Artemisia Ordosica Polysaccharides Enhance Antioxidant Capacity of Peripheral Blood Lymphocytes in Poultry Through Nrf2/Keap1 and TLR4/NF-κB Signal Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of AOP
2.2. Collection and Separation Cultivation of Peripheral Blood Lymphocytes
2.3. Treatment of PBLs with Signaling Molecules Inhibitors
2.4. Cell Viability Measurement
2.5. Assay of Antioxidant Indicators in Cell Sample
2.6. RNA Preparation and Fluorescence Quantitative Real-Time PCR
2.7. Data Processing and Statistical Analysis
3. Results
3.1. Effects of AOP on the Cell Viability of PBLs
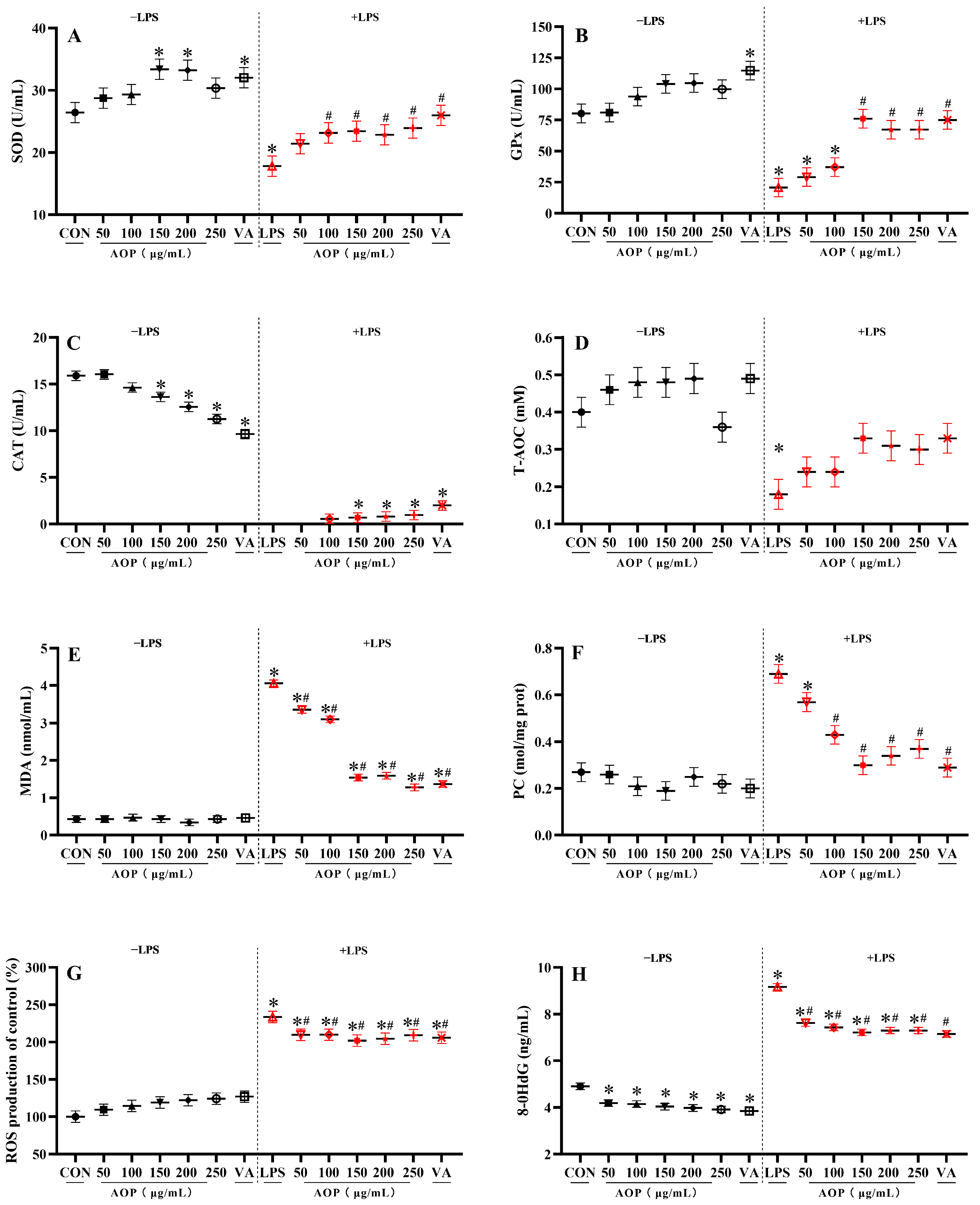
3.2. Effects of AOP on Antioxidant Enzyme Activity and Oxidative Stress Metabolites in PBLs
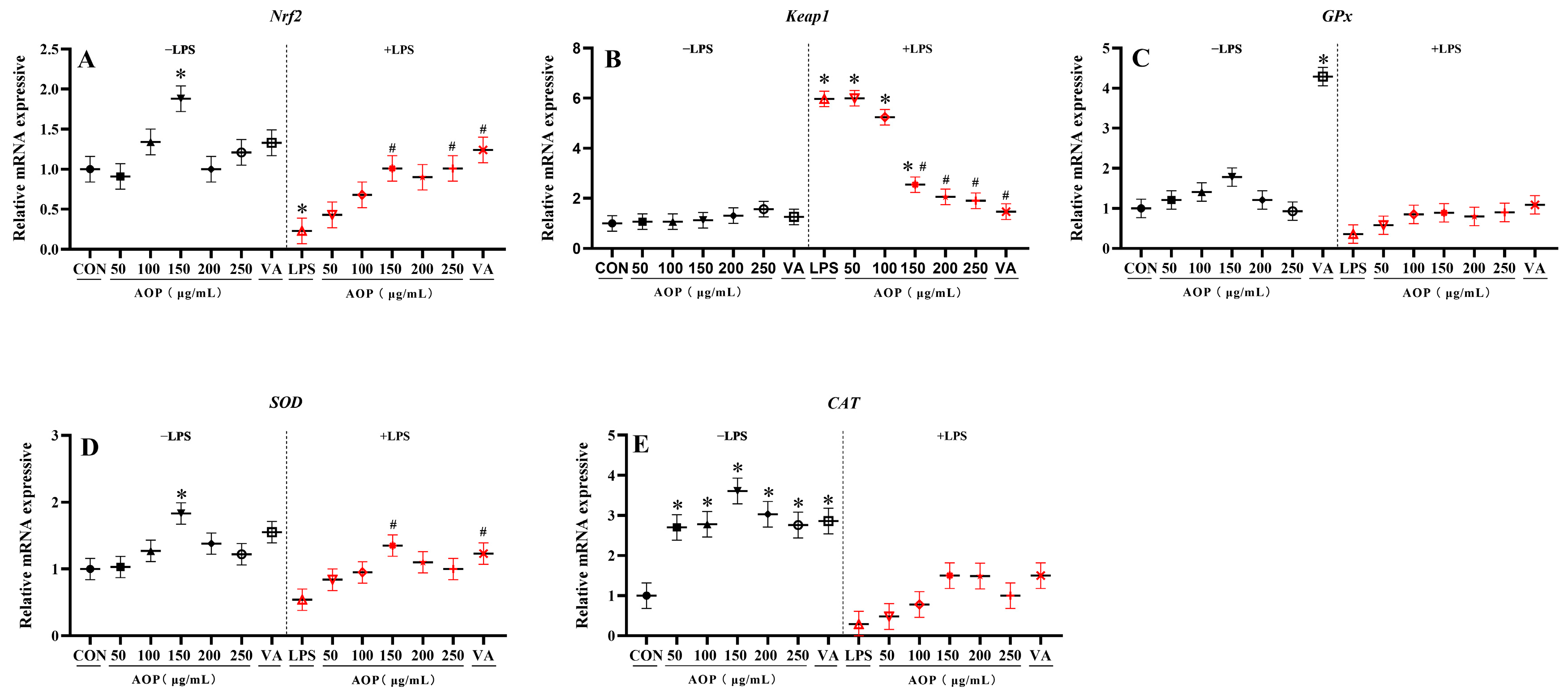
3.3. Effects of AOP on Nrf2 Signaling Pathway-Related Gene Expression in PBLs
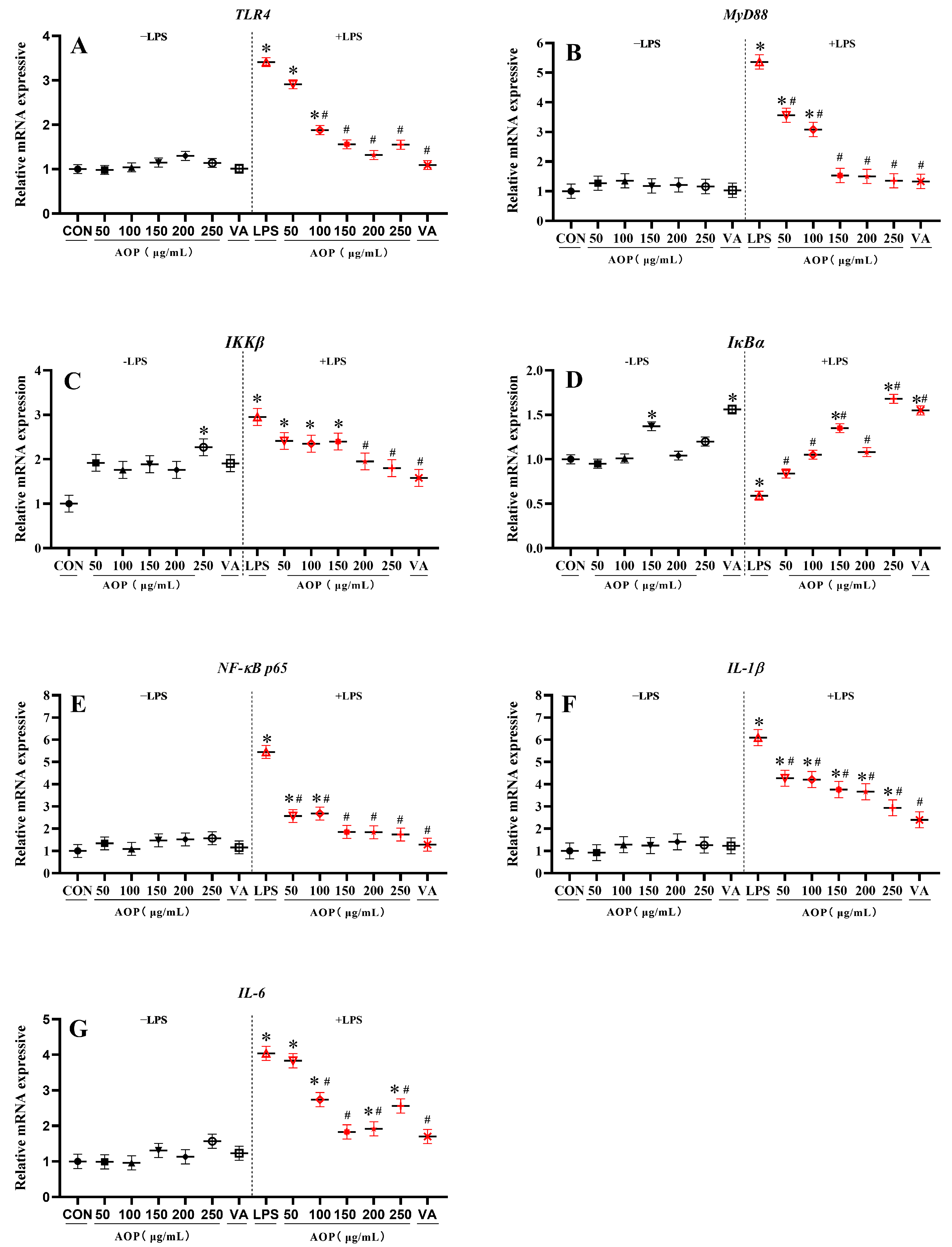
3.4. Effects of AOP on NF-κB Signaling Pathway-Related Gene Expression in PBLs
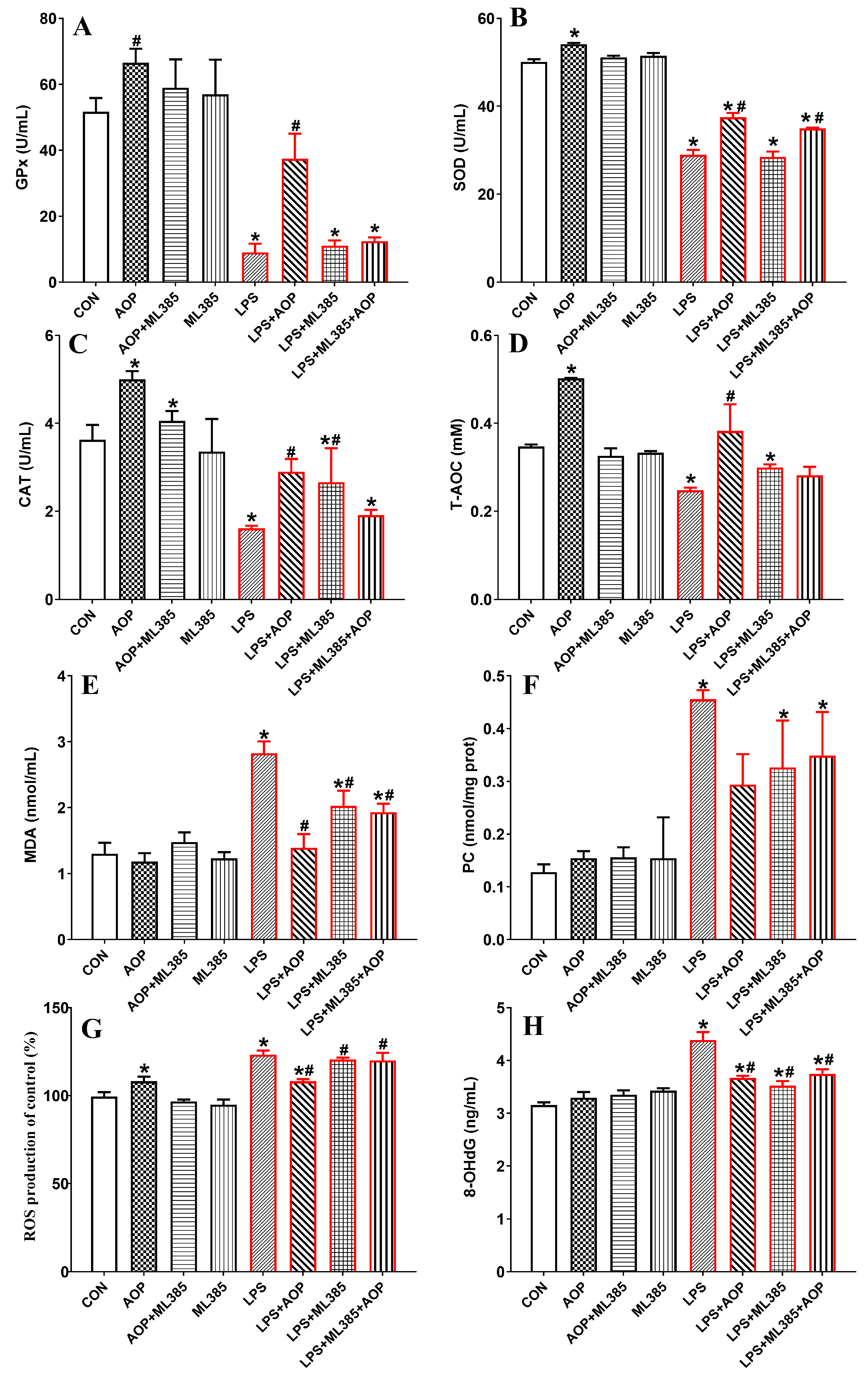
3.5. Effects of AOP on Antioxidative Enzymes and Oxidative Stress Metabolites in PBLs Challenged by LPS and Blocked by ML385
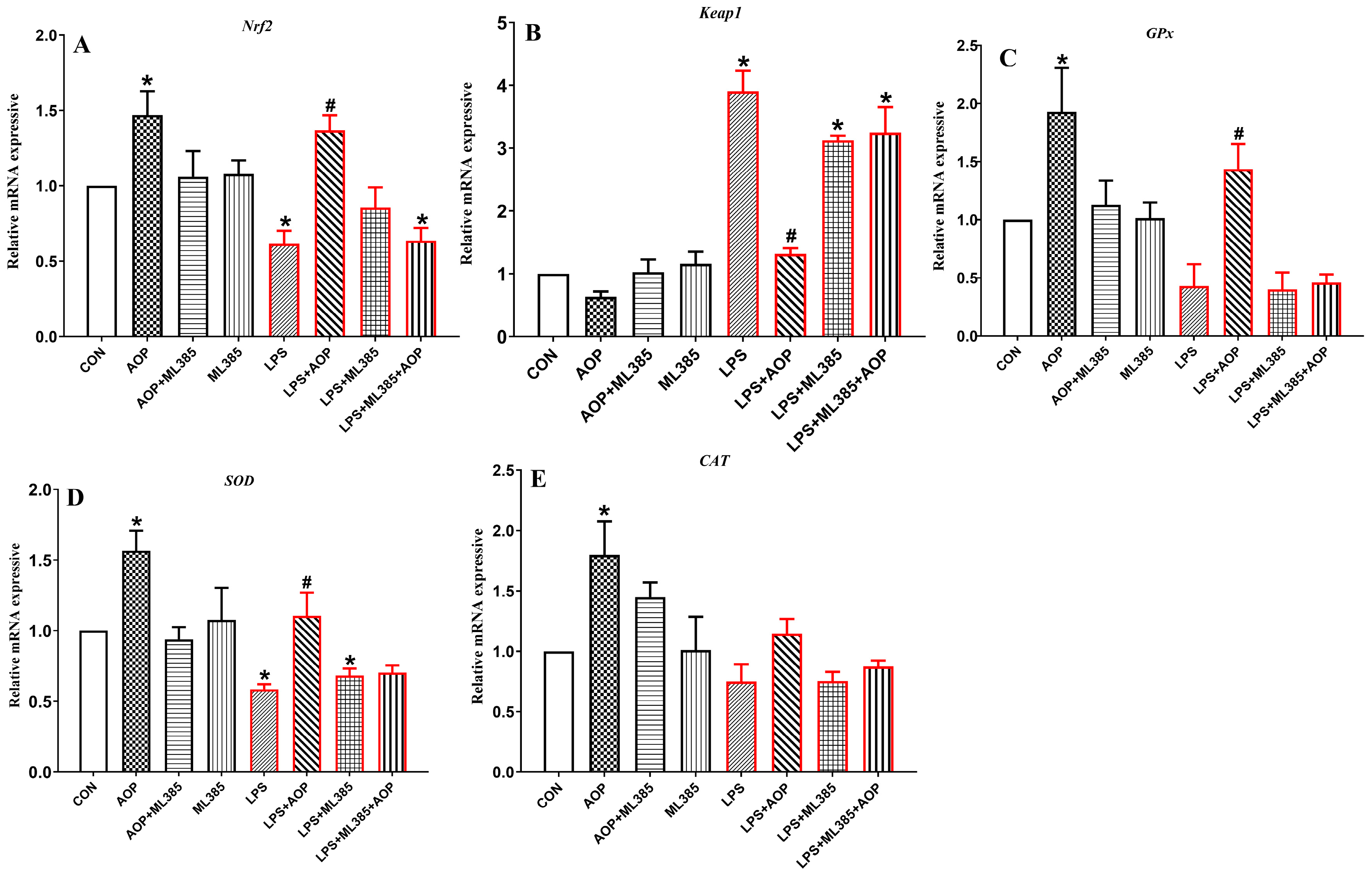
3.6. Effects of AOP on the Gene Expression of Nrf2 Signaling Pathway-Related Factors in PBLs Challenged by LPS and Blocked by ML385
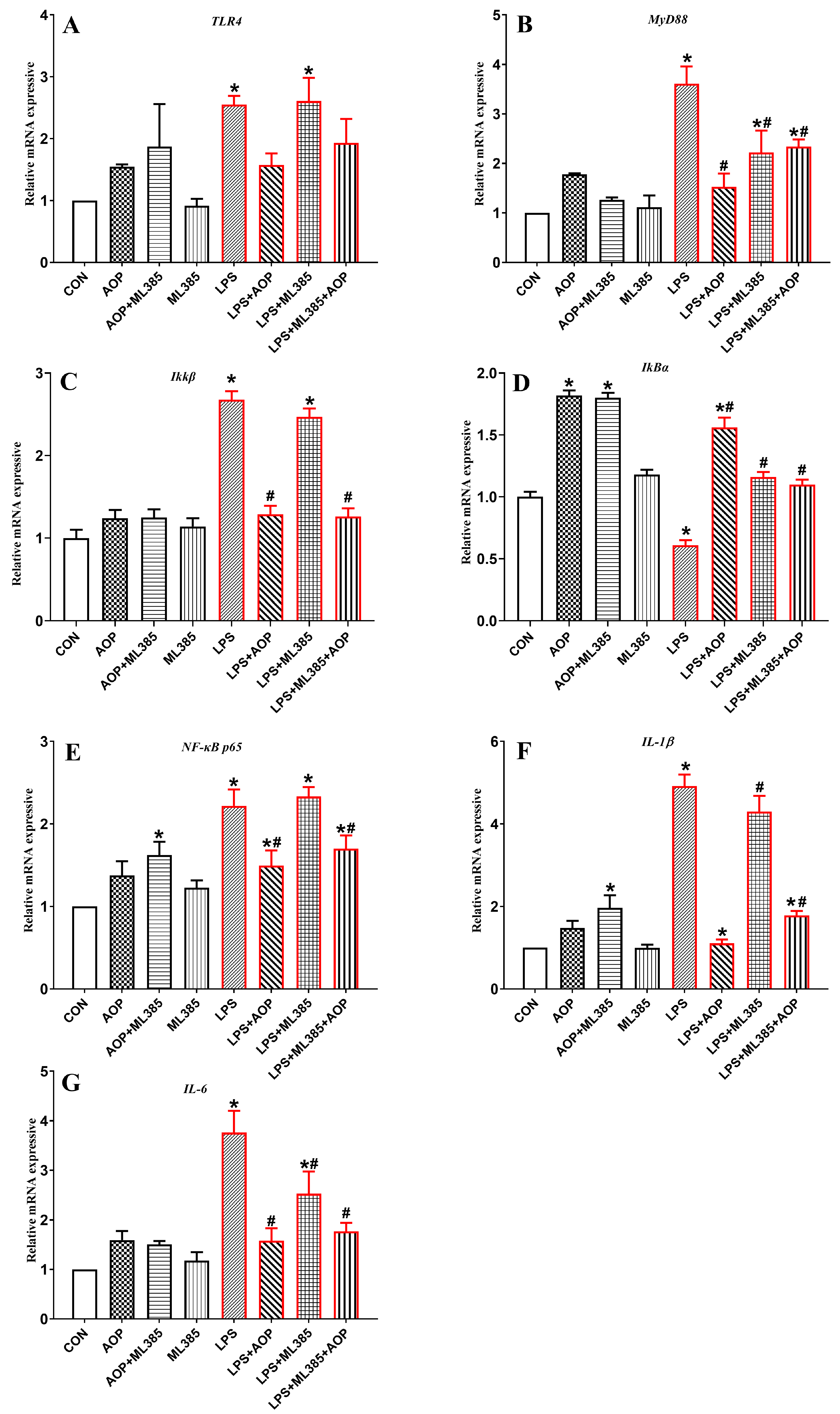
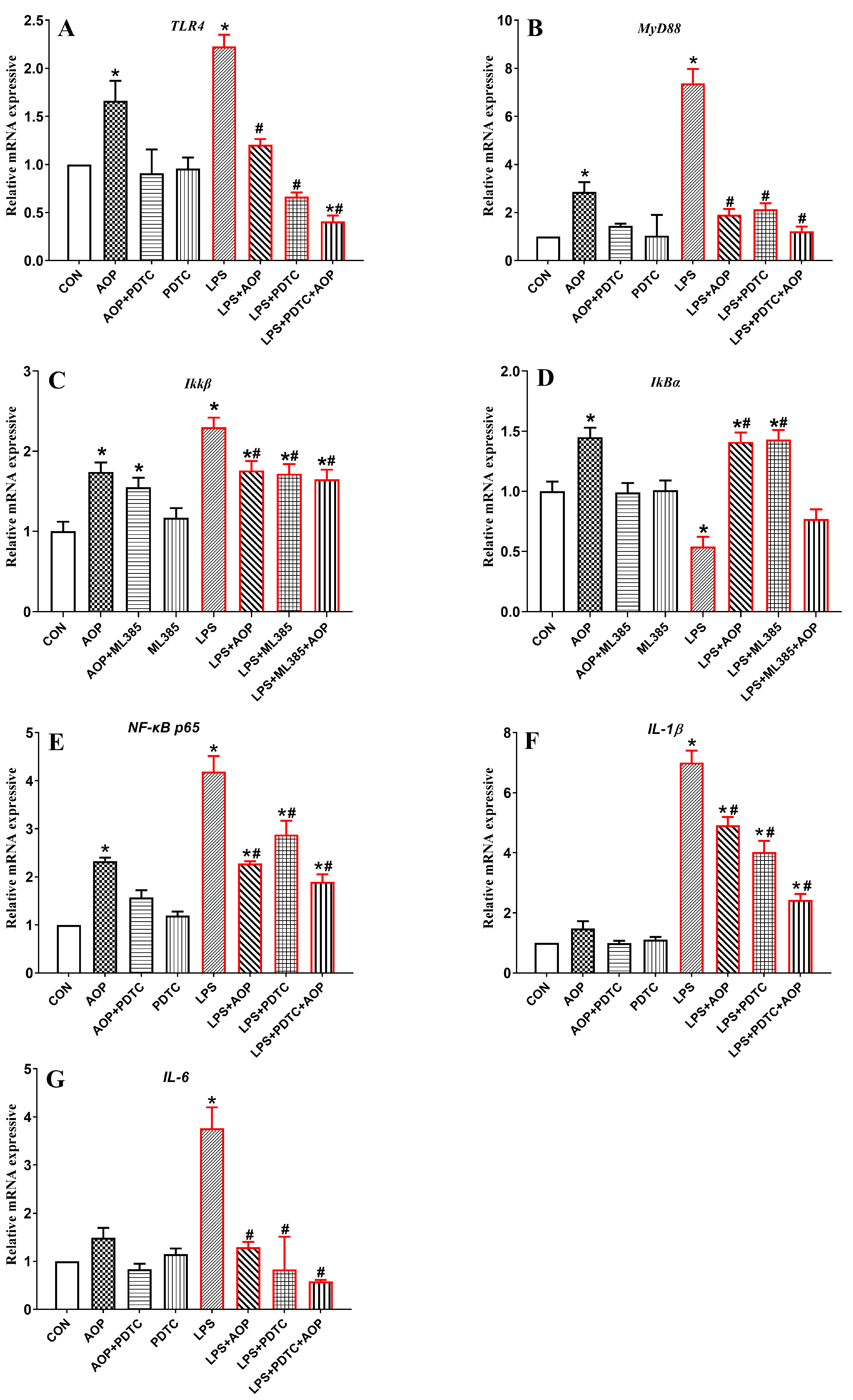
