Antioxidant Function and Application of Plant-Derived Peptides
Abstract
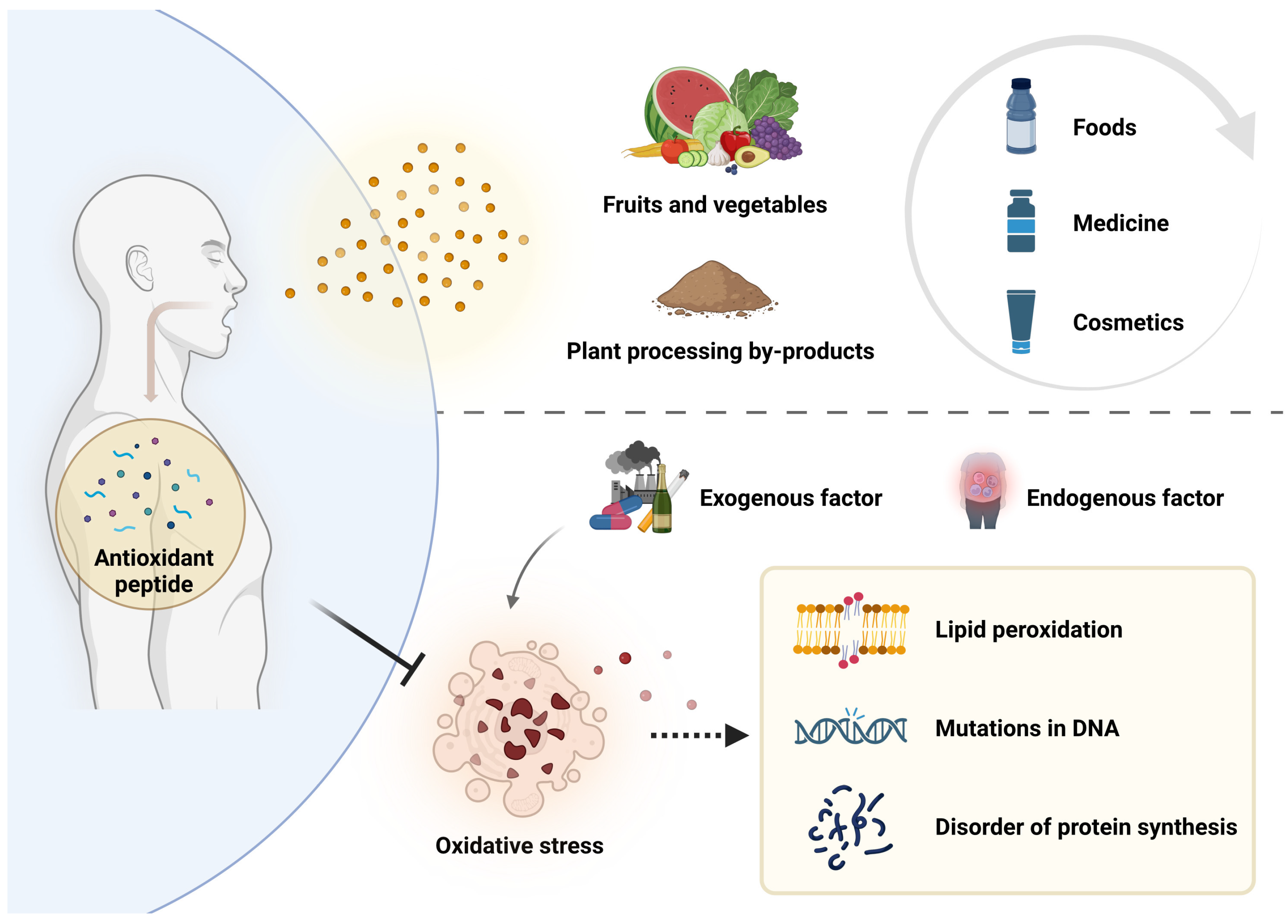
:1. Introduction
2. Sources of Plant Antioxidant Peptides
3. Structure and Activity of Plant Antioxidant Peptides
4. Other Biological Functions of Plant Antioxidant Peptides
| Amino Acid Sequence | Source | Other Biological Functions | Reference |
|---|---|---|---|
| —— | Cicer arietinum seed | Hypoglycemic | [45] |
| —— | Amaranthus hypochondriacus seed | Anti-thrombotic | [46] |
| —— | Dioscorea polystachya stem | Anti-hypertensive | [47] |
| —— | Oryza sativa by-product | Anti-hypertensive | [48,49] |
| —— | Triticum aestivum by-product | Anti-hypertensive | [50] |
| IF | Solanum tuberosum stem | Anti-hypertensive | [19,51] |
| PWLNFK FSIAWPR GSHWPFGGK | Chenopodium quinoa seed | Anti-hypertensive | [52] |
| PADVTPEEKPEV | Helianthus annuus seed | Anti-inflammatory | [32] |
| —— | Glycine max seed | Anti-aging Anti-hypertensive Anti-inflammatory | [50,53] |
| LY GHS RALP | Brassica rapa seed | Anti-hypertensive Anti-inflammatory | [28] |
| QGRPWG PSRADIY AYNIPVNIAR CTLEW VQTL LGYEN GGW VYY LLPF | Juglans regia by-product | Anticancer Anti-hypertensive Improvement in cognitive and memory disorders | [33,44,54,55] |
5. Mechanisms of Action of Plant Antioxidant Peptides
5.1. Direct Scavenging of Free Radicals
5.2. Chelation of Pro-Oxidant Metal Ions
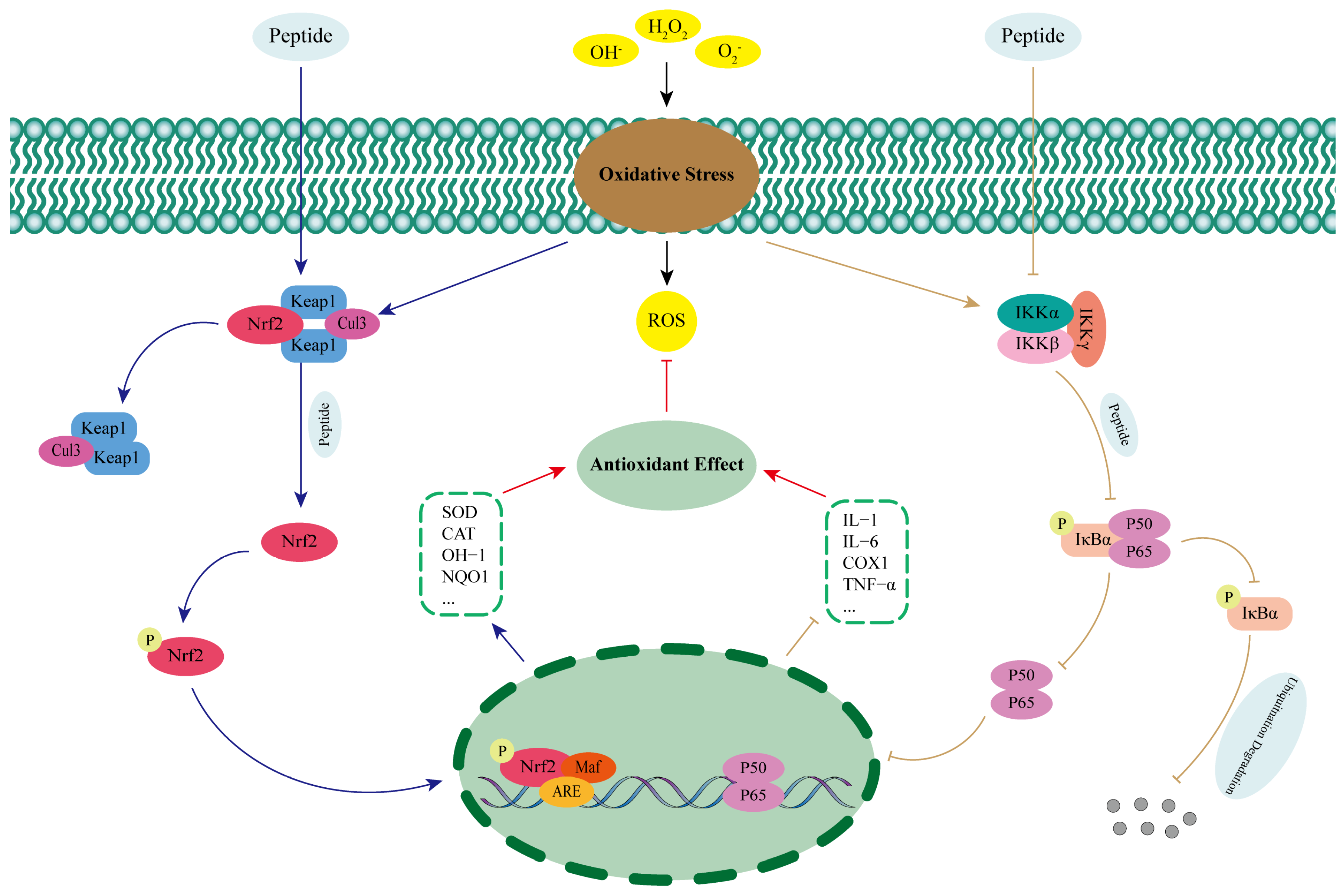
5.3. Enhancement of the Antioxidant Defence System
6. Integrated Application of Plant Antioxidant Peptides (Figure 3)
6.1. Food Field
6.1.1. Used as an Antioxidant
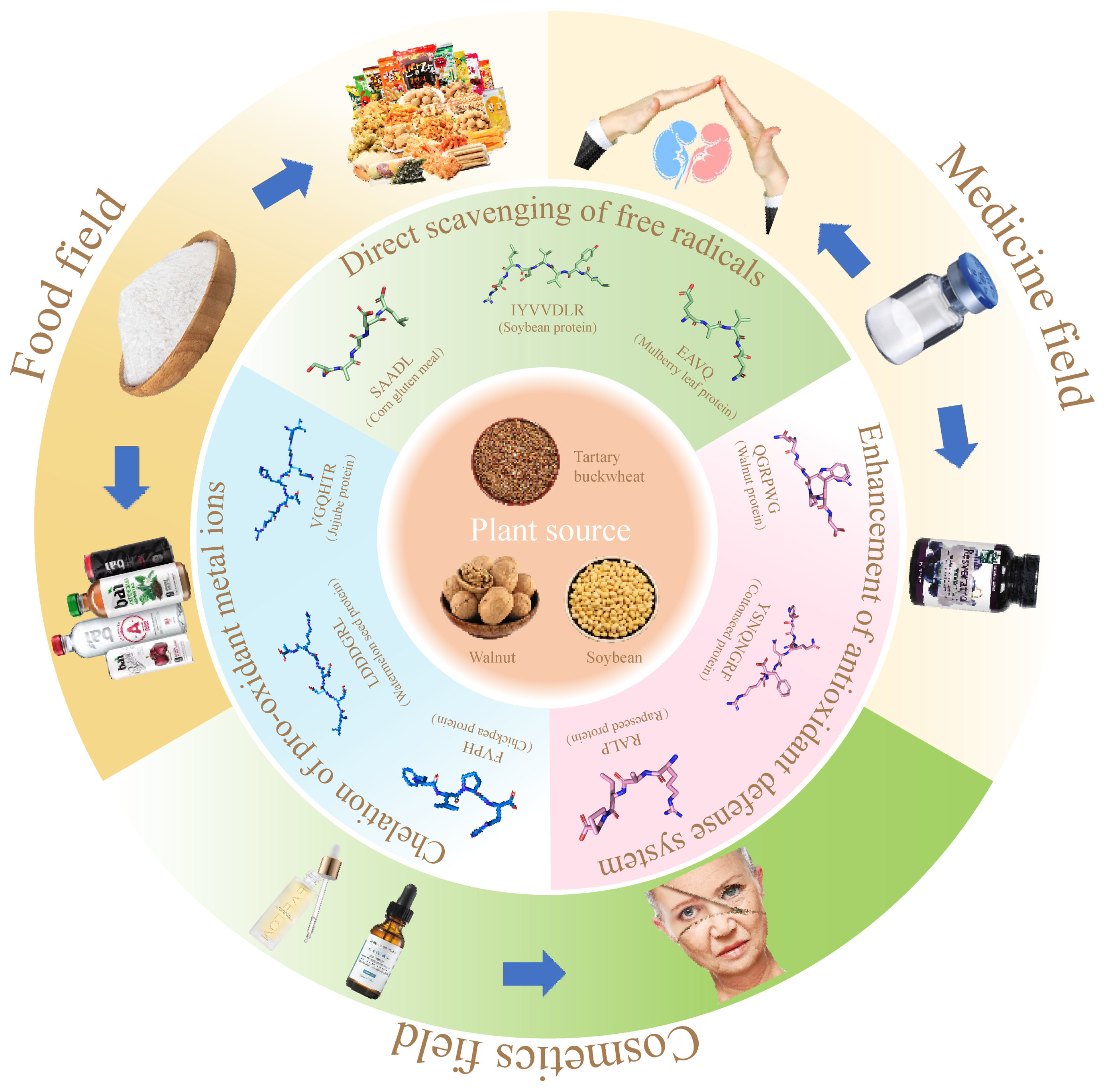
6.1.2. Development of Functional Products
6.2. Medicine Field
6.3. Cosmetics Field
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meghea, A. Nanoparticles Induce Oxidative and Endoplasmic Reticulum Stresses: Antioxidant Therapeutic Defenses. MRS Bull. 2020, 45, 868. [Google Scholar] [CrossRef]
- Rahman, M.S.; Choi, Y.H.; Choi, Y.S.; Alam, M.B.; Lee, S.H.; Yoo, J.C. A novel antioxidant peptide, purified from Bacillus amyloliquefaciens, showed strong antioxidant potential via Nrf-2 mediated heme oxygenase-1 expression. Food Chem. 2018, 239, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and anti-freezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Jiang, B.; Miao, M.; Mu, W. The effects of an antioxidative pentapeptide derived from chickpea protein hydrolysates on oxidative stress in Caco-2 and HT-29 cell lines. J. Funct. Foods 2014, 7, 719–726. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- García-Nebot, M.J.; Recio, I.; Hernández-Ledesma, B. Antioxidant activity and protective effects of peptide lunasin against oxidative stress in intestinal Caco-2 cells. Food Chem. Toxicol. 2014, 65, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Wang, W.; Kang, P.M. Oxidative Stress and Antioxidant Treatments in Cardiovascular Diseases. Antioxidants 2020, 9, 1292. [Google Scholar] [CrossRef]
- Gurer-Orhan, H.; Ince, E.; Konyar, D.; Saso, L.; Suzen, S. The Role of Oxidative Stress Modulators in Breast Cancer. Curr. Med. Chem. 2018, 25, 4084–4101. [Google Scholar] [CrossRef]
- Puspita, L.; Chung, S.Y.; Shim, J.W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain 2017, 10, 53. [Google Scholar] [CrossRef]
- Sun, Z.; Tang, Z.; Yang, X.; Liu, Q.S.; Zhang, J.; Zhou, Q.; Jiang, G. 3-tert-Butyl-4-hydroxyanisole Impairs Hepatic Lipid Metabolism in Male Mice Fed with a High-Fat Diet. Environ. Sci. Technol. 2022, 56, 3204–3213. [Google Scholar] [CrossRef]
- Gerhart, A.K.; Janz, D.M. Toxicity of Aqueous L-Selenomethionine and Tert-Butyl Hydroperoxide Exposure to Zebrafish (Danio rerio) Embryos Following Tert-Butyl Hydroquinone Treatment. Toxics 2019, 7, 44. [Google Scholar] [CrossRef]
- Apone, F.; Barbulova, A.; Colucci, M.G. Plant and Microalgae Derived Peptides Are Advantageously Employed as Bioactive Compounds in Cosmetics. Front. Plant Sci. 2019, 10, 756. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Jahandideh, F.; Wu, J. Food-derived bioactive peptides on inflammation and oxidative stress. BioMed Res. Int. 2014, 2014, 608979. [Google Scholar] [CrossRef]
- Fang, L.; Ren, D.; Cui, L.; Liu, C.; Wang, J.; Liu, W.; Min, W.; Liu, J. Antifatigue, Antioxidant and Immunoregulatory Effects of Peptides Hydrolyzed from Manchurian Walnut (Juglans mandshurica Maxim.) on Mice. Grain Oil Sci. Technol. 2019, 1, 44–52. [Google Scholar] [CrossRef]
- Long, Y.; Tao, H.; Wang, S.; Xing, B.; Wang, Z.; Liu, K.; Shao, Q.; Gao, F. Identification and Functional Validation of Two Novel Antioxidant Peptides in Saffron. Antioxidants 2024, 13, 378. [Google Scholar] [CrossRef]
