Expressions of Immune Prophenoloxidase (proPO) System-Related Genes Under Oxidative Stress in the Gonads and Stomach of the Mud Crab (Macrophthalmus japonicus) Exposed to Endocrine-Disrupting Chemicals
Abstract
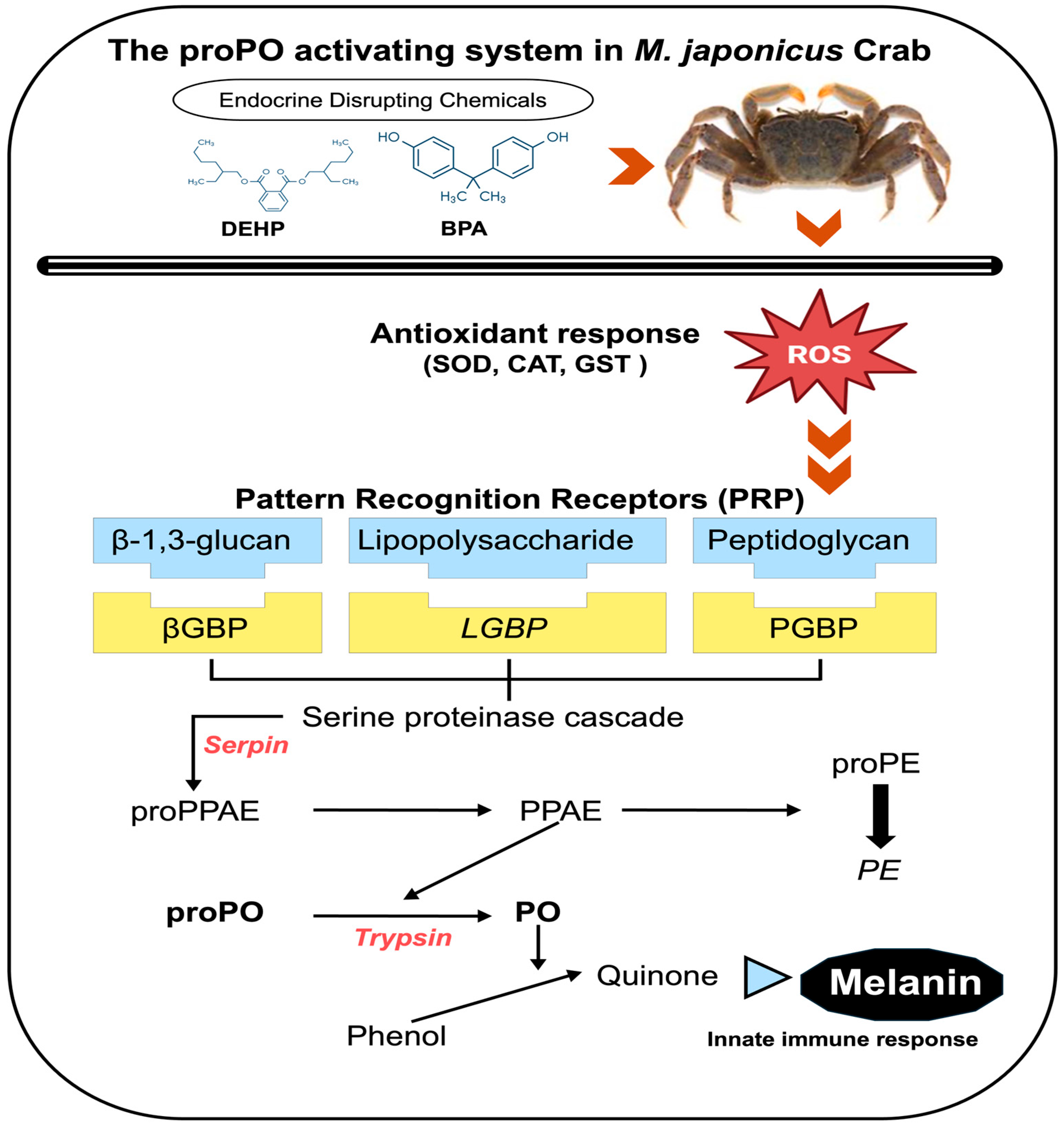
:1. Introduction
2. Materials and Methods
2.1. Test Organism
2.2. Chemicals, Reagents, and Exposure Experiment Design
2.3. Total RNA Extraction and cDNA Synthesis
2.4. Gene Expression Quantification in M. japonicus
2.5. Data Analysis
3. Results
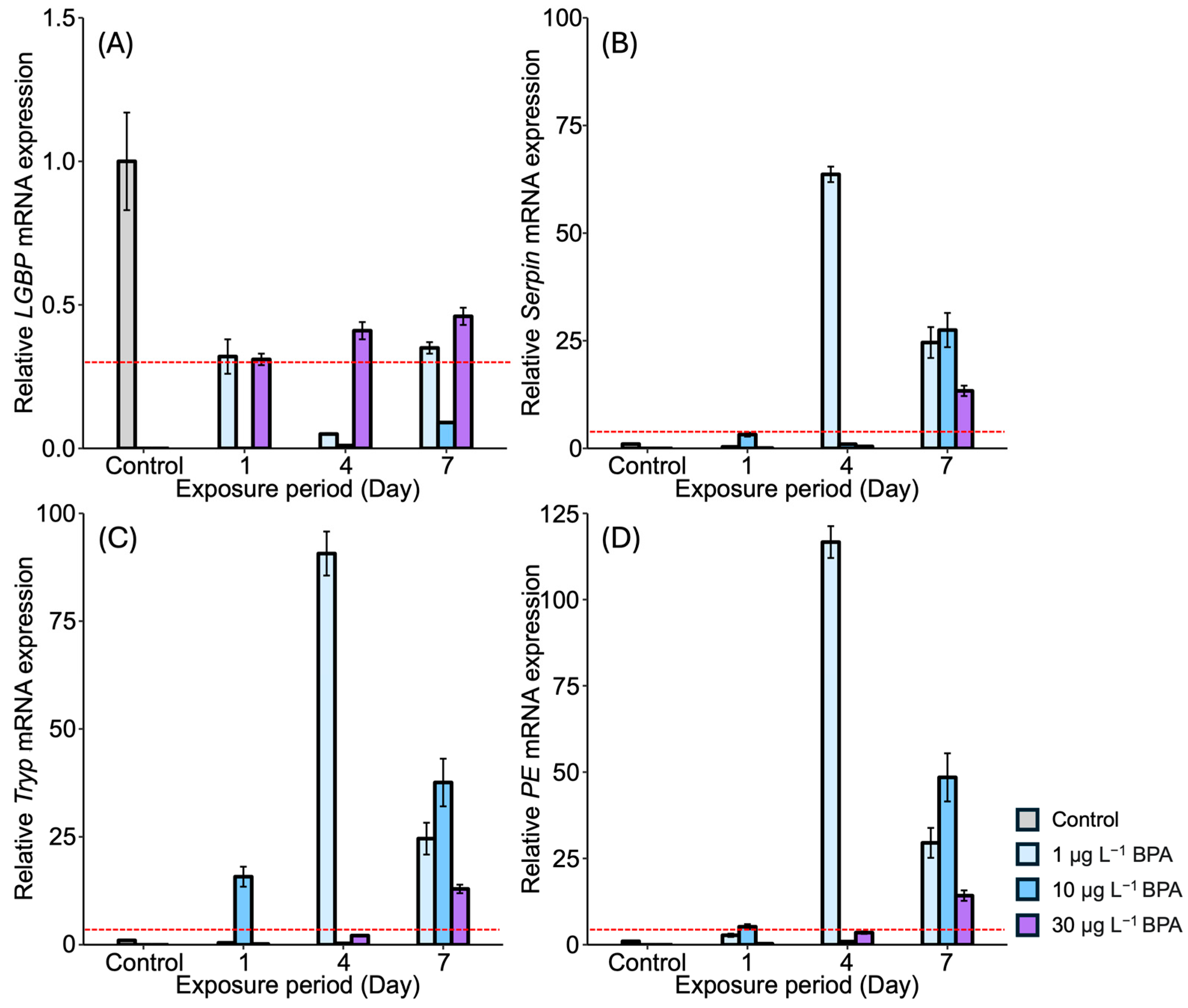
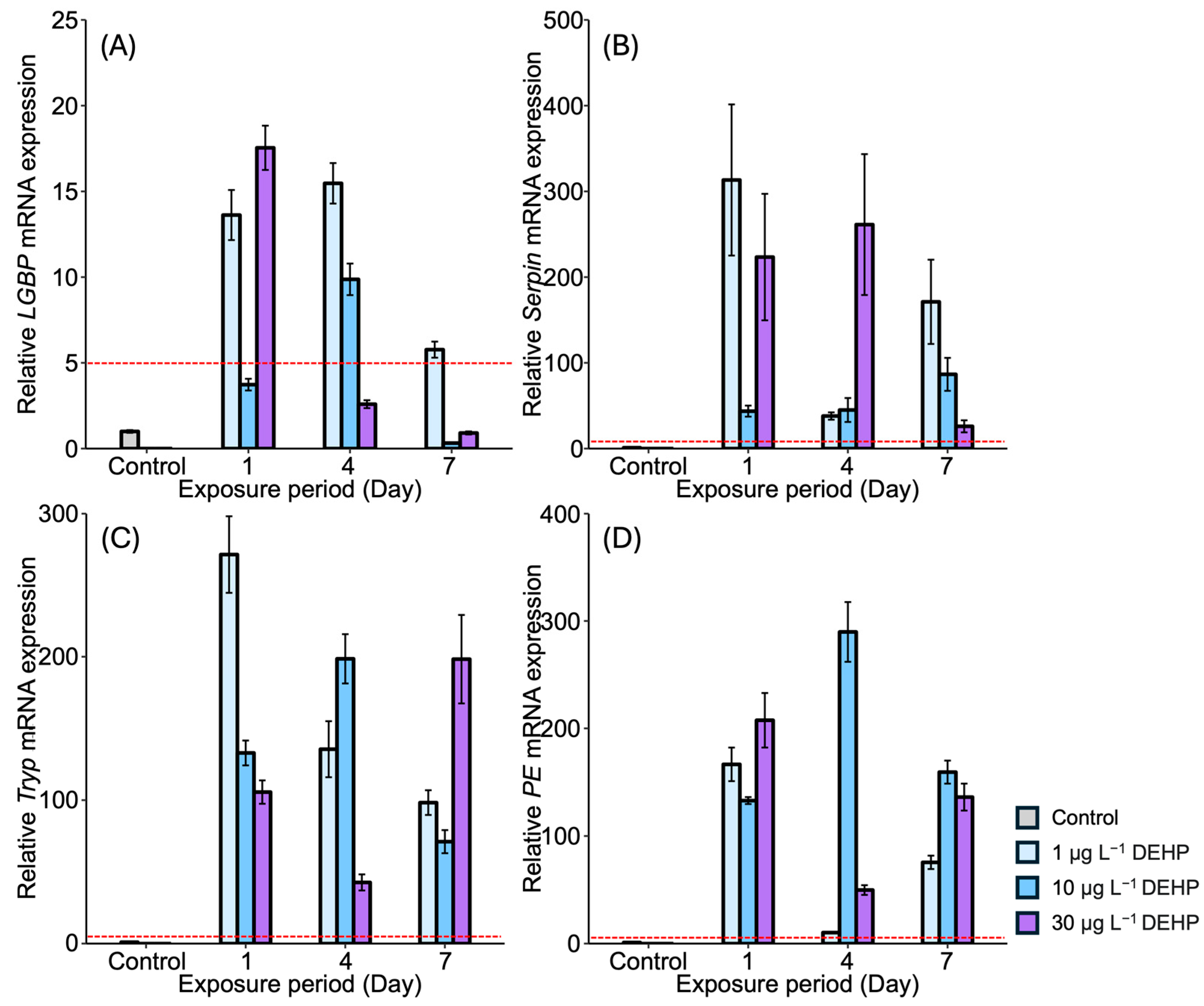
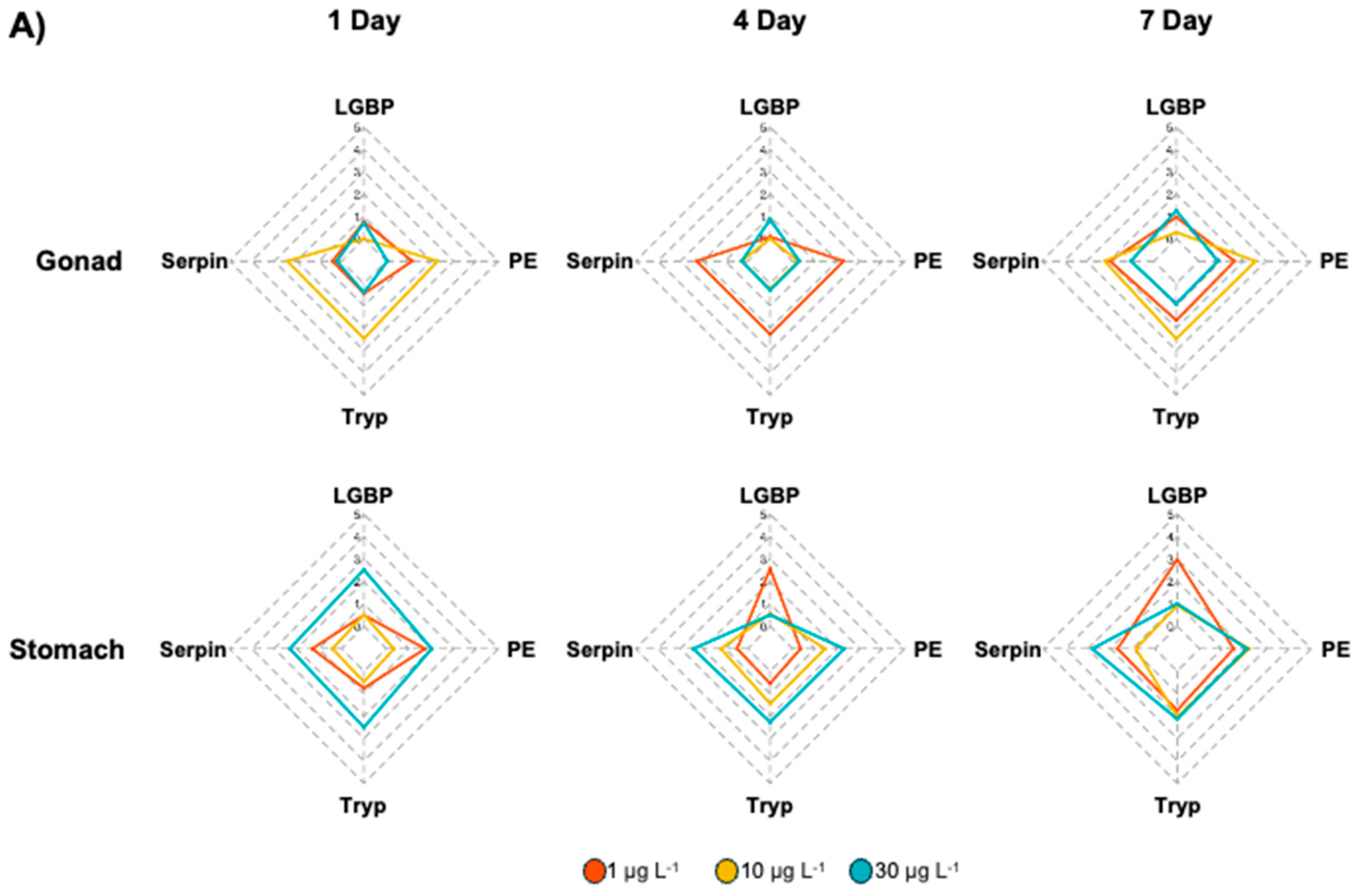
3.1. Expression of proPO System-Related Genes in Gonads After Exposure to EDCs
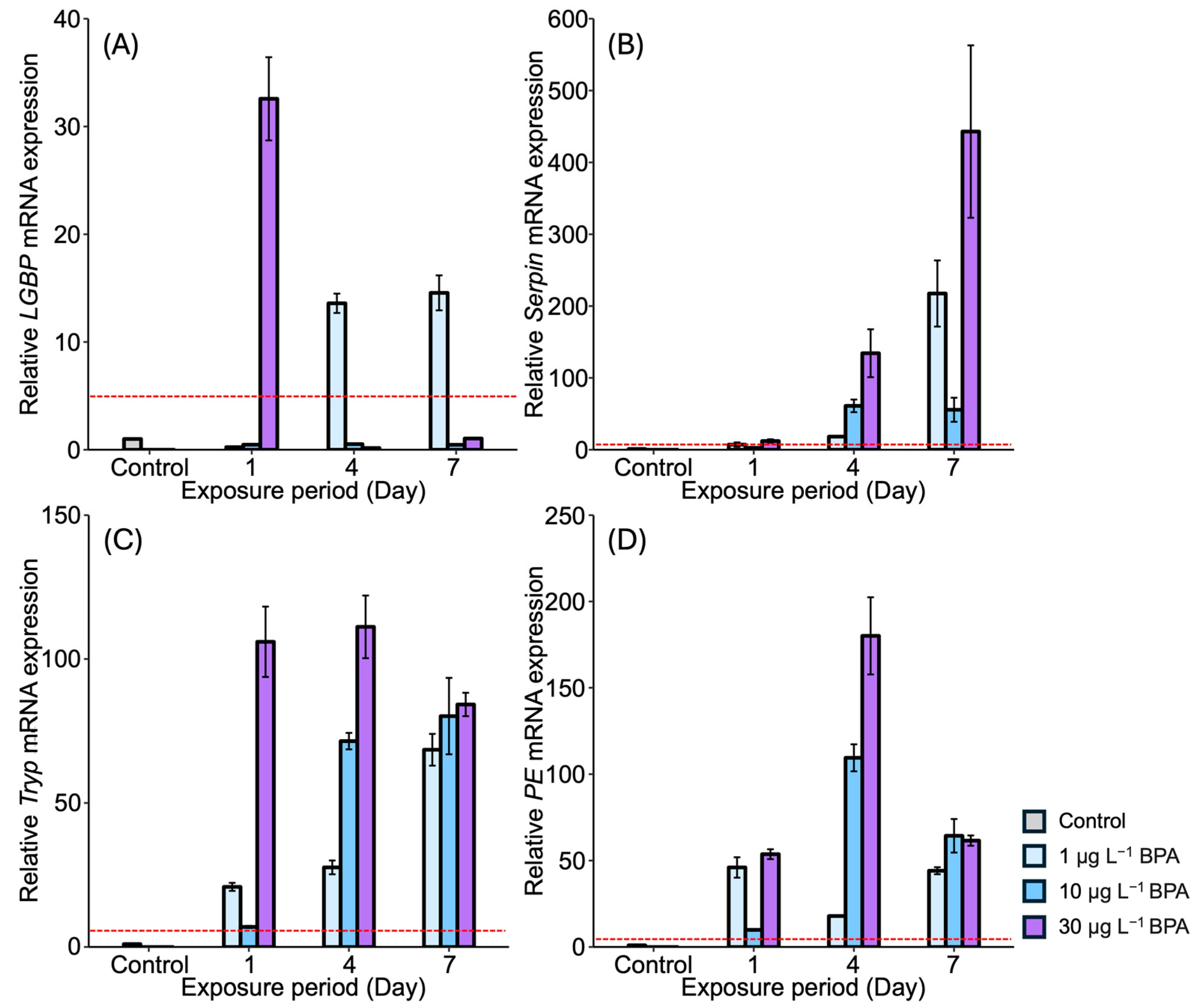
3.2. Expression of proPO System-Related Genes in the Stomach After EDC Exposure
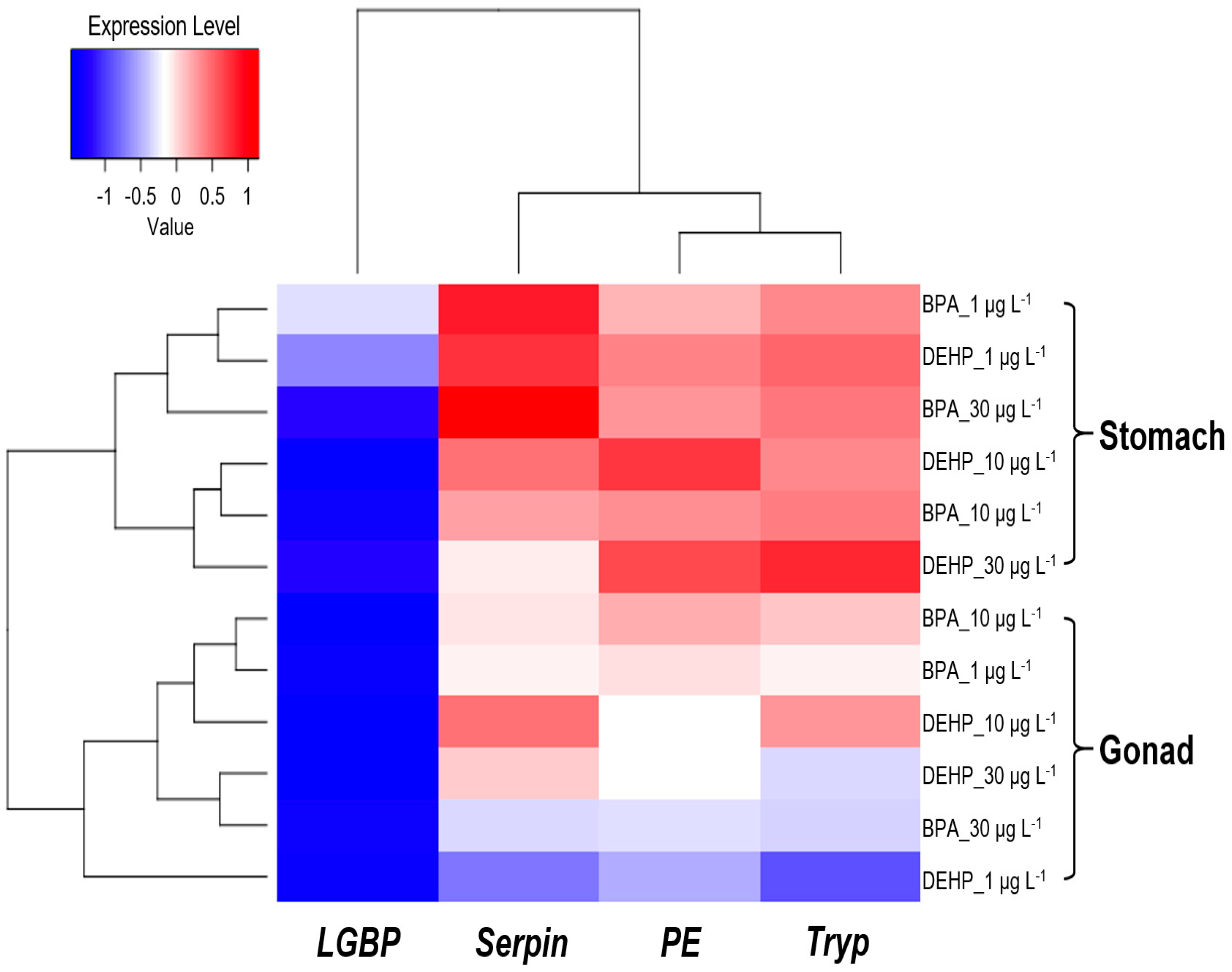
3.3. Integrated Biomarker Response (IBR) Index and Heatmap Analysis on M. japonicus Exposed to BPA and DEHP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alrumman, S.; Keshk, S.; El Kott, A. Water pollution: Source & treatment. Am. J. Environ. Eng. 2016, 6, 88–98. [Google Scholar]
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Vos, J.G.; Dybing, E.; Greim, H.A.; Ladefoged, O.; Lambré, C.; Tarazona, J.V.; Brandt, I.; Vethaal, A.D. Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situation. Crit. Rev. Toxicol. 2000, 30, 71–133. [Google Scholar] [CrossRef]
- Darbre, P.D. The history of endocrine-disrupting chemicals. Curr. Opin. Endocr. Metab. Res. 2019, 7, 26–33. [Google Scholar] [CrossRef]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity—A review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Lin, C.; Lee, C.J.; Mao, W.M.; Nadim, F. Identifying the potential sources of di-(2-ethylhexyl) phthalate contamination in the sediment of the Houjing River in southern Taiwan. J. Hazard. Mater. 2009, 161, 270–275. [Google Scholar] [CrossRef]
- Rowdhwal, S.S.S.; Chen, J. Toxic Effects of Di-2-ethylhexyl Phthalate: An Overview. Biomed. Res. Int. 2018, 2018, 1750368. [Google Scholar] [CrossRef]
- Park, K.; Kim, W.S.; Kwak, I.S. Endocrine-disrupting chemicals impair the innate immune T prophenoloxidase system in the intertidal mud crab, Macrophthalmus japonicus. Fish. Shellfish Immunol. 2019, 87, 322–332. [Google Scholar] [CrossRef]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef]