3.7. Effects of AOP on the Gene Expression of NF-κB Signaling Pathway-Related Factors in PBLs Challenged by LPS and Blocked by ML385
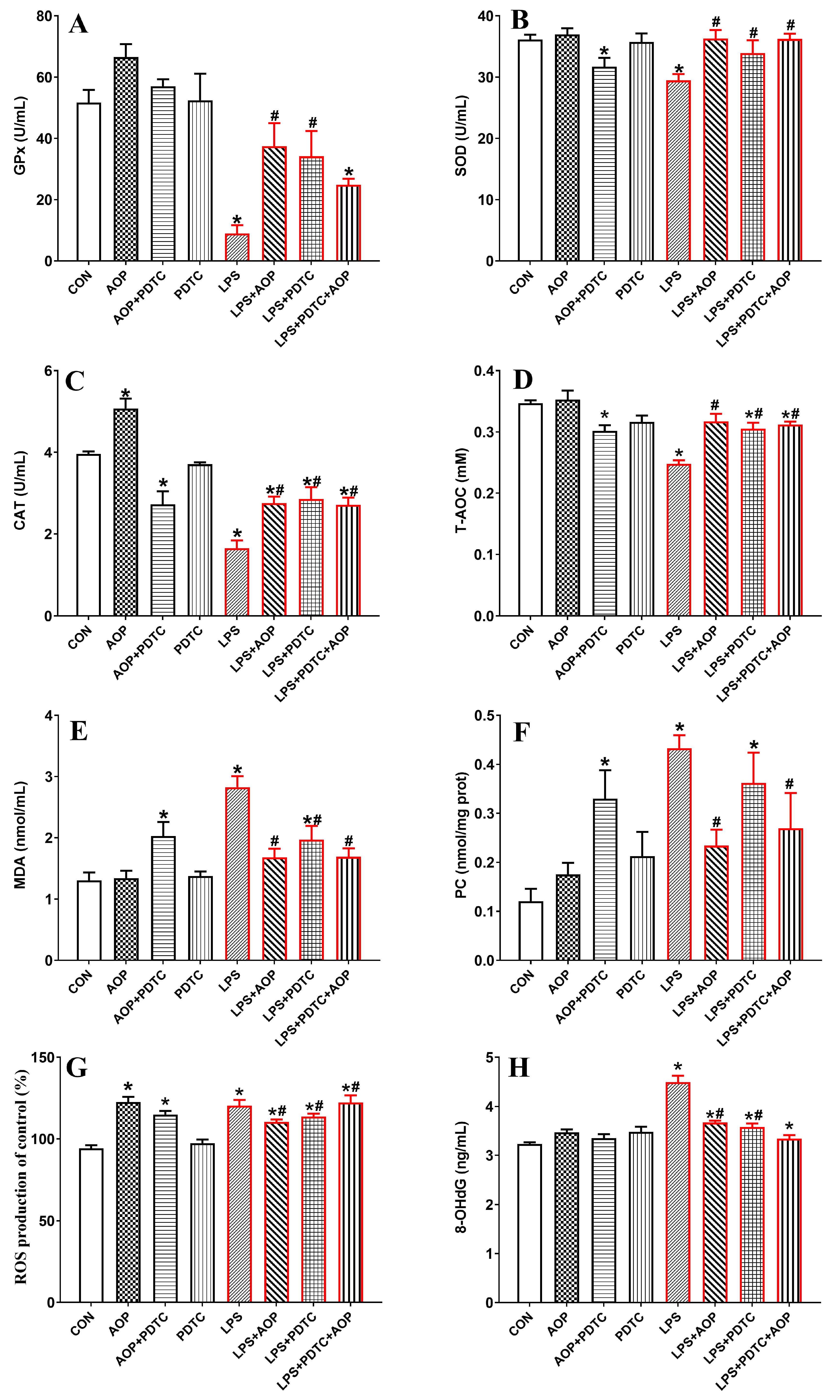
3.8. Effects of AOP on Antioxidative Enzymes and Oxidative Stress Metabolites in PBLs Challenged by LPS and Blocked by PDTC
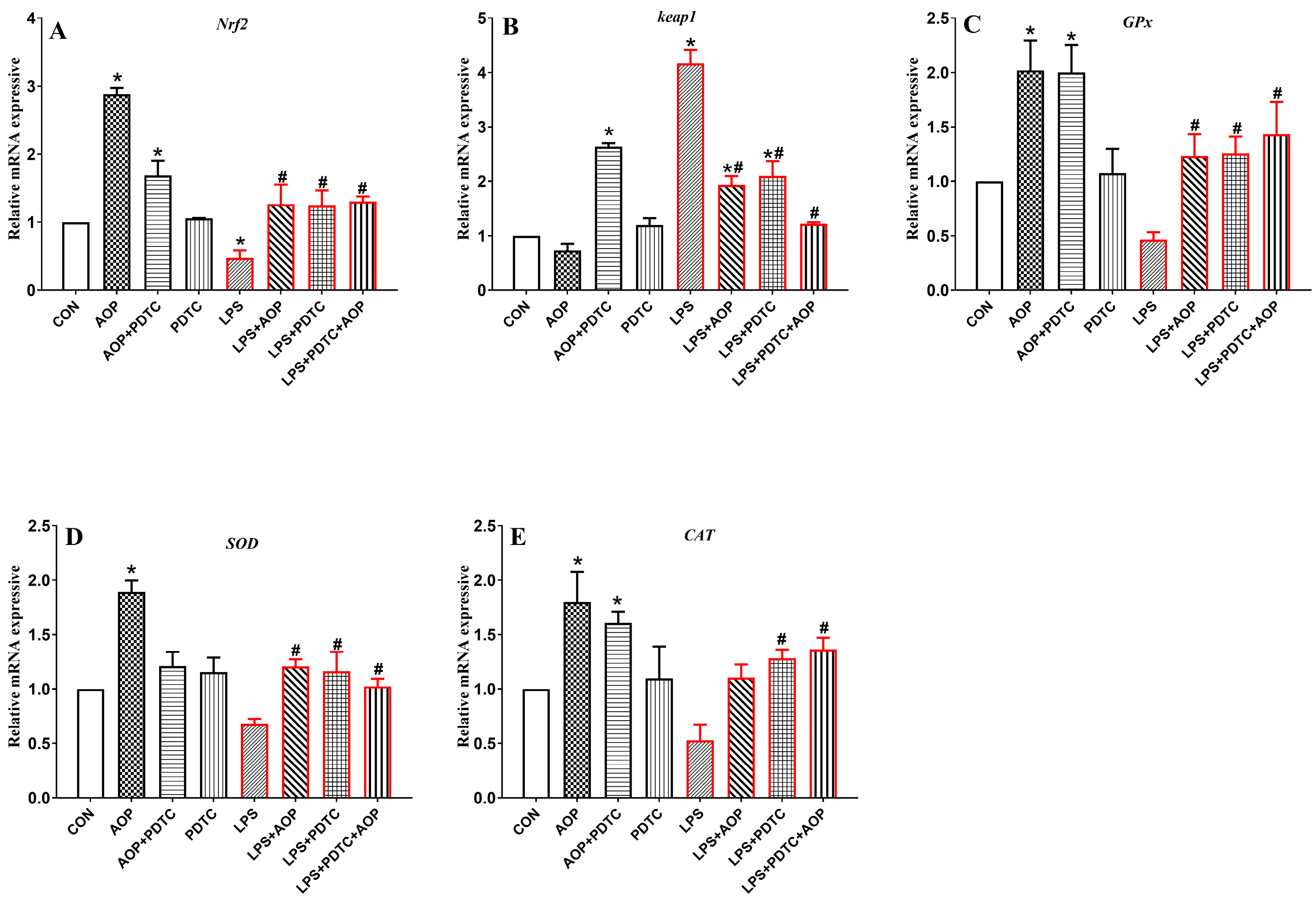
3.9. Effects of AOP on the Gene Expression of Nrf2 Signaling Pathway-Related Factors in PBLs Challenged by LPS and Blocked by PDTC
3.10. Effects of AOP on the Gene Expression of NF-κB Signaling Pathway-Related Factors in PBLs
4. Discussion
4.1. Alleviating Effect of AOP on Oxidative Stress of PBLs
4.2. AOP Alleviated Oxidative Stress Through Nrf2/Keap1 and TLR4/NF-κB Pathway
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S. From Challenge to Opportunity: Addressing Oxidative Stress in Animal Husbandry. Antioxidants 2023, 12, 1543. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; McCarl, B.; Fei, C. Climate Change and Livestock Production: A Literature Review. Atmosphere 2022, 13, 140. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Gusti, A.M.T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10, 595. [Google Scholar] [CrossRef]
- Xiao, J.L.; Liu, H.Y.; Sun, C.C.; Tang, C.F. Regulation of Keap1-Nrf2 signaling in health and diseases. Mol. Biol. Rep. 2024, 51, 809. [Google Scholar] [CrossRef]
