Oxidative Stress Induced by Antivirals: Implications for Adverse Outcomes During Pregnancy and in Newborns
Abstract
1. Introduction
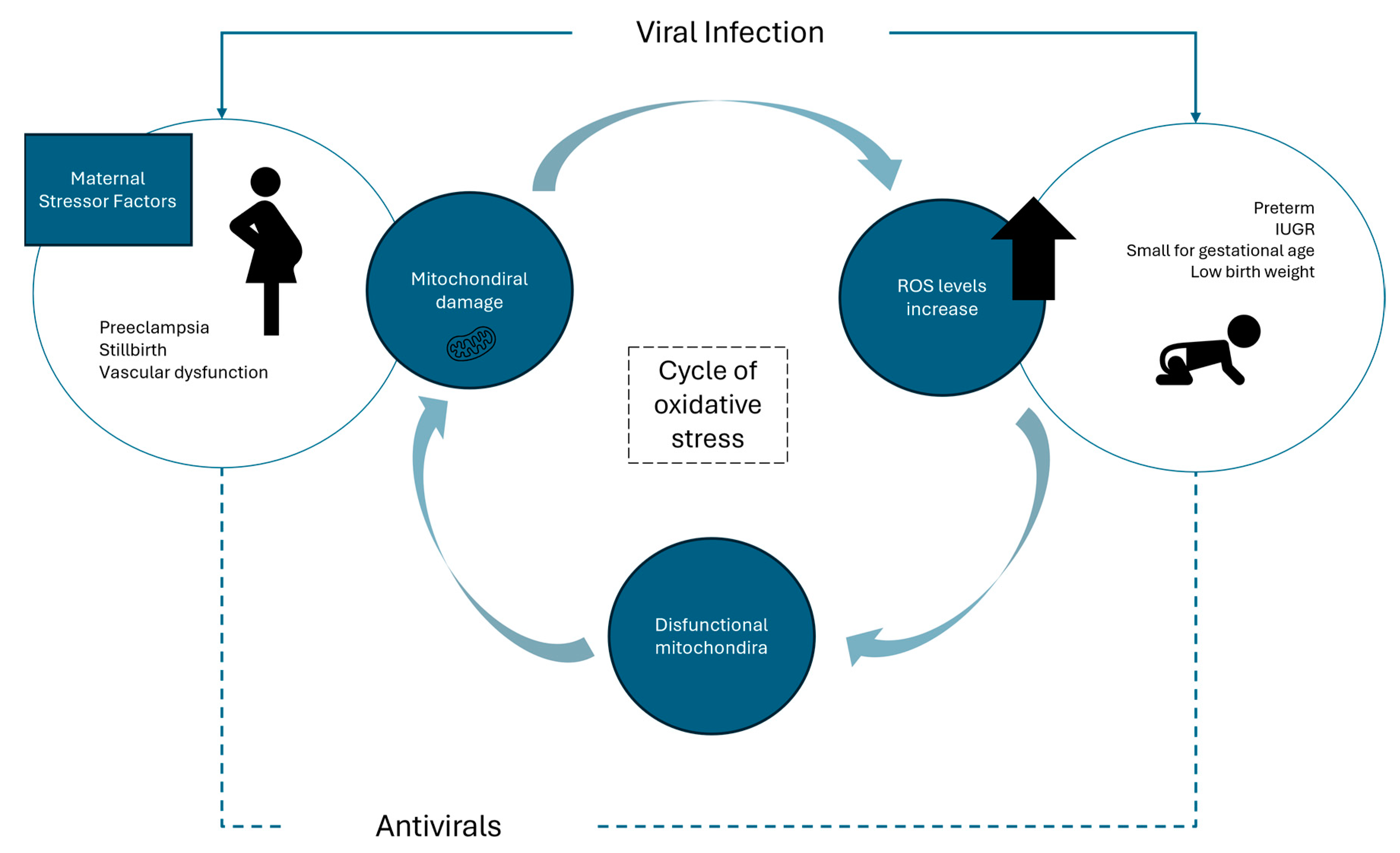
2. Viral Infections and Their Role in Oxidative Stress
3. The Link Between Oxidative Stress and Antiviral Agents
3.1. Impact of Antiviral Medications on Antioxidant Mechanisms
3.2. Challenges in Assessing Oxidative Stress Caused by Antivirals
4. Impact of Antivirals During Pregnancy and Adverse Outcomes in Newborns
4.1. Oxidative Balance During Pregnancy and Viral Infection
4.2. Pre-Existing Maternal Health Conditions and Antiviral Therapy
4.3. Adverse Outcomes in Newborns Caused by Viral and Antiviral-Induced Oxidative Stress
| Biomarker | Type of Oxidative Damage | Significance | Sample Type | References |
|---|---|---|---|---|
| Isoprostanes | Lipid peroxidation | Reflects oxidative damage to lipids; used as a marker of oxidative stress in preterm infants | Cord blood, urine, saliva | [133,134] |
| Advanced Oxidation Protein Products (AOPP) | Protein oxidation | Measures protein damage caused by ROS; associated with various neonatal diseases | Cord blood, plasma, saliva | [133,135] |
| Non-Protein-Bound Iron (NPBI) | Iron-mediated oxidative damage | Indicator of free iron availability, which can catalyze ROS production, linked to oxidative damage | Cord blood, urine | [133] |
| 8-Hydroxy-2′-deoxyguanosine (8-OHdG) | DNA oxidation | Marker of oxidative DNA damage; can indicate long-term risks like cancer or neurodevelopmental disorders | Urine, blood | [136] |
| Malondialdehyde (MDA) | Lipid peroxidation | Byproduct of lipid peroxidation, associated with cellular damage and oxidative stress in newborns | Cord blood, plasma, urine | [134] |
| Glutathione (GSH)/Glutathione Disulfide (GSSG) | Redox balance marker | Reflects the cellular oxidative stress status by measuring the balance between reduced and oxidized glutathione | Blood, cord blood, saliva | [133] |
| Total Antioxidant Capacity (TAC) | Antioxidant defense capacity | Assesses the body’s overall ability to neutralize ROS; useful in determining oxidative stress status | Blood, urine, saliva | [135] |
| Mechanism | Antiviral Class/Example | Effects in Pregnancy | Implications for Newborns |
|---|---|---|---|
| Directly Generate ROS | Zidovudine (NRTI), efavirenz (NNRTI), protease inhibitors | Mitochondrial dysfunction, increased oxidative stress, potential fetal growth restriction | Increased ROS production, potential for low birth weight, and neurodevelopmental risks |
| Impair Antioxidant Defenses | Integrase inhibitors (dolutegravir, raltegravir), neuraminidase inhibitors (oseltamivir) | Disruption of redox balance, compromised placental antioxidant activity | Reduced antioxidant capacity, higher risk of oxidative stress-related conditions |
5. Efficacy and Safety of Specific Antioxidants in Reducing Oxidative Stress During Pregnancy
6. Potential Strategies to Mitigate Antiviral-Induced Oxidative Stress
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Money, D.M. Antiviral and Antiretroviral Use in Pregnancy. Obstet. Gynecol. Clin. N. Am. 2003, 30, 731–749. [Google Scholar] [CrossRef] [PubMed]
- Waitt, C.; Astill, D.; Zavala, E.; Karron, R.A.; Faden, R.R.; Stratton, P.; Temkin, S.M.; Clayton, J.A. Clinical Trials and Pregnancy. Commun. Med. 2022, 2, 132. [Google Scholar] [CrossRef] [PubMed]
- Sewell, C.A.; Sheehan, S.M.; Gill, M.S.; Henry, L.M.; Bucci-Rechtweg, C.; Gyamfi-Bannerman, C.; Lyerly, A.D.; McKinney, L.C.; Hatfield, K.P.; Baer, G.R.; et al. Scientific, Ethical, and Legal Considerations for the Inclusion of Pregnant People in Clinical Trials. Am. J. Obstet. Gynecol. 2022, 227, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Recommendations for the Use of Antiretroviral Drugs During Pregnancy. Available online: https://clinicalinfo.hiv.gov/en/guidelines/perinatal/recommendations-arv-drugs-pregnancy-overview#:~:text=Overview,-Panel’s%20Recommendations&text=All%20pregnant%20people%20with%20HIV,and%20sexual%20transmission%20(AI) (accessed on 1 May 2024).
- Kolding, L.; Eken, H.; Uldbjerg, N. Drug Exposure during Pregnancy and Fetal Cardiac Function—A Systematic Review. J. Perinat. Med. 2020, 48, 199–208. [Google Scholar] [CrossRef]
