Ole-Oxy, a Semi-Synthetic Analog of Oleuropein, Ameliorates Acute Skin and Colon Inflammation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
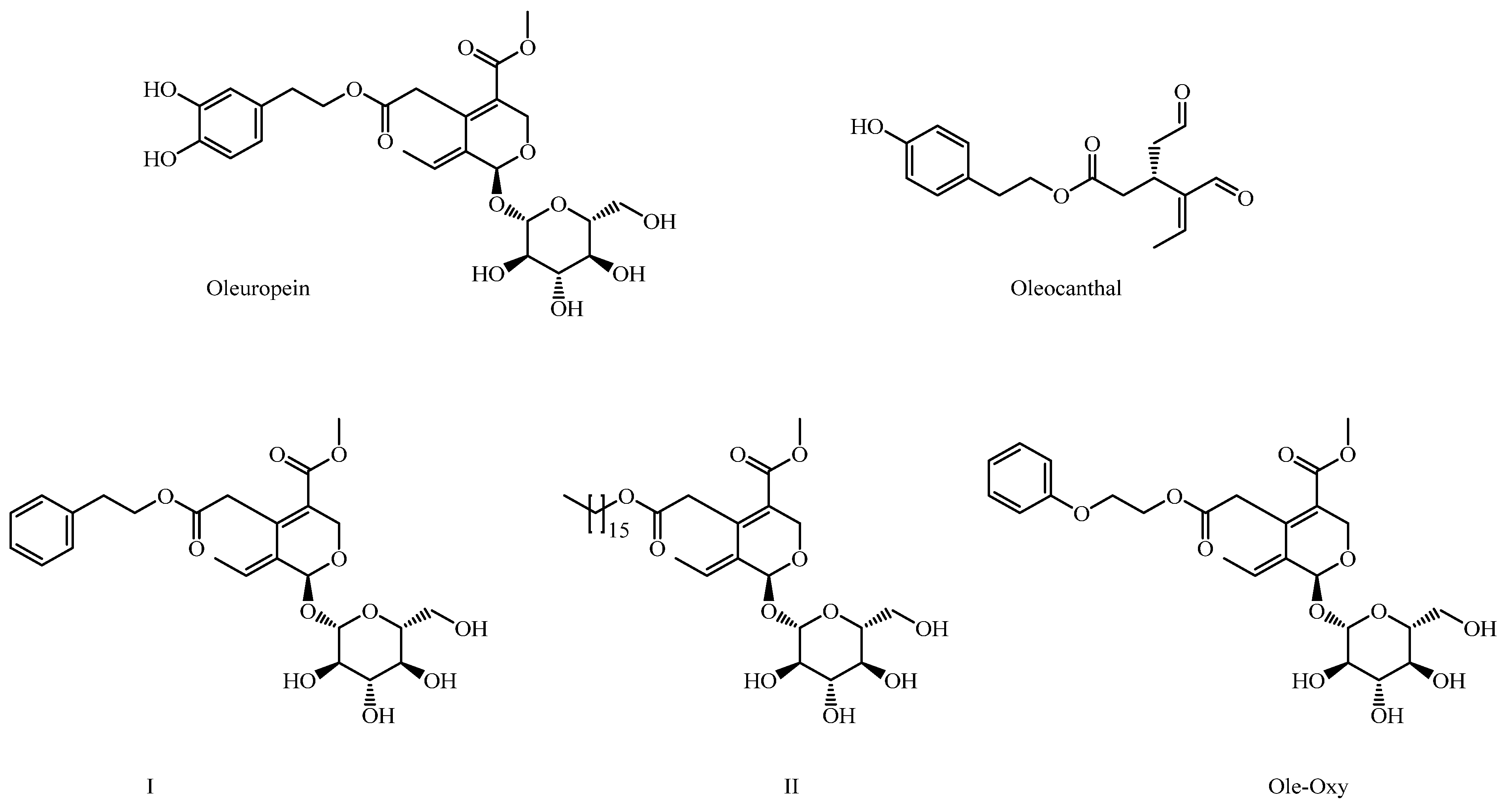
2.2. Chemistry
2.2.1. Synthesis of Oleoside or (4S,5E,6S)-4-(Carboxymethyl)-5-ethylidene-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4H-pyran-3-carboxylic Acid (1)
2.2.2. Synthesis of Methyl (4S,5E,6S)-4-[2-[2-(Phenoxy)ethoxy]-2-oxoethyl]-5-ethylidene-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4H-pyran-3-carboxylate (Ole-Oxy)
2.3. Cells and Cell Cultures
2.4. Cell Viability Assay
2.5. Assessment of Cytokine Levels
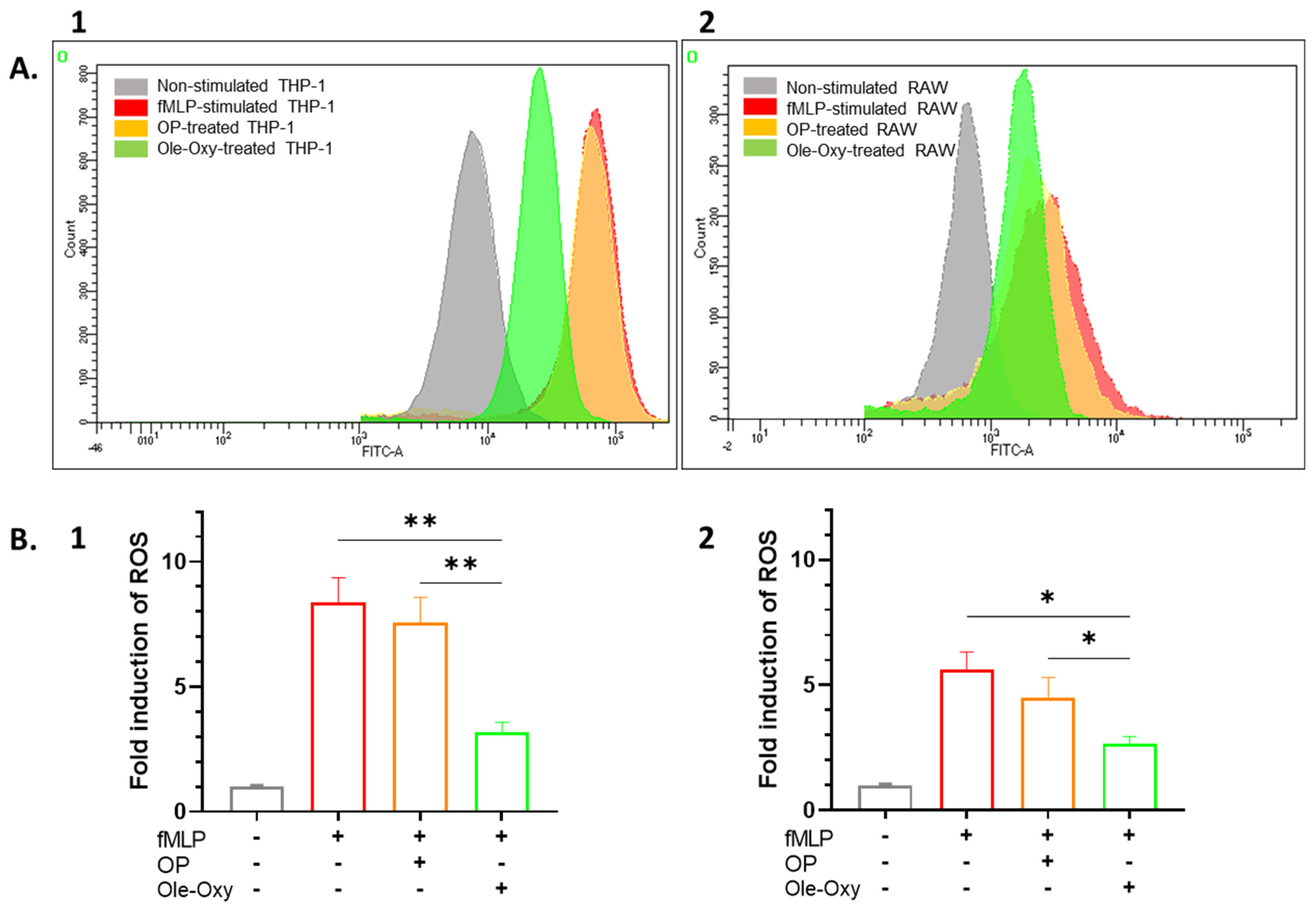
2.6. Measurement of Reactive Oxygen Species (ROS)
2.7. Mouse Models
2.8. Toxicity Studies of Ole-Oxy
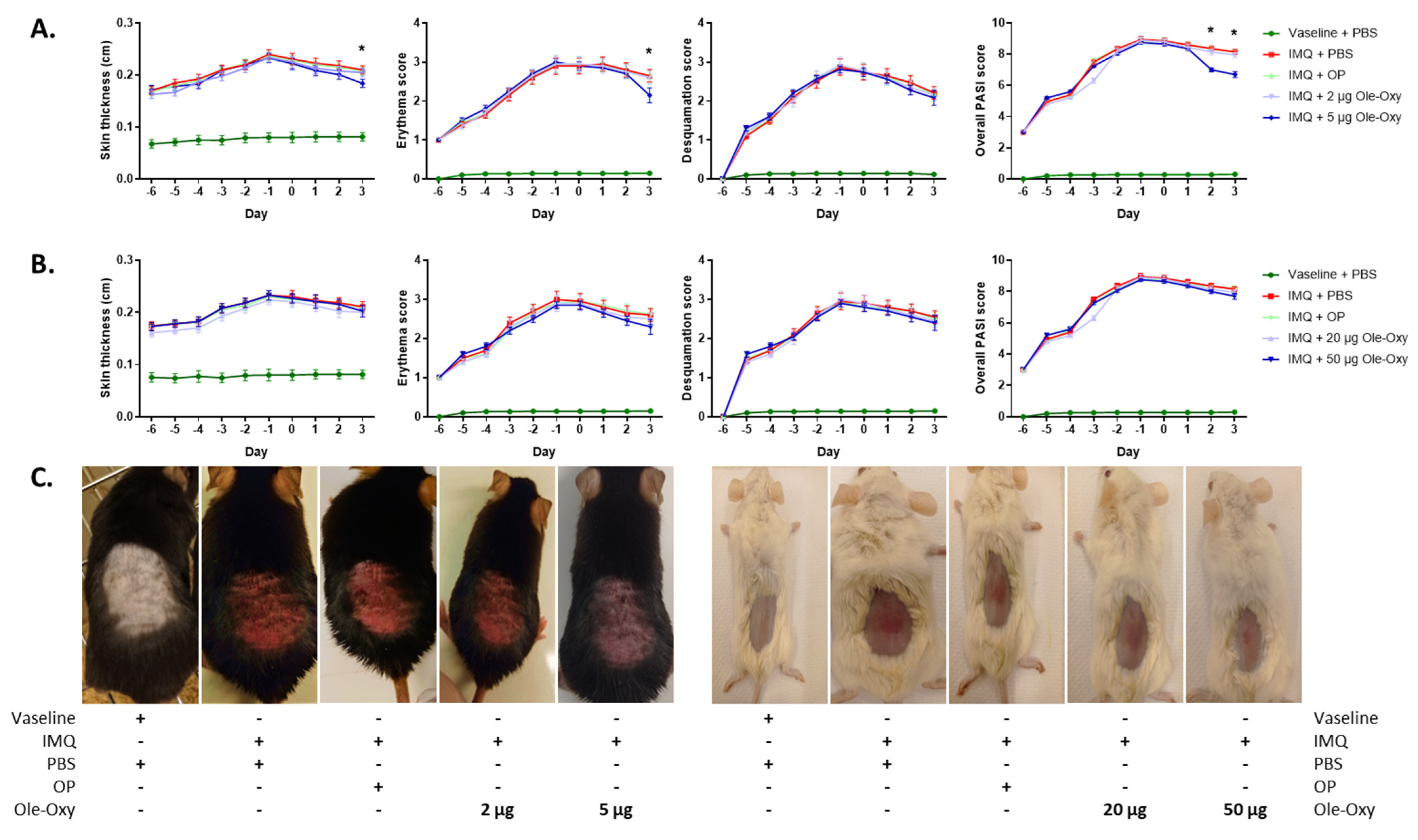
2.9. Skin Inflammation Model
2.10. Colon Inflammation Model
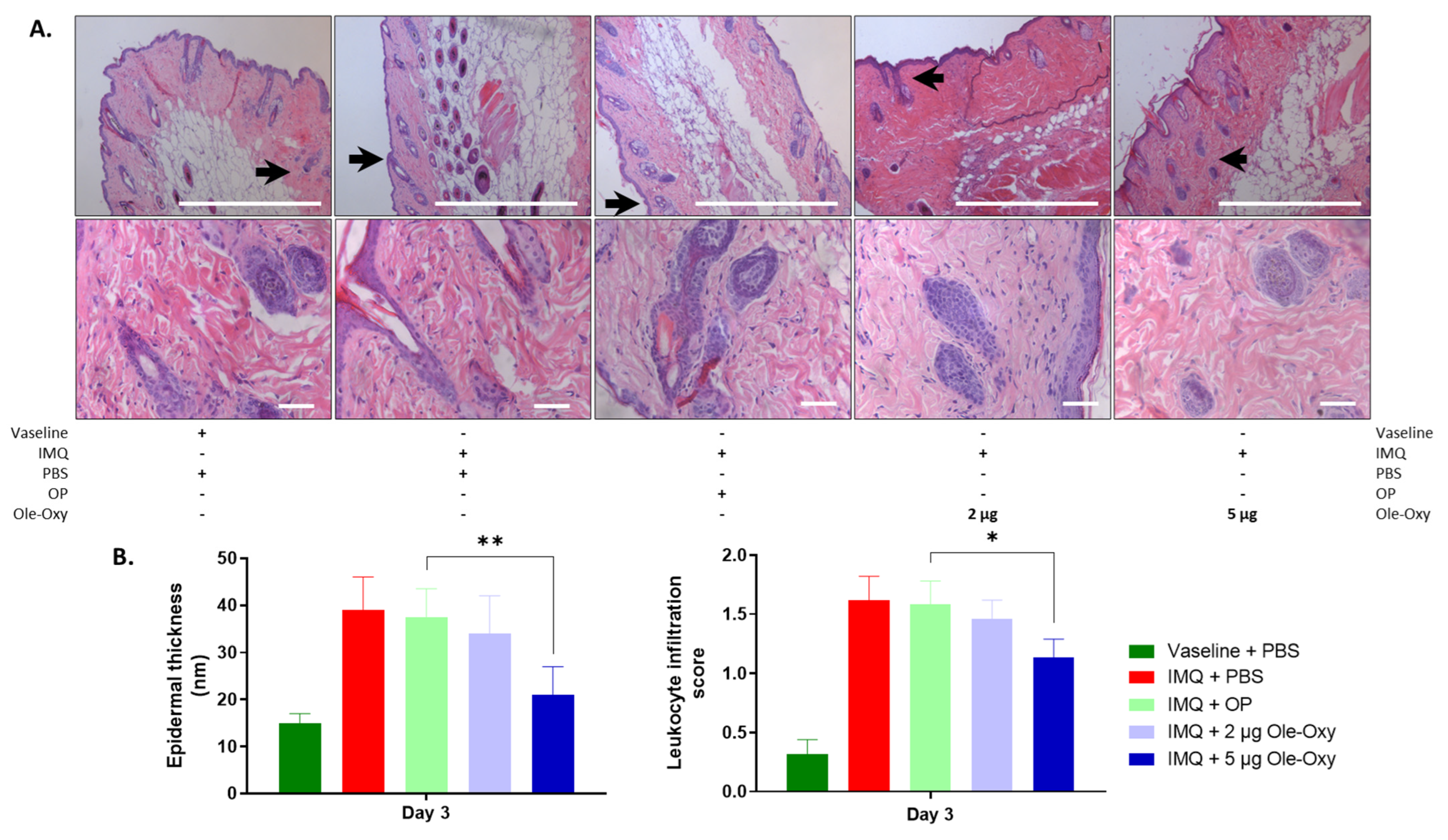
2.11. Tissue Collection and Histological Staining
2.12. Statistical Analysis
3. Results
3.1. Chemical Synthesis of Ole-Oxy
3.2. Ole-Oxy Reduces Inflammatory Responses in Cultured Human and Mouse Macrophages
3.3. Ole-Oxy Shows Higher Antioxidant Activity Compared to OP
3.4. Toxicity Assessment of Ole-Oxy
3.5. Ole-Oxy Reduces Imiquimod-Induced Acute Skin Inflammation
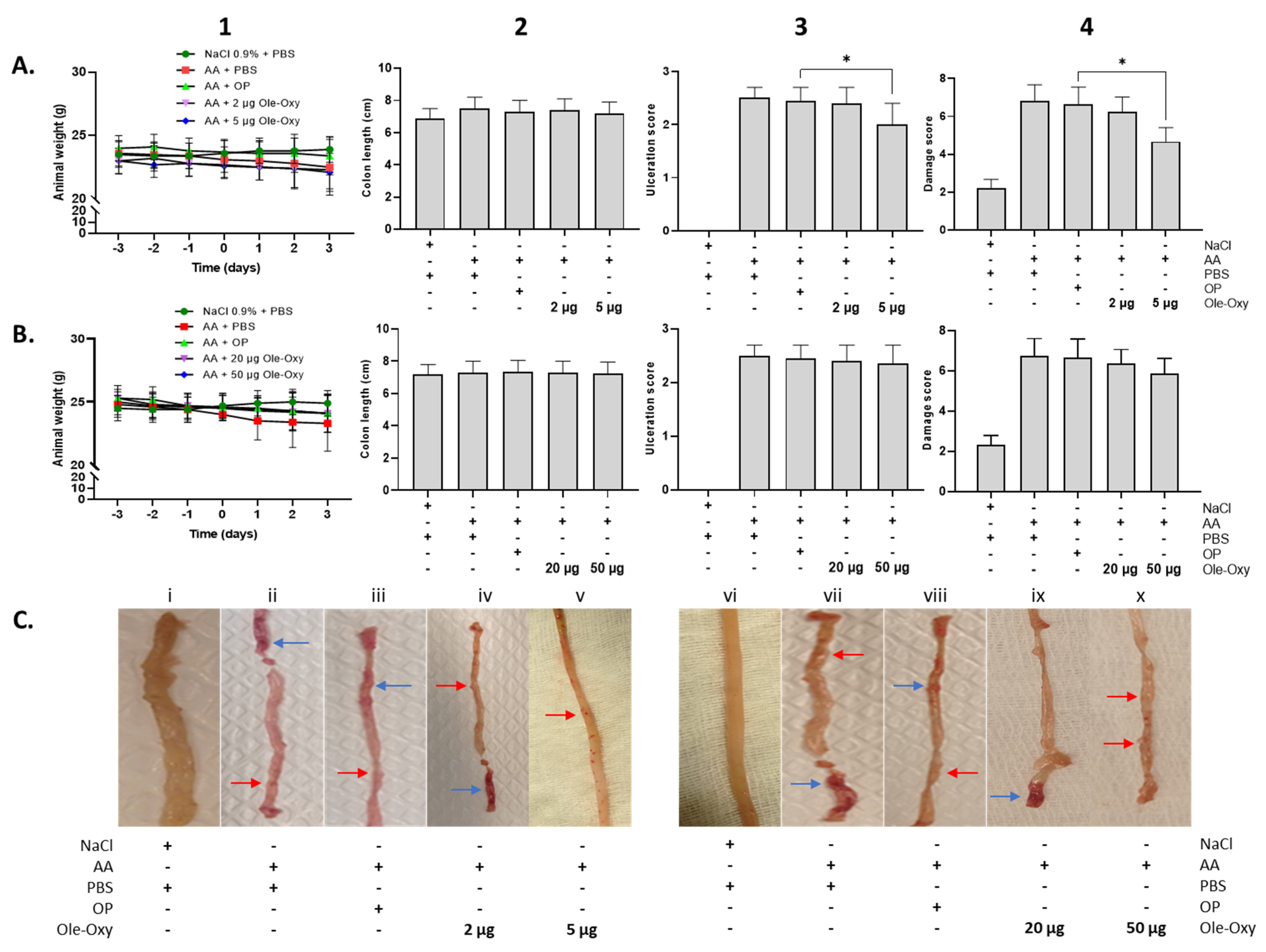
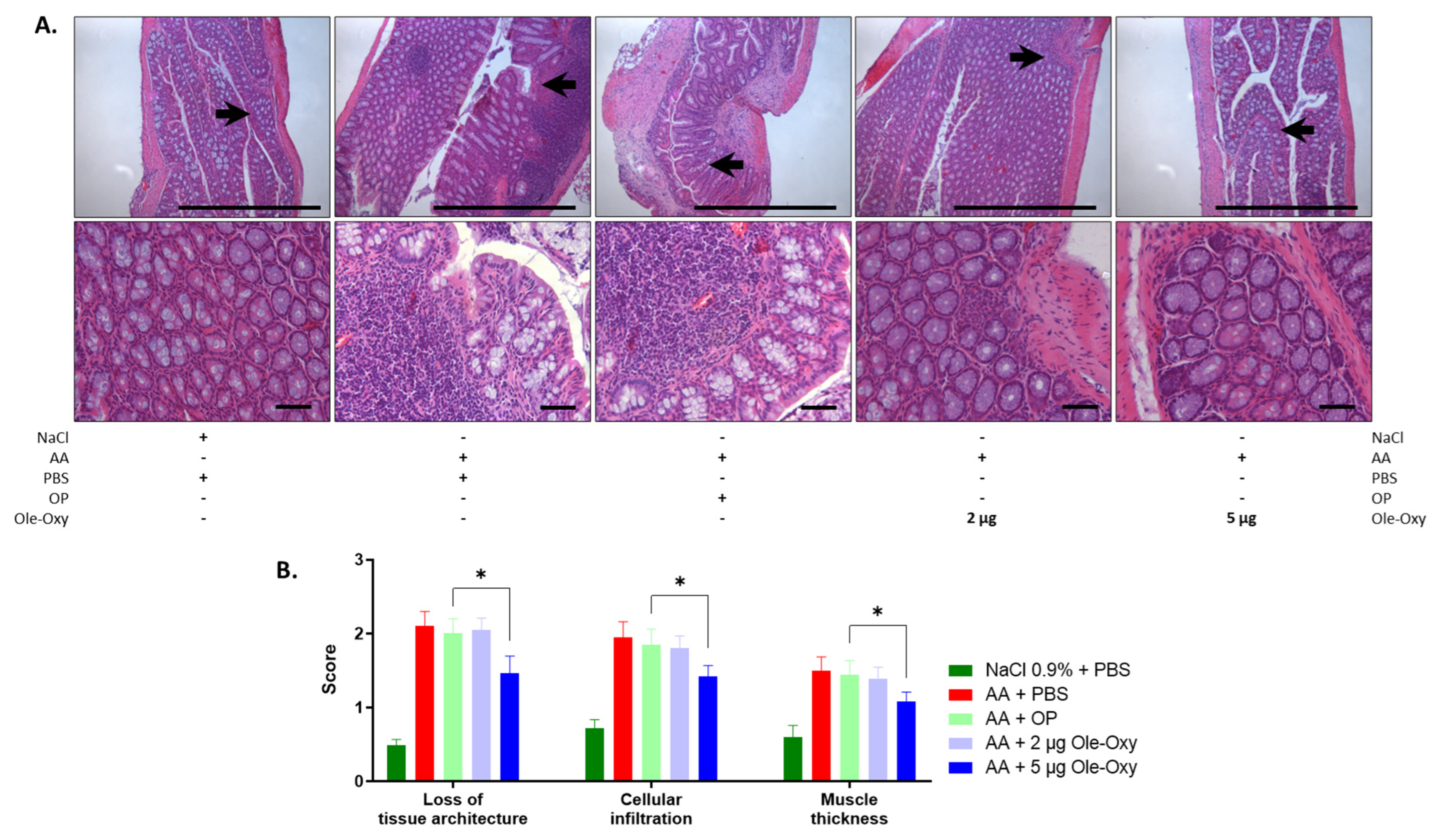