- Wang, H.; Pan, L.; Si, L.; Ji, R.W.; Cao, Y.H. Effects of Nrf2-Keap1 signaling pathway on antioxidant defense system and oxidative damage in the clams Ruditapes philippinarum exposure to PAHs. Environ. Sci. Pollut. Res. 2021, 28, 33060–33071. [Google Scholar] [CrossRef]
- Yue, J.; Guo, P.; Jin, Y.; Li, M.; Hu, X.; Wang, W.; Wei, X.; Qi, S. Momordica charantia polysaccharide ameliorates D-galactose-induced aging through the Nrf2/β-Catenin signaling pathway. Metab. Brain Dis. 2023, 38, 1067–1077. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Jiao, H.X.; Sun, J.Z.; Obinwanne Okoye, C.; Zhang, H.X.; Li, Y.; Lu, X.C.; Wang, Q.Q.; Liu, J. Structure-activity relationships of bioactive polysaccharides extracted from macroalgae towards biomedical application: A review. Carbohyd. Polym. 2024, 324, 121533. [Google Scholar] [CrossRef]
- Yang, Q.; Chang, S.L.; Tian, Y.M.; Li, W.; Ren, J.L. Glucan polysaccharides isolated from Lactarius hatsudake Tanaka mushroom: Structural characterization and in vitro bioactivities. Carbohyd. Polym. 2024, 337, 122171. [Google Scholar] [CrossRef]
- Wen, C.; Wu, J.P.; Zhao, X.Y.; Li, Z.X.; Jie, Y.; Shao, T.L.; Hou, X.F.; Zhou, L.T.; Wang, C.F.; Wang, G.D.; et al. Structural elucidation of an active polysaccharide from Radix Puerariae lobatae and its protection against acute alcoholic liver disease. Carbohyd. Polym. 2024, 325, 121565. [Google Scholar] [CrossRef]
