ju, X.; Zhang, J.; Yin, S.; Shi, J.; He, R.; Yuan, Q. Transepithelial Transport of YWDHNNPQIR and Its Metabolic Fate with Cytoprotection against Oxidative Stress in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2017, 65, 2056–2065. [Google Scholar] [CrossRef]
- Xue, Z.; Wen, H.; Zhai, L.; Yu, Y.; Li, Y.; Yu, W.; Cheng, A.; Wang, C.; Kou, X. Antioxidant activity and anti-proliferative effect of a bioactive peptide from chickpea (Cicer arietinum L.). Food Res. Int. 2015, 77, 75–81. [Google Scholar] [CrossRef]
- Marcela, G.M.; Eva, R.G.; Del Carmen, R.M.; Rosalva, M.E. Evaluation of the Antioxidant and Antiproliferative Effects of Three Peptide Fractions of Germinated Soybeans on Breast and Cervical Cancer Cell Lines. Plant Foods Hum. Nutr. 2016, 71, 368–374. [Google Scholar] [CrossRef]
- Ji, Z.; Mao, J.; Chen, S.; Mao, J. Antioxidant and anti-inflammatory activity of peptides from foxtail millet (Setaria italica) prolamins in HaCaT cells and RAW264.7 murine macrophages. Food Biosci. 2020, 36, 100636. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Q.; Lu, Q. Purification, structural analysis, and stability of antioxidant peptides from purple wheat bran. BMC Chem. 2020, 14, 58. [Google Scholar] [CrossRef]
- Intiquilla, A.; Jimenez-Aliaga, K.; Zavaleta, A.I.; Gamboa, A.; Caro, N.; Diaz, M.; Gotteland, M.; Abugoch, L.; Tapia, C. Nanoencapsulation of antioxidant peptides from Lupinus mutabilis in chitosan nanoparticles obtained by ionic gelling and spray freeze drying intended for colonic delivery. Food Biosci. 2022, 50 Pt A, 102055. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Wang, Z.; Ren, F.; Zhu, X.; Chen, B.; Liu, H.; Wuyun, T. Screening and preparation of highly active antioxidant peptides of apricot and their inhibitory effect on ultraviolet radiation. Food Chem. 2024, 463, 141336. [Google Scholar] [CrossRef]
- Mo, Q.; You, S.; Fu, H.; Wang, D.; Zhang, J.; Wang, C.; Li, M. Purification and Identification of Antioxidant Peptides from Rice Fermentation of Lactobacillus plantarum and Their Protective Effects on UVA-Induced Oxidative Stress in Skin. Antioxidants 2022, 11, 2333. [Google Scholar] [CrossRef] [PubMed]
- Liv, Y.Q.; Strappe, P.; Shang, W.T.; Zhou, Z.K. Functional peptides derived from rice bran proteins. Crit. Rev. Food Sci. Nutr. 2019, 59, 349–356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).