- Memarpoor-Yazdi, M.; Mahaki, H.; Zare-Zardini, H. Antioxidant activity of protein hydrolysates and purified peptides from Zizyphus jujuba fruits. J. Funct. Foods 2013, 5, 62–70. [Google Scholar] [CrossRef]
- Sompinit, K.; Lersiripong, S.; Reamtong, O.; Pattarayingsakul, W.; Patikarnmonthon, N.; Panbangred, W. In vitro study on novel bioactive peptides with antioxidant and antihypertensive properties from edible rhizomes. LWT-Food Sci. Technol. 2020, 134, 110227. [Google Scholar] [CrossRef]
- Tsai, B.C.; Hsieh, D.J.; Lin, W.T.; Tamilselvi, S.; Day, C.H.; Ho, T.J.; Chang, R.L.; Viswanadha, V.P.; Kuo, C.H.; Huang, C.Y. Functional potato bioactive peptide intensifies Nrf2-dependent antioxidant defense against renal damage in hypertensive rats. Food Res. Int. 2020, 129, 108862. [Google Scholar] [CrossRef]
- Sun, C.; Tang, X.; Ren, Y.; Wang, E.; Shi, L.; Wu, X.; Wu, H. Novel Antioxidant Peptides Purified from Mulberry (Morus atropurpurea Roxb.) Leaf Protein Hydrolysates with Hemolysis Inhibition Ability and Cellular Antioxidant Activity. J. Agric. Food Chem. 2019, 67, 7650–7659. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Chen, Y.; Sun, H.; Liu, Y. Preparation and characterization of lipophilic antioxidative peptides derived from mung bean protein. Food Chem. 2022, 395, 133535. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Li, Y.; Wang, H.; Wang, Z.; Qi, B.; Sui, X.; Jiang, L. Purification and Characterization of Antioxidant Peptides from Alcalase-Hydrolyzed Soybean (Glycine max L.) Hydrolysate and Their Cytoprotective Effects in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2019, 67, 5772–5781. [Google Scholar] [CrossRef]
- Gao, J.; Li, T.; Chen, D.; Gu, H.; Mao, X. Identification and molecular docking of antioxidant peptides from hemp seed protein hydrolysates. LWT-Food Sci. Technol. 2021, 147, 111453. [Google Scholar] [CrossRef]
- Zhou, Y.; She, X.; Chen, Z.; Wei, Y.; Xiao, Y.; Zhou, X. Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn) protein-derived antioxidant peptides: Mechanisms of action and structure-activity relationship in Caco-2 cell models. Food Sci. Hum. Wellness 2022, 11, 1580–1590. [Google Scholar] [CrossRef]
- Liang, L.-L.; Cai, S.-Y.; Gao, M.; Chu, X.-M.; Pan, X.-Y.; Gong, K.-K.; Xiao, C.-W.; Chen, Y.; Zhao, Y.-Q.; Wang, B.; et al. Purification of antioxidant peptides of Moringa oleifera seeds and their protective effects on H2O2 oxidative damaged Chang liver cells. J. Funct. Foods 2020, 64, 103698. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Purification and identification of novel antioxidant peptides from watermelon seed protein hydrolysates and their cytoprotective effects on H2O2-induced oxidative stress. Food Chem. 2020, 327, 127059. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Contreras, M.D.M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef]
- He, R.; Wang, Y.; Yang, Y.; Wang, Z.; Ju, X.; Yuan, J. Rapeseed protein-derived ACE inhibitory peptides LY, RALP and GHS show antioxidant and anti-inflammatory effects on spontaneously hypertensive rats. J. Funct. Foods 2019, 55, 211–219. [Google Scholar] [CrossRef]
- Martineau-Côté, D.; Achouri, A.; Karboune, S.; L’Hocine, L. Antioxidant and Angiotensin-Converting Enzyme Inhibitory Activity of Faba Bean-Derived Peptides after In Vitro Gastrointestinal Digestion: Insight into Their Mechanism of Action. J. Agric. Food Chem. 2024, 72, 6432–6443. [Google Scholar] [CrossRef]
- Chai, T.T.; Xiao, J.; Mohana Dass, S.; Teoh, J.Y.; Ee, K.Y.; Ng, W.J.; Wong, F.C. Identification of antioxidant peptides derived from tropical jackfruit seed and investigation of the stability profiles. Food Chem. 2021, 340, 127876. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Sun, Q.; Song, G.; Huang, J. Extraction, identification and structure-activity relationship of antioxidant peptides from sesame (Sesamum indicum L.) protein hydrolysate. Food Res. Int. 2019, 116, 707–716. [Google Scholar] [CrossRef]
- Tonolo, F.; Coletta, S.; Fiorese, F.; Grinzato, A.; Albanesi, M.; Folda, A.; Ferro, S.; De Mario, A.; Piazza, I.; Mammucari, C.; et al. Sunflower seed-derived bioactive peptides show antioxidant and anti-inflammatory activity: From in silico simulation to the animal model. Food Chem. 2024, 439, 138124. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; John, A.; Jiang, Y.; Zhu, H.; Yang, B.; Wen, L. Structure identification of walnut peptides and evaluation of cellular antioxidant activity. Food Chem. 2022, 388, 132943. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Zhuang, Y.; Li, Y.; Tian, H.; Shi, P.; Li, G. Isolation of Novel ACE-Inhibitory and Antioxidant Peptides from Quinoa Bran Albumin Assisted with an In Silico Approach: Characterization, In Vivo Antihypertension, and Molecular Docking. Molecules 2019, 24, 4562. [Google Scholar] [CrossRef]