3.6. Ole-Oxy Reduces Acetic Acid-Induced Acute Colon Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive astrocytes: Production, function, and therapeutic potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Hunter, L.J.; Wood, D.M.; Dargan, P.I. The patterns of toxicity and management of acute nonsteroidal anti-inflammatory drug (NSAID) overdose. Open Access Emerg. Med. 2011, 3, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef]
- de Bock, M.; Thorstensen, E.B.; Derraik, J.G.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef]
- Samara, P.; Christoforidou, N.; Lemus, C.; Argyropoulou, A.; Ioannou, K.; Vougogiannopoulou, K.; Aligiannis, N.; Paronis, E.; Gaboriaud-Kolar, N.; Tsitsilonis, O.; et al. New semi-synthetic analogs of OP show improved anticancer activity in vitro and in vivo. Eur. J. Med. Chem. 2017, 137, 11–29. [Google Scholar] [CrossRef]
- Ioannou, K.; Cheng, K.F.; Crichlow, G.V.; Birmpilis, A.I.; Lolis, E.J.; Tsitsilonis, O.E.; Al-Abed, Y. ISO-66, a novel inhibitor of macrophage migration, shows efficacy in melanoma and colon cancer models. Int. J. Oncol. 2014, 45, 1457–1468. [Google Scholar] [CrossRef]
- Ray, A.; Dittel, B.N. Isolation of mouse peritoneal cavity cells. J. Vis. Exp. 2010, 35, 1488. [Google Scholar] [CrossRef]