- Bérard, A.; Sheehy, O.; Zhao, J.; Vinet, É.; Bernatsky, S.; Abrahamowicz, M. SSRI and SNRI Use during Pregnancy and the Risk of Persistent Pulmonary Hypertension of the Newborn. Br. J. Clin. Pharmacol. 2017, 83, 1126–1133. [Google Scholar] [CrossRef]
- Odufalu, F.-D.; Long, M.; Lin, K.; Mahadevan, U. Exposure to Corticosteroids in Pregnancy Is Associated with Adverse Perinatal Outcomes among Infants of Mothers with Inflammatory Bowel Disease: Results from the PIANO Registry. Gut 2022, 71, 1766–1772. [Google Scholar] [CrossRef]
- Ross, E.J.; Graham, D.L.; Money, K.M.; Stanwood, G.D. Developmental Consequences of Fetal Exposure to Drugs: What We Know and What We Still Must Learn. Neuropsychopharmacology 2015, 40, 61–87. [Google Scholar] [CrossRef]
- Hudson, R.E.; Metz, T.D.; Ward, R.M.; McKnite, A.M.; Enioutina, E.Y.; Sherwin, C.M.; Watt, K.M.; Job, K.M. Drug Exposure during Pregnancy: Current Understanding and Approaches to Measure Maternal-Fetal Drug Exposure. Front. Pharmacol. 2023, 14, 1111601. [Google Scholar] [CrossRef]
- Ibrahim, A.; Khoo, M.I.; Ismail, E.H.E.; Hussain, N.H.N.; Zin, A.A.M.; Noordin, L.; Abdullah, S.; Mahdy, Z.A.; Lah, N.A.Z.N. Oxidative Stress Biomarkers in Pregnancy: A Systematic Review. Reprod. Biol. Endocrinol. 2024, 22, 93. [Google Scholar] [CrossRef]
- Pereira, A.C.; Martel, F. Oxidative Stress in Pregnancy and Fertility Pathologies. Cell Biol. Toxicol. 2014, 30, 301–312. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Y.; Yang, Y.; Du, Z.; Fan, Y.; Zhao, Y.; Yuan, S. Oxidative Stress on Vessels at the Maternal-Fetal Interface for Female Reproductive System Disorders: Update. Front. Endocrinol. 2023, 14, 1118121. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernandez, J.; Ochoa, J.J.; De Paco Matallana, C.; Caño, A.; Martín-Alvarez, E.; Sanchez-Romero, J.; Toledano, J.M.; Puche-Juarez, M.; Prados, S.; Ruiz-Duran, S.; et al. COVID-19 during Gestation: Maternal Implications of Evoked Oxidative Stress and Iron Metabolism Impairment. Antioxidants 2022, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Mandò, C.; Savasi, V.M.; Anelli, G.M.; Corti, S.; Serati, A.; Lisso, F.; Tasca, C.; Novielli, C.; Cetin, I. Mitochondrial and Oxidative Unbalance in Placentas from Mothers with SARS-CoV-2 Infection. Antioxidants 2021, 10, 1517. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Vornic, I.; Buciu, V.; Furau, C.G.; Gaje, P.N.; Ceausu, R.A.; Dumitru, C.-S.; Barb, A.C.; Novacescu, D.; Cumpanas, A.A.; Latcu, S.C.; et al. Oxidative Stress and Placental Pathogenesis: A Contemporary Overview of Potential Biomarkers and Emerging Therapeutics. Int. J. Mol. Sci. 2024, 25, 12195. [Google Scholar] [CrossRef]
- Garcia-Flores, V.; Romero, R.; Xu, Y.; Theis, K.R.; Arenas-Hernandez, M.; Miller, D.; Peyvandipour, A.; Bhatti, G.; Galaz, J.; Gershater, M.; et al. Maternal-Fetal Immune Responses in Pregnant Women Infected with SARS-CoV-2. Nat. Commun. 2022, 13, 320. [Google Scholar] [CrossRef]
- Argueta, L.B.; Lacko, L.A.; Bram, Y.; Tada, T.; Carrau, L.; Rendeiro, A.F.; Zhang, T.; Uhl, S.; Lubor, B.C.; Chandar, V.; et al. Inflammatory Responses in the Placenta upon SARS-CoV-2 Infection Late in Pregnancy. iScience 2022, 25, 104223. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Nüsken, E.; Appel, S.; Saschin, L.; Kuiper-Makris, C.; Oberholz, L.; Schömig, C.; Tauscher, A.; Dötsch, J.; Kribs, A.; Alejandre Alcazar, M.A.; et al. Intrauterine Growth Restriction: Need to Improve Diagnostic Accuracy and Evidence for a Key Role of Oxidative Stress in Neonatal and Long-Term Sequelae. Cells 2024, 13, 501. [Google Scholar] [CrossRef]
- Lee, C. Therapeutic Modulation of Virus-Induced Oxidative Stress via the Nrf2-Dependent Antioxidative Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 6208067. [Google Scholar] [CrossRef]
- Gain, C.; Song, S.; Angtuaco, T.; Satta, S.; Kelesidis, T. The Role of Oxidative Stress in the Pathogenesis of Infections with Coronaviruses. Front. Microbiol. 2023, 13, 1111930. [Google Scholar] [CrossRef] [PubMed]
- Silwal, P.; Kim, J.K.; Kim, Y.J.; Jo, E.-K. Mitochondrial Reactive Oxygen Species: Double-Edged Weapon in Host Defense and Pathological Inflammation During Infection. Front. Immunol. 2020, 11, 1649. [Google Scholar] [CrossRef]
