Oxalis erythrorhiza Gillies ex Hooker et Arnott (Oxalidaceae): Chemical Analysis, Biological In Vitro and In Vivo Properties and Behavioral Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material, Decoction (DOe) and Methanolic Extract (MGEOe)
2.3. Ultra-High-Resolution Liquid Chromatography Analysis (UHPLC-ESI-QTOF-MS)
2.4. The Total Phenol and Flavonoid Content
2.5. In Vitro Studies
2.5.1. Antioxidant Activity
Radical Scavenging Capacity Assay of 2,2-Diphenyl-1-picrylhydrazyl (DPPH)
Ferric-Reducing Antioxidant Power Assay (FRAP)
Trolox Equivalent Antioxidant Activity Assay (TEAC)
Inhibition of Lipid Peroxidation (ILP) in Erythrocytes
2.5.2. Anti-Inflammatory Effects: Cyclooxygenase (COX) Inhibition Method
2.5.3. Antitumoral and Cytotoxic Assays
Cell Culture Procedure
Methyl Thiazolyl Tetrazolium (MTT) Assay
2.6. Experimental Procedure In Vivo
2.6.1. Determination of GTT and Serum Parameters
2.6.2. TBAR Assay Method
2.6.3. Behavioral Tests Procedure
Open Field and Elevated Plus Maze Tests
Novel Object Location Test
2.6.4. Statistical Analysis
3. Results
3.1. Metabolite Profiling: UHPLC-ESI-QTOF-MS Analysis of Doe and MGEOe
3.2. In Vitro Studies
3.2.1. Total Phenolic and Flavonoid Contents, Antioxidant Activity and Inhibition Enzyme COX of DOe and MGEOe
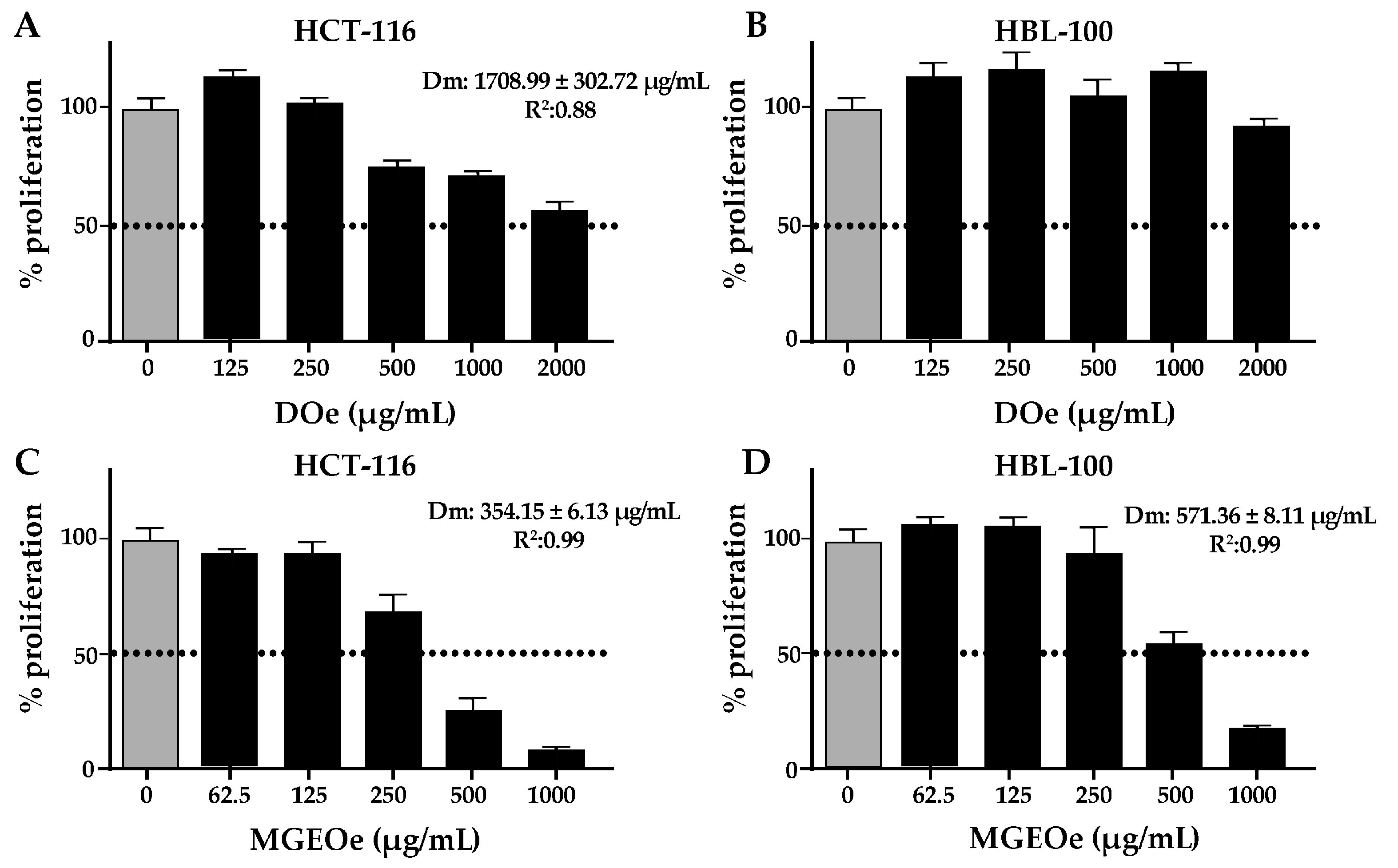
3.2.2. Cytotoxicity Study of DOe and MGEOe
3.3. In Vivo Study
3.3.1. BW Gain and Average Beverage Volume
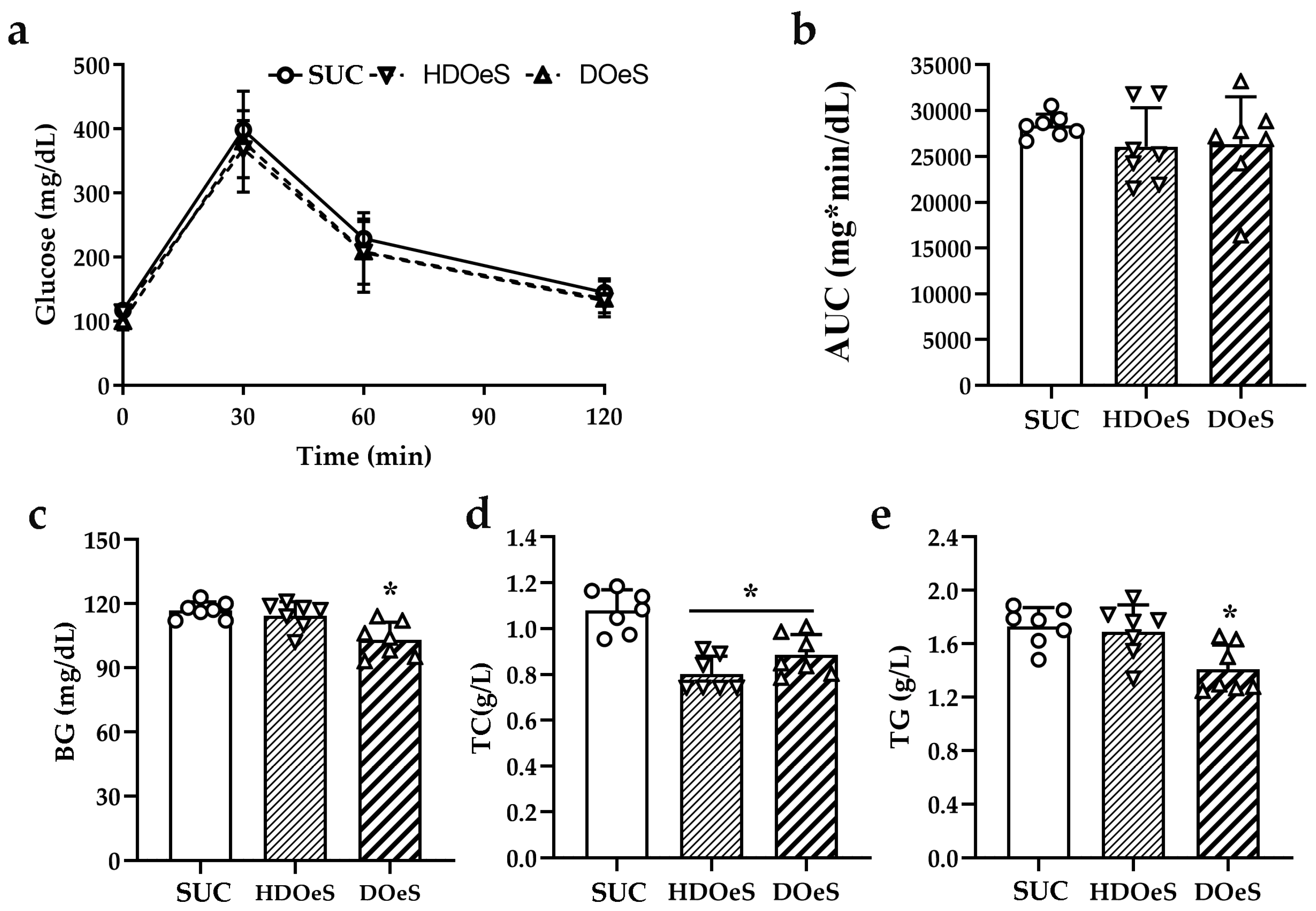
3.3.2. GTT and Serum Parameters
3.3.3. TBAR Assay
3.3.4. Behavioral Tests
OFT and EPM
NOL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbinta-Patrascu, M.E.; Bita, B.; Negut, I. From Nature to Technology: Exploring the Potential of Plant-Based Materials and Modified Plants in Biomimetics, Bionics, and Green Innovations. Biomimetics 2024, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Bustos, D.A.; Tapia, A.A.; Feresin, G.E.; Ariza Espinar, L. Ethnopharmacobotanical survey of Bauchazeta district, San Juan Province, Argentina. Fitoterapia 1996, 67, 411–415. [Google Scholar]
- Lourteig, A. Oxalidaceae. In Flora Patagónica, Colección Científica; Correa, M.N., Ed.; Instituto Nacional de Tecnología Agropecuaria: Buenos Aires, Argentina, 1988; Volume 8, pp. 1–29. [Google Scholar]
- Feresin, G.E.; Tapia, A.; López, S.N.; Zacchino, S.A. Antimicrobial Activity of Plants Used in Traditional Medicine of San Juan Province, Argentine. J. Ethnopharmacol. 2001, 78, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Feresin, G.E.; Tapia, A.; Sortino, M.; Zacchino, S.; Arias, A.R.D.; Inchausti, A.; Yaluff, G.; Rodriguez, J.; Theoduloz, C.; Schmeda-Hirschmann, G. Bioactive Alkyl Phenols and Embelin from Oxalis erythrorhiza. J. Ethnopharmacol. 2003, 88, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Gudasi, S.; Gharge, S.; Koli, R.; Patil, K. Antioxidant properties and cytotoxic effects of Oxalis corniculata on human Hepatocarci-noma (Hep-G2) cell line: An in vitro and in silico evaluation. Futur. J. Pharm. Sci. 2023, 9, 25. [Google Scholar] [CrossRef]
- Gholipour, A.R.; Jafari, L.; Ramezanpour, M.; Evazalipour, M.; Chavoshi, M.; Yousefbeyk, F.; Kargar Moghaddam, S.J.; Yekta Kooshali, M.H.; Ramezanpour, N.; Daei, P.; et al. Apoptosis Effects of Oxalis corniculata L. Extract on Human MCF-7 Breast Cancer Cell Line. Galen Med. J. 2022, 11, e2484. [Google Scholar]
- Zhang, J.; Shen, W.; He, H. Exploring the action mechanism of Oxalis corniculata L. decoction in treating osteoarthritis utilizing liquid chromatography–mass spectrometry technology combined with network pharmacology. Medicine 2024, 103, e39515. [Google Scholar] [CrossRef]