- Wang, L.; Ma, M.; Yu, Z.; Du, S.K. Preparation and identification of antioxidant peptides from cottonseed proteins. Food Chem. 2021, 352, 129399. [Google Scholar] [CrossRef]
- Wang, M.; Li, C.; Li, H.; Wu, Z.; Chen, B.; Lei, Y.; Shen, Y. In Vitro and In Silico Antioxidant Activity of Novel Peptides Prepared from Paeonia Ostii ‘Feng Dan’ Hydrolysate. Antioxidants 2019, 8, 433. [Google Scholar] [CrossRef]
- Yu, X.; Chen, Y.; Qi, Z.; Chen, Q.; Cao, Y.; Kong, Q. Preparation and identification of a novel peptide with high antioxidant activity from corn gluten meal. Food Chem. 2023, 424, 136389. [Google Scholar] [CrossRef]
- Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Anwar, F.; Abu Bakar, F.; Philip, R.; Saari, N. Identification and characterization of papain-generated antioxidant peptides from palm kernel cake proteins. Food Res. Int. 2014, 62, 726–734. [Google Scholar] [CrossRef]
- Chen, M.L.; Ning, P.; Jiao, Y.; Xu, Z.; Cheng, Y.H. Extraction of antioxidant peptides from rice dreg protein hydrolysate via an angling method. Food Chem. 2021, 337, 128069. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Z.; Zhao, W. Identification and action mechanism of novel antioxidative peptides from copra meal protein. LWT-Food Sci. Technol. 2023, 188, 115425. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Jung, W.K.; Je, J.Y.; Kim, S.K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, M.; Wang, Z.; Aluko, R.E.; He, R. Antihypertensive and antioxidant activities of enzymatic wheat bran protein hydrolysates. J. Food Biochem. 2020, 44, e13090. [Google Scholar] [CrossRef]
- Wei, W.; Wu, Q.; Wang, S.; Dong, C.; Shao, S.; Zhang, Z.; Zhang, X.; Zhang, X.; Kan, J.; Liu, F. Treatment with walnut peptide ameliorates memory impairment in zebrafish and rats: Promoting the expression of neurotrophic factors and suppressing oxidative stress. Food Funct. 2024, 15, 8043–8052. [Google Scholar] [CrossRef]
- Li, P.; Chen, G.; Liang, R.; Cai, K.; Chen, Z.; Yang, N.; Huang, W.; Xie, Z.; Chen, Y.; Liao, Q. Identification and Function Analysis of Novel Hypoglycemic and Antioxidant Peptides from Chickpea. Plant Foods Hum. Nutr. 2024. [Google Scholar] [CrossRef]
- Sabbione, A.C.; Ibañez, S.M.; Martínez, E.N.; Añón, M.C.; Scilingo, A.A. Antithrombotic and Antioxidant Activity of Amaranth Hydrolysate Obtained by Activation of an Endogenous Protease. Plant Foods Hum. Nutr. 2016, 71, 174–182. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, N.; Kai, N.; Tanoue, Y. Functional properties of autolysate and enzymatic hydrolysates from yam tsukuneimo (Dioscorea opposita Thunb.) tuber mucilage tororo: Antioxidative activity and antihypertensive activity. J. Food Sci. Technol. 2014, 51, 3838–3845. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT-Food Sci. Technol. 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F.; Xu, Y.X.; Tian, T.G.; Xu, Y.P. Antihypertensive and antioxidant effects of food-derived bioactive peptides in spontaneously hypertensive rats. Food Sci. Nutr. 2024, 00, 1–11. [Google Scholar] [CrossRef]
- Pihlanto, A.; Akkanen, S.; Korhonen, H.J. ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum). Food Chem. 2008, 109, 104–112. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Oliveira, L.; Martinez-Villaluenga, C.; Frias, J.; Elena Cartea, M.; Francisco, M.; Cristianini, M.; Peñas, E. High pressure-assisted enzymatic hydrolysis potentiates the production of quinoa protein hydrolysates with antioxidant and ACE-inhibitory activities. Food Chem. 2024, 447, 138887. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Xiang, J.; Li, B.; Zhao, M.; Hou, T. Selenium-containing soybean antioxidant peptides: Preparation and comprehensive comparison of different selenium supplements. Food Chem. 2021, 358, 129888. [Google Scholar] [CrossRef]
- Ma, S.; Huang, D.; Zhai, M.; Yang, L.; Peng, S.; Chen, C.; Feng, X.; Weng, Q.; Zhang, B.; Xu, M. Isolation of a novel bio-peptide from walnut residual protein inducing apoptosis and autophagy on cancer cells. BMC Complement. Altern. Med. 2015, 15, 413. [Google Scholar] [CrossRef]
- Chen, S.; Huan, P.; Ma, T.; Zhong, Y.; Ning, D.; Zhuang, Y. Walnut peptide relieves hypertension and associated kidney and heart injury by regulating the renin-angiotensin-aldosterone system and intestinal microbiota. J. Sci. Food Agric. 2024. [Google Scholar] [CrossRef]