- Alatery, A.; Basta, S. An efficient culture method for generating large quantities of mature mouse splenic macrophages. J. Immunol. Methods. 2008, 338, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Starr, T.; Bauler, T.J.; Malik-Kale, P.; Steele-Mortimer, O. The phorbol 12-myristate-13-acetate differentiation protocol is critical to the interaction of THP-1 macrophages with Salmonella Typhimurium. PLoS ONE 2018, 13, e0193601. [Google Scholar] [CrossRef] [PubMed]
- Gatto, F.; Cagliani, R.; Catelani, T.; Guarnieri, D.; Moglianetti, M.; Pompa, P.P.; Bardi, G. PMA-Induced THP-1 Macrophage Differentiation is Not Impaired by Citrate-Coated Platinum Nanoparticles. Nanomaterials 2017, 7, 332. [Google Scholar] [CrossRef]
- Farzam-Kia, N.; Moratalla, A.C.; Lemaître, F.; Levert, A.; Da Cal, S.; Margarido, C.; Carpentier Solorio, Y.; Arbour, N. GM-CSF distinctly impacts human monocytes and macrophages via ERK1/2-dependent pathways. Immunol. Lett. 2023, 261, 47–55. [Google Scholar] [CrossRef]
- Meng, F.; Lowell, C.A. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J. Exp. Med. 1997, 185, 1661–1670. [Google Scholar] [CrossRef]
- Paronis, E.; Katsimpoulas, M.; Kadoglou, N.P.E.; Provost, C.; Stasinopoulou, M.; Spyropoulos, C.; Poulaki, E.; Prignon, A.; Kakisis, I.; Kostomitsopoulos, N.G.; et al. Cilostazol mediates immune responses and affects angiogenesis during the acute phase of hind limb ischemia in a mouse model. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 273–285. [Google Scholar] [CrossRef]
