Hydrogen Sulfide Modulates Astrocytic Toxicity in Mouse Spinal Cord Cultures: Implications for Amyotrophic Lateral Sclerosis
Abstract
:1. Introduction
2. Material and Methods
2.1. Spinal Cord Culture and Treatments
2.2. Immunophenotyping by Flow Cytometry
2.3. Immunofluorescence
2.4. Morphological Assessment
2.5. Protein Extraction and Western Blot
2.6. JC-1 and TMRM Dye Staining
2.7. Mitochondrial Bioenergetics
2.8. Cell Sorting and Isolation of ACSA-1 and 2+ Cells
2.9. RNA Extraction and qRT-PCR
2.10. Statistics
3. Results
3.1. Hydrogen Sulfide Affects Astrocyte Morphology and Activation Status
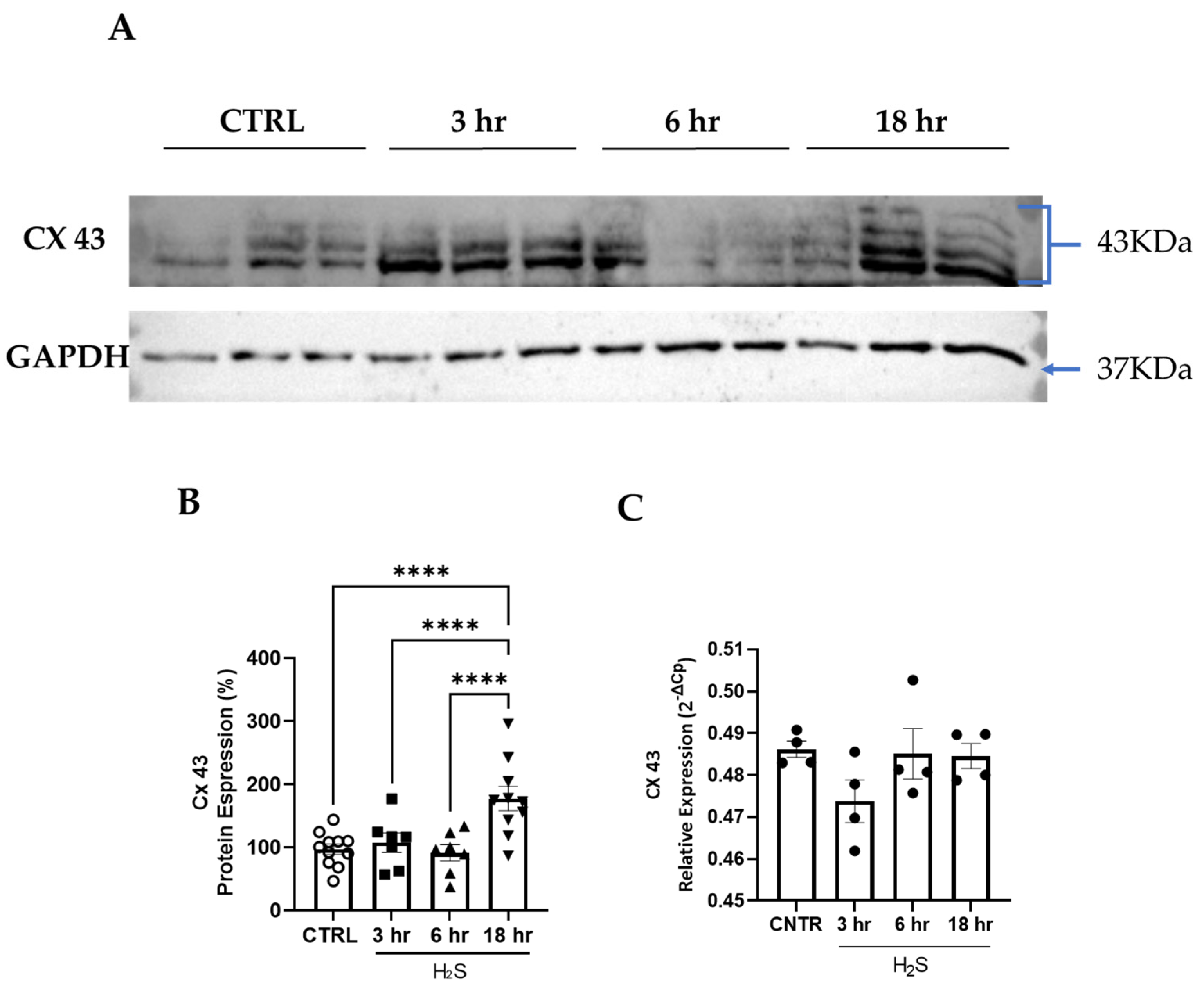
3.2. Cx43 Expression Is Increased in Spinal Cord Cultures Challenged with Hydrogen Sulfide
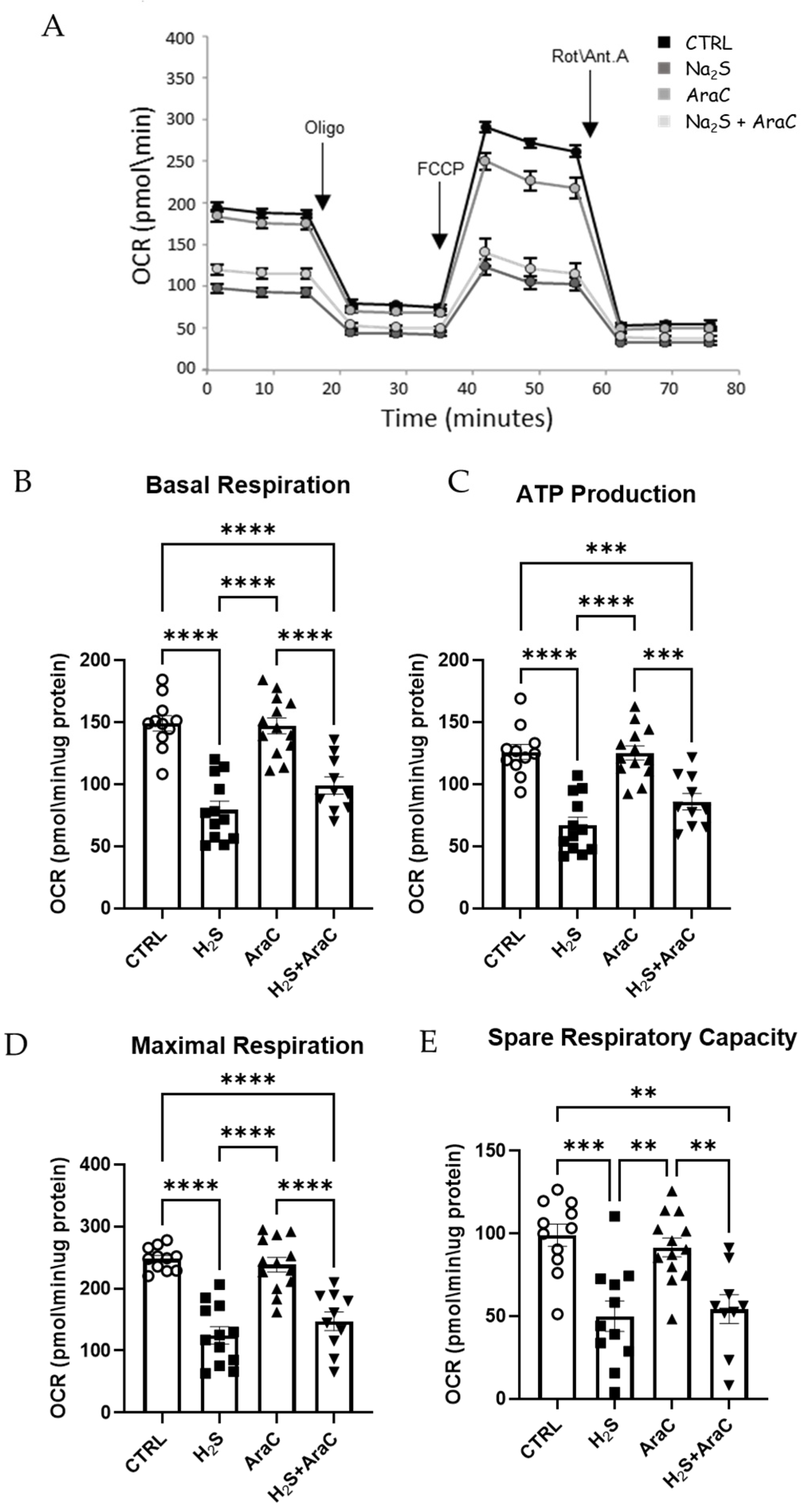
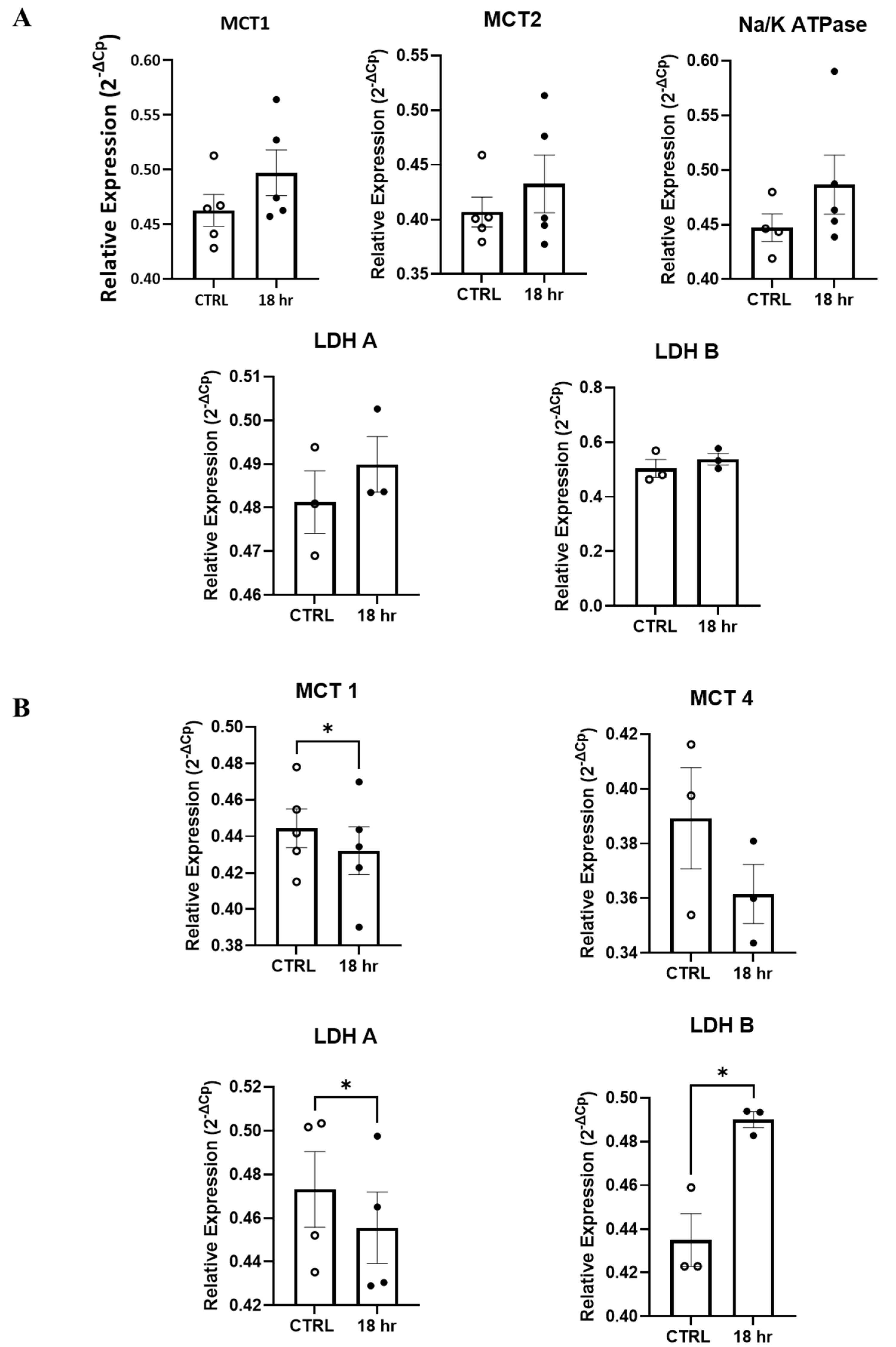
3.3. Mitochondrial Function Is Impaired by H2S in Primary Spinal Cord Cultures
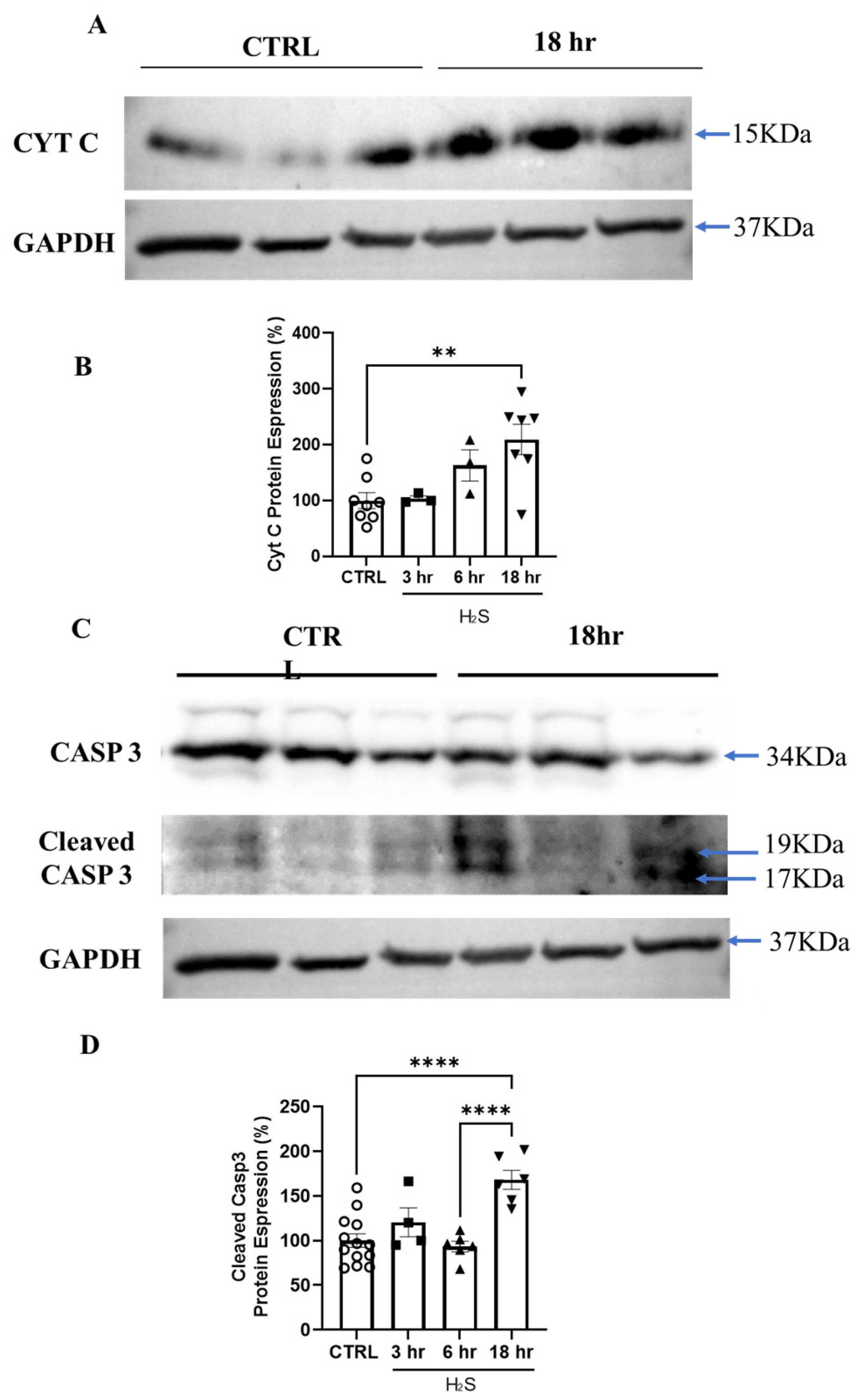
3.4. Hydrogen Sulfide Activates Death by Apoptosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kimura, H. Hydrogen Sulfide (H2S) and Polysulfide (H2Sn) Signaling: The First 25 Years. Biomolecules 2021, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Bełtowski, J. Synthesis, Metabolism, and Signaling Mechanisms of Hydrogen Sulfide: An Overview. In Vascular Effects of Hydrogen Sulfide; Humana: New York, NY, USA, 2019; pp. 1–8. [Google Scholar]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen Sulfide Signaling in Mitochondria and Disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef] [PubMed]
- Shefa, U.; Kim, D.; Kim, M.-S.; Jeong, N.Y.; Jung, J. Roles of Gasotransmitters in Synaptic Plasticity and Neuropsychiatric Conditions. Neural Plast. 2018, 2018, 1824713. [Google Scholar] [CrossRef]
- Lee, M.; Schwab, C.; Yu, S.; McGeer, E.; McGeer, P.L. Astrocytes Produce the Antiinflammatory and Neuroprotective Agent Hydrogen Sulfide. Neurobiol. Aging 2009, 30, 1523–1534. [Google Scholar] [CrossRef]
- Spalloni, A.; de Stefano, S.; Gimenez, J.; Greco, V.; Mercuri, N.B.; Chiurchiù, V.; Longone, P. The Ying and Yang of Hydrogen Sulfide as a Paracrine/Autocrine Agent in Neurodegeneration: Focus on Amyotrophic Lateral Sclerosis. Cells 2023, 12, 1691. [Google Scholar] [CrossRef]