- Valentino, R.; D’Esposito, V.; Ariemma, F.; Cimmino, I.; Beguinot, F.; Formisano, P. Bisphenol A environmental exposure and the detrimental effects on human metabolic health: Is it necessary to revise the risk assessment in vulnerable population? J. Endocrinol. Investig. 2016, 39, 259–263. [Google Scholar] [CrossRef]
- Seoane, M.; Cid, Á.; Esperanza, M. Toxicity of bisphenol A on marine microalgae: Single- and multispecies bioassays based on equivalent initial cell biovolume. Sci. Total. Environ. 2021, 767, 144363. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Li, H.; Yuan, S.; Li, J.; Liu, C. Greater toxic potency of bisphenol AF than bisphenol A in growth, reproduction, and transcription of genes in Daphnia magna. Environ. Sci. Pollut. Res. Int. 2021, 28, 25218–25227. [Google Scholar] [CrossRef]
- Yin, X.; Zeb, R.; Wei, H.; Cai, L. Acute exposure of di(2-ethylhexyl) phthalate (DEHP) induces immune signal regulation and ferroptosis in oryzias melastigma. Chemosphere 2021, 265, 129053. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, N.; Zhang, Y.; Hu, H.; Zhao, M.; Jin, H. Occurrence and partitioning of bisphenol analogues, triclocarban, and triclosan in seawater and sediment from East China Sea. Chemosphere 2022, 287, 132218. [Google Scholar] [CrossRef]
- Yamazaki, E.; Yamashita, N.; Taniyasu, S.; Lam, J.; Lam, P.K.; Moon, H.B.; Jeong, Y.; Kannan, P.; Achyuthan, H.; Munuswamy, N.; et al. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf. 2015, 122, 565–572. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Jalón-Rojas, I.; Wang, X.H.; Fredj, E.; Zhang, D.; Feng, L.; Li, X. Assessing the potential risk and relationship between microplastics and phthalates in surface seawater of a heavily human-impacted metropolitan bay in northern China. Ecotoxicol. Environ. Saf. 2020, 204, 111067. [Google Scholar] [CrossRef]
- Sha, Y.; Xia, X.; Yang, Z.; Huang, G.H. Distribution of PAEs in the middle and lower reaches of the Yellow River, China. Environ. Monit. Assess. 2007, 124, 277–287. [Google Scholar] [CrossRef]
- Paluselli, A.; Aminot, Y.; Galgani, F.; Net, S.; Sempere, R. Occurrence of phthalate acid esters (PAEs) in the northwestern Mediterranean Sea and the Rhone River. Prog. Oceanogr. 2018, 163, 221–231. [Google Scholar] [CrossRef]