- Pang, Y.R.; Peng, Z.G.; Ding, K. An in-depth review: Unraveling the extraction, structure, bio-functionalities, target molecules, and applications of pectic polysaccharides. Carbohyd. Polym. 2024, 343, 122457. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, A.; Moujir, L.M.; Sousa, P.M.C.; Seca, A.M.L. Research Advances on Health Effects of Edible Artemisia Species and Some Sesquiterpene Lactones Constituents. Foods 2021, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Wang, J.H.; Zhou, C.Y.; Chen, J.M.; Zhang, N.; Zhao, N.; Han, X.Y.; Niu, Y.X.; Feng, Y.B.; Du, G.H. Ethno-medicinal study of Artemisia ordosica Krasch. (traditional Chinese/Mongolian medicine) extracts for the treatment of allergic rhinitis and nasosinusitis. J. Ethnopharmacol. 2020, 10, 112262. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; She, Z.; Su, C.; Zhao, Q.; Wang, S.; Xiao, B. The effects and the mechanisms of naringenin from Artemisia ordosica Krasch on allergic rhinitis based on mast cell degranulation model and network pharmacology. J. Pharm. Pharmacol. 2022, 74, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.Y.; Zheng, Y.K.; Yang, S.; Zhang, L.H.; Guo, S.W.; Shi, L.L.; Xu, Y.Q.; Jin, X.; Yan, S.M.; Shi, B.L. Artemisia ordosica Polysaccharide Alleviated Lipopolysaccharide-induced Oxidative Stress of Broilers via Nrf2/Keap1 and TLR4/NF-κB Pathway. Ecotox Environ. Safe 2022, 223, 112566. [Google Scholar] [CrossRef]