- Karachaliou, C.E.; Liolios, C.; Triantis, C.; Zikos, C.; Samara, P.; Tsitsilonis, O.E.; Kalbacher, H.; Voelter, W.; Papadopoulos, M.; Pirmettis, I.; et al. Specific in vitro binding of a new (99m)Tc-radiolabeled derivative of the C-terminal decapeptide of prothymosin alpha on human neutrophils. Int. J. Pharm. 2015, 486, 1–12. [Google Scholar] [CrossRef]
- Faour, W.H.; Fayyad-Kazan, H.; El Zein, N. fMLP-dependent activation of Akt and ERK1/2 through ROS/Rho A pathways is mediated through restricted activation of the FPRL1 (FPR2) receptor. Inflamm. Res. 2018, 67, 711–722. [Google Scholar] [CrossRef]
- Horváth, S.; Komlódi, R.; Perkecz, A.; Pintér, E.; Gyulai, R.; Kemény, Á. Methodological refinement of Aldara-induced psoriasiform dermatitis model in mice. Sci. Rep. 2019, 9, 3685. [Google Scholar] [CrossRef]
- Bhor, U.; Pande, S. Scoring systems in dermatology. Indian. J. Dermatol. Venereol. Leprol. 2006, 72, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Guazelli, C.F.S.; Fattori, V.; Ferraz, C.R.; Borghi, S.M.; Casagrande, R.; Baracat, M.M.; Verri, W.A., Jr. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem. Biol. Interact. 2021, 333, 109315. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, G.; Malekshahi, H.; Miraghaee, S.; Madani, H.; Babaei, A. Improving animal model of induced colitis by acetic acid in terms of fibrosis and inflammation incidence in the colon. J. Investig. Surg. 2022, 35, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Birmpilis, A.I.; Karachaliou, C.E.; Samara, P.; Ioannou, K.; Selemenakis, P.; Kostopoulos, I.V.; Kavrochorianou, N.; Kalbacher, H.; Livaniou, E.; Haralambous, S.; et al. Antitumor reactive T-cell responses are enhanced in vivo by DAMP prothymosin alpha and its C-terminal decapeptide. Cancers 2019, 11, 1764. [Google Scholar] [CrossRef]
- Sarikaki, G.; Christoforidou, N.; Gaboriaud-Kolar, N.; Smith, A.B., 3rd; Kostakis, I.K.; Skaltsounis, A.L. Biomimetic synthesis of Oleocanthal, Oleacein, and their analogs starting from OP, a major compound of olive leaves. J. Nat. Prod. 2020, 83, 1735–1739. [Google Scholar] [CrossRef]
- Wynn, T.; Chawla, A.; Pollard, J. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Chiong, H.S.; Yong, Y.K.; Ahmad, Z.; Sulaiman, M.R.; Zakaria, Z.A.; Yuen, K.H.; Hakim, M.N. Cytoprotective and enhanced anti-inflammatory activities of liposomal piroxicam formulation in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Nanomed. 2013, 8, 1245–1255. [Google Scholar] [CrossRef]
- Verreck, F.A.; de Boer, T.; Langenberg, D.M.; van der Zanden, L.; Ottenhoff, T.H. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J. Leukoc. Biol. 2006, 79, 285–293. [Google Scholar] [CrossRef]
- Zhang, X.; Goncalves, R.; Mosser, D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008, 83, 14.1.1–14.1.14. [Google Scholar] [CrossRef]