- Rogers, J.A.; Metz, L.; Yong, V.W. Review: Endocrine disrupting chemicals and immune responses: A focus on bisphenol-A and its potential mechanisms. Mol. Immunol. 2013, 53, 421–430. [Google Scholar] [CrossRef]
- Huang, R.G.; Li, X.B.; Wang, Y.Y.; Wu, H.; Li, K.D.; Jin, X.; Du, Y.J.; Wang, H.; Qian, F.Y.; Li, B.Z. Endocrine-disrupting chemicals and autoimmune diseases. Environ. Res. 2023, 231, 116222. [Google Scholar] [CrossRef]
- Vivier, E.; Malissen, B. Innate and adaptive immunity: Specificities and signaling hierarchies revisited. Nat. Immunol. 2005, 6, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Amparyup, P.; Charoensapsri, W.; Tassanakajon, A. Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish. Shellfish Immunol. 2013, 34, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Binggeli, O.; Neyen, C.; Poidevin, M.; Lemaitre, B. Prophenoloxidase Activation Is Required for Survival to Microbial Infections in Drosophila. PLoS Pathog. 2014, 10, e1004067. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Söderhäll, K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004, 198, 116–126. [Google Scholar] [CrossRef]
- Wu, F.; Falfushynska, H.; Delwig, O.; Piontkivska, H.; Sokolova, I.M. Interactive effects of salinity variation and exposure to ZnO nanoparticles on the innate immune system of a sentinel marine bivalve, Mytilus edulis. Sci. Total. Environ. 2020, 712, 136473. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Jin, Q.; Zhu, F. Effects of the aquatic pollutant sulfamethoxazole on the innate immunity and antioxidant capacity of the mud crab Scylla paramamosain. Chemosphere 2024, 349, 140775. [Google Scholar] [CrossRef]
- Kitaura, J.; Nishida, M.; Wada, K. Genetic and behavioral diversity in the Macrophthalmus japonicus species complex (Crustacea: Brachyura: Ocypodidae). Mar. Biol. 2002, 140, 1–8. [Google Scholar]
- Henmi, Y. Life-history patterns in two forms of Macrophthalmus japonicus (Crustacea: Brachyura). Mar. Biol. 1989, 101, 53–60. [Google Scholar] [CrossRef]
- Davis, J.A.; Wille, M.; Hecht, T.; Sorgeloos, P. Optimal first feed organism for South African mud crab Scylla serrata (Forskål) larvae. Aquac. Int. 2005, 13, 187–201. [Google Scholar] [CrossRef]
- Mohapatra, A.; Mohanty, R.K.; Mohanty, S.K.; Bhatta, K.S.; Das, N.R. Fisheries enhancement and biodiversity assessment of fish, prawn and mud crab in Chilika lagoon through hydrological intervention. Wetl. Ecol. Manag. 2007, 15, 229–251. [Google Scholar] [CrossRef]