- Power, O.; Jakeman, P.; FitzGerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2013, 44, 797–820. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Zhang, T.; Zhang, Z. Research progress on the mechanism of antioxidant peptide. J. Food Saf. Qual. 2022, 13, 3981–3988. [Google Scholar] [CrossRef]
- Moayedi, A.; Mora, L.; Aristoy, M.C.; Hashemi, M.; Safari, M.; Toldrá, F. ACE-Inhibitory and Antioxidant Activities of Peptide Fragments Obtained from Tomato Processing By-Products Fermented Using Bacillus subtilis: Effect of Amino Acid Composition and Peptides Molecular Mass Distribution. Appl. Biochem. Biotechnol. 2017, 181, 48–64. [Google Scholar] [CrossRef]
- Bamdad, F.; Chen, L. Antioxidant capacities of fractionated barley hordein hydrolysates in relation to peptide structures. Mol. Nutr. Food Res. 2013, 57, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Bamdad, F.; Ahmed, S.; Chen, L. Specifically designed peptide structures effectively suppressed oxidative reactions in chemical and cellular systems. J. Funct. Foods 2015, 18, 35–46. [Google Scholar] [CrossRef]
- Huo, J.; Luo, X.; Huang, M.; Wu, J.; Zhang, J.; Liu, X.; Li, H.; Sun, X. Identification and Antioxidant Activity of a Novel Peptide from Baijiu. Int. J. Pept. Res. Ther. 2020, 26, 1199–1210. [Google Scholar] [CrossRef]
- Díaz, M.; Dunn, C.M.; McClements, D.J.; Decker, E.A. Use of caseinophosphopeptides as natural antioxidants in oil-in-water emulsions. J. Agric. Food Chem. 2003, 51, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Alaiz, M.; Vioque, J. Affinity purification and characterisation of chelating peptides from chickpea protein hydrolysates. Food Chem. 2011, 129, 485–490. [Google Scholar] [CrossRef]
- Qi, J.H.; Dong, F.X. The relevant targets of anti-oxidative stress: A review. J. Drug Target. 2021, 29, 677–686. [Google Scholar] [CrossRef]
- Puchalska, P.; Marina, M.L.; García, M.C. Isolation and identification of antioxidant peptides from commercial soybean-based infant formulas. Food Chem. 2014, 148, 147–154. [Google Scholar] [CrossRef]
- Chen, M.-F.; Zhang, Y.Y.; He, M.D.; Li, C.Y.; Zhou, C.X.; Hong, P.Z.; Qian, Z.-J. Antioxidant Peptide Purified from Enzymatic Hydrolysates of Isochrysis Zhanjiangensis and Its Protective Effect against Ethanol Induced Oxidative Stress of HepG2 Cells. Biotechnol. Bioprocess Eng. 2019, 24, 308–317. [Google Scholar] [CrossRef]
- Ren, L.K.; Yang, Y.; Ma, C.M.; Fan, J.; Bian, X.; Liu, B.X.; Wang, D.F.; Zhu, P.Y.; Fu, Y.; Zhang, N. Identification and in silico analysis of novel antioxidant peptides in broken rice protein hydrolysate and its cytoprotective effect against H2O2-induced 2BS cell model. Food Res. Int. 2022, 162, 112108. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.; Cadenas, S. The Antioxidant Transcription Factor Nrf2 in Cardiac Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2021, 22, 11939. [Google Scholar] [CrossRef] [PubMed]
- Adelusi, T.I.; Du, L.; Hao, M.; Zhou, X.; Xuan, Q.; Apu, C.; Sun, Y.; Lu, Q.; Yin, X. Keap1/Nrf2/ARE signaling unfolds therapeutic targets for redox imbalanced-mediated diseases and diabetic nephropathy. Biomed. Pharmacother. 2020, 123, 109732. [Google Scholar] [CrossRef]
- Zborowski, V.A.; Heck, S.O.; Vencato, M.; Pinton, S.; Marques, L.S.; Nogueira, C.W. Keap1/Nrf2/HO-1 signaling pathway contributes to p-chlorodiphenyl diselenide antidepressant-like action in diabetic mice. Psychopharmacology 2020, 237, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Dong, Q.; Yu, C.; Chen, H.; Zhao, Y.; Zhang, B.; Yu, P.; Chen, M. Advances in Research on the Activity Evaluation, Mechanism and Structure-Activity Relationships of Natural Antioxidant Peptides. Antioxidants 2024, 13, 479. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yi, Z.; Chen, X.; Pan, N.; Su, X.; Shi, H.; Liu, Z. Screening and mechanistic study of antioxidant peptides from Bangia fusco-purpurea targeting the Keap1–Nrf2 pathway. Food Biosci. 2024, 59, 104122. [Google Scholar] [CrossRef]
- Ren, L.K.; Fan, J.; Yang, Y.; Liu, X.F.; Wang, B.; Bian, X.; Wang, D.F.; Xu, Y.; Liu, B.X.; Zhu, P.Y.; et al. Identification, in silico selection, and mechanism study of novel antioxidant peptides derived from the rice bran protein hydrolysates. Food Chem. 2023, 408, 135230. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D.; Wolenski, F.S. NF-κB: Where did it come from and why? Immunol. Rev. 2012, 246, 14–35. [Google Scholar] [CrossRef]