- Miyamoto, R.; Otsuguro, K.; Yamaguchi, S.; Ito, S. Neuronal Regulation of Expression of Hydrogen Sulfide-Producing Enzyme Cystathionine β-Synthase in Rat Spinal Cord Astrocytes. Neurosci. Res. 2015, 97, 52–59. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen Sulfide as a Neuromodulator. Mol. Neurobiol. 2002, 26, 13–20. [Google Scholar] [CrossRef]
- Seydi, E.; Irandoost, Z.; Khansari, M.G.; Naserzadeh, P.; Tanbakosazan, F.; Pourahmad, J. Toxicity of Hydrogen Sulfide on Rat Brain Neurons. Drug Res. 2022, 72, 197–202. [Google Scholar] [CrossRef]
- Módis, K.; Coletta, C.; Erdélyi, K.; Papapetropoulos, A.; Szabo, C. Intramitochondrial Hydrogen Sulfide Production by 3-mercaptopyruvate Sulfurtransferase Maintains Mitochondrial Electron Flow and Supports Cellular Bioenergetics. FASEB J. 2013, 27, 601–611. [Google Scholar] [CrossRef]
- Szabo, C.; Ransy, C.; Módis, K.; Andriamihaja, M.; Murghes, B.; Coletta, C.; Olah, G.; Yanagi, K.; Bouillaud, F. Regulation of Mitochondrial Bioenergetic Function by Hydrogen Sulfide. Part I. Biochemical and Physiological Mechanisms. Br. J. Pharmacol. 2014, 171, 2099–2122. [Google Scholar] [CrossRef]
- Hanna, D.; Kumar, R.; Banerjee, R. A Metabolic Paradigm for Hydrogen Sulfide Signaling via Electron Transport Chain Plasticity. Antioxid. Redox Signal. 2023, 38, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Nii, T.; Eguchi, R.; Yamaguchi, S.; Otsuguro, K. Hydrogen Sulfide Induces Ca2+ Release from the Endoplasmic Reticulum and Suppresses ATP-Induced Ca2+ Signaling in Rat Spinal Cord Astrocytes. Eur. J. Pharmacol. 2021, 891, 173684. [Google Scholar] [CrossRef] [PubMed]
- Stoklund Dittlau, K.; Van Den Bosch, L. Why should we care about astrocytes in a motor neuron disease? Front. Mol. Med. 2023, 3, 1047540. [Google Scholar] [CrossRef] [PubMed]
- Van Harten, A.C.M.; Phatnani, H.; Przedborski, S. Non-cell-autonomous pathogenic mechanisms in amyotrophic lateral sclerosis. Trends Neurosci. 2021, 44, 658–668. [Google Scholar] [CrossRef]
- Davoli, A.; Greco, V.; Spalloni, A.; Guatteo, E.; Neri, C.; Rizzo, G.R.; Cordella, A.; Romigi, A.; Cortese, C.; Bernardini, S.; et al. Evidence of Hydrogen Sulfide Involvement in Amyotrophic Lateral Sclerosis. Ann. Neurol. 2015, 77, 697–709. [Google Scholar] [CrossRef]