- Xing, Y.Y.; Zheng, Y.K.; Yang, S.; Zhang, L.H.; Guo, S.W.; Shi, L.L.; Xu, Y.Q.; Jin, X.; Yan, S.M.; Shi, B.L. Artemisia ordosica polysaccharide ameliorated LPS-induced growth inhibition and intestinal injury in broilers through enhancing immune-regulation and antioxidant capacity. J. Nutr. Biochem. 2023, 115, 109284. [Google Scholar] [CrossRef]
- Du, H.D.; Xing, Y.Y.; Xu, Y.Q.; Jin, X.; Yan, S.M.; Shi, B.L. Dietary Artemisia Ordosica Polysaccharide Enhances Spleen and Intestinal Immune Response of Broiler Chickens. Biology 2023, 12, 1390. [Google Scholar] [CrossRef]
- Zhu, X.; Han, X.; Wang, J. Sufentanil-induced Nrf2 protein ameliorates cerebral ischemia-reperfusion injury through suppressing neural ferroptosis. Int. J. Biol. Macromol. 2024, 279, 135109. [Google Scholar] [CrossRef]
- Chen, D.; Lou, Q.; Song, X.J.; Kang, F.; Liu, A.; Zheng, C.; Li, Y.; Wang, D.; Qun, S.; Zhang, Z.; et al. Microglia govern the extinction of acute stress-induced anxiety-like behaviors in male mice. Nat. Commun. 2024, 15, 449. [Google Scholar] [CrossRef]
- Tariq, M.; Chen, R.; Yuan, H.; Liu, Y.; Wu, Y.; Wang, J.; Xia, C. De Novo Transcriptomic Analysis of Peripheral Blood Lymphocytes from the Chinese Goose: Gene Discovery and Immune System Pathway Description. PLoS ONE 2015, 10, e0121015. [Google Scholar] [CrossRef]