- Jifar, W.W.; Debele, G.R.; Kanfe, S.G.; Mule, C.T. Evaluation of in vivo Antidiabetic, Antidyslipidemic and in vitro Anti-Oxidant Activity of Extract and Solvent Fractions of Discopodium penninervum Hoschst Leaf in Mice: Normoglycemic and Streptozocin-Induced Model. J. Exp. Pharmacol. 2022, 28, 317–330. [Google Scholar] [CrossRef]
- Kabach, I.; Bouchmaa, N.; Zouaoui, Z.; Ennoury, A.; El Asri, S.; Laabar, A.; Oumeslakht, L.; Cacciola, F.; El Majdoub, Y.O.; Mondello, L.; et al. Phytochemical Profile and Antioxidant Capacity, α-Amylase and α-Glucosidase Inhibitory Activities of Oxalis Pes-Caprae Extracts in Alloxan-Induced Diabetic Mice. Biomed. Pharmacother. 2023, 160, 114393. [Google Scholar] [CrossRef]
- Abhilash, P.A.; Nisha, P.; Prathapan, A.; Nampoothiri, S.V.; Lijo Cherian, O.; Sunitha, T.K.; Raghu, K.G. Cardioprotective Effects of Aqueous Extract of Oxalis Corniculata in Experimental Myocardial Infarction. Exp. Toxicol. Pathol. 2011, 63, 535–540. [Google Scholar] [CrossRef]
- Gupta, G.; Kazmi, I.; Afzal, M.; Rahman, M.; Anwar, F. Anxiolytic Effect of Oxalis Corniculata (Oxalidaceae) in Mice. Asian Pac. J. Trop. Dis. 2012, 2, S837–S840. [Google Scholar] [CrossRef]
- Aruna, K.; Rajeswari, P.D.R.; Sankar, S.R. The Effect of Oxalis Corniculata Extract against the Behavioral Changes Induced by 1-Methyl- 4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) in Mice. J. Appl. Pharm. Sci. 2017, 7, 148–153. [Google Scholar]
- Manrique, S.; Gómez, J.; Piñeiro, M.; Sampietro, B.A.; Peschiutta, M.L.; Tapia, A.; Simirgiotis, M.J.; Lima, B. Zuccagnia punctata Cav., a Potential Environmentally Friendly and Sustainable Bionematicide for the Control of Argentinean Horticultural Crops. Plants 2023, 12, 4104. [Google Scholar] [CrossRef] [PubMed]
- Luna, L.; Simirgiotis, M.J.; Lima, B.; Bórquez, J.; Feresin, G.E.; Tapia, A. UHPLC-MS Metabolome Fingerprinting: The Isolation of Main Compounds and Antioxidant Activity of the Andean Species Tetraglochin ameghinoi (Speg.) Speg. Molecules 2018, 23, 793. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.; Simirgiotis, M.J.; Lima, B.; Paredes, J.D.; Villegas Gabutti, C.M.; Gamarra-Luques, C.; Bórquez, J.; Luna, L.; Wendel, G.H.; Maria, A.O.; et al. Antioxidant, gastroprotective, cytotoxic activities and UHPLC PDA-Q orbitrap mass spectrometry identification of metabolites in Baccharis grisebachii decoction. Molecules 2019, 24, 1085. [Google Scholar] [CrossRef] [PubMed]
- Kulmacz, R.J.; Lands, W.E. Requirements for Hydroperoxide by the Cyclooxygenase and Peroxidase Activities of Prostaglandin H Synthase. Prostaglandins 1983, 25, 531–540. [Google Scholar] [CrossRef]
- Torres Carro, R.; D’Almeida, R.E.; Isla, M.I.; Alberto, M.R. Antioxidant and Anti-Inflammatory Activities of Frankenia Triandra (J. Rémy) Extracts. S. Afr. J. Bot. 2016, 104, 208–214. [Google Scholar] [CrossRef]
- Chou, T.C.; Martin, N. CompuSyn for Dose–Effect Analysis and for Quantitation of Synergism and Antagonism. Software and Manual; ComboSyn: Paramus, NJ, USA, 2004. [Google Scholar]
- Rey, M.; Coirini, H.; Marchena, A.; González-Deniselle, M.C.; Kruse, M.S. Effects of metformin on behavioral alterations produced by chronic sucrose consumption in male rats. J. Neuroendocrinol. 2024, 36, e13362. [Google Scholar] [CrossRef]
- Rey, M.; Kruse, M.S.; Magrini-Huamán, R.N.; Coirini, H. High-Fat Diets and LXRs Expression in Rat Liver and Hypothalamus. Cell. Mol. Neurobiol. 2019, 39, 963–974. [Google Scholar] [CrossRef]