- Nardo, G.; Trolese, M.C.; Tortarolo, M.; Vallarola, A.; Freschi, M.; Pasetto, L.; Bonetto, V.; Bendotti, C. New Insights on the Mechanisms of Disease Course Variability in ALS from Mutant SOD1 Mouse Models. Brain Pathol. 2016, 26, 237–247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spalloni, A.; Albo, F.; Ferrari, F.; Mercuri, N.; Bernardi, G.; Zona, C.; Longone, P. Cu/Zn-Superoxide Dismutase (GLY93→ALA) Mutation Alters AMPA Receptor Subunit Expression and Function and Potentiates Kainate-Mediated Toxicity in Motor Neurons in Culture. Neurobiol. Dis. 2004, 15, 340–350. [Google Scholar] [CrossRef]
- Greco, V.; Spalloni, A.; Corasolla Carregari, V.; Pieroni, L.; Persichilli, S.; Mercuri, N.; Urbani, A.; Longone, P. Proteomics and Toxicity Analysis of Spinal-Cord Primary Cultures upon Hydrogen Sulfide Treatment. Antioxidants 2018, 7, 87. [Google Scholar] [CrossRef]
- Krashia, P.; Cordella, A.; Nobili, A.; La Barbera, L.; Federici, M.; Leuti, A.; Campanelli, F.; Natale, G.; Marino, G.; Calabrese, V.; et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat. Commun. 2019, 10, 3945, Erratum in Nat. Commun. 2019, 10, 4725. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, F.; Zaccaria, F.; Ninni, A.; Ceci, V.; Turchi, R.; Apolloni, S.; Milani, M.; Della Valle, I.; Tiberi, M.; Chiurchiù, V.; et al. Frataxin deficiency shifts metabolism to promote reactive microglia via glucose catabolism. Life Sci. Alliance 2024, 7, e202402609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Nutini, M.; Frazzini, V.; Marini, C.; Spalloni, A.; Sensi, S.L.; Longone, P. Zinc pre-treatment enhances NMDAR-mediated excitotoxicity in cultured cortical neurons from SOD1(G93A) mouse, a model of amyotrophic lateral sclerosis. Neuropharmacology 2011, 60, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial Membrane Potential Probes and the Proton Gradient: A Practical Usage Guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.C.; Bakowska, J.C. Determination of Mitochondrial Membrane Potential and Reactive Oxygen Species in Live Rat Cortical Neurons. J. Vis. Exp. 2011, 51, e2704. [Google Scholar] [CrossRef]
- Salvatori, I.; Ferri, A.; Scaricamazza, S.; Giovannelli, I.; Serrano, A.; Rossi, S.; D’Ambrosi, N.; Cozzolino, M.; Di Giulio, A.; Moreno, S.; et al. Differential Toxicity of TAR DNA-binding Protein 43 Isoforms Depends on Their Submitochondrial Localization in Neuronal Cells. J. Neurochem. 2018, 146, 585–597. [Google Scholar] [CrossRef]