- Wu, S.; Liao, X.; Zhu, Z.; Huang, R.; Chen, M.; Huang, A.; Zhang, J.; Wu, Q.; Wang, J.; Ding, Y. Antioxidant and anti-inflammation effects of dietary phytochemicals: The Nrf2/NF-κB signalling pathway and upstream factors of Nrf2. Phytochemistry 2022, 204, 113429. [Google Scholar] [CrossRef]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Quan, Z.; Xiao, P.; Duan, J.A. New Insights into Antioxidant Peptides: An Overview of Efficient Screening, Evaluation Models, Molecular Mechanisms, and Applications. Antioxidants 2024, 13, 203. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, Z.; Bu, L.; An, C.; Wang, D.; Liu, X.; Zhang, J.; Yang, W.; Deng, B.; Xie, J.; et al. Rapeseed protein-derived antioxidant peptide RAP alleviates renal fibrosis through MAPK/NF-κB signaling pathways in diabetic nephropathy. Drug Des. Dev. Ther. 2018, 12, 1255–1268. [Google Scholar] [CrossRef]
- Tong, L.T.; Ju, Z.; Liu, L.; Wang, L.; Zhou, X.; Xiao, T.; Zhou, S. Rice-derived peptide AAGALPS inhibits TNF-α-induced inflammation and oxidative stress in vascular endothelial cells. Food Sci. Nutr. 2020, 8, 659–667. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Durand, E.; Beaubier, S.; Ilic, I.; Fine, F.; Kapel, R.; Villeneuve, P. Production and antioxidant capacity of bioactive peptides from plant biomass to counteract lipid oxidation. Curr. Res. Food Sci. 2021, 4, 365–397. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M.; Badakné, K.; Farmani, J.; Aluko, R.E. Antioxidant peptides: Overview of production, properties, and applications in food systems. Compr. Rev. Food Sci. Food Saf. 2023, 22, 46–106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lao, F.; Pan, X.; Wu, J. Food Protein-Derived Antioxidant Peptides: Molecular Mechanism, Stability and Bioavailability. Biomolecules 2022, 12, 1622. [Google Scholar] [CrossRef]
- Hu, R.; Chen, G.; Li, Y. Production and Characterization of Antioxidative Hydrolysates and Peptides from Corn Gluten Meal Using Papain, Ficin, and Bromelain. Molecules 2020, 25, 4091. [Google Scholar] [CrossRef]
- Fan, H.; Liu, H.; Zhang, Y.; Zhang, S.; Liu, T.; Wang, D. Review on plant-derived bioactive peptides: Biological activities, mechanism of action and utilizations in food development. J. Future Foods 2022, 2, 143–159. [Google Scholar] [CrossRef]
- Chen, P.; Huang, P.; Liang, Y.; Wang, Q.; Miao, J. The antioxidant peptides from walnut protein hydrolysates and their protective activity against alcoholic injury. Food Funct. 2024, 15, 5315–5328. [Google Scholar] [CrossRef]
- Samaei, S.P.; Ghorbani, M.; Tagliazucchi, D.; Martini, S.; Gotti, R.; Themelis, T.; Tesini, F.; Gianotti, A.; Gallina Toschi, T.; Babini, E. Functional, nutritional, antioxidant, sensory properties and comparative peptidomic profile of faba bean (Vicia faba, L.) seed protein hydrolysates and fortified apple juice. Food Chem. 2020, 330, 127120. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, W.; Lin, Y.; Du, C.; Huang, D.; Chen, S.; Yu, T.; Cong, X. Antioxidant and anti-fatigue activities of selenium-enriched peptides isolated from Cardamine violifolia protein hydrolysate. J. Funct. Foods 2021, 79, 104412. [Google Scholar] [CrossRef]
- Gong, X.; An, Q.; Le, L.; Geng, F.; Jiang, L.; Yan, J.; Xiang, D.; Peng, L.; Zou, L.; Zhao, G.; et al. Prospects of cereal protein-derived bioactive peptides: Sources, bioactivities diversity, and production. Crit. Rev. Food Sci. Nutr. 2022, 62, 2855–2871. [Google Scholar] [CrossRef]
- Choudhury, D.; Tuncel, M.; Levi, M. Diabetic nephropathy—A multifaceted target of new therapies. Discov. Med. 2010, 10, 406–415. [Google Scholar] [PubMed]
- He, X.; Kuang, G.; Zuo, Y.; Li, S.; Zhou, S.; Ou, C. The Role of Non-coding RNAs in Diabetic Nephropathy-Related Oxidative Stress. Front. Med. 2021, 8, 626423. [Google Scholar] [CrossRef]
- Xu, F.; Wang, L.; Ju, X.; Zhang, J.; Yin, S.; Shi, J.; He, R.; Yuan, Q. Transepithelial Transport of YWDHNNPQIR and Its Metabolic Fate with Cytoprotection against Oxidative Stress in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2017, 65, 2056–2065. [Google Scholar] [CrossRef]
- Xue, Z.; Wen, H.; Zhai, L.; Yu, Y.; Li, Y.; Yu, W.; Cheng, A.; Wang, C.; Kou, X. Antioxidant activity and anti-proliferative effect of a bioactive peptide from chickpea (Cicer arietinum L.). Food Res. Int. 2015, 77, 75–81. [Google Scholar] [CrossRef]
- Marcela, G.M.; Eva, R.G.; Del Carmen, R.M.; Rosalva, M.E. Evaluation of the Antioxidant and Antiproliferative Effects of Three Peptide Fractions of Germinated Soybeans on Breast and Cervical Cancer Cell Lines. Plant Foods Hum. Nutr. 2016, 71, 368–374. [Google Scholar] [CrossRef]
