Microplastic-Enhanced Cadmium Toxicity: A Growing Threat to the Sea Grape, Caulerpa lentillifera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Growth Data and Morphological Characteristics Collection
2.4. Determination of Cd Concentration
2.5. Determination of Antioxidant Capacity
2.6. Transcriptome Analysis
2.7. Determination of Chlorophyll Content
2.8. Determination of Soluble Sugar Content
2.9. Data Processing and Analysis
3. Results
3.1. Comparison of Growth Conditions
3.2. Analysis of Cd2⁺ Content in Thalli
3.3. Comparison of Antioxidant Capacity
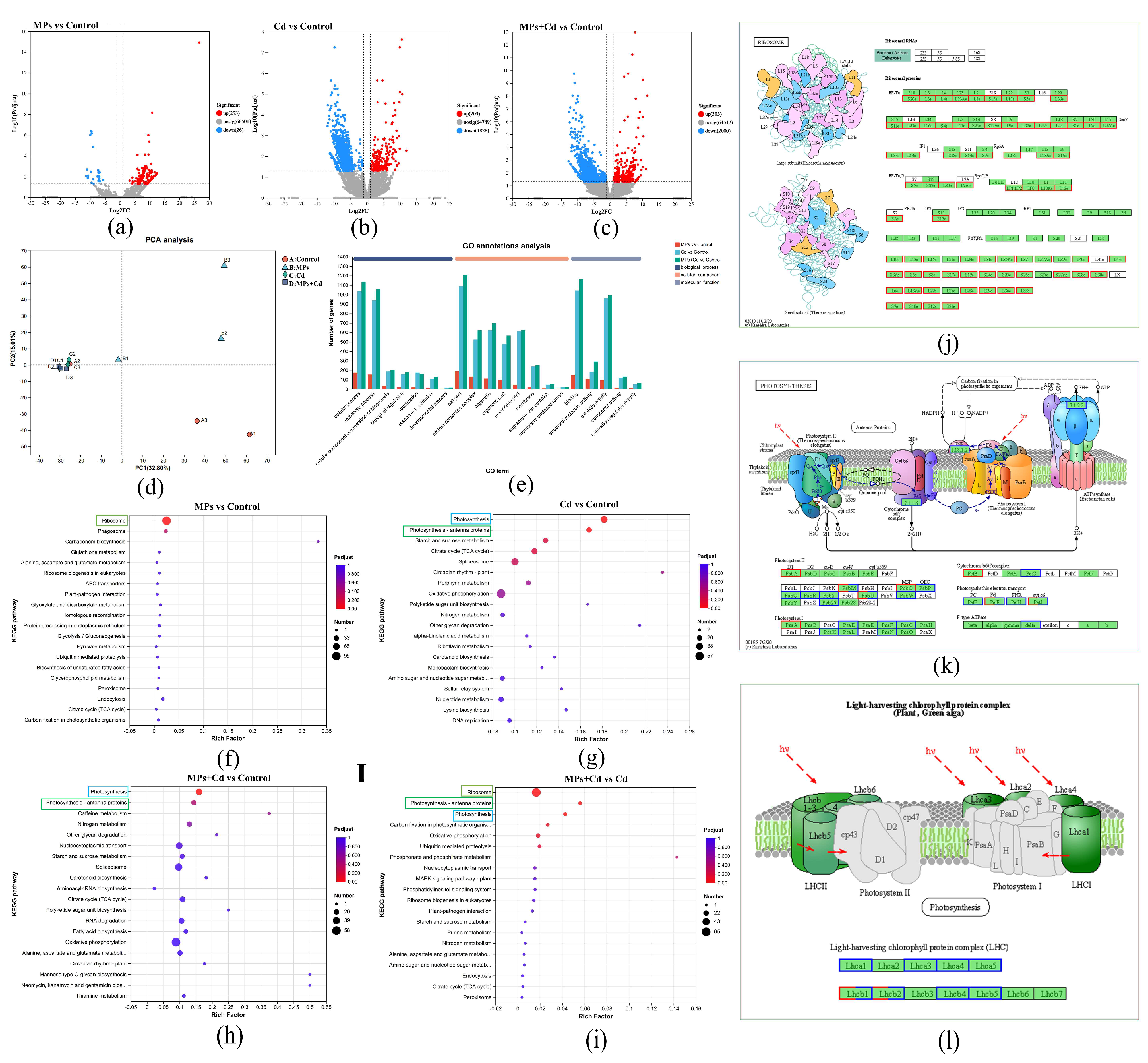
3.4. Sequencing and Gene Expression Analysis
3.5. Functional Enrichment Analysis of DEGs
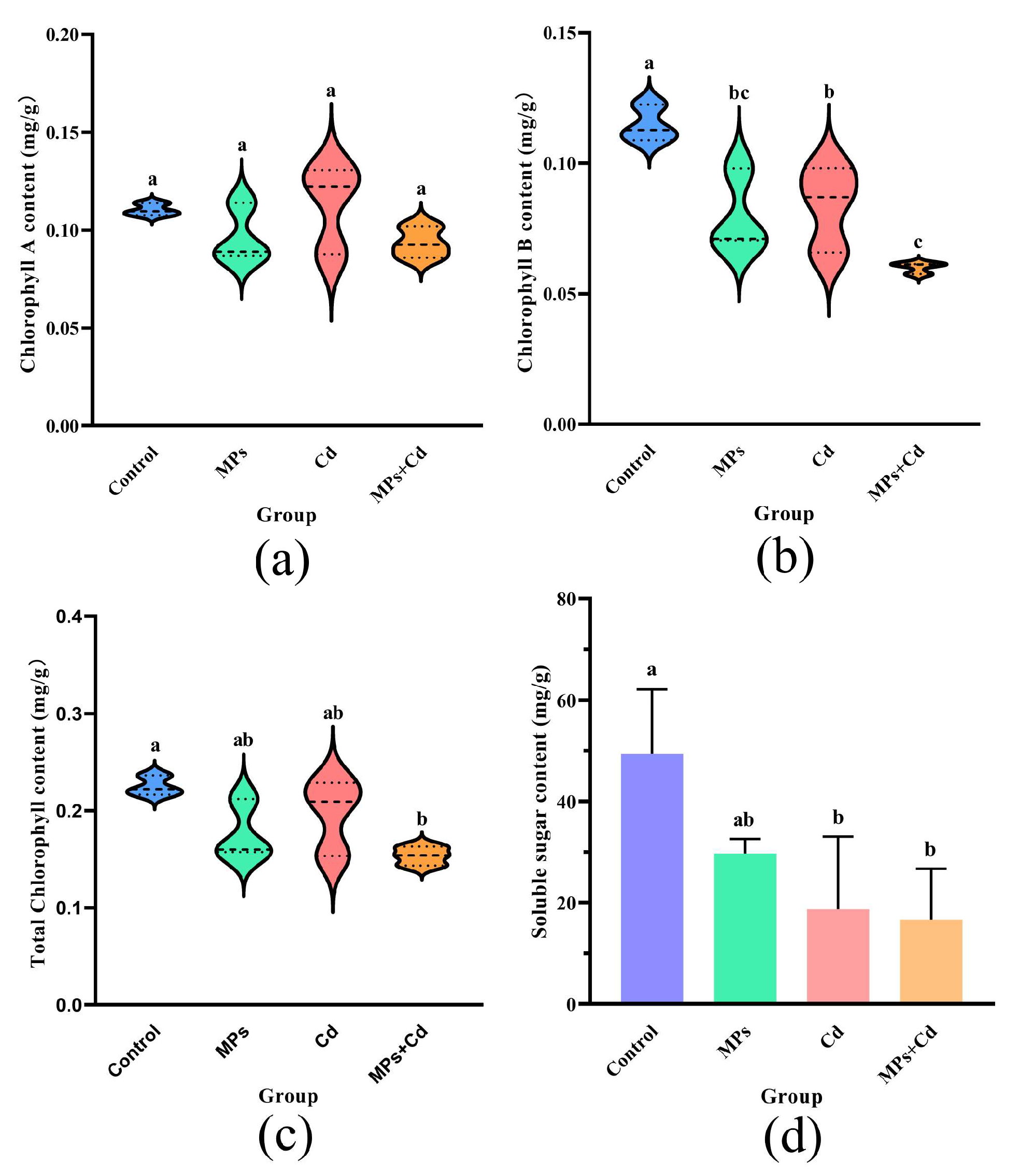
3.6. Chlorophyll Content
3.7. Soluble Sugar Content
4. Discussion
4.1. Influence of Microplastics on Ribosomal Activity and Growth of C. lentillifera
4.2. Oxidative Stress and Photosynthetic Inhibition Induced by Cadmium Exposure
4.3. Synergistic Effects of Microplastics and Cadmium on Gene Expression and Cellular Function
4.4. Ecological Risks and Industrial Implications of Combined Pollutants in Marine Environments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrison, R.M. Marine Pollution and Human Health; Royal Society of Chemistry: London, UK, 2011; Volume 33. [Google Scholar]
- Yang, Q.; Ma, L.; Qiu, K.; Feng, Z.; Wang, Y.; Zhong, Z.; Cheng, F.; Zhai, T.; Zeng, J.; Huang, W. Characterization and risk assessment of microplastics in laver from the Yueqing Bay. Mar. Environ. Res. 2024, 193, 106258. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.; Jalil, A.; Dutta, K.; Rajendran, S.; Vasseghian, Y.; Karimi-Maleh, H.; Soto-Moscoso, M. Algal degradation of microplastic from the environment: Mechanism, challenges, and future prospects. Algal Res. 2022, 67, 102848. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Huang, W.; Wang, Y.; Wang, Y.; Cao, L.; Yang, Z.; Dou, S. Microplastic pollution in the environment and organisms of Xiangshan Bay, East China Sea: An area of intensive mariculture. Water Res. 2022, 212, 118117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, W.; Yan, P.; Wang, J.; Yan, S.; Liu, X.; Aurangzeib, M. Microplastic migration and distribution in the terrestrial and aquatic environments: A threat to biotic safety. J. Environ. Manag. 2023, 333, 117412. [Google Scholar] [CrossRef]