- Ramljak, S.; Schmitz, M.; Repond, C.; Zerr, I.; Pellerin, L. Altered MRNA and Protein Expression of Monocarboxylate Transporter MCT1 in the Cerebral Cortex and Cerebellum of Prion Protein Knockout Mice. Int. J. Mol. Sci. 2021, 22, 1566. [Google Scholar] [CrossRef]
- Gomes, C.; Sequeira, C.; Barbosa, M.; Cunha, C.; Vaz, A.R.; Brites, D. Astrocyte Regional Diversity in ALS Includes Distinct Aberrant Phenotypes with Common and Causal Pathological Processes. Exp. Cell Res. 2020, 395, 112209. [Google Scholar] [CrossRef]
- Rash, J.E.; Yasumura, T.; Dudek, F.E.; Nagy, J.I. Cell-Specific Expression of Connexins and Evidence of Restricted Gap Junctional Coupling between Glial Cells and between Neurons. J. Neurosci. 2001, 21, 1983–2000. [Google Scholar] [CrossRef]
- Axelsen, L.N.; Calloe, K.; Holstein-Rathlou, N.-H.; Nielsen, M.S. Managing the Complexity of Communication: Regulation of Gap Junctions by Post-Translational Modification. Front. Pharmacol. 2013, 4, 130. [Google Scholar] [CrossRef]
- Módis, K.; Bos, E.M.; Calzia, E.; van Goor, H.; Coletta, C.; Papapetropoulos, A.; Hellmich, M.R.; Radermacher, P.; Bouillaud, F.; Szabo, C. Regulation of Mitochondrial Bioenergetic Function by Hydrogen Sulfide. Part II. Pathophysiological and Therapeutic Aspects. Br. J. Pharmacol. 2014, 171, 2123–2146. [Google Scholar] [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Falkowska, A.; Gutowska, I.; Goschorska, M.; Nowacki, P.; Chlubek, D.; Baranowska-Bosiacka, I. Energy Metabolism of the Brain, Including the Cooperation between Astrocytes and Neurons, Especially in the Context of Glycogen Metabolism. Int. J. Mol. Sci. 2015, 16, 25959–25981. [Google Scholar] [CrossRef] [PubMed]
- Alberini, C.M.; Cruz, E.; Descalzi, G.; Bessières, B.; Gao, V. Astrocyte Glycogen and Lactate: New Insights into Learning and Memory Mechanisms. Glia 2018, 66, 1244–1262. [Google Scholar] [CrossRef]
- Beard, E.; Lengacher, S.; Dias, S.; Magistretti, P.J.; Finsterwald, C. Astrocytes as Key Regulators of Brain Energy Metabolism: New Therapeutic Perspectives. Front. Physiol. 2022, 12, 825816. [Google Scholar] [CrossRef]
- Pellerin, L.; Bouzier-Sore, A.; Aubert, A.; Serres, S.; Merle, M.; Costalat, R.; Magistretti, P.J. Activity-dependent Regulation of Energy Metabolism by Astrocytes: An Update. Glia 2007, 55, 1251–1262. [Google Scholar] [CrossRef]