- Rey, M.; Kruse, M.S.; Magrini-Huamán, R.N.; Gómez, J.; Simirgiotis, M.J.; Tapia, A.; Feresin, G.E.; Coirini, H. Tessaria absinthioides (Hook. & Arn.) DC. (Asteraceae) Decoction Improves the Hypercholesterolemia and Alters the Expression of LXRs in Rat Liver and Hypothalamus. Metabolites 2021, 11, 579. [Google Scholar] [CrossRef]
- Rey, M.; Kruse, M.S.; Gómez, J.; Simirgiotis, M.J.; Tapia, A.; Coirini, H. Ultra-High-Resolution Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry Analysis of Tessaria absinthioides (Hook. & Arn.) DC. (Asteraceae) and Antioxidant and Hypocholesterolemic Properties. Antioxidants 2024, 13, 50. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Sturman, O.; Germain, P.L.; Bohacek, J. Exploratory Rearing: A Context- and Stress-Sensitive Behavior Recorded in the Open-Field Test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Veloso, A.W.N.; Filgueiras, G.B.; Lorenzo, P.; Estanislau, C. Modulation of Grooming Behavior in Rats by Different Test Situations. Psychol. Neurosci. 2016, 9, 91–104. [Google Scholar] [CrossRef]
- Cruz, A.D.M.; Frei, F.; Graeff, F.G. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol. Biochem. Behav. 1994, 49, 171–176. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Johnson, N.J. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol. Biochem. Behav. 1995, 52, 297–303. [Google Scholar] [CrossRef]
- Espejo, E.F. Effects of weekly or daily exposure to the elevated plus-maze in male mice. Behav. Brain Res. 1997, 87, 233–238. [Google Scholar] [CrossRef]
- Holmes, A.; Rodgers, R.J. Responses of Swiss-Webster mice to repeated plus-maze experience: Further evidence for a qualitative shift in emotional state? Pharmacol. Biochem. Behav. 1998, 60, 473–488. [Google Scholar] [CrossRef]
- Gómez, J.; Simirgiotis, M.J.; Lima, B.; Gamarra-Luques, C.; Bórquez, J.; Caballero, D.; Feresin, G.E.; Tapia, A. UHPLC–Q/Orbitrap/MS/MS fingerprinting, free radical scavenging, and antimicrobial activity of Tessaria absinthiodes (Hook. &arn.) DC. (Asteraceae) lyophilized decoction from Argentina and Chile. Antioxidants 2019, 8, 593. [Google Scholar] [CrossRef]
- Gómez, J.; Simirgiotis, M.J.; Manrique, S.; Piñeiro, M.; Lima, B.; Bórquez, J.; Feresin, G.E.; Tapia, A. UHPLC-ESI-OT-MS Phenolics Profiling, Free Radical Scavenging, Antibacterial and Nematicidal Activities of “Yellow-Brown Resins” from Larrea spp. Antioxidants 2021, 10, 185. [Google Scholar] [CrossRef]
- Gómez, J.; Simirgiotis, M.J.; Manrique, S.; Lima, B.; Bórquez, J.; Feresin, G.E.; Tapia, A. UHPLC-HESI-OT-MS-MS biomolecules profiling, antioxidant and antibacterial activity of the “orange-yellow resin” from Zuccagnia punctata Cav. Antioxidants 2020, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.C.; Palacios, J.; Barrientos, R.E.; Gómez, J.; Castagnini, J.M.; Barba, F.J.; Tapia, A.; Paredes, A.; Cifuentes, F.; Simirgiotis, M.J. UHPLC-MS Phenolic Fingerprinting, Aorta Endothelium Relaxation Effect, Antioxidant, and Enzyme Inhibition Activities of Azara dentata Ruiz & Pav Berries. Foods 2023, 12, 643. [Google Scholar] [CrossRef] [PubMed]