- Wang, T.; Yang, X.; Ouyang, S.; Huang, W.; Ma, G.; Liu, S.; Zhu, Y.; Zhang, Y.; Li, H.; Yu, H. The native submerged plant, Hydrilla verticillata outperforms its exotic confamilial with exposure to polyamide microplastic pollution: Implication for wetland revegetation and potential driving mechanism. Aquat. Toxicol. 2024, 273, 107029. [Google Scholar] [CrossRef]
- Karlsson, T.M.; Vethaak, A.D.; Almroth, B.C.; Ariese, F.; van Velzen, M.; Hassellöv, M.; Leslie, H.A. Screening for microplastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation. Mar. Pollut. Bull. 2017, 122, 403–408. [Google Scholar] [CrossRef]
- Kováčik, J.; Babula, P.; Peterkova, V.; Hedbavny, J. Long-term impact of cadmium shows little damage in Scenedesmus acutiformis cultures. Algal Res. 2017, 25, 184–190. [Google Scholar] [CrossRef]
- Naik, S.; Pradhan, U.; Karthikeyan, P.; Begum, M.; Panda, U.S.; Mishra, P.; Murthy, M.R. Heavy metal pollution causes mass mortality of fish in a tropical estuary in the southwestern Bay of Bengal. Mar. Environ. Res. 2024, 199, 106595. [Google Scholar] [CrossRef]
- Sankar, T.; Zynudheen, A.; Anandan, R.; Nair, P.V. Distribution of organochlorine pesticides and heavy metal residues in fish and shellfish from Calicut region, Kerala, India. Chemosphere 2006, 65, 583–590. [Google Scholar] [CrossRef]
- Rainbow, P.; Luoma, S. Metal toxicity, uptake and bioaccumulation in aquatic invertebrates—Modelling zinc in crustaceans. Aquat. Toxicol. 2011, 105, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.T.T.; Hang, N.T.T.; Nguyen, X.C.; Nguyen, N.T.M.; Truong, H.B.; Liu, C.; La, D.D.; Kim, S.S.; Nguyen, D.D. Toxic metals in rice among Asian countries: A review of occurrence and potential human health risks. Food Chem. 2024, 460, 140479. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, N.; Zhu, Y.; Wu, Z.; Ye, Z.; Christakos, G.; Wu, J. Seasonal effects of fish, seaweed and abalone cultures on dissolved organic matter and carbon sequestration potential in Sansha Bay, China. Sci. Total Environ. 2024, 945, 174144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhou, W.; Shi, Y.; Yang, S.; Tang, X.; Xiong, Y.; Gu, Z. Active Components and Skin Care Mechanism of Sea Grape (Caulerpa lentillifera) Extract. J. Biobased Mater. Bioenergy 2024, 18, 868–877. [Google Scholar] [CrossRef]