- Leoville, A.; Lagarde, R.; Grondin, H.; Faivre, L.; Rasoanirina, E.; Teichert, N. Influence of environmental conditions on the distribution of burrows of the mud crab, Scylla serrata, in a fringing mangrove ecosystem. Reg. Stud. Mar. Sci. 2021, 43, 101684. [Google Scholar] [CrossRef]
- Hsieh, S.L.; Hsieh, S.; Xu, R.Q.; Chen, Y.T.; Chen, C.W.; Singhania, R.R.; Chen, Y.C.; Tsai, T.H.; Dong, C.D. Toxicological effects of polystyrene nanoplastics on marine organisms on marine organisms. Environ. Technol. Innov. 2023, 30, 103073. [Google Scholar] [CrossRef]
- Yifei, Y.; Zhixiong, Z.; Luna, C.; Qihui, C.; Zuoyuan, W.; Xinqi, L.; Zhexiang, L.; Fei, Z.; Xiujuan, Z. Marine pollutant Phenanthrene (PHE) exposure causes immunosuppression of hemocytes in crustacean species, Scylla paramamosain. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 275, 109761. [Google Scholar] [CrossRef]
- Park, K.; Moon, B.S.; Kwak, I.S. Responses of multifunctional immune complement component 1q (C1q) and apoptosis-related genes in Macrophthalmus japonicus tissues and human cells of following exposure to environmental pollutants. Cell. Stress. Chaperones. 2023, 28, 959–968. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Avellan, A.; Duarte, A.; Rocha-Santos, T. Organic contaminants in marine sediments and seawater: A review for drawing environmental diagnostics and searching for informative predictors. Sci. Total. Environ. 2022, 808, 152012. [Google Scholar] [CrossRef] [PubMed]
- Neves, R.A.; Miralha, A.; Guimarães, T.B.; Sorrentino, R.; Calderari, M.R.; Santos, L.N. Phthalates contamination in the coastal and marine sediments of Rio de Janeiro, Brazil. Mar. Pollut. Bull. 2023, 190, 114819. [Google Scholar] [CrossRef]
- Rolfo, A.; Nuzzo, A.M.; De Amicis, R.; Moretti, L.; Bertoli, S.; Leone, A. Fetal-Maternal Exposure to Endocrine Disruptors: Correlation with Diet Intake and Pregnancy Outcomes. Nutrients 2020, 12, 1744. [Google Scholar] [CrossRef] [PubMed]
- Hamid, N.; Junaid, M.; Pei, D.S. Combined toxicity of endocrine-disrupting chemicals: A review. Ecotoxicol. Environ. Saf. 2021, 215, 112136. [Google Scholar] [CrossRef]
- Zhang, X.; Flaws, J.A.; Spinella, M.J.; Irudayaraj, J. The relationship between typical environmental endocrine disruptors and kidney disease. Toxics 2022, 11, 32. [Google Scholar] [CrossRef]