- Persia, F.A.; Troncoso, M.E.; Rinaldini, E.; Simirgiotis, M.; Tapia, A.; Bórquez, J.; Mackern-Oberti, J.P.; Hapon, M.B.; Gamarra-Luques, C. UHPLC–Q/Orbitrap/MS/MS Fingerprinting and Antitumoral Effects of Prosopis Strombulifera (LAM.) BENTH. Queous Extract on Allograft Colorectal and Melanoma Cancer Models. Heliyon 2020, 6, e03353. [Google Scholar] [CrossRef] [PubMed]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Xiao Hui, Z. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Bender, O.; Atalay, A. Chapter 28—Polyphenol chlorogenic acid, antioxidant profile, and breast cancer. In Cancer, 2nd ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 311–321. [Google Scholar]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.; Wu, W.; Liu, Q.; Liu, X. Effect of isoorientin on intra-cellular antioxidant defense mechanisms in hepatoma and liver cell lines. Biomed. Pharmacother. 2016, 81, 356–362. [Google Scholar] [CrossRef]
- Ziqubu, K.; Dludla, P.V.; Joubert, E.; Muller, C.J.; Louw, J.; Tiano, L.; Mazibuko-Mbeje, S.E. Isoorientin: A dietary flavone with the potential to ameliorate diverse metabolic complications. Pharmacol. Res. 2020, 158, 104867. [Google Scholar] [CrossRef]
- Yuan, L.; Han, X.; Li, W.; Ren, D.; Yang, X. Isoorientin prevents hyperlipidemia and liver injury by regulating lipid metabolism, antioxidant capability, and inflammatory cytokine release in high-fructose-fed mice. J. Agric. Food Chem. 2016, 64, 2682–2689. [Google Scholar] [CrossRef]
- He, M.; Min, J.W.; Kong, W.L.; He, X.H.; Li, J.X.; Peng, B.W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Wang, H.; Pan, C.W.; Lin, M.X. Isovitexin alleviates liver injury induced by lipopolysaccharide/d-galactosamine by activating Nrf2 and inhibiting NF-?B activation. Microb. Pathog. 2018, 119, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, L. Antioxidant, antiepileptic, and anticholinergic properties of 4?, 5, 7-Trihydroxy-3, 6-dimethoxyflavone as natural phenolic compound: A toxicology approach. Toxin. Rev. 2021, 40, 292–299. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, J.; Ding, Y.; Ma, Y.; Shang, P.; Liu, T.; Wen, A. Alpha-boswellic acid protects against ethanol-induced gastric injury in rats: Involvement of nuclear factor erythroid-2-related factor 2/heme oxygenase-1 pathway. J. Pharm. Pharmacol. 2016, 68, 514–522. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Calder, P.C. A Review of the Functional Effects of Pine Nut Oil, Pinolenic Acid and Its Derivative Eicosatrienoic Acid and Their Potential Health Benefits. Prog. Lipid Res. 2021, 82, 101097. [Google Scholar] [CrossRef]
- El Omari, N.; Jaouadi, I.; Lahyaoui, M.; Benali, T.; Taha, D.; Bakrim, S.; El Menyiy, N.; El Kamari, F.; Zengin, G.; Bangar, S.P.; et al. Natural Sources, Pharmacological Properties, and Health Benefits of Daucosterol: Versatility of Actions. Appl. Sci. 2022, 12, 5779. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Shafiee, S.; Burcher, J.T.; Lagoa, R.; Farzaei, M.H.; Bishayee, A. Anticancer Potential of Apigenin and Isovitexin with Focus on Oncogenic Metabolism in Cancer Stem Cells. Metabolites 2023, 13, 404. [Google Scholar] [CrossRef]
- Joray, M.B.; Villafañez, F.; González, M.L.; Crespo, M.I.; Laiolo, J.; Palacios, S.M.; Carpinella, M.C. P53 tumor suppressor is required for efficient execution of the death program following treatment with a cytotoxic limonoid obtained from Melia azedarach. Food Chem. Toxicol. 2017, 109, 888–897. [Google Scholar] [CrossRef]
- Roy, N.K.; Deka, A.; Bordoloi, D.; Mishra, S.; Kumar, A.P.; Sethi, G.; Kunnumakkara, A.B. The potential role of boswellic acids in cancer prevention and treatment. Cancer Lett. 2016, 377, 74–86. [Google Scholar] [CrossRef]