- Mabeau, S.; Fleurence, J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, T.; Wang, J.; Huang, W.; Wang, R.; Xu, J.; Fu, G.; Gao, G. Spatio-temporal features of microplastics pollution in macroalgae growing in an important mariculture area, China. Sci. Total Environ. 2020, 719, 137490. [Google Scholar] [CrossRef]
- Liranzo-Gómez, R.E.; Gómez, A.M.; Gómez, B.; González-Hernández, Y.; Jauregui-Haza, U.J. Characterization of Sargassum accumulated on Dominican beaches in 2021: Analysis of heavy, alkaline and alkaline-earth metals, proteins and fats. Mar. Pollut. Bull. 2023, 193, 115120. [Google Scholar] [CrossRef]
- Li, Q.; Su, L.; Ma, C.; Feng, Z.; Shi, H. Plastic debris in coastal macroalgae. Environ. Res. 2022, 205, 112464. [Google Scholar] [CrossRef]
- Wickramasinghe, W.A.D.; Mubiana, V.K.; Blust, R. The effects of heavy metal concentration on bio-accumulation, productivity and pigment content of two species of marine macro algae. Sri Lanka J. Aquat. Sci. 2017, 22, 1–8. [Google Scholar] [CrossRef]
- Znad, H.; Awual, M.R.; Martini, S. The utilization of algae and seaweed biomass for bioremediation of heavy metal-contaminated wastewater. Molecules 2022, 27, 1275. [Google Scholar] [CrossRef]
- Li, Z.; Fu, D.; Lü, S.; Liu, Z. Interaction between macroalgae and microplastics: Caulerpa lentillifera and Gracilaria tenuistipitata as microplastic bio-elimination vectors. J. Oceanol. Limnol. 2023, 41, 2249–2261. [Google Scholar] [CrossRef]
- Baumann, H.A.; Morrison, L.; Stengel, D.B. Metal accumulation and toxicity measured by PAM—Chlorophyll fluorescence in seven species of marine macroalgae. Ecotoxicol. Environ. Saf. 2009, 72, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, Y.; Jiang, W.; Chen, J.; Chen, Y.; Zhang, X.; Wang, G. Microplastics with cadmium inhibit the growth of Vallisneria natans (Lour.) Hara rather than reduce cadmium toxicity. Chemosphere 2021, 266, 128979. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Licciano, M.; Giotta, L.; Petrocelli, A.; Cecere, E.; Quarta, E. Microplastic evidence and removal from the seaweed bioremediator Chaetomorpha linum. In Proceedings of the 2022 IEEE International Workshop on Metrology for the Sea; Learning to Measure Sea Health Parameters (MetroSea), Milazzo, Italy, 3–5 October 2022; pp. 353–357. [Google Scholar]
- Seng, N.; Lai, S.; Fong, J.; Saleh, M.F.; Cheng, C.; Cheok, Z.Y.; Todd, P.A. Early evidence of microplastics on seagrass and macroalgae. Mar. Freshw. Res. 2020, 71, 922–928. [Google Scholar] [CrossRef]