- Prasada Kabekkodu, S.; Chakrabarty, S.; Jayaram, P.; Mallya, S.; Thangaraj, K.; Singh, K.K.; Satyamoorthy, K. Severe Acute Respiratory Syndrome Coronaviruses Contributing to Mitochondrial Dysfunction: Implications for Post-COVID Complications. Mitochondrion 2023, 69, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Schank, M.; Zhao, J.; Wang, L.; Nguyen, L.N.T.; Cao, D.; Dang, X.; Khanal, S.; Zhang, J.; Zhang, Y.; Wu, X.Y.; et al. Oxidative Stress Induces Mitochondrial Compromise in CD4 T Cells From Chronically HCV-Infected Individuals. Front. Immunol. 2021, 12, 760707. [Google Scholar] [CrossRef]
- Shimizu, I.; Shimamoto, N.; Saiki, K.; Furujo, M.; Osaw, K. Lipid Peroxidation in Hepatic Fibrosis. In Lipid Peroxidation; InTech: London, UK, 2012. [Google Scholar]
- Zhou, Y.; Long, D.; Zhao, Y.; Li, S.; Liang, Y.; Wan, L.; Zhang, J.; Xue, F.; Feng, L. Oxidative Stress-Mediated Mitochondrial Fission Promotes Hepatic Stellate Cell Activation via Stimulating Oxidative Phosphorylation. Cell Death Dis. 2022, 13, 689. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Gandhi, C.R. Stellate Cell in Hepatic Inflammation and Acute Injury. J. Cell Physiol. 2023, 238, 1226–1236. [Google Scholar] [CrossRef]
- Baghaei, K.; Mazhari, S.; Tokhanbigli, S.; Parsamanesh, G.; Alavifard, H.; Schaafsma, D.; Ghavami, S. Therapeutic Potential of Targeting Regulatory Mechanisms of Hepatic Stellate Cell Activation in Liver Fibrosis. Drug Discov. Today 2022, 27, 1044–1061. [Google Scholar] [CrossRef]
- Ivanov, A.; Bartosch, B.; Smirnova, O.; Isaguliants, M.; Kochetkov, S. HCV and Oxidative Stress in the Liver. Viruses 2013, 5, 439–469. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, F.; Liu, T.; Chen, F.; Liu, S.; Yang, J. The Role of Oxidative Stress in Influenza Virus Infection. Microbes Infect. 2017, 19, 580–586. [Google Scholar] [CrossRef]
- Kim, C.-U.; Lim, D.; Kim, Y.S.; Ku, B.; Kim, D.-J. Influenza Viral Matrix 1 Protein Aggravates Viral Pathogenicity by Inducing TLR4-Mediated Reactive Oxygen Species Production and Apoptotic Cell Death. Cell Death Dis. 2023, 14, 228. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell. Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.; Byrnes, S.; Cochrane, C.; Roche, M.; Estes, J.D.; Selemidis, S.; Angelovich, T.A.; Churchill, M.J. The Role of Oxidative Stress in HIV-Associated Neurocognitive Disorders. Brain Behav. Immun. Health 2021, 13, 100235. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Raftari, M.; Cesário, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef]
- Cuadrado, A.; Pajares, M.; Benito, C.; Jiménez-Villegas, J.; Escoll, M.; Fernández-Ginés, R.; Garcia Yagüe, A.J.; Lastra, D.; Manda, G.; Rojo, A.I.; et al. Can Activation of NRF2 Be a Strategy against COVID-19? Trends Pharmacol. Sci. 2020, 41, 598–610. [Google Scholar] [CrossRef]
- Kombe Kombe, A.J.; Fotoohabadi, L.; Nanduri, R.; Gerasimova, Y.; Daskou, M.; Gain, C.; Sharma, E.; Wong, M.; Kelesidis, T. The Role of the Nrf2 Pathway in Airway Tissue Damage Due to Viral Respiratory Infections. Int. J. Mol. Sci. 2024, 25, 7042. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, L.; Ghotbabadi, Z.R.; Gholipour, A.; Ehymayed, H.M.; Najafiyan, B.; Amirlou, P.; Yasamineh, S.; Gholizadeh, O.; Emtiazi, N. A State-of-the-Art Review on the NRF2 in Hepatitis Virus-Associated Liver Cancer. Cell Commun. Signal. 2023, 21, 318. [Google Scholar] [CrossRef]
- Waqas, F.H.; Shehata, M.; Elgaher, W.A.M.; Lacour, A.; Kurmasheva, N.; Begnini, F.; Kiib, A.E.; Dahlmann, J.; Chen, C.; Pavlou, A.; et al. NRF2 Activators Inhibit Influenza A Virus Replication by Interfering with Nucleo-Cytoplasmic Export of Viral RNPs in an NRF2-Independent Manner. PLoS Pathog. 2023, 19, e1011506. [Google Scholar] [CrossRef]
- Hamad, R.S.; Al-kuraishy, H.M.; Alexiou, A.; Papadakis, M.; Ahmed, E.A.; Saad, H.M.; Batiha, G.E.-S. SARS-CoV-2 Infection and Dysregulation of Nuclear Factor Erythroid-2-Related Factor 2 (Nrf2) Pathway. Cell Stress. Chaperones 2023, 28, 657–673. [Google Scholar] [CrossRef]
- Ramezani, A.; Nahad, M.P.; Faghihloo, E. The Role of Nrf2 Transcription Factor in Viral Infection. J. Cell Biochem. 2018, 119, 6366–6382. [Google Scholar] [CrossRef]
- Herengt, A.; Thyrsted, J.; Holm, C.K. NRF2 in Viral Infection. Antioxidants 2021, 10, 1491. [Google Scholar] [CrossRef] [PubMed]