- Zhao, C.; She, T.; Wang, L.; Su, Y.; Qu, L.; Gao, Y.; Xu, S.; Cai, S.; Shou, C. Daucosterol Inhibits Cancer Cell Proliferation by Inducing Autophagy through Reactive Oxygen Species-Dependent Manner. Life Sci. 2015, 137, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Liu, X.; Li, X.; Zheng, Y.; Liu, B.; Xiao, Y. Daucosterol Inhibits the Proliferation, Migration, and Invasion of Hepatocellular Carcinoma Cells via Wnt/β-Catenin Signaling. Molecules 2017, 22, 862. [Google Scholar] [CrossRef] [PubMed]
- Zingue, S.; Gbaweng Yaya, A.J.; Michel, T.; Ndinteh, D.T.; Rutz, J.; Auberon, F.; Maxeiner, S.; Chun, F.K.H.; Tchinda, A.T.; Njamen, D.; et al. Bioguided Identification of Daucosterol, a Compound That Contributes to the Cytotoxicity Effects of Crateva Adansonii DC (Capparaceae) to Prostate Cancer Cells. J. Ethnopharmacol. 2020, 247, 112251. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Y.; Mi, S.; Fan, Q.; Sun, X.; Deng, B.; Wu, G.; Li, Y.; Zhou, Q.; Ruan, Z. Hepatoprotective Effect of Chlorogenic Acid against Chronic Liver Injury in Inflammatory Rats. J. Funct. Foods 2019, 62, 103540. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Bae, J.S. Vascular Barrier Protective Effects of Orientin and Isoorientin in LPS-Induced Inflammation in Vitro and in Vivo. Vascul. Pharmacol. 2014, 62, 3–14. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Tan, X.; Liu, J.; Zhi, Y.; Yi, L.; Bai, S.; Du, Q.; Li, Q.X.; Dong, Y. Isoorientin Inhibits Inflammation in Macrophages and Endotoxemia Mice by Regulating Glycogen Synthase Kinase 3β. Mediators Inflamm. 2020, 2020, 8704146. [Google Scholar] [CrossRef]
- Lin, C.M.; Huang, S.T.; Liang, Y.C.; Lin, M.S.; Shih, C.M.; Chang, Y.C.; Chen, T.Y.; Chen, C.T. Isovitexin Suppresses Lipopolysaccharide-Mediated Inducible Nitric Oxide Synthase through Inhibition of NF-Kappa B in Mouse Macrophages. Planta Med. 2005, 71, 748–753. [Google Scholar] [CrossRef]
- Takala, R.; Ramji, D.P.; Choy, E. The Beneficial Effects of PineNuts and Its Major Fatty Acid, Pinolenic Acid, on Inflammation and Metabolic Perturbations inInflammatory Disorders. Int. J. Mol. Sci. 2023, 24, 1171. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Evangelista, S.; Cirillo, R.; Lippi, A.; Maggi, C.A.; Manzini, S. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediators Inflamm. 2000, 9, 223–228. [Google Scholar] [CrossRef]
- Seo, S.; Oh, S.; Shin, Y.; Jung, S.; Kim, Y. Reduction of body weight by rutin is associated with an increase of brown adipose tissue mitochondrial biogenesis in high-fat diet induced obese rat (LB430). FASEB J. 2014, 28, LB430. [Google Scholar] [CrossRef]
- Watanabe, T.; Kobayashi, S.; Yamaguchi, T.; Hibi, M.; Fukuhara, I.; Osaki, N. Coffee Abundant in Chlorogenic Acids Reduces Abdominal Fat in Overweight Adults: A Randomized, Double-Blind, Controlled Trial. Nutrients 2019, 16, 1617. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, J.; Kim, Y. Effect of Deglycosylated Rutin by Acid Hydrolysis on Obesity and Hyperlipidemia in High-Fat Diet-Induced Obese Mice. Nutrients 2020, 25, 1539. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, Z.; Zhai, Y.; Yan, X.; Zhou, W.; Liu, H.; Guan, L.; Peng, L. Apigenin Alleviates Obesity-Associated Metabolic Syndrome by Regulating the Composition of the Gut Microbiome. Front. Microbiol. 2022, 12, 805827. [Google Scholar] [CrossRef]
- Kanchanasurakit, S.; Saokaew, S.; Phisalprapa, P.; Duangjai, A. Chlorogenic Acid in Green Bean Coffee on Body Weight: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Syst. Rev. 2023, 12, 163. [Google Scholar] [CrossRef]