- Pu, C.; Liu, Y.; Ma, J.; Li, J.; Sun, R.; Zhou, Y.; Zhang, C. The effects of bisphenol S exposure on the growth, physiological and biochemical indices, and ecdysteroid receptor gene expression in red swamp crayfish, Procambarus clarkii. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 276, 109811. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Park, K.; Kim, J.H.; Kwak, I.S. Effect of endocrine-disrupting chemicals on the expression of a calcium ion channel receptor (ryanodine receptor) in the mud crab (Macrophthalmus japonicus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 283, 109972. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cao, Y.; Wang, S.; Cai, J.; Zhang, Z. DEHP induces immunosuppression through disturbing inflammatory factors T and CYPs system homeostasis in common carp neutrophils. Fish. Shellfish Immunol. 2020, 96, 26–31. [Google Scholar] [CrossRef]
- Melnick, R.; Lucier, G.; Wolfe, M.; Hall, R.; Stancel, G.; Prins, G.; Gallo, M.; Reuhl, K.; Ho, S.M.; Brown, T.; et al. Summary of the National Toxicology Program’s report of the endocrine disruptors low-dose peer review. Environ. Health Perspect. 2002, 110, 427–431. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar]
- Lyu, L.; Tao, Y.; Abaakil, K.; Gu, Y.; Zhong, G.; Hu, Y.; Zhang, Y. Novel insights into DEHP-induced zebrafish spleen damage: Cellular apoptosis, mitochondrial dysfunction, and innate immunity. Sci. Total. Environ. 2024, 912, 169324. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.P.; Junaid, M.; Xin, G.Y.; Wang, Y.; Ma, Y.B.; Pei, D.S. Disruption of intestinal homeostasis through altered responses of the microbial community, energy metabolites, and immune system in zebrafish after chronic exposure to DEHP. Front. Microbiol. 2021, 12, 729530. [Google Scholar] [CrossRef]
- Yen, P.L.; Yang, C.R.; Huang, M.L.; Lin, T.A.; Liao, V.H.C. Chronic exposure to di (2-ethylhexyl) phthalate (DEHP) weakens innate immunity and leads to immunosenescence in C. elegans. Environ. Toxicol. Pharmacol. 2023, 98, 104071. [Google Scholar] [CrossRef] [PubMed]
- Linghu, D.; Zhu, Z.; Zhang, D.; Luo, Y.; Ma, J.; Li, T.; Sun, Z.; Xie, Z.; Sun, J.; Cao, C. Diethylhexyl phthalate induces immune dysregulation and is an environmental immune disruptor. J. Hazard. Mater. 2024, 480, 136244. [Google Scholar] [CrossRef]