- Appleyard, C.B.; Wallace, J.L. Reactivation of hapten-induced colitis and its prevention by anti-inflammatory drugs. Am. J. Physiol. 1995, 269, G119–G125. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.R.; Craik, D.J.; Martin, J.L. Functional group contributions to drug-receptor interactions. J. Med. Chem. 1984, 27, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, L. Chapter 14—The role of functional groups in drug–receptor interactions. In The Practice of Medicinal Chemistry, 4th ed.; Wermuth, C.G., Aldous, D., Raboisson, P., Rognan, D., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 359–378. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Jiang, J.; Huang, J.; Chen, H.; Pan, L.; Nesterov, D.S.; Ma, Z.; Pombeiro, A.J.L. A new concept of enhancing the anticancer activity of manganese terpyridine complex by oxygen-containing substituent modification. Int. J. Mol. Sci. 2023, 24, 3903. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Zhang, G.; Ghosh, S. Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J. Endotoxin Res. 2000, 6, 453–457. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hwang, J.H.; Lee, H.T. Differential susceptibility to lipopolysaccharide affects the activation of toll-like-receptor 4 signaling in THP-1 cells and PMA-differentiated THP-1 cells. Innate Immun. 2022, 28, 122–129. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Malm Tillgren, S.; Nieto-Fontarigo, J.J.; Cerps, S.; Ramu, S.; Menzel, M.; Mahmutovic Persson, I.; Meissner, A.; Akbarshahi, H.; Uller, L. C57Bl/6N mice have an attenuated lung inflammatory response to dsRNA compared to C57Bl/6J and BALB/c mice. J. Inflamm. 2023, 20, 6. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Tsurutani, M.; Horie, H.; Ogawa, K. Cell properties of lung tissue-resident macrophages propagated by co-culture with lung fibroblastic cells from C57BL/6 and BALB/c mice. Biomedicines 2021, 9, 1241. [Google Scholar] [CrossRef]
- Barr, J.T.; Tran, T.B.; Rock, B.M.; Wahlstrom, J.L.; Dahal, U.P. Strain-dependent variability of early discovery small molecule pharmacokinetics in mice: Does Strain Matter? Drug Metab. Dispos. 2020, 48, 613–621. [Google Scholar] [CrossRef]
- Watanabe, H.; Numata, K.; Ito, T.; Takagi, K.; Matsukawa, A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 2004, 22, 460–466. [Google Scholar] [CrossRef]
- Morrison, D.K.; Davis, R.J. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 2003, 19, 91–118. [Google Scholar] [CrossRef]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: A systems medicine approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef]
- Hancock, J.T.; Desikan, R.; Neill, S.J. Role of reactive oxygen species in cell signalling pathways. Biochem. Soc. Trans. 2001, 29 Pt 2, 345–350. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelis, N.V.; Paronis, E.; Sarikaki, G.; Kyriakopoulos, A.; Agapaki, A.; Niotopoulou, P.