- Glover, H.L.; Schreiner, A.; Dewson, G.; Tait, S.W.G. Mitochondria and Cell Death. Nat. Cell Biol. 2024, 26, 1434–1446. [Google Scholar] [CrossRef]
- Jürgensmeier, J.M.; Xie, Z.; Deveraux, Q.; Ellerby, L.; Bredesen, D.; Reed, J.C. Bax Directly Induces Release of Cytochrome c from Isolated Mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 4997–5002. [Google Scholar] [CrossRef]
- Scorrano, L.; Korsmeyer, S.J. Mechanisms of Cytochrome c Release by Proapoptotic BCL-2 Family Members. Biochem. Biophys. Res. Commun. 2003, 304, 437–444. [Google Scholar] [CrossRef]
- Stefanis, L. Caspase-Dependent and -Independent Neuronal Death: Two Distinct Pathways to Neuronal Injury. Neuroscientist 2005, 11, 50–62. [Google Scholar] [CrossRef]
- Khan, H.; Bangar, A.; Grewal, A.K.; Bansal, P.; Singh, T.G. Caspase-Mediated Regulation of the Distinct Signaling Pathways and Mechanisms in Neuronal Survival. Int. Immunopharmacol. 2022, 110, 108951. [Google Scholar] [CrossRef]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef] [PubMed]
- De Pittà, M.; Brunel, N.; Volterra, A. Astrocytes: Orchestrating Synaptic Plasticity? Neuroscience 2016, 323, 43–61. [Google Scholar] [CrossRef]
- Li, S.; Graham, E.S.; Unsworth, C.P. Extracellular ATP Release Predominantly Mediates Ca2+ Communication Locally in Highly Organised, Stellate-Like Patterned Networks of Adult Human Astrocytes. PLoS ONE 2023, 18, e0289350. [Google Scholar] [CrossRef]
- Pérez-Alvarez, A.; Araque, A. Astrocyte-Neuron Interaction at Tripartite Synapses. Curr. Drug Targets 2013, 14, 1220–1224. [Google Scholar] [CrossRef]
- Calma, A.D.; Pavey, N.; Menon, P.; Vucic, S. Neuroinflammation in amyotrophic lateral sclerosis: Pathogenic insights and therapeutic implications. Curr. Opin. Neurol. 2024, 37, 585–592. [Google Scholar] [CrossRef]
- Hamby, M.E.; Sofroniew, M.V. Reactive Astrocytes As Therapeutic Targets for CNS Disorders. Neurotherapeutics 2010, 7, 494–506. [Google Scholar] [CrossRef]
- Tsugane, M.; Nagai, Y.; Kimura, Y.; Oka, J.-I.; Kimura, H. Differentiated Astrocytes Acquire Sensitivity to Hydrogen Sulfide That Is Diminished by the Transformation into Reactive Astrocytes. Antioxid. Redox Signal. 2007, 9, 257–269. [Google Scholar] [CrossRef]
- Parpura, V.; Heneka, M.T.; Montana, V.; Oliet, S.H.R.; Schousboe, A.; Haydon, P.G.; Stout, R.F.; Spray, D.C.; Reichenbach, A.; Pannicke, T.; et al. Glial Cells in (Patho)Physiology. J. Neurochem. 2012, 121, 4–27. [Google Scholar] [CrossRef]