- Han, Y.; Shi, W.; Tang, Y.; Zhou, W.; Sun, H.; Zhang, J.; Yan, M.; Hu, L.; Liu, G. Microplastics and bisphenol A hamper gonadal development of whiteleg shrimp (Litopenaeus vannamei) by interfering with metabolism and disrupting hormone regulation. Sci. Total Environ. 2022, 810, 152354. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, W.; Sun, S.; Du, X.; Han, Y.; Shi, W.; Liu, G. Immunotoxicity and neurotoxicity of bisphenol A and microplastics alone or in combination to a bivalve species, Tegillarca granosa. Environ. Pollut. 2020, 265, 115115. [Google Scholar] [CrossRef]
- Donaghy, L.; Hong, H.K.; Jauzein, C.; Choi, K.S. The known and unknown sources of reactive oxygen and nitrogen species in haemocytes of marine bivalve molluscs. Fish. Shellfish Immunol. 2015, 42, 91–97. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, K.S.; Yang, H.S.; Kang, H.S.; Hong, H.K. In vitro impact of Bisphenol A on the immune functions of primary cultured hemocytes of Pacific abalone (Haliotis discus hannai). Mar. Pollut. Bull. 2024, 206, 116770. [Google Scholar] [CrossRef]
- Montero, V.; Chinchilla, Y.; Gómez, L.; Flores, A.; Medaglia, A.; Guillén, R.; Montero, E. Human health risk assessment for consumption of microplastics and plasticizing substances through marine species. Environ. Res. 2023, 237, 116843. [Google Scholar] [CrossRef]
- Park, K.; Kim, W.S.; Choi, B.; Kwak, I.S. Expression levels of the immune-related p38 mitogen-activated protein kinase transcript in response to environmental pollutants on Macrophthalmus japonicus crab. Genes 2020, 11, 958. [Google Scholar] [CrossRef]
- Kim, W.S.; Kwak, I.S. EDCs trigger immune-neurotransmitter related gene expression, and cause histological damage in sensitive mud crab Macrophthalmus japonicus gills and hepatopancreas. Fish. Shellfish Immunol. 2022, 122, 484–494. [Google Scholar] [CrossRef]
- Taguchi, T.; Mukai, K. Innate immunity signalling and membrane trafficking. Curr. Opin. Cell. Biol. 2019, 59, 1–7. [Google Scholar] [CrossRef]
- De Winther, M.P.; Palaga, T. Epigenetic Regulation of Innate Immunity. Front. Immunol. 2021, 12, 713758. [Google Scholar] [CrossRef]
- Meekins, D.A.; Kanost, M.R.; Michel, K. Serpins in arthropod biology. Semin. Cell. Dev. Biol. 2017, 62, 105–119. [Google Scholar] [CrossRef]