- Cornish, E.F.; Filipovic, I.; Åsenius, F.; Williams, D.J.; McDonnell, T. Innate Immune Responses to Acute Viral Infection During Pregnancy. Front. Immunol. 2020, 11, 572567. [Google Scholar] [CrossRef] [PubMed]
- Megli, C.J.; Coyne, C.B. Infections at the Maternal–Fetal Interface: An Overview of Pathogenesis and Defence. Nat. Rev. Microbiol. 2022, 20, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Holguín, V.J.; González-García, L.D.; Velázquez-Cervantes, M.A.; Arévalo-Romero, H.; De Jesús-González, L.A.; Helguera-Repetto, A.C.; León-Reyes, G.; Salazar, M.I.; Cedillo-Barrón, L.; León-Juárez, M. Collateral Damage in the Placenta during Viral Infection in Pregnancy: A Possible Mechanism for Vertical Transmission and an Adverse Pregnancy Outcome. Diseases 2024, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, S.; Rizzo, S.; Schiuma, G.; Speltri, G.; Di Luca, D.; Rizzo, R.; Bortolotti, D. Gestational Viral Infections: Role of Host Immune System. Microorganisms 2023, 11, 1637. [Google Scholar] [CrossRef]
- Espino, A.; El Costa, H.; Tabiasco, J.; Al-Daccak, R.; Jabrane-Ferrat, N. Innate Immune Response to Viral Infections at the Maternal-Fetal Interface in Human Pregnancy. Front. Med. 2021, 8, 674645. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Piani, F.; Crescimanno, C.; Ciavattini, A.; Giannubilo, S.R.; Marzioni, D. Modulation of NRF2/KEAP1 Signaling in Preeclampsia. Cells 2023, 12, 1545. [Google Scholar] [CrossRef]
- Fields, N.J.; Palmer, K.R.; Nisi, A.; Marshall, S.A. Preeclampsia to COVID-19: A Journey towards Improved Placental and Vascular Function Using Sulforaphane. Placenta 2023, 141, 84–93. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Khan, M.Z.; Khan, A.; Huang, B.; Wei, R.; Kou, X.; Wang, X.; Chen, W.; Li, L.; Zahoor, M.; Wang, C. Bioactive Compounds Protect Mammalian Reproductive Cells from Xenobiotics and Heat Stress-Induced Oxidative Distress via Nrf2 Signaling Activation: A Narrative Review. Antioxidants 2024, 13, 597. [Google Scholar] [CrossRef]
- Zinovkin, R.A.; Zamyatnin, A.A. Mitochondria-Targeted Drugs. Curr. Mol. Pharmacol. 2019, 12, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Stoker, M.L.; Newport, E.; Hulit, J.C.; West, A.P.; Morten, K.J. Impact of Pharmacological Agents on Mitochondrial Function: A Growing Opportunity? Biochem. Soc. Trans. 2019, 47, 1757–1772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Meng, Y.; Wang, K.; Zhang, X.; Chen, W.; Sheng, J.; Qiu, Y.; Diao, H.; Li, L. Inflammation and Antiviral Immune Response Associated With Severe Progression of COVID-19. Front. Immunol. 2021, 12, 631226. [Google Scholar] [CrossRef]
- Sebastiani, G.; Navarro-Tapia, E.; Almeida-Toledano, L.; Serra-Delgado, M.; Paltrinieri, A.L.; García-Algar, Ó.; Andreu-Fernández, V. Effects of Antioxidant Intake on Fetal Development and Maternal/Neonatal Health during Pregnancy. Antioxidants 2022, 11, 648. [Google Scholar] [CrossRef]
- Chow, E.J.; Beigi, R.H.; Riley, L.E.; Uyeki, T.M. Clinical Effectiveness and Safety of Antivirals for Influenza in Pregnancy. Open Forum Infect. Dis. 2021, 8, ofab138. [Google Scholar] [CrossRef]
- Pan, X.; Chen, J.; Zhou, L.; Ou, X.; He, F.; Liu, Y.; Zheng, S.; Wang, H.; Cao, B.; Wang, Z.; et al. Efficacy and Safety of Continuous Antiviral Therapy from Preconception to Prevent Perinatal Transmission of Hepatitis B Virus. Sci. Rep. 2020, 10, 13631. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Cui, J.-J.; OuYang, Q.-Y.; Zhan, Y.; Wang, Y.-M.; Xu, X.-Y.; Yu, L.-L.; Yin, H.; Wang, Y.; Luo, C.-H.; et al. Complex Analysis of the Personalized Pharmacotherapy in the Management of COVID-19 Patients and Suggestions for Applications of Predictive, Preventive, and Personalized Medicine Attitude. EPMA J. 2021, 12, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Dopazo, J.; Maya-Miles, D.; García, F.; Lorusso, N.; Calleja, M.Á.; Pareja, M.J.; López-Miranda, J.; Rodríguez-Baño, J.; Padillo, J.; Túnez, I.; et al. Implementing Personalized Medicine in COVID-19 in Andalusia: An Opportunity to Transform the Healthcare System. J. Pers. Med. 2021, 11, 475. [Google Scholar] [CrossRef]
- Akbari, H.; Taghizadeh-Hesary, F. COVID-19 Induced Liver Injury from a New Perspective: Mitochondria. Mitochondrion 2023, 70, 103–110. [Google Scholar] [CrossRef]