- Tripathi, P.; Rodriguez-Muela, N.; Klim, J.R.; de Boer, A.S.; Agrawal, S.; Sandoe, J.; Lopes, C.S.; Ogliari, K.S.; Williams, L.A.; Shear, M.; et al. Reactive Astrocytes Promote ALS-like Degeneration and Intracellular Protein Aggregation in Human Motor Neurons by Disrupting Autophagy through TGF-Β1. Stem Cell Rep. 2017, 9, 667–680. [Google Scholar] [CrossRef]
- Ding, Z.-B.; Song, L.-J.; Wang, Q.; Kumar, G.; Yan, Y.-Q.; Ma, C.-G. Astrocytes: A Double-Edged Sword in Neurodegenerative Diseases. Neural Regen. Res. 2021, 16, 1702. [Google Scholar] [CrossRef]
- Almad, A.A.; Doreswamy, A.; Gross, S.K.; Richard, J.; Huo, Y.; Haughey, N.; Maragakis, N.J. Connexin 43 in Astrocytes Contributes to Motor Neuron Toxicity in Amyotrophic Lateral Sclerosis. Glia 2016, 64, 1154–1169. [Google Scholar] [CrossRef] [PubMed]
- Almad, A.A.; Taga, A.; Joseph, J.; Gross, S.K.; Welsh, C.; Patankar, A.; Richard, J.-P.; Rust, K.; Pokharel, A.; Plott, C.; et al. Cx43 Hemichannels Contribute to Astrocyte-Mediated Toxicity in Sporadic and Familial ALS. Proc. Natl. Acad. Sci. USA 2022, 119, e2107391119. [Google Scholar] [CrossRef] [PubMed]
- Spalloni, A.; Greco, V.; Ciriminna, G.; Corasolla Carregari, V.; Marini, F.; Pieroni, L.; Mercuri, N.B.; Urbani, A.; Longone, P. Impact of Pharmacological Inhibition of Hydrogen Sulphide Production in the SOD1G93A-ALS Mouse Model. Int. J. Mol. Sci. 2019, 20, 2550. [Google Scholar] [CrossRef] [PubMed]
- Cassina, P.; Peluffo, H.; Pehar, M.; Martinez-Palma, L.; Ressia, A.; Beckman, J.S.; Estévez, A.G.; Barbeito, L. Peroxynitrite Triggers a Phenotypic Transformation in Spinal Cord Astrocytes That Induces Motor Neuron Apoptosis. J. Neurosci. Res. 2002, 67, 21–29. [Google Scholar] [CrossRef]
- Rash, J.E.; Yasumura, T.; Davidson, K.G.V.; Furman, C.S.; Dudek, F.E.; Nagy, J.I. Identification of Cells Expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in Gap Junctions of Rat Brain and Spinal Cord. Cell Commun. Adhes. 2001, 8, 315–320. [Google Scholar] [CrossRef]
- Sohl, G. Gap Junctions and the Connexin Protein Family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, M.; Cai, C.; Zhu, S.; Zhang, C.; Wang, Q.; Zhai, Y.; Ji, X.; Wu, D. Role of Hydrogen Sulphide in Physiological and Pathological Angiogenesis. Cell Prolif. 2023, 56, e13374. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Samiei, M.; Maleki Dizaj, S.; Vinken, M. The Role and Therapeutic Potential of Connexins, Pannexins and Their Channels in Parkinson’s Disease. Cell Signal 2019, 58, 111–118. [Google Scholar] [CrossRef]