- Jiang, N.; Yang, T.; Han, H.; Shui, J.; Hou, M.; Wei, W.; Kumar, G.; Song, L.; Ma, C.; Li, X.; et al. Exploring Research Trend and Hotspots on Oxidative Stress in Ischemic Stroke (2001–2022): Insights from Bibliometric. Mol. Neurobiol. 2024, 61, 6200–6216. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Chen, J.; Hou, R.; Tian, W.; Liu, K.; Wang, D.; Lu, Y.; Liu, J.; Wu, Y.; Hu, Y. The antioxidant action and mechanism of selenizing Schisandra chinensis polysaccharide in chicken embryo hepatocyte. Int. J. Biol. Macromol. 2017, 98, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Li, K.; Min, Y.; Qiu, B.; Huang, X.; Luo, J.; Qi, L.; Kang, M.; Xia, P.; Qiao, H.; et al. Rehmannia glutinosa Polysaccharides: Optimization of the Decolorization Process and Antioxidant and Anti-Inflammatory Effects in LPS-Stimulated Porcine Intestinal Epithelial Cells. Antioxidants 2023, 12, 914. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X.; Dong, H. Current advances in the anti-inflammatory effects and mechanisms of natural polysaccharides. Crit. Rev. Food Sci. Nutr. 2023, 63, 5890–5910. [Google Scholar] [CrossRef]
- Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ma, J.; Li, T.; Li, P.; Yan, D.; Zhu, J.; Zhang, X. Advances in the Preparation, Structure and Bioactivity of Polysaccharides from Lycium ruthenicum Murr.: A Review. Foods 