- Heikkilä, E.; Hermant, A.; Thevenet, J.; Bermont, F.; Kulkarni, S.S.; Ratajczak, J.; Santo-Domingo, J.; Dioum, E.H.; Canto, C.; Barron, D.; et al. The plant product quinic acid activates Ca2+-dependent mitochondrial function and promotes insulin secretion from pancreatic beta cells. Br. J. Pharmacol. 2019, 176, 3250–3263. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2021, 11, 592654. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic Acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Alternat. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, L.; Wen, M.; Che, H.; Du, L.; Wang, J.; Xue, C.; Xu, J.; Wang, Y. Eicosapentaenoic acid-enriched phospholipids improve atherosclerosis by mediating cholesterol metabolism. J. Funct. Foods 2017, 32, 90–97. [Google Scholar] [CrossRef]
- Clandinin, M.T.; Cook, S.L.; Konard, S.D.; French, M.A. The effect of palmitic acid on lipoprotein cholesterol levels. Int. J. Food Sci. Nutr. 2000, 51 (Suppl. S1), S61–S71. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Dicko, A.; Younos, C.; Soulimani, R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007, 262, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Foudah, A.I.; Alqarni, M.H.; Alam, A.; Devi, S.; Salkini, M.A.; Alam, P. Rutin Improves Anxiety and Reserpine-Induced Depression in Rats. Molecules 2022, 27, 7313. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.D.; da Silva, C.P.; Iglesias, B.B.; Beleboni, R.O. Vitexin Possesses Anticonvulsant and Anxiolytic-Like Effects in Murine Animal Models. Front. Pharmacol. 2020, 11, 1181. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Nisbett, K.E.; Vendruscolo, L.F.; Koob, G.F. Indulging Curiosity: Preliminary Evidence of an Anxiolytic-like Effect of Castor Oil and Ricinoleic Acid. Nutrients 2024, 16, 1527. [Google Scholar] [CrossRef]
- Munawaroh, F.; Arfian, N.; Saputri, L.A.A.W.S.; Kencana, S.M.S.; Sari, D.C.R. Chlorogenic acid may improve memory function and decrease inflamation of frontal lobe in diabetic rat. Med. J. Malays. 2023, 78, 476–483. [Google Scholar]
- Xu, P.X.; Wang, S.W.; Yu, X.L.; Su, Y.J.; Wang, T.; Zhou, W.W.; Zhang, H.; Wang, Y.J.; Liu, R.T. Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing A? oligomer level and attenuating oxidative stress and neuroinflammation. Behav. Brain Res. 2014, 264, 173–180. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaokun, O.O. The Beneficial Role of Apigenin against Cognitive and Neurobehavioural Dysfunction: A Systematic Review of Preclinical Investigations. Biomedicines 2024, 12, 178. [Google Scholar] [CrossRef]
- Ji, Z.H.; Xu, Z.Q.; Zhao, H.; Yu, X.Y. Neuroprotective effect and mechanism of daucosterolpalmitate in ameliorating learning and memory impairment in a rat model of Alzheimer’s disease. Steroids 2017, 119, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Cortez Tornello, P.R.; Feresin, G.E.; Tapia, A.; Dzieciuch, M.; Cuadrado, T.R.; Abraham, G.A. Effect of processing techniques on new poly (ε-caprolactone)-embelin microparticles of biomedical interest. Adv. Polym. Technol. 2018, 37, 1570–1580. [Google Scholar] [CrossRef]
- Wan, Z.; Gan, L.; Wang, W.N.; Jiang, Y. Rapid membrane surface functionalization with Ag nanoparticles via coupling electrospray and polymeric solvent bonding for enhanced antifouling and catalytic performance: Deposition and interfacial reaction mechanisms. J. Colloid Interface Sci. 2023, 639, 203–213. [Google Scholar] [CrossRef]
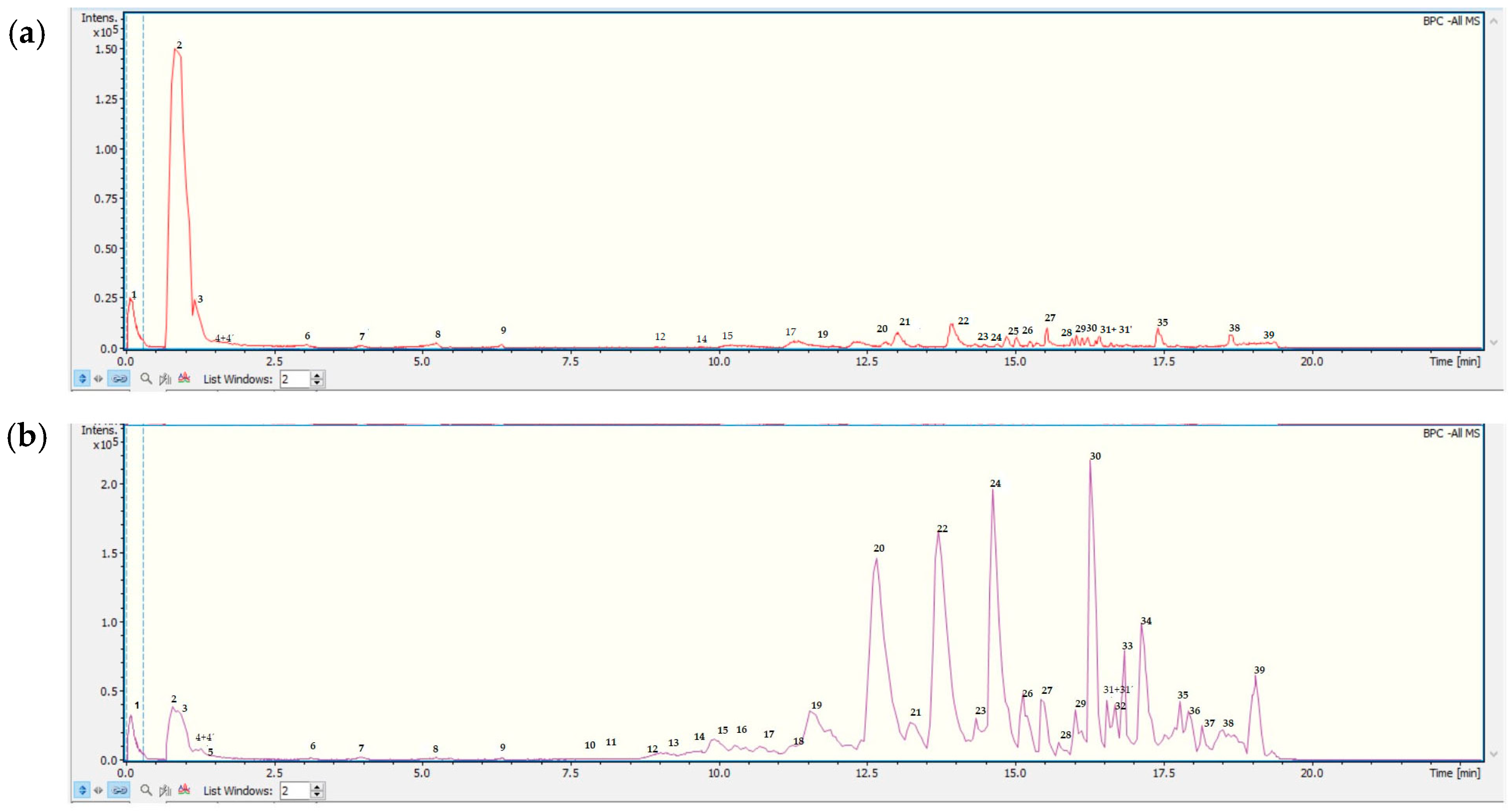
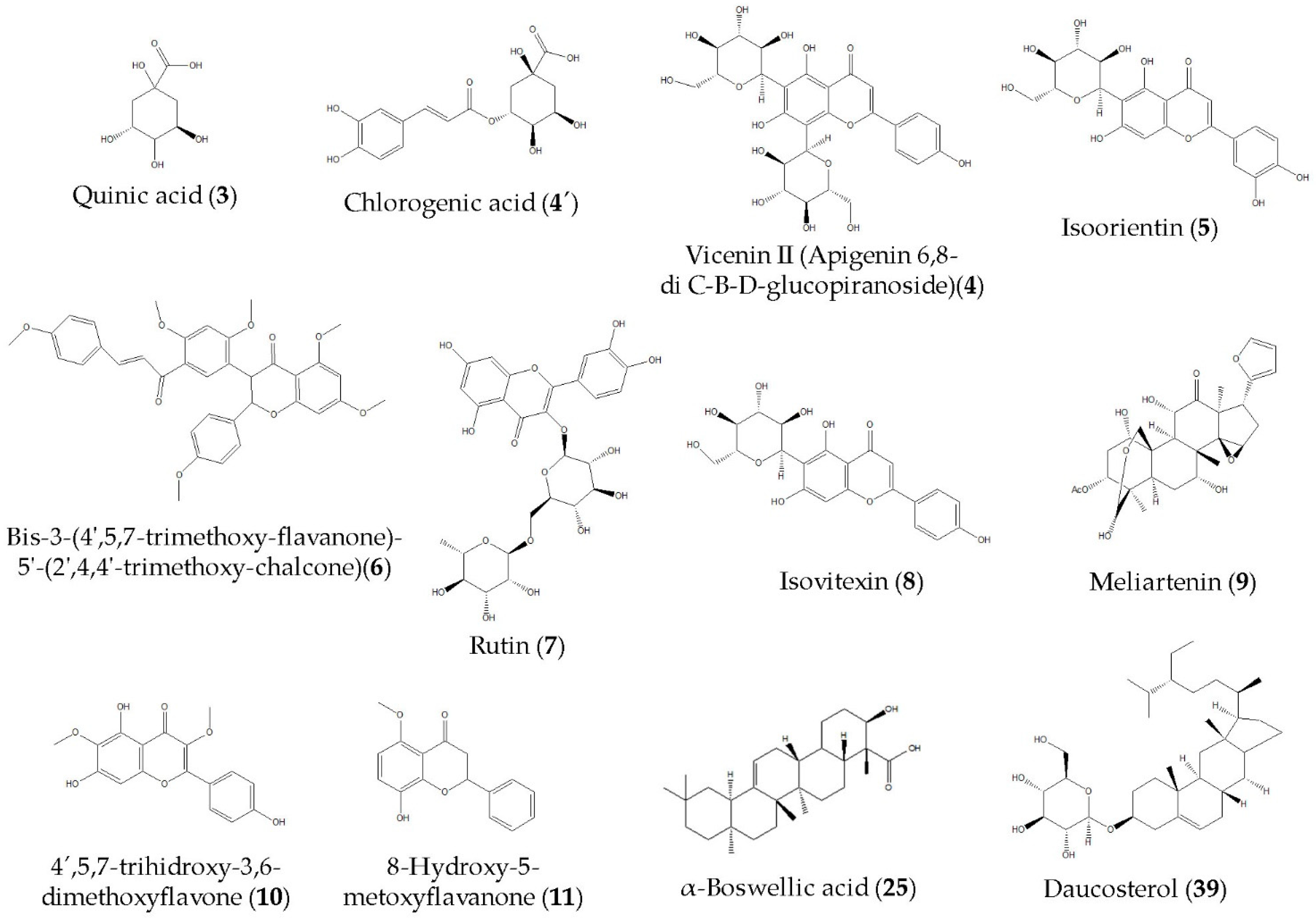



| Peak | Tentative Identification | [M-H]- | Retention Time (min) | Measured Mass (m/z) | Molecular Formula | Accuracy (ppm) | Metabolite Type | MS Ions (m/z) |
|---|---|---|---|---|---|---|---|---|