- FakhriRavari, A.; Malakouti, M. Remdesivir and the Liver: A Concise Narrative Review of Remdesivir-Associated Hepatotoxicity in Patients Hospitalized Due to COVID-19. Pharmacoepidemiology 2024, 3, 69–81. [Google Scholar] [CrossRef]
- Aleem, A.; Mahadevaiah, G.; Shariff, N.; Kothadia, J.P. Hepatic Manifestations of COVID-19 and Effect of Remdesivir on Liver Function in Patients with COVID-19 Illness. Bayl. Univ. Med. Cent. Proc. 2021, 34, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.; Ramamoorthy, H.; Isaac, B. Depletion of the Cellular Antioxidant System Contributes to Tenofovir Disoproxil Fumarate—Induced Mitochondrial Damage and Increased Oxido-Nitrosative Stress in the Kidney. J. Biomed. Sci. 2013, 20, 61. [Google Scholar] [CrossRef]
- Ramamoorthy, H.; Abraham, P.; Isaac, B.; Selvakumar, D. Role for NF-ΚB Inflammatory Signalling Pathway in Tenofovir Disoproxil Fumarate (TDF) Induced Renal Damage in Rats. Food Chem. Toxicol. 2017, 99, 103–118. [Google Scholar] [CrossRef]
- Cheng, P.-N.; Sun, H.-Y.; Feng, I.-C.; Wang, S.-T.; Chiu, Y.-C.; Chiu, H.-C.; Chien, S.-C.; Young, K.-C. Reversibility of Some Oxidative Stress Markers in Chronic Hepatitis C Patients after Receiving Direct-Acting Antiviral Agents. J. Virus Erad. 2023, 9, 100318. [Google Scholar] [CrossRef]
- Reyskens, K.M.S.E.; Essop, M.F. HIV Protease Inhibitors and Onset of Cardiovascular Diseases: A Central Role for Oxidative Stress and Dysregulation of the Ubiquitin–Proteasome System. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2014, 1842, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Proskurnina, E.V.; Izmailov, D.Y.; Sozarukova, M.M.; Zhuravleva, T.A.; Leneva, I.A.; Poromov, A.A. Antioxidant Potential of Antiviral Drug Umifenovir. Molecules 2020, 25, 1577. [Google Scholar] [CrossRef] [PubMed]
- Camini, F.C.; da Silva, T.F.; da Silva Caetano, C.C.; Almeida, L.T.; Ferraz, A.C.; Alves Vitoreti, V.M.; de Mello Silva, B.; de Queiroz Silva, S.; de Magalhães, J.C.; de Brito Magalhães, C.L. Antiviral Activity of Silymarin against Mayaro Virus and Protective Effect in Virus-Induced Oxidative Stress. Antiviral Res. 2018, 158, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Fedoreyev, S.A.; Krylova, N.V.; Mishchenko, N.P.; Vasileva, E.A.; Pislyagin, E.A.; Iunikhina, O.V.; Lavrov, V.F.; Svitich, O.A.; Ebralidze, L.K.; Leonova, G.N. Antiviral and Antioxidant Properties of Echinochrome A. Mar. Drugs 2018, 16, 509. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Chen, H.; Chen, Z.; Tian, Y. Antioxidants: Potential Antiviral Agents for Japanese Encephalitis Virus Infection. Int. J. Infect. Dis. 2014, 24, 30–36. [Google Scholar] [CrossRef]
- Vasil’ev, A.N.; Deriabin, P.G.; Galegov, G.A. Antiviral Activity of Recombinant Interferon-Alpha-2b in Combination with Certain Antioxidant. Antibiot. Khimioter 2011, 56, 27–32. [Google Scholar]
- Sutter, J.; Brettschneider, J.; Wigdahl, B.; Bruggeman, P.J.; Krebs, F.C.; Miller, V. Non-Thermal Plasma Reduces HSV-1 Infection of and Replication in HaCaT Keratinocytes In Vitro. Int. J. Mol. Sci. 2024, 25, 3839. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-Induced Oxidative Stress and Toxicity. J. Toxicol. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef]
- Díaz-Castro, J.; Toledano, J.M.; Sanchez-Romero, J.; Aguilar, A.C.; Martín-Alvarez, E.; Puche-Juarez, M.; Moreno-Fernandez, J.; Pinar-Gonzalez, M.; Prados, S.; Carrillo, M.P.; et al. COVID-19 and Pregnancy: A Dangerous Mix for Bone Turnover and Metabolism Biomarkers in Placenta and Colostrum. J. Clin. Med. 2024, 13, 2124. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, A.; Cosma, S.; Nuzzo, A.M.; Salio, C.; Moretti, L.; Sassoè-Pognetto, M.; Carosso, A.R.; Borella, F.; Cutrin, J.C.; Benedetto, C. Increased Placental Anti-Oxidant Response in Asymptomatic and Symptomatic COVID-19 Third-Trimester Pregnancies. Biomedicines 2022, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.W.; Candia, A.A.; Sferruzzi-Perri, A.N. Placental Inflammation, Oxidative Stress, and Fetal Outcomes in Maternal Obesity. Trends Endocrinol. Metab. 2024, 35, 638–647. [Google Scholar] [CrossRef]
- Raffaeli, G.; Manzoni, F.; Cortesi, V.; Cavallaro, G.; Mosca, F.; Ghirardello, S. Iron Homeostasis Disruption and Oxidative Stress in Preterm Newborns. Nutrients 2020, 12, 1554. [Google Scholar] [CrossRef]