- Ji, Z.; Mao, J.; Chen, S.; Mao, J. Antioxidant and anti-inflammatory activity of peptides from foxtail millet (Setaria italica) prolamins in HaCaT cells and RAW264.7 murine macrophages. Food Biosci. 2020, 36, 100636. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Q.; Lu, Q. Purification, structural analysis, and stability of antioxidant peptides from purple wheat bran. BMC Chem. 2020, 14, 58. [Google Scholar] [CrossRef]
- Intiquilla, A.; Jimenez-Aliaga, K.; Zavaleta, A.I.; Gamboa, A.; Caro, N.; Diaz, M.; Gotteland, M.; Abugoch, L.; Tapia, C. Nanoencapsulation of antioxidant peptides from Lupinus mutabilis in chitosan nanoparticles obtained by ionic gelling and spray freeze drying intended for colonic delivery. Food Biosci. 2022, 50 Pt A, 102055. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Wang, Z.; Ren, F.; Zhu, X.; Chen, B.; Liu, H.; Wuyun, T. Screening and preparation of highly active antioxidant peptides of apricot and their inhibitory effect on ultraviolet radiation. Food Chem. 2024, 463, 141336. [Google Scholar] [CrossRef]
- Mo, Q.; You, S.; Fu, H.; Wang, D.; Zhang, J.; Wang, C.; Li, M. Purification and Identification of Antioxidant Peptides from Rice Fermentation of Lactobacillus plantarum and Their Protective Effects on UVA-Induced Oxidative Stress in Skin. Antioxidants 2022, 11, 2333. [Google Scholar] [CrossRef] [PubMed]
- Liv, Y.Q.; Strappe, P.; Shang, W.T.; Zhou, Z.K. Functional peptides derived from rice bran proteins. Crit. Rev. Food Sci. Nutr. 2019, 59, 349–356. [Google Scholar] [CrossRef]
| Amino Acid Sequence | Source | Genus and Species | Reference |
|---|---|---|---|
| VDPYFNK DGGSDYLGK | Flower | Crocus sativus | [16] |
| GWLK VGQHTR | Fruit | Ziziphus jujuba | [17] |
| VTYM | Root and stem | Zingiber officinale | [18] |
| IF | Stem | Solanum tuberosum | [19] |
| SVL RDY EAVQ | Leaf | Morus alba | [20] |
| AH LY TW LW ELT LHK LHV LAH LLPH ALLPH | Seed | Vigna radiata | [21] |
| IYVFVR IYVVDLR VVFVDRL VIYVVDLR | Seed | Glycine max | [22] |
| LDLVKPQ YGRDEISV | Seed | Cannabis sativa | [23] |
| CTGFVAVR LRGENDQR GFIVQAQDLK SFFLAGQSQQGR | Seed | Fagopyrum tataricum | [24] |
| SF QY | Seed | Moringa oleifera | [25] |
| RDPEER KELEEK LDDDGRL DAAGRLQE GFAGDDAPRA | Seed | Citrullus lanatus | [26] |
| FVPH ALEPDHR SAEHGSLH TETWNPNHPEL | Seed | Cicer arietinum | [27] |
| LY GHS RALP | Seed | Brassica rapa | [28] |
| TETWNPNHPEL | Seed | Vicia faba | [29] |
| VGPWQK | Seed | Artocarpus heterophyllus | [30] |
| RDRHQKIG TDRHQKLR SYPTECRMR RENIDKPSRA MNDRVNQGE | Seed | Sesamum indicum | [31] |
| AGEQGFEYVTFR GGVPRSGEQEQQ | Seed | Sesamum indicum | [31] |
| DVAMPVPK VETGVIKPG TTHTNPPPEAE PADVTPEEKPEV LTHPQHQQQGPSTG | Seed | Helianthus annuus | [32] |
| QGRPWG PSRADIY AYNIPVNIAR | By-product | Juglans regia | [33] |
| QFLLAGR ASPKPSSA RGQVIYVL | By-product | Chenopodium quinoa | [34] |
| QWDRQ WDTRGQ YSNQNGRF | By-product | Gossypium hirsutum | [35] |
| FSAP EAAY QEPLLR PVETVR VLRPPLS | By-product | Paeonia ostii | [36] |
| IEFL RYLL SAADL | By-product | Zea mays | [37] |
| WAF GGIF AWFS YLLLK YGIKVGYAIP LPWRPATNVF | By-product | Elaeis guineensis | [38] |
| LLLRW GDMNP | By-product | Oryza sativa | [39] |
| EEGER GMEEER | By-product | Cocos nucifera | [40] |
| Amino Acid Sequence | Source | Experimental Result | Structural Feature | Reference |
|---|---|---|---|---|
| VDPYFNK DGGSDYLGK | Crocus sativus flower | Scavenging capacity for ABTS free radicals (IC50): 0.1657 mg/mL VDPYFNK; 0.2930 mg/mL DGGSDYLGK. Scavenging capacity for DPPH free radicals (IC50): 0.6411 mg/mL VDPYFNK; 0.3901 mg/mL DGGSDYLGK. Reducing capacity for Fe3+: 0.167 µmol/mL~0.063 µmol/mL VDPYFNK; 0.144 µmol/mL~0.0549 µmol/mL DGGSDYLGK. Effects on HepG2 oxidative stress cell model: enhancement of SOD, CAT, and other antioxidant enzyme activities and reduction in intracellular MDA content. | Contains hydrophobic and aromatic amino acids. | [16] |
| GWLK VGQHTR | Zizyphus jujuba fruit | Scavenging capacity for DPPH free radicals (EC50): 0.5 ± 0.051 mg/mL GWLK; 0.6 ± 0.065 mg/mL VGQHTR. Chelating capacity with Fe2+ (EC50): 0.86 mg/mL GWLK; 1.18 mg/mL VGQHTR. | Rich in hydrophobic, aromatic, and basic amino acids. | [17] |
| VTYM | Zingiber officinale root and stem | Scavenging capacity for ABTS free radicals (EC50): 19.9 ± 2.1 μmol/L. Scavenging capacity for DPPH free radicals (EC50): 24.0 ± 3.7 μmol/L. | Contains hydrophobic and aromatic amino acids. | [18] |
| SVL RDY EAVQ | Morus alba leaf | Effect on cellular antioxidant activity (CAA): 1706 μM QE/100 g SVL; 2204 μM QE/100 g RDY; 1501 μM QE/100 g EAVQ. | Contains hydrophobic amino acids and the presence of Tyr at the C-terminus of the peptide chain. | [20] |