- Brix da Costa, B.; Stuthmann, L.E.; Cordes, A.J.; Du, H.T.; Kunzmann, A.; Springer, K. Culturing delicacies: Potential to integrate the gastropod Babylonia areolata into pond cultures of Caulerpa lentillifera. Aquac. Rep. 2023, 33, 101793. [Google Scholar] [CrossRef]
- Guo, H.; Yao, J.; Sun, Z.; Duan, D. Effect of temperature, irradiance on the growth of the green alga Caulerpa lentillifera (Bryopsidophyceae, Chlorophyta). J. Appl. Phycol. 2015, 27, 879–885. [Google Scholar] [CrossRef]
- Syakilla, N.; George, R.; Chye, F.Y.; Pindi, W.; Mantihal, S.; Wahab, N.A.; Fadzwi, F.M.; Gu, P.H.; Matanjun, P. A review on nutrients, phytochemicals, and health benefits of green seaweed, Caulerpa lentillifera. Foods 2022, 11, 2832. [Google Scholar] [CrossRef]
- Strady, E.; Dang, T.H.; Dao, T.D.; Dinh, H.N.; Do, T.T.D.; Duong, T.N.; Duong, T.T.; Hoang, D.A.; Kieu-Le, T.C.; Le, T.P.Q. Baseline assessment of microplastic concentrations in marine and freshwater environments of a developing Southeast Asian country, Viet Nam. Mar. Pollut. Bull. 2021, 162, 111870. [Google Scholar] [CrossRef]
- Cheung, P.K.; Cheung, L.T.O.; Fok, L. Seasonal variation in the abundance of marine plastic debris in the estuary of a subtropical macro-scale drainage basin in South China. Sci. Total Environ. 2016, 562, 658–665. [Google Scholar] [CrossRef]
- Mart, L.; Nürnberg, H. The distribution of cadmium in the sea. In Cadmium in the Environment; Springer: Berlin/Heidelberg, Germany, 1986; pp. 28–40. [Google Scholar]
- Gu, X.; Xu, L.; Wang, Z.; Ming, X.; Dang, P.; Ouyang, W.; Lin, C.; Liu, X.; He, M.; Wang, B. Assessment of cadmium pollution and subsequent ecological and health risks in Jiaozhou Bay of the Yellow Sea. Sci. Total Environ. 2021, 774, 145016. [Google Scholar] [CrossRef]
- Tjahjono, A.; Suwarno, D. The spatial distribution of heavy metal lead and cadmium pollution and coliform abundance of waters and surface sediment in Demak. J. Ecol. Eng. 2018, 19, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Cárdenas, A.; van Pelt, F.N.; O’Halloran, J.; Jansen, M.A. Adsorption, uptake and toxicity of micro-and nanoplastics: Effects on terrestrial plants and aquatic macrophytes. Environ. Pollut. 2021, 284, 117183. [Google Scholar] [CrossRef] [PubMed]