2024, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yang, F.; Fu, R.; Trinh, B.; Sun, N.; Liu, J.; Kumar, A.; Baglieri, J.; Siruno, J.; Le, M.; et al. Collagenolysis-dependent DDR1 signalling dictates pancreatic cancer outcome. Nature 2022, 610, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Ruan, D.D.; Wu, W.Z.; Wu, M.; Wu, Q.Y.; Wang, H.L.; Ji, Y.Y.; Zhang, Y.P.; Lin, X.F.; Fang, Z.T.; et al. Potential regulatory role of the Nrf2/HMGB1/TLR4/NF-κB signaling pathway in lupus nephritis. Pediatr. Rheumatol. 2023, 21, 130. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Z.; Li, P.; Gao, Y.; Shi, Y. Bakuchiol regulates TLR4/MyD88/NF-κB and Keap1/Nrf2/HO-1 pathways to protect against LPS-induced acute lung injury in vitro and in vivo. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 3301–3312. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.; Lee, J.H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Perez, V.I. Molecular Mechanisms of Nrf2 in Inflammation: Interactions between Nrf2 and Inflammatory Mediators. In Nrf2 and its Modulation in Inflammation; Progress in Inflammation Research; Deng, H., Ed.; Springer: Cham, Switzerland, 2020; Volume 85. [Google Scholar] [CrossRef]