- Oseghale, O.; Vlahos, R.; O’Leary, J.J.; Brooks, R.D.; Brooks, D.A.; Liong, S.; Selemidis, S. Influenza Virus Infection during Pregnancy as a Trigger of Acute and Chronic Complications. Viruses 2022, 14, 2729. [Google Scholar] [CrossRef] [PubMed]
- Kotsias, F.; Hoffmann, E.; Amigorena, S.; Savina, A. Reactive Oxygen Species Production in the Phagosome: Impact on Antigen Presentation in Dendritic Cells. Antioxid. Redox Signal 2013, 18, 714–729. [Google Scholar] [CrossRef] [PubMed]
- To, E.E.; Erlich, J.R.; Liong, F.; Liong, S.; Luong, R.; Oseghale, O.; Miles, M.A.; Papagianis, P.C.; Quinn, K.M.; Bozinovski, S.; et al. Therapeutic Targeting of Endosome and Mitochondrial Reactive Oxygen Species Protects Mice From Influenza Virus Morbidity. Front. Pharmacol. 2022, 13, 870156. [Google Scholar] [CrossRef]
- Martinez Manfio, V.; Tasca, K.I.; Garcia, J.L.; de Oliveira Góis, J.; Correa, C.R.; de Souza, L.d.R. Redox Imbalance Is Related to HIV and Pregnancy. PLoS ONE 2021, 16, e0251619. [Google Scholar] [CrossRef]
- Riggs, P.K.; Anderson, A.M.; Tang, B.; Rubin, L.H.; Morgello, S.; Marra, C.M.; Gelman, B.B.; Clifford, D.B.; Franklin, D.; Heaton, R.K.; et al. Elevated Plasma Protein Carbonyl Concentration Is Associated with More Abnormal White Matter in People with HIV. Viruses 2023, 15, 2410. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, S.; Krishnaswamy, S.; Giles, M.L. Unravelling the Mechanisms by Which Chronic Hepatitis B Infection Is Associated with an Increased Risk of Gestational Diabetes. Front. Glob. Womens Health 2023, 4, 1184090. [Google Scholar] [CrossRef]
- Pergam, S.A.; Wang, C.C.; Gardella, C.M.; Sandison, T.G.; Phipps, W.T.; Hawes, S.E. Pregnancy Complications Associated with Hepatitis C: Data from a 2003-2005 Washington State Birth Cohort. Am. J. Obstet. Gynecol. 2008, 199, 38.e1–38.e9. [Google Scholar] [CrossRef]
- Afraie, M.; Moradi, G.; Zamani, K.; Azami, M.; Moradi, Y. The Effect of Hepatitis B Virus on the Risk of Pregnancy Outcomes: A Systematic Review and Meta-Analysis of Cohort Studies. Virol. J. 2023, 20, 213. [Google Scholar] [CrossRef]
- Bai, H. Relationship of Hepatitis B Virus Infection of Placental Barrier and Hepatitis B Virus Intra-Uterine Transmission Mechanism. World J. Gastroenterol. 2007, 13, 3625. [Google Scholar] [CrossRef]
- León-Juárez, M.; Martínez–Castillo, M.; González-García, L.D.; Helguera-Repetto, A.C.; Zaga-Clavellina, V.; García-Cordero, J.; Flores-Pliego, A.; Herrera-Salazar, A.; Vázquez-Martínez, E.R.; Reyes-Muñoz, E. Cellular and Molecular Mechanisms of Viral Infection in the Human Placenta. Pathog. Dis. 2017, 75, ftx093. [Google Scholar] [CrossRef]
- Rose, P.C.; Nel, E.D.; Cotton, M.F.; Pitcher, R.D.; Otwombe, K.; Browne, S.H.; Innes, S. Prevalence and Risk Factors for Hepatic Steatosis in Children With Perinatal HIV on Early Antiretroviral Therapy Compared to HIV-Exposed Uninfected and HIV-Unexposed Children. Front. Pediatr. 2022, 10, 893579. [Google Scholar] [CrossRef] [PubMed]
- Koi, H.; Zhang, J.; Makrigiannakis, A.; Getsios, S.; MacCalman, C.D.; Strauss, J.F.; Parry, S. Syncytiotrophoblast Is a Barrier to Maternal-Fetal Transmission of Herpes Simplex Virus1. Biol. Reprod. 2002, 67, 1572–1579. [Google Scholar] [CrossRef]
- Mimura, N.; Nagamatsu, T.; Morita, K.; Taguchi, A.; Toya, T.; Kumasawa, K.; Iriyama, T.; Kawana, K.; Inoue, N.; Fujii, T.; et al. Suppression of Human Trophoblast Syncytialization by Human Cytomegalovirus Infection. Placenta 2022, 117, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Kim, J.-S. Chronic Inflammation of the Placenta: Definition, Classification, Pathogenesis, and Clinical Significance. Am. J. Obstet. Gynecol. 2015, 213, S53–S69. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, K.; O’Keefe, M. Placental Cytomegalovirus Infection. Arch. Pathol. Lab. Med. 2019, 143, 639–642. [Google Scholar] [CrossRef]
- Pereira, L.; Petitt, M.; Tabata, T. Cytomegalovirus Infection and Antibody Protection of the Developing Placenta. Clin. Infect. Dis. 2013, 57, S174–S177. [Google Scholar] [CrossRef][Green Version]
- Hamilton, S.T.; Scott, G.; Naing, Z.; Iwasenko, J.; Hall, B.; Graf, N.; Arbuckle, S.; Craig, M.E.; Rawlinson, W.D. Human Cytomegalovirus-Induces Cytokine Changes in the Placenta with Implications for Adverse Pregnancy Outcomes. PLoS ONE 2012, 7, e52899. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an Antioxidant: Under Promises but over Delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Perrone, S.; Negro, S.; Tataranno, M.L.; Buonocore, G. Oxidative Stress and Antioxidant Strategies in Newborns. J. Matern.-Fetal Neonatal Med. 2010, 23, 63–65. [Google Scholar] [CrossRef]