- Park, K.; Kwak, I.S. Expression of Chironomus riparius serine-type endopeptidase gene under di-(2-ethylhexyl)-phthalate (DEHP) exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 151, 349–354. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Lin, Q.; Zhu, M.; Mei, F.; Jian, S.; Zhao, D. Role of peroxinectin in the antibacterial immune response of the Chinese mitten crab, Eriocheir sinensis. Fish. Shellfish Immunol. 2022, 123, 496–505. [Google Scholar] [CrossRef]
- Jiang, C.; Feng, M.; Fan, R.; Wang, C.; Shu, G.; Qiu, Y.; Lou, H.; Dai, L.; Zhao, H.; Ding, F.; et al. Molecular cloning and functional characterization of peroxinectin from red swamp crayfish, Procambarus clarkii. Fish. Shellfish Immunol. 2023, 143, 109206. [Google Scholar] [CrossRef]
- Tassanakajon, A.; Rimphanitchayakit, V.; Visetnan, S.; Amparyup, P.; Somboonwiwat, K.; Charoensapsri, W.; Tang, S. Shrimp humoral responses against pathogens: Antimicrobial peptides and melanization. Dev. Comp. Immunol. 2023, 143, 109206. [Google Scholar] [CrossRef]
- Alvarez, J.V.; Chung, J.S. Cloning of prophenoloxidase from hemocytes of the blue crab, Callinectes sapidus and its expression and enzyme activity during the molt cycle. Fish. Shellfish Immunol. 2013, 35, 1349–1358. [Google Scholar] [CrossRef]
- Adachi, K.; Endo, H.; Watanabe, T.; Nishioka, T.; Hirata, T. Hemocyanin in the exoskeleton of crustaceans: Enzymatic properties and immunolocalization. Pigment. Cell. Res. 2005, 18, 136–143. [Google Scholar] [CrossRef]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends. Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pan, J.; Wang, X.; Cai, X.; Lin, Z.; Shi, Q.; Li, E.; Qin, J.G.; Chen, L. N-acetylcysteine provides protection against the toxicity of dietary T-2 toxin in juvenile Chinese mitten crab (Eriocheir sinensis). Aquaculture 2021, 538, 736531. [Google Scholar] [CrossRef]


| Gene | Primer Sequence (5′–3′) | Amplicon Size (bp) | Efficiency (%) | Accession Number |
|---|---|---|---|---|
| LGBP_F | AATGGCTTCTTCCCTGACGG | 131 | 100.0 | KJ653260 |
| LGBP_R | CTGATCTTGCCCTCACCCTG | |||
| Serpin_F | TTTGGAACGTGGGAGTATGC | 74 | 93.0 | MH41109 |
| Serpin_R | TGCACATTGGGAATCGCATG | |||
| Tryp_F | CCTAGAGGTCGGGGTCAAGA | 91 | 99.5 | KJ653261 |
| Tryp_R | CCTATCCAGCTCGAGCAGTG | |||
| PE_F | CTGACCACCATACACACGCT | 98 | 90.0 | KF804082 |
| PE_R | TGGAACACTTGCTCGTCCTG | |||
| GAPDH_F | TGCTGATGCACCCATGTTTG | 147 | 102.5 | KJ653265 |
| GAPDH_R | AGGCCCTGGACAATCTCAAAG |