- Geddie, A.W.; Hall, S.G. An introduction to copper and zinc pollution in macroalgae: For use in remediation and nutritional applications. J. Appl. Phycol. 2019, 31, 691–708. [Google Scholar] [CrossRef]
- Ma, H.; Zou, D.; Wen, J.; Ji, Z.; Gong, J.; Liu, C. The impact of elevated atmospheric CO2 on cadmium toxicity in Pyropia haitanensis (Rhodophyta). Environ. Sci. Pollut. Res. 2018, 25, 33361–33369. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Sun, R.; Dai, Z.; He, L.; Li, C. Distribution and risk assessment of microplastics in typical ecosystems in the South China Sea. Sci. Total Environ. 2023, 883, 163678. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Shen, Y.; Peng, W.; Chen, N.; Gan, Y.; Xiao, Q.; Liu, J.; Lu, Y.; Lin, W.; Han, Z. Metabolic and transcriptional responses demonstrating enhanced thermal tolerance in domesticated abalone. Sci. Total Environ. 2023, 872, 162060. [Google Scholar] [CrossRef]
- Xue, S.; Barna, M. Specialized ribosomes: A new frontier in gene regulation and organismal biology. Nat. Rev. Mol. Cell Biol. 2012, 13, 355–369. [Google Scholar] [CrossRef]
- Adeli, K. Translational control mechanisms in metabolic regulation: Critical role of RNA binding proteins, microRNAs, and cytoplasmic RNA granules. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1051–E1064. [Google Scholar] [CrossRef]
- Lempiäinen, H.; Shore, D. Growth control and ribosome biogenesis. Curr. Opin. Cell Biol. 2009, 21, 855–863. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, X.; Liu, Y.; Li, W.; Cui, W.; Sun, Y.; Zhang, S.; Wang, F.; Xing, B. Response of peanut plant and soil N-fixing bacterial communities to conventional and biodegradable microplastics. J. Hazard. Mater. 2023, 459, 132142. [Google Scholar] [CrossRef]
- Zhu, W.; Lu, S.; Jiang, H.; Wang, P.; He, C.; Bian, H.; Wang, J. Phenanthrene Mitigated the Toxicological Effects of Polystyrene Micro/Nano Plastics on Rice (Oryza Sativa L.). SSRN 2023. [Google Scholar] [CrossRef]
- Gao, W.; Wu, D.; Zhang, D.; Geng, Z.; Tong, M.; Duan, Y.; Xia, W.; Chu, J.; Yao, X. Comparative analysis of the effects of microplastics and nitrogen on maize and wheat: Growth, redox homeostasis, photosynthesis, and AsA-GSH cycle. Sci. Total Environ. 2024, 932, 172555. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Yin, H.; Xiao, T.; Xiao, E.; Dang, Z.; Pan, K. Effects of Microplastics on Endophytes in Different Niches of Chinese Flowering Cabbage (Brassica campestris). J. Agric. Food Chem. 2024, 72, 4679–4688. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, A.; Rasheed, R.; Ashraf, M.A.; Qureshi, F.F.; Hussain, I.; Iqbal, M. Effect of heavy metals on growth, physiological and biochemical responses of plants. In Plants and Their Interaction to Environmental Pollution; Elsevier: Amsterdam, The Netherlands, 2023; pp. 139–159. [Google Scholar]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int. J. Plant Prod. 2009, 3, 65–76. [Google Scholar] [CrossRef]
- Kramer, D.M.; Evans, J.R. The importance of energy balance in improving photosynthetic productivity. Plant Physiol. 2011, 155, 70–78. [Google Scholar] [CrossRef]
- Vecchi, V.; Barera, S.; Bassi, R.; Dall’Osto, L. Potential and challenges of improving photosynthesis in algae. Plants 2020, 9, 67. [Google Scholar] [CrossRef]
- Dubey, R.S. Photosynthesis in plants under stressful conditions. In Handbook of Photosynthesis; CRC Press: Boca Raton, FL, USA, 2018; pp. 629–649. [Google Scholar]
- Goyal, D.; Yadav, A.; Prasad, M.; Singh, T.B.; Shrivastav, P.; Ali, A.; Dantu, P.K.; Mishra, S. Effect of heavy metals on plant growth: An overview. In Contaminants in Agriculture: Sources, Impacts and Management; Springer: Cham, Switzerland, 2020; pp. 79–101. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Mates, J. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Foyer, C.H. The contribution of photosynthetic oxygen metabolism to oxidative stress in plants. In Oxidative Stress Plants; CRC Press: Boca Raton, FL, USA, 2002; pp. 33–68. [Google Scholar]
- Shao, H.-b.; Chu, L.-y.; Shao, M.-a.; Jaleel, C.A.; Hong-mei, M. Higher plant antioxidants and redox signaling under environmental stresses. Comptes Rendus. Biol. 2008, 331, 433–441. [Google Scholar] [CrossRef]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Teng, X.; Li, C.; Li, H.; Lu, H. Transcriptome Analysis of Gene Expression Patterns of Populus tomentosa in Response to Oxidative Stress. Bot. Res. 2018, 7, 186–195. [Google Scholar]