| 1 | Naformiate (internal standard) | NaC2H2O4 | 0.12 | 112.9829 | 112.9856 | 3.10 | A | |
| 2 | Monohexosyldiglyceride | C12H18O12 | 0.78 | 353.0786 | 354.0859 | 17.08 | Fatty acid | |
| 3 | Quinic acid | C7H12O6 | 0.84 | 191.0563 | 192.0636 | 2.26 | Acid | 93.0345 |
| 4 | Vicenin II (apigenin 6,8-di C-B-D-glucopiranoside) | C27H30O15 | 1.20 | 593.2185 | 594.2199 | 2.45 | C-glycosil flavone | 353.1079 |
| 4′ | Chlorogenic acid | C16H18O9 | 1.20 | 353.0886 | 354.0959 | 2.45 | Phenolic acid | 191.0513 |
| 5 | Luteolin-6-C-glucoside (Isoorientin) | C21H20O11 | 1.50 | 447.0953 | 448.1025 | 3.93 | C-glycosil flavone | 429.1389 357.1038 327.0886 298.0861 |
| 6 | Bis-3-(4′,5,7-trimethoxy-flavanone)-5′-(2′,4,4′-trimethoxy-chalcone) | C36H34O9 | 3.13 | 609.2154 | 610.2130 | 3.89 | Flavone | 447.1456 341.1492 112.9882 |
| 7 | Rutin | C27H30O16 | 4.42 | 609.1477 | 610.1550 | 4.67 | Flavone | 301.0333 300.0258 |
| 8 | Isovitexin | C21H20O10 | 4.48 | 431.1006 | 432.1078 | 5.07 | Isoflavone | 311.0563 283.0608 |
| 9 | Meliartenin | C28H36O10 | 6.25 | 531.2313 | 532.2236 | 3.05 | Furane | 486.2168 385.3007 |
| 10 | 4′,5,7-trihydroxy-3,6-dimethoxyflavone | C17H14O7 | 7.54 | 329.0677 | 330.0750 | 3.31 | Flavone | 311.1599 255.0120 |
| 11 | 8-hydroxy-5-methoxyflavanone | C16H14O4 | 7.93 | 269.0817 | 270.0894 | 4.06 | Flavone | 254.0580 |
| 12 | 17-hydroxylinolenic acid | C18H30O3 | 8.45 | 293.2134 | 294.2207 | 4.00 | Aromatic | 439.0996 311.1636 |
| 13 | Diffractaic acid | C20H22O7 | 9.62 | 373.1304 | 374.1376 | 4.47 | Aromatic | 282.0879 135.0810 |
| 14 | 9-HPODE octadecenedioic acid | C18H32O4 | 9.80 | 311.223 | 312.2305 | 0.80 | Fatty acid | 269.2072 257.2058 |
| 15 | 9-hydroxy-10,12-octadecadienoic acid | C18H32O3 | 10.50 | 295.2283 | 296.2351 | 1.62 | Fatty acid | 269.2077 |
| 16 | 13-HPODE octadecenedioic acid | C18H32O4 | 10.56 | 311.2235 | 312.2308 | 3.49 | Fatty acid | 257.2058 |
| 17 | (9Z,12R)-12-hydroxyoctadec-9-enoic acid | C18H34O3 | 11.52 | 311.2235 | 312.2308 | 5.76 | Fatty acid | 257.2057 |
| 18 | Ricinoleic acid | C18H34O3 | 11.88 | 297.2437 | 298.2513 | 0.44 | Fatty acid | 253.5113 |
| 19 | 5-(octadecyloxy) isophthalic acid | C26H41O5 | 11.57 | 433.2866 | 434.2960 | −21.04 | Phenolic acid | |
| 20 | Ricinoleic acid isomer | C18H34O3 | 12.54 | 297.2427 | 298.2513 | 5.04 | Fatty acid | 283.0608 311.0561 |
| 21 | Lesquerolic acid | C20H38O3 | 13.22 | 325.2745 | 326.2748 | 8.33 | Fatty acid | 299.2586 227.2255 |
| 22 | Pinolenic acid | C18H30O2 | 14.23 | 277.2184 | 278.2257 | 4.04 | Fatty acid | 283.2152 |
| 23 | 9-octadecenoic acid (Z)-, 2-hydroxyethyl ester | C20H38O3 | 14.31 | 325.2767 | 326.2748 | 5.74 | Fatty acid | 307.2263 299.2586 293.2044 |
| 24 | Roccellaric acid | C19H34O4 | 14.62 | 325.2396 | 326.2464 | 2.70 | Fatty acid | 281.2495 |
| 25 | Alpha-boswellic acid | C30H48O3 | 14.85 | 455.3540 | 456.3613 | 1.37 | Fatty acid | 227.2255 |
| 26 | Eicosapentaenoic acid | C20H30O2 | 15.02 | 301.2184 | 302.2257 | 1.79 | Fatty acid | 251.2660 |
| 27 | Pristimerin | C30H40O4 | 15.02 | 463.2860 | 464.2933 | 1.44 | Terpene | 449.2673 433.2385 |
| 28 | Dodecanoic acid,2-ethylhexanoic acid,propane-1,2,3-triol | C23H48O7 | 15.58 | 435.3275 | 436.3327 | −11.95 | Fatty acid | 255.2610 |
| 29 | Palmitic acid | C16H32O2 | 16.13 | 255.2338 | 256.2411 | 3.29 | Fatty acid | 112.9879 |
| 30 | FAHFA 18:1/2:0 hydroxy fatty acid ester: icos-10-enedioic acid | C20H36O4 | 16.28 | 339.2553 | 340.2625 | 3.48 | Fatty acid | 281.2811 |
| 31 | 22-oxodocosanoic acid | C22H41O3 | 16.39 | 353.3116 | 354.3061 | 15.40 | Fatty acid | 339.2952 |