- Hoos, M.D.; Richardson, B.M.; Foster, M.W.; Everhart, A.; Thompson, J.W.; Moseley, M.A.; Colton, C.A. Longitudinal Study of Differential Protein Expression in an Alzheimer’s Mouse Model Lacking Inducible Nitric Oxide Synthase. J. Proteome Res. 2013, 12, 4462–4477. [Google Scholar] [CrossRef]
- Nagai, M.; Re, D.B.; Nagata, T.; Chalazonitis, A.; Jessell, T.M.; Wichterle, H.; Przedborski, S. Astrocytes Expressing ALS-Linked Mutated SOD1 Release Factors Selectively Toxic to Motor Neurons. Nat. Neurosci. 2007, 10, 615–622. [Google Scholar] [CrossRef]
- Ross, J.M.; Öberg, J.; Brené, S.; Coppotelli, G.; Terzioglu, M.; Pernold, K.; Goiny, M.; Sitnikov, R.; Kehr, J.; Trifunovic, A.; et al. High Brain Lactate Is a Hallmark of Aging and Caused by a Shift in the Lactate Dehydrogenase A/B Ratio. Proc. Natl. Acad. Sci. USA 2010, 107, 20087–20092. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Lee, D.; Xiong, W.-C. Lactate Metabolism, Signaling, and Function in Brain Development, Synaptic Plasticity, Angiogenesis, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 13398. [Google Scholar] [CrossRef] [PubMed]
- Kleene, R.; Loers, G.; Langer, J.; Frobert, Y.; Buck, F.; Schachner, M. Prion Protein Regulates Glutamate-Dependent Lactate Transport of Astrocytes. J. Neurosci. 2007, 27, 12331–12340. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. Correction to: ‘The Warburg Effect: How Does It Benefit Cancer Cells? ’ Trends Biochem. Sci. 2016, 41, 287. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]



| Antibody | Source | (#CAT) | Dilution |
|---|---|---|---|
| Alfa-Tubulin | Sigma-Aldrich (Milan, Italy) | (T6199) | 1:500 (IF) |
| Caspase-3 | Invitrogen (Rodano, Italy) | (PA5-77887) | 1:100 (WB) |
| Bax | Abcam (Cambridge, UK) | (Ab5714) | 1:1000 (WB) |
| Connexin 43 | Sigma-Aldrich | (C6219) | 1:500 (WB) |
| 1:200 (IF) | |||
| Cytocrome C | Invitrogen | (33-8500) | 1:100 (WB) |
| Galectin 3 | Invitrogen | (A3A12) | 1:200 (IF) |
| GAPDH | Calbiochem (Milan, Italy) | (CB1001) | 1: 60.000 (WB) |
| GFAP | Immunological Sciences (Rome, Italy) | (AB-10678) | 1:500 (IF) |