-M.; Knai, C.C.; Alexakos, P.; Liagkas, O.; Mavreas, K.F.; et al. Ole-Oxy, a Semi-Synthetic Analog of Oleuropein, Ameliorates Acute Skin and Colon Inflammation in Mice. Antioxidants 2024, 13, 1422. https://doi.org/10.3390/antiox13111422
Angelis NV, Paronis E, Sarikaki G, Kyriakopoulos A, Agapaki A, Niotopoulou P-M, Knai CC, Alexakos P, Liagkas O, Mavreas KF, et al. Ole-Oxy, a Semi-Synthetic Analog of Oleuropein, Ameliorates Acute Skin and Colon Inflammation in Mice. Antioxidants. 2024; 13(11):1422. https://doi.org/10.3390/antiox13111422
Chicago/Turabian StyleAngelis, Nikolaos V., Efthymios Paronis, Georgia Sarikaki, Antonios Kyriakopoulos, Anna Agapaki, Pigi-Maria Niotopoulou, Christina C. Knai, Pavlos Alexakos, Odyssefs Liagkas, Konstantinos F. Mavreas, and et al. 2024. "Ole-Oxy, a Semi-Synthetic Analog of Oleuropein, Ameliorates Acute Skin and Colon Inflammation in Mice" Antioxidants 13, no. 11: 1422. https://doi.org/10.3390/antiox13111422
APA StyleAngelis, N. V., Paronis, E., Sarikaki, G., Kyriakopoulos, A., Agapaki, A., Niotopoulou, P.-M., Knai, C. C., Alexakos, P., Liagkas, O., Mavreas, K. F., Baxevanis, C. N., Skaltsounis, A.-L., Tsitsilonis, O. E., & Kostakis, I. K. (2024). Ole-Oxy, a Semi-Synthetic Analog of Oleuropein, Ameliorates Acute Skin and Colon Inflammation in Mice. Antioxidants, 13(11), 1422. https://doi.org/10.3390/antiox13111422