| AH LY TW LW ELT LHK LHV LAH LLPH ALLPH | Vigna radiata seed | Scavenging capacity of mung bean hydrolyzed protein for ABTS free radicals (IC50): 18.06 ± 0.18 μg/mL. Scavenging capacity of mung bean hydrolyzed protein for DPPH free radicals (IC50): 11.10 ± 0.02 μg/mL. Chelating ability of mung bean hydrolyzed protein with Fe2+ (IC50): 3.78 ± 0.59 μg/mL. | Rich in hydrophobic amino acids and His. | [21] |
| IYVFVR IYVVDLR VVFVDRL VIYVVDLR | Glycine max seed | Scavenging capacity for ABTS free radicals: 3.22 ± 0.1 mM TE/mg IYVFVR; 3.72 ± 0.3 mM TE/mg IYVVDLR; 3.14 ± 0.1 mM TE/mg VVFVDRL; 3.34 ± 0.2 mM TE/mg VIYVVDLR. Scavenging capacity for DPPH free radicals: 14.4 ± 0.6 μM TE/mg IYVFVR; 17.8 ± 0.9 μM TE/mg IYVVDLR; 14.9 ± 0.7 μM TE/mg VVFVDRL; 16.1 ± 0.5 μM TE/mg VIYVVDLR. Reducing capacity for Fe3+: 62.9 ± 0.5 mM Fe2+/mg IYVFVR; 68.9 ± 1.4 mM Fe2+/mg IYVVDLR; 63.1 ± 1.2 mM Fe2+/mg VVFVDRL; 53.4 ± 1.2 mM Fe2+/mg VIYVVDLR. Absorption capacity for oxygen free radicals: 136 ± 3.8 μM TE/mg IYVFVR; 139 ± 1.0 μM TE/mg IYVVDLR; 136 ± 1.9 μM TE/mg VVFVDRL; 140 ± 2.0 μM TE/mg VIYVVDLR. | Rich in hydrophobic and antioxidant amino acids; the presence of repeated amino acids in the antioxidant peptide sequence. | [22] |
| LDLVKPQ YGRDEISV | Cannabis sativa seed | Clearance rate of hemp seed hydrolyzed protein for ABTS free radicals: 52.3 ± 0.1%. Chelation rate of hemp seed hydrolyzed protein for Fe2+: 52.9 ± 0.9%. Clearance rate of hemp seed hydrolyzed protein for hydroxyl radical: 50.9 ± 1.3%. Effects of hemp seed hydrolyzed protein on HepG2 oxidative stress cell model: enhancement of SOD, CAT, and GSH-Px and other antioxidant enzyme activities. | Lower molecular weight; contains hydrophobic and aromatic amino acids; Val is present at the C-terminus of the peptide chain; Tyr is present at the N- or C-terminus of the peptide chain. | [23] |
| CTGFVAVR LRGENDQR GFIVQAQDLK SFFLAGQSQQGR | Fagopyrum tataricum seed | Effects of tartary buckwheat hydrolyzed protein on Caco-2 oxidative stress cell model: enhancement of SOD, CAT, and other antioxidant enzyme activities and reduction in intracellular MDA content. | Val is present at the C-terminus of the peptide chain; higher hydrophilic amino acid content; Cys is present at the N-terminus of the peptide chain. | [24] |
| SF QY | Moringa oleifera seed | Scavenging capacity for ABTS free radicals (EC50): 0.32 ± 0.022 mg/mL SF; 0.33 ± 0.041 mg/mL QY. Scavenging capacity for DPPH free radicals (EC50): 1.37 ± 0.086 mg/mL SF; 0.75 ± 0.065 mg/mL QY. Effects on Chang liver oxidative stress cell model: enhancement of SOD, CAT, and other antioxidant enzyme activity and reduction in intracellular ALT, AST, and MDA content. | Shorter peptide chains; contains hydrophobic and aromatic amino acids. | [25] |
| RDPEER KELEEK LDDDGRL DAAGRLQE GFAGDDAPRA | Citrullus lanatus seed | Scavenging capacity for ABTS free radicals (IC50): 0.54 ± 0.02~1.23 ± 0.03 mg/mL. Scavenging capacity for DPPH free radicals (IC50): 0.216 ± 0.01~0.435 ± 0.03 mg/mL. Absorption capacity for oxidative free radicals: 82.36 ± 1.2~130.67 ± 2.2 μM TE/mg. Effects on HepG2 oxidative stress cell model: enhancement of SOD, CAT, and GSH-Px and other antioxidant enzyme activities and reduction in intracellular MDA content. | Lower molecular weight; contains acidic, hydrophobic, and antioxidant amino acids; the presence of repeated amino acids in the antioxidant peptide sequence; Leu is present at the N-terminus of the peptide chain. | [26] |
| FVPH ALEPDHR SAEHGSLH TETWNPNHPEL | Cicer arietinum seed | —— | Rich in acidic, basic, and hydrophobic amino acids; hydrophobic amino acids are present at the N- or C-terminus of the peptide chain; His and Arg are present at the C-terminus of the peptide chain. | [27] |
| VGPWQK | Artocarpus heterophyllus seed | Scavenging capacity for ABTS free radicals (EC50): 1.00 ± 0.00 mg/mL. | Contains hydrophobic amino acids and is present at the N-terminal and C-terminal ends of the peptide chain. | [30] |
| RDRHQKIG TDRHQKLR SYPTECRMR RENIDKPSRA MNDRVNQGE AGEQGFEYVTFR GGVPRSGEQEQQ | Sesamum indicum seed | Scavenging capacity for ABTS free radicals (IC50): 6.414 ± 0.292 mg/mL RDRHQKIG; 1.145 ± 0.042 mg/mL TDRHQKLR;10.004 ± 0.000 mg/mL SYPTECRMR; 2.842 ± 0.073 mg/mL RENIDKPSRA; 7.390 ± 0.387 mg/mL MNDRVNQGE; 3.958 ± 0.036 mg/mL AGEQGFEYVTFR;10.720 ± 0.047 mg/mL GGVPRSGEQEQQ. Scavenging capacity for DPPH free radicals (IC50): 4.648 ± 0.021 mg/mL RDRHQKIG; 6.353 ± 0.035 mg/mL TDRHQKLR; 10.105 ± 0.018 mg/mL SYPTECRMR; 23.650 ± 0.117 mg/mL RENIDKPSRA; 6.763 ± 0.084 mg/mL MNDRVNQGE; 1.141 ± 0.012 mg/mL AGEQGFEYVTFR; 10.601 ± 0.023 mg/mL GGVPRSGEQEQQ. | Contains Cys, Met, and aromatic amino acids; larger amino acids at the C-terminal end of the peptide chain; the presence of electrostatic interaction. | [31] |