- Ando, M.; Magi, S.; Seki, M.; Suzuki, Y.; Kasukawa, T.; Lefaudeux, D.; Hoffmann, A.; Okada, M. IκBα is required for full transcriptional induction of some NFκB-regulated genes in response to TNF in MCF-7 cells. NPJ Syst. Biol. Appl. 2021, 7, 42. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Zheng, Y.; Chen, L.; Xu, Y.; Jin, X.; Hong, L.; Yan, S.; Shi, B. Artemisia Ordosica Polysaccharides Enhance Antioxidant Capacity of Peripheral Blood Lymphocytes in Poultry Through Nrf2/Keap1 and TLR4/NF-κB Signal Pathway. Antioxidants 2024, 13, 1308. https://doi.org/10.3390/antiox13111308
Xing Y, Zheng Y, Chen L, Xu Y, Jin X, Hong L, Yan S, Shi B. Artemisia Ordosica Polysaccharides Enhance Antioxidant Capacity of Peripheral Blood Lymphocytes in Poultry Through Nrf2/Keap1 and TLR4/NF-κB Signal Pathway. Antioxidants. 2024; 13(11):1308. https://doi.org/10.3390/antiox13111308
Chicago/Turabian StyleXing, Yuanyuan, Yankai Zheng, Lu Chen, Yuanqing Xu, Xiao Jin, Li Hong, Sumei Yan, and Binlin Shi. 2024. "Artemisia Ordosica Polysaccharides Enhance Antioxidant Capacity of Peripheral Blood Lymphocytes in Poultry Through Nrf2/Keap1 and TLR4/NF-κB Signal Pathway" Antioxidants 13, no. 11: 1308. https://doi.org/10.3390/antiox13111308
APA StyleXing, Y., Zheng, Y., Chen, L., Xu, Y., Jin, X., Hong, L., Yan, S., & Shi, B. (2024). Artemisia Ordosica Polysaccharides Enhance Antioxidant Capacity of Peripheral Blood Lymphocytes in Poultry Through Nrf2/Keap1 and TLR4/NF-κB Signal Pathway. Antioxidants, 13(11), 1308. https://doi.org/10.3390/antiox13111308