- Jiménez-Osorio, A.S.; Carreón-Torres, E.; Correa-Solís, E.; Ángel-García, J.; Arias-Rico, J.; Jiménez-Garza, O.; Morales-Castillejos, L.; Díaz-Zuleta, H.A.; Baltazar-Tellez, R.M.; Sánchez-Padilla, M.L.; et al. Inflammation and Oxidative Stress Induced by Obesity, Gestational Diabetes, and Preeclampsia in Pregnancy: Role of High-Density Lipoproteins as Vectors for Bioactive Compounds. Antioxidants 2023, 12, 1894. [Google Scholar] [CrossRef]
- Saucedo, R.; Ortega-Camarillo, C.; Ferreira-Hermosillo, A.; Díaz-Velázquez, M.F.; Meixueiro-Calderón, C.; Valencia-Ortega, J. Role of Oxidative Stress and Inflammation in Gestational Diabetes Mellitus. Antioxidants 2023, 12, 1812. [Google Scholar] [CrossRef] [PubMed]
- Foka, F.E.T.; Mufhandu, H.T. Current ARTs, Virologic Failure, and Implications for AIDS Management: A Systematic Review. Viruses 2023, 15, 1732. [Google Scholar] [CrossRef]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative Stress in Preterm Newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef]
- Perrone, S.; Manti, S.; Petrolini, C.; Dell’Orto, V.G.; Boscarino, G.; Ceccotti, C.; Bertini, M.; Buonocore, G.; Esposito, S.M.R.; Gitto, E. Oxygen for the Newborn: Friend or Foe? Children 2023, 10, 579. [Google Scholar] [CrossRef]
- Martin, A.; Faes, C.; Debevec, T.; Rytz, C.; Millet, G.; Pialoux, V. Preterm Birth and Oxidative Stress: Effects of Acute Physical Exercise and Hypoxia Physiological Responses. Redox Biol. 2018, 17, 315–322. [Google Scholar] [CrossRef]
- Capasso, L.; Vento, G.; Loddo, C.; Tirone, C.; Iavarone, F.; Raimondi, F.; Dani, C.; Fanos, V. Oxidative Stress and Bronchopulmonary Dysplasia: Evidences From Microbiomics, Metabolomics, and Proteomics. Front. Pediatr. 2019, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, L.; Perrone, S.; Viola, V.; Marseglia, L.; Di Rosa, G.; Gitto, E. Oxidative Stress and Respiratory Diseases in Preterm Newborns. Int. J. Mol. Sci. 2021, 22, 12504. [Google Scholar] [CrossRef]
- Millán, I.; Piñero-Ramos, J.D.; Lara, I.; Parra-Llorca, A.; Torres-Cuevas, I.; Vento, M. Oxidative Stress in the Newborn Period: Useful Biomarkers in the Clinical Setting. Antioxidants 2018, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, C.; Parisi, F.; Cetin, I. Impact of Maternal Environment and Inflammation on Fetal Neurodevelopment. Antioxidants 2024, 13, 453. [Google Scholar] [CrossRef]
- Kneeland, R.E.; Fatemi, S.H. Viral Infection, Inflammation and Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 42, 35–48. [Google Scholar] [CrossRef]
- Andescavage, N.N.; Limperopoulos, C. Placental Abnormalities in Congenital Heart Disease. Transl. Pediatr. 2021, 10, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- Dawood, F.S.; Kittikraisak, W.; Patel, A.; Rentz Hunt, D.; Suntarattiwong, P.; Wesley, M.G.; Thompson, M.G.; Soto, G.; Mundhada, S.; Arriola, C.S.; et al. Incidence of Influenza during Pregnancy and Association with Pregnancy and Perinatal Outcomes in Three Middle-Income Countries: A Multisite Prospective Longitudinal Cohort Study. Lancet Infect. Dis. 2021, 21, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Allison, B.J.; Castillo-Melendez, M.; Jenkin, G.; Polglase, G.R.; Miller, S.L. Neonatal Morbidities of Fetal Growth Restriction: Pathophysiology and Impact. Front. Endocrinol. 2019, 10, 55. [Google Scholar] [CrossRef]
- Newsome, K.; Alverson, C.J.; Williams, J.; McIntyre, A.F.; Fine, A.D.; Wasserman, C.; Lofy, K.H.; Acosta, M.; Louie, J.K.; Jones-Vessey, K.; et al. Outcomes of Infants Born to Women with Influenza A(H1N1)Pdm09. Birth Defects Res. 2019, 111, 88–95. [Google Scholar] [CrossRef]
- Wang, R.; Yan, W.; Du, M.; Tao, L.; Liu, J. The Effect of Influenza Virus Infection on Pregnancy Outcomes: A Systematic Review and Meta-Analysis of Cohort Studies. Int. J. Infect. Dis. 2021, 105, 567–578. [Google Scholar] [CrossRef]
- Allgäuer, L.; Cabungcal, J.-H.; Yzydorczyk, C.; Do, K.Q.; Dwir, D. Low Protein-Induced Intrauterine Growth Restriction as a Risk Factor for Schizophrenia Phenotype in a Rat Model: Assessing the Role of Oxidative Stress and Neuroinflammation Interaction. Transl. Psychiatry 2023, 13, 30. [Google Scholar] [CrossRef]