| Chemical | Organ | Concentration (μg/L) | proPO System-Related Gene | IBR Value | ||||
|---|---|---|---|---|---|---|---|---|
| LGBP | Serpin | Tryp | PE | Mean | ||||
| BPA | Gonad | Control | 2.67 | 0.00 | 0.15 | 0.20 | 0.75 | 2.45 |
| 1 | 0.96 | 1.96 | 1.65 | 1.60 | 1.54 | 1.17 | ||
| 10 | 0.29 | 2.20 | 2.48 | 2.53 | 1.87 | 4.31 | ||
| 30 | 1.25 | 1.03 | 0.91 | 0.85 | 1.01 | 2.61 | ||
| Stomach | Control | 1.00 | 0.58 | 0 | 0.05 | 0.41 | 0.46 | |
| 1 | 2.98 | 1.67 | 1.74 | 1.52 | 1.98 | 1.38 | ||
| 10 | 0.92 | 0.85 | 2.04 | 2.22 | 1.51 | 3.90 | ||
| 30 | 1.01 | 2.81 | 2.14 | 2.12 | 2.02 | 4.24 | ||
| DEHP | Gonad | Control | 2.65 | 0.46 | 0.56 | 0 | 0.92 | 2.58 |
| 1 | 1.17 | 0.54 | 0.63 | 0.82 | 0.79 | 1.35 | ||
| 10 | 0.63 | 2.62 | 2.69 | 2.08 | 2.00 | 1.93 | ||
| 30 | 0.45 | 1.29 | 1.02 | 2.01 | 1.19 | 3.78 | ||
| Stomach | Control | 0.90 | 0.37 | 0.18 | 0 | 0.37 | 0.34 | |
| 1 | 2.79 | 2.62 | 1.37 | 1.05 | 1.96 | 1.31 | ||
| 10 | 0.64 | 1.50 | 1.04 | 2.24 | 1.35 | 3.40 | ||
| 30 | 0.87 | 0.70 | 2.60 | 1.91 | 1.52 | 2.97 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Park, K.; Kim, W.-S.; Kwak, I.-S. Expressions of Immune Prophenoloxidase (proPO) System-Related Genes Under Oxidative Stress in the Gonads and Stomach of the Mud Crab (Macrophthalmus japonicus) Exposed to Endocrine-Disrupting Chemicals. Antioxidants 2024, 13, 1433. https://doi.org/10.3390/antiox13121433
Kim J-H, Park K, Kim W-S, Kwak I-S. Expressions of Immune Prophenoloxidase (proPO) System-Related Genes Under Oxidative Stress in the Gonads and Stomach of the Mud Crab (Macrophthalmus japonicus) Exposed to Endocrine-Disrupting Chemicals. Antioxidants. 2024; 13(12):1433. https://doi.org/10.3390/antiox13121433
Chicago/Turabian StyleKim, Ji-Hoon, Kiyun Park, Won-Seok Kim, and Ihn-Sil Kwak. 2024. "Expressions of Immune Prophenoloxidase (proPO) System-Related Genes Under Oxidative Stress in the Gonads and Stomach of the Mud Crab (Macrophthalmus japonicus) Exposed to Endocrine-Disrupting Chemicals" Antioxidants 13, no. 12: 1433. https://doi.org/10.3390/antiox13121433
APA StyleKim, J.-H., Park, K., Kim, W.-S., & Kwak, I.-S. (2024). Expressions of Immune Prophenoloxidase (proPO) System-Related Genes Under Oxidative Stress in the Gonads and Stomach of the Mud Crab (Macrophthalmus japonicus) Exposed to Endocrine-Disrupting Chemicals. Antioxidants, 13(12), 1433. https://doi.org/10.3390/antiox13121433