| QGRPWG PSRADIY AYNIPVNIAR | Juglans regia by-product | Scavenging capacity of walnut meal hydrolyzed protein for DPPH free radicals (IC50): 674.2 μg/mL. Absorption capacity of walnut meal hydrolyzed protein for oxygen free radicals: 591.4 μmol TE/g. | Rich in hydrophobic amino acids; the PWG sequence is present at positions 4–6 of the peptide chain. | [33] |
| QFLLAGR ASPKPSSA RGQVIYVL | Chenopodium quinoa by-product | Clearance rate of quinoa bran hydrolyzed protein for ABTS free radicals: 58.29~74.28%. Chelation rate of quinoa bran hydrolyzed protein for Fe2+: 32.54~82.48%. Scavenging capacity of quinoa bran hydrolyzed protein for hydroxyl radicals: 61.69~117.46 µM. | Shorter peptide chains; contains hydrophobic and aromatic amino acids, with hydrophobic amino acids predominating. | [34] |
| QWDRQ WDTRGQ YSNQNGRF | Gossypium hirsutum by-product | Scavenging capacity of cottonseed meal hydrolyzed protein for ABST free radicals (EC50): 2.05 ± 0.02 mg/mL. Scavenging capacity of cottonseed meal hydrolyzed protein for DPPH free radicals (EC50): 0.49 ± 0.02 mg/mL. Chelating capacity of cottonseed meal for Fe2+ (EC50): 0.99 ± 0.03 mg/mL. Scavenging capacity of cottonseed meal hydrolyzed protein for hydroxyl radicals (EC50): 2.21 ± 0.12 mg/mL. | Rich in acidic/alkaline, hydrophobic, and aromatic amino acids. | [35] |
| FSAP EAAY QEPLLR PVETVR VLRPPLS | Paeonia ostii by-product | Clearance rate for ABTS free radicals: 98.5% ± 1.1% EAAY. Clearance rate for hydroxyl radicals: 61.9% ± 1.3% EAAY. | Contains hydrophobic and aromatic amino acids; the presence of repeated amino acids in the antioxidant peptide sequence; Ala and Tyr are present at the c-terminus of the peptide chain. | [36] |
| IEFL RYLL SAADL | Zea mays by-product | Scavenging capacity for ABTS free radicals (IC50): 0.122 mg/mL RYLL. Scavenging capacity for DPPH free radicals (EC50): 0.180 mg/mL RYLL. | Lower molecular weight; contains hydrophobic and aromatic amino acids, with hydrophobic amino acids predominating; the presence of repeated amino acids in the antioxidant peptide sequence. | [37] |
| WAF GGIF AWFS YLLLK YGIKVGYAIP LPWRPATNVF | Elaeis guineensis by-product | Scavenging capacity for DPPH free radicals (IC50): 1.310 μM WAF; 0.350 μM GGIF; 1.360 μM AWFS; 0.948 μM YLLLK; 1.090 μM YGIKVGYAIP; 1.070 μM LPWRPATNVF. Chelating capacity with Fe2+ (IC50): 2.363 μM WAF;1.498 μM GGIF; 0.002 μM AWFS; 0.218 μM YLLLK;0.087 μM YGIKVGYAIP; 0.001 μM LPWRPATNVF. | Lower molecular weight; contains hydrophobic and aromatic amino acids. | [38] |
| LLLRW GDMNP | Oryza sativa by-product | Scavenging capacity for DPPH free radicals (IC50): 0.131 ± 0.008 mg/mL LLLRW; 0.120 ± 0.007 mg/mL GDMNP. Scavenging capacity for superoxide anion radicals (IC50): 0.430 ± 0.012 mg/mL LLLRW; 0.400 ± 0.008 mg/mL GDMNP. Scavenging capacity for hydroxyl radicals (IC50): 0.380 ± 0.012 mg/mL LLLRW; 0.370 ± 0.009 mg/mL GDMNP. Chelating capacity with Fe2+ (IC50): 0.024 ± 0.008 mg/mL LLLRW; 0.068 ± 0.010 mg/mL GDMNP. | Rich in hydrophobic and aromatic amino acids. | [39] |
| EEGER GMEEER | Cocos nucifera by-product | Scavenging capacity for ABTS free radicals (IC50): 6.54 ± 0.14 mmol/L EEGER; 0.46 ± 0.067 mmol/L GMEEER. Scavenging capacity for DPPH free radicals (IC50): 11.06 ± 0.18 mmol/L EEGER; 6.98 ± 0.29 mmol/L GMEEER. | Contains hydrophobic amino acids; Arg is present at the C-terminus of the peptide chain. | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Xu, Z.; Li, Y.; Fan, Y.; Zhou, Y.; Song, K.; Meng, L. Antioxidant Function and Application of Plant-Derived Peptides. Antioxidants 2024, 13, 1203. https://doi.org/10.3390/antiox13101203
Zhu Z, Xu Z, Li Y, Fan Y, Zhou Y, Song K, Meng L. Antioxidant Function and Application of Plant-Derived Peptides. Antioxidants. 2024; 13(10):1203. https://doi.org/10.3390/antiox13101203
Chicago/Turabian StyleZhu, Zhengqing, Ziwu Xu, Yuhang Li, Yutong Fan, Yingqian Zhou, Kaixin Song, and Lei Meng. 2024. "Antioxidant Function and Application of Plant-Derived Peptides" Antioxidants 13, no. 10: 1203. https://doi.org/10.3390/antiox13101203
APA StyleZhu, Z., Xu, Z., Li, Y., Fan, Y., Zhou, Y., Song, K., & Meng, L. (2024). Antioxidant Function and Application of Plant-Derived Peptides. Antioxidants, 13(10), 1203. https://doi.org/10.3390/antiox13101203