- Bag, P. Light harvesting in fluctuating environments: Evolution and function of antenna proteins across photosynthetic lineage. Plants 2021, 10, 1184. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V.; Johnson, M.P.; Duffy, C.D. The photoprotective molecular switch in the photosystem II antenna. Biochim. Biophys. Acta (BBA)-Bioenerg. 2012, 1817, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Pedriza, A.; Jaumot, J. Interaction of environmental pollutants with microplastics: A critical review of sorption factors, bioaccumulation and ecotoxicological effects. Toxics 2020, 8, 40. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Liang, X.; Lu, S.; Ren, J.; Zhang, Y.; Han, Z.; Gao, B.; Sun, K. Effects of microplastics on soil carbon pool and terrestrial plant performance. Carbon Res. 2024, 3, 1–23. [Google Scholar] [CrossRef]
- Rassaei, F. Impact of polystyrene microplastics on cadmium uptake in corn (Zea mays L.) in a cadmium-contaminated calcareous soil. Environ. Prog. Sustain. Energy 2024, 43, e14230. [Google Scholar] [CrossRef]
- Wu, X.; Yin, S.; Liu, Y.; Zhu, Y.; Jiang, T.; Liang, S.; Bian, S.; Cao, Y.; Wang, G.; Yang, J. Molecular mechanisms and physiological responses of rice leaves co-exposed to submicron-plastics and cadmium: Implication for food quality and security. J. Hazard. Mater. 2024, 463, 132957. [Google Scholar] [CrossRef]
- Harmon, S.M.; Chen, Q.; Ma, C.; Ji, M.; Yan, X.; Ji, R.; Shi, H. The effects of microplastic pollution on aquatic organisms. In Microplastic Contamination in Aquatic Environments; Elsevier: Amsterdam, The Netherlands, 2024; pp. 355–379. [Google Scholar]
- Shaaban, M.; Wang, X.-L.; Song, P.; Hou, X.; Wei, Z. Microplastic Pollution and E-Waste: Unraveling Sources, Mechanisms, and Impacts Across Environments. Curr. Opin. Green Sustain. Chem. 2024, 46, 100891. [Google Scholar] [CrossRef]
- Duan, L.-Y.; Zhang, Y.; Li, Y.-Y.; Li, X.-Q.; Liu, Y.-Q.; Li, B.L.; Ding, C.-Y.; Ren, X.-M.; Duan, P.-F.; Han, H. Effects of combined microplastic and cadmium pollution on sorghum growth, Cd accumulation, and rhizosphere microbial functions. Ecotoxicol. Environ. Saf. 2024, 277, 116380. [Google Scholar] [CrossRef]
- Peng, H.; Lin, Z.; Lu, D.; Yu, B.; Li, H.; Yao, J. How do polystyrene microplastics affect the adsorption of copper in soil? Sci. Total Environ. 2024, 924, 171545. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, O.; Aboko, J.; Ankapong, E.; Marfo, J.T.; Awuah-Boateng, N.Y.; Sarpong, K.; Dartey, E. A systematic review of heavy metals contamination in cosmetics. Cutan. Ocul. Toxicol. 2024, 43, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, R.Y., Jr.; Vleza, J.F.; Ompoc, J.; Oporto, M.D.; Mobo, D.F. Baseline Assessment of Marine Resources Caught by Fishermen in Selected Coastal Barangays of Cawayan, Masbate, Philippines. Int. J. Multidiscip. Appl. Bus. Educ. Res. 2023, 4, 2785–2701. [Google Scholar] [CrossRef]