| IBA 1 | Wako (DBA, Milan, Italy) | (019-19741) | 1:500 (IF) |
| SMI 32 | Covance (Campoverde, Milan, Italy) | (SM1-32P) | 1:500 (IF) |
| S100B | Sigma-Aldrich | (SAB5700647) | 1:100 (IF) |
| Name | Forward Sequence | Reverse Sequence |
|---|---|---|
| LDHA NM-010699.2 | CAGTGGCTTTGCCAAAAACCGAGT | CCATCAGGTAACGGAACCGCG |
| LDHB NM-008492.3 | CCTGCTGACTTTGCAGTGGCTCC | TCGCCGCGGCAGCCTCATCAT |
| MCT1 NM-009196.4 | TTGGACCCCAGAGGTTCTCC | AGGCGGCCTAAAAGTGGTG |
| MCT2 NM-001428808.1 | CAGCAACAGCGTGATAGAGCTT | TGGTTGCAGGTTGAATGCTAAT |
| MCT4 NM-001038653.1 | CGGCTGGCGGTAACAGAGTA | CGGCCTCGGACCTGAGTATT |
| Na+/K+-ATPase α2 NM-178405.3 | GAGACGCGCAATATCTGTTTCTT | ACCTGTGGCAATCACAATGC |
|
CX 43 NM-010288.4 | CCCGAACTCTCCTTTTCCTT | TGGGCACCTCTCTTTCACTT |
| Ribosomal protein L34 NM-001005859.4 | GGTGCTCAGAGGCACTCAGGATG | GTGCTTTCCCAACCTTCTGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Stefano, S.; Tiberi, M.; Salvatori, I.; De Bardi, M.; Gimenez, J.; Pirshayan, M.; Greco, V.; Borsellino, G.; Ferri, A.; Valle, C.; et al. Hydrogen Sulfide Modulates Astrocytic Toxicity in Mouse Spinal Cord Cultures: Implications for Amyotrophic Lateral Sclerosis. Antioxidants 2024, 13, 1241. https://doi.org/10.3390/antiox13101241
De Stefano S, Tiberi M, Salvatori I, De Bardi M, Gimenez J, Pirshayan M, Greco V, Borsellino G, Ferri A, Valle C, et al. Hydrogen Sulfide Modulates Astrocytic Toxicity in Mouse Spinal Cord Cultures: Implications for Amyotrophic Lateral Sclerosis. Antioxidants. 2024; 13(10):1241. https://doi.org/10.3390/antiox13101241
Chicago/Turabian StyleDe Stefano, Susanna, Marta Tiberi, Illari Salvatori, Marco De Bardi, Juliette Gimenez, Mahsa Pirshayan, Viviana Greco, Giovanna Borsellino, Alberto Ferri, Cristiana Valle, and et al. 2024. "Hydrogen Sulfide Modulates Astrocytic Toxicity in Mouse Spinal Cord Cultures: Implications for Amyotrophic Lateral Sclerosis" Antioxidants 13, no. 10: 1241. https://doi.org/10.3390/antiox13101241
APA StyleDe Stefano, S., Tiberi, M., Salvatori, I., De Bardi, M., Gimenez, J., Pirshayan, M., Greco, V., Borsellino, G., Ferri, A., Valle, C., Mercuri, N. B., Chiurchiù, V., Spalloni, A., & Longone, P. (2024). Hydrogen Sulfide Modulates Astrocytic Toxicity in Mouse Spinal Cord Cultures: Implications for Amyotrophic Lateral Sclerosis. Antioxidants, 13(10), 1241. https://doi.org/10.3390/antiox13101241