| 31′ | PI 34:21-phosphatidyl-1D-myo-inositol | C43H79O13P | 16.39 | 833.5264 | 834.5337 | 9.47 | Fatty acid | 717.4680 533.4085 403.2977 323.2977 |
| 32 | Isopropyl linoleate | C21H39O2 | 16.45 | 323.2969 | 324.2956 | 4.12 | Fatty acid | 275.0326 |
| 33 | PI(16:0/13-HODE) hydroxyoctadecadienoic acid | C43H79O14P | 17.02 | 849.5204 | 850.5276 | 8.12 | Fatty acid | 353.3115 |
| 34 | Muricatenol | C37H68O6 | 17.43 | 607.4909 | 607.4943 | −5.49 | Fatty acid | 599.4280 574.4127 409.3437 |
| 35 | 28-O-acetylbetulin-3-yl-β-D-glucopyranoside | C38H62O8 | 17.52 | 645.4363 | 646.4372 | −1.33 | Fatty acid | 607.4901 573.4062 |
| 36 | 3β-O-acetyl-28-O-lup-20(29)-ene | C38H62O8 | 17.74 | 645.4363 | 645.4367 | −1.31 | Sterol | 409.3437 |
| 37 | 1-stearyl-2-cholesterylcarbonoyl-3-trityl glycerol | C40H59O3 | 18.56 | 586.4309 | 587.4391 | −14.02 | Fatty acid | 281.2111 |
| 38 | Aipolic acid | C32H52O5 | 18.51 | 515.3647 | 515.3589 | 11.09 | Terpene | 361.3160 |
| 39 | Daucosterol | C35H60O6 | 19.12 | 575.4261 | 576.4317 | −9.66 | Sterol | 477.3778 |
| Spectrophotometric Quantification of Compounds of Interest | ||
|---|---|---|
| Total phenols (mg GAE/g extracts) | DOe | 69.24 ± 5.78 |
| MGEOe | 65.45 ± 0.06 | |
| Flavonoids (mg QE/g extracts) | DOe | 43.31 ± 4.13 |
| MGEOe | 31.90 ± 0.05 | |
| Antioxidant and Inhibition COX-2 Assays | ||
| DPPH (EC50 in µg extracts/mL) | DOe | 70.11 ± 0.07 |
| MGEOe | 62.07 ± 1.74 | |
| FRAP (mg TE/g of extracts) | DOe | 4.95 ± 0.08 |
| MGEOe | 3.25 ± 0.06 | |
| TEAC (mg TE/g of extracts) | DOe | 0.67 ± 0.05 |
| MGEOe | 1.24 ± 0.05 | |
| Percentage ILP (at 250 µg extract/mL) | DOe | 83.81 ± 3.59 |
| MGEOe | 90.46 ± 0.03 | |
| Percentage inhibition COX-2 (at 72 µg/mL) | DOe | 86.97± 0.05 |
| Cerebral Cortex | Hippocampus | Hypothalamus | |
|---|---|---|---|
| SUC | 40.45 ± 13.82 | 34.67 ± 18.11 | 60.24 ± 7.83 |
| HDOeS | 44.39 ± 14.95 | 32.26 ± 7.27 | 46.64 ± 12.56 |
| DOeS | 46.21 ± 17.85 | 46.97 ± 10.65 | 56.20 ± 9.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, J.; Simirgiotis, M.J.; Kruse, M.S.; Gamarra-Luques, C.; Lima, B.; Zaragosa, J.; Piñeiro, M.; Tapia, A.; Coirini, H.; Rey, M. Oxalis erythrorhiza Gillies ex Hooker et Arnott (Oxalidaceae): Chemical Analysis, Biological In Vitro and In Vivo Properties and Behavioral Effects. Antioxidants 2024, 13, 1494. https://doi.org/10.3390/antiox13121494
Gómez J, Simirgiotis MJ, Kruse MS, Gamarra-Luques C, Lima B, Zaragosa J, Piñeiro M, Tapia A, Coirini H, Rey M. Oxalis erythrorhiza Gillies ex Hooker et Arnott (Oxalidaceae): Chemical Analysis, Biological In Vitro and In Vivo Properties and Behavioral Effects. Antioxidants. 2024; 13(12):1494. https://doi.org/10.3390/antiox13121494
Chicago/Turabian StyleGómez, Jessica, Mario J. Simirgiotis, María Sol Kruse, Carlos Gamarra-Luques, Beatriz Lima, José Zaragosa, Mauricio Piñeiro, Alejandro Tapia, Héctor Coirini, and Mariana Rey. 2024. "Oxalis erythrorhiza Gillies ex Hooker et Arnott (Oxalidaceae): Chemical Analysis, Biological In Vitro and In Vivo Properties and Behavioral Effects" Antioxidants 13, no. 12: 1494. https://doi.org/10.3390/antiox13121494
APA StyleGómez, J., Simirgiotis, M. J., Kruse, M. S., Gamarra-Luques, C., Lima, B., Zaragosa, J., Piñeiro, M., Tapia, A., Coirini, H., & Rey, M. (2024). Oxalis erythrorhiza Gillies ex Hooker et Arnott (Oxalidaceae): Chemical Analysis, Biological In Vitro and In Vivo Properties and Behavioral Effects. Antioxidants, 13(12), 1494. https://doi.org/10.3390/antiox13121494