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Mir, S.A.; Dar, Z.A.; Dar, S.A. Concerns and threats of contamination on aquatic ecosystems. In Bioremediation and Biotechnology: Sustainable Approaches to Pollution Degradation; Springer: Cham, Switzerland, 2020; pp. 1–26. [Google Scholar] [CrossRef]



| Growth Performance | Control | MPs | Cd | MPs + Cd |
|---|---|---|---|---|
| FLE (cm) | 7.25 ± 0.30 b | 8.75 ± 0.62 a | 6.25 ± 0.15 c | 5.04 ± 0.47 d |
| FLS (cm) | 5.77 ± 1.81 b | 13.36 ± 1.04 a | 2.43 ± 1.06 c | 0.40 ± 0.17 c |
| NTB (individual) | 13.00 ± 3.00 a | 10.67 ± 2.08 ab | 6.33 ± 2.31 b | 5.67 ± 3.79 b |
| IW (g) | 3.47 ± 0.65 a | 3.87 ± 0.29 a | 3.49 ± 0.51 a | 3.71 ± 0.51 a |
| FW (g) | 5.95 ± 0.45 a | 8.48 ± 1.11 b | 4.35 ± 0.88 bc | 4.52 ± 0.55 c |
| WGR (%) | 74.14 ± 7.56 b | 119.10 ± 38.00 a | 24.23 ± 13.44 c | 24.53 ± 29.82 c |
| SGR (%/day) | 3.93 ± 0.29 a | 5.56 ± 1.33 a | 1.52 ± 0.77 b | 1.42 ± 1.82 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Zheng, H.; Wu, Y.; Lin, J.; Ma, X.; Xing, Y.; Ou, H.; Vasquez, H.E.; Zheng, X.; Yu, F.; et al. Microplastic-Enhanced Cadmium Toxicity: A Growing Threat to the Sea Grape, Caulerpa lentillifera. Antioxidants 2024, 13, 1268. https://doi.org/10.3390/antiox13101268
Zhou W, Zheng H, Wu Y, Lin J, Ma X, Xing Y, Ou H, Vasquez HE, Zheng X, Yu F, et al. Microplastic-Enhanced Cadmium Toxicity: A Growing Threat to the Sea Grape, Caulerpa lentillifera. Antioxidants. 2024; 13(10):1268. https://doi.org/10.3390/antiox13101268
Chicago/Turabian StyleZhou, Weilong, Haolong Zheng, Yingyin Wu, Junyi Lin, Xiaofei Ma, Yixuan Xing, Huilong Ou, Hebert Ely Vasquez, Xing Zheng, Feng Yu, and et al. 2024. "Microplastic-Enhanced Cadmium Toxicity: A Growing Threat to the Sea Grape, Caulerpa lentillifera" Antioxidants 13, no. 10: 1268. https://doi.org/10.3390/antiox13101268
APA StyleZhou, W., Zheng, H., Wu, Y., Lin, J., Ma, X., Xing, Y., Ou, H., Vasquez, H. E., Zheng, X., Yu, F., & Gu, Z. (2024). Microplastic-Enhanced Cadmium Toxicity: A Growing Threat to the Sea Grape, Caulerpa lentillifera. Antioxidants, 13(10), 1268. https://doi.org/10.3390/antiox13101268