- Di Martino, D.D.; Avagliano, L.; Ferrazzi, E.; Fusè, F.; Sterpi, V.; Parasiliti, M.; Stampalija, T.; Zullino, S.; Farina, A.; Bulfamante, G.P.; et al. Hypertensive Disorders of Pregnancy and Fetal Growth Restriction: Clinical Characteristics and Placental Lesions and Possible Preventive Nutritional Targets. Nutrients 2022, 14, 3276. [Google Scholar] [CrossRef] [PubMed]
- Liong, S.; Choy, K.H.C.; De Luca, S.N.; Liong, F.; Coward-Smith, M.; Oseghale, O.; Miles, M.A.; Vlahos, R.; Valant, C.; Nithianantharajah, J.; et al. Brain Region-Specific Alterations in Gene Expression Trajectories in the Offspring Born from Influenza A Virus Infected Mice. Brain Behav. Immun. 2024, 120, 488–498. [Google Scholar] [CrossRef]
- Hung, T.-H.; Skepper, J.N.; Burton, G.J. In Vitro Ischemia-Reperfusion Injury in Term Human Placenta as a Model for Oxidative Stress in Pathological Pregnancies. Am. J. Pathol. 2001, 159, 1031–1043. [Google Scholar] [CrossRef]
- Lanzieri, T.M.; Leung, J.; Caviness, A.C.; Chung, W.; Flores, M.; Blum, P.; Bialek, S.R.; Miller, J.A.; Vinson, S.S.; Turcich, M.R.; et al. Long-Term Outcomes of Children with Symptomatic Congenital Cytomegalovirus Disease. J. Perinatol. 2017, 37, 875–880. [Google Scholar] [CrossRef]
- Fisher, S.; Genbacev, O.; Maidji, E.; Pereira, L. Human Cytomegalovirus Infection of Placental Cytotrophoblasts In Vitro and In Utero: Implications for Transmission and Pathogenesis. J. Virol. 2000, 74, 6808–6820. [Google Scholar] [CrossRef] [PubMed]
- Njue, A.; Coyne, C.; Margulis, A.V.; Wang, D.; Marks, M.A.; Russell, K.; Das, R.; Sinha, A. The Role of Congenital Cytomegalovirus Infection in Adverse Birth Outcomes: A Review of the Potential Mechanisms. Viruses 2020, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Fasoulakis, Z.; Koutras, A.; Antsaklis, P.; Theodora, M.; Valsamaki, A.; Daskalakis, G.; Kontomanolis, E.N. Intrauterine Growth Restriction Due to Gestational Diabetes: From Pathophysiology to Diagnosis and Management. Medicina 2023, 59, 1139. [Google Scholar] [CrossRef]
- Das, A. Cytomegalovirus-Induced Hepatitis in an Immunocompetent Patient. Am. J. Case Rep. 2014, 15, 447–449. [Google Scholar] [CrossRef]
- Müller, L.; Di Benedetto, S. Immunosenescence and Cytomegalovirus: Exploring Their Connection in the Context of Aging, Health, and Disease. Int. J. Mol. Sci. 2024, 25, 753. [Google Scholar] [CrossRef]
- Hernández, S.; Catalán-García, M.; Morén, C.; García-Otero, L.; López, M.; Guitart-Mampel, M.; Milisenda, J.; Coll, O.; Cardellach, F.; Gratacós, E.; et al. Placental Mitochondrial Toxicity, Oxidative Stress, Apoptosis, and Adverse Perinatal Outcomes in HIV Pregnancies Under Antiretroviral Treatment Containing Zidovudine. JAIDS J. Acquir. Immune Defic. Syndr. 2017, 75, e113–e119. [Google Scholar] [CrossRef]
- Elias, A.; Nelson, B.; Oputiri, D.; Geoffrey, O.-B.P. Antiretroviral Toxicity and Oxidative Stress. Am. J. Pharmacol. Toxicol. 2013, 8, 187–196. [Google Scholar] [CrossRef]
- Greer, L.G.; Sheffield, J.S.; Rogers, V.L.; Roberts, S.W.; McIntire, D.D.; Wendel, G.D. Maternal and Neonatal Outcomes After Antepartum Treatment of Influenza With Antiviral Medications. Obstet. Gynecol. 2010, 115, 711–716. [Google Scholar] [CrossRef]
- Kadambari, S.; Griffiths, P.D.; Sharland, M. The Novel Antiviral Pipeline to Treat Severe Neonatal Viral Infections. Pediatr. Infect. Dis. J. 2013, 32, 682–683. [Google Scholar] [CrossRef]
- Niu, Y.; Herrera, E.A.; Evans, R.D.; Giussani, D.A. Antioxidant Treatment Improves Neonatal Survival and Prevents Impaired Cardiac Function at Adulthood Following Neonatal Glucocorticoid Therapy. J. Physiol. 2013, 591, 5083–5093. [Google Scholar] [CrossRef]
- Perrone, S.; Laschi, E.; Buonocore, G. Oxidative Stress Biomarkers in the Perinatal Period: Diagnostic and Prognostic Value. Semin. Fetal Neonatal Med. 2020, 25, 101087. [Google Scholar] [CrossRef] [PubMed]
- Peña-Bautista, C.; Durand, T.; Vigor, C.; Oger, C.; Galano, J.-M.; Cháfer-Pericás, C. Non-Invasive Assessment of Oxidative Stress in Preterm Infants. Free Radic. Biol. Med. 2019, 142, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Longini, M.; Belvisi, E.; Proietti, F.; Bazzini, F.; Buonocore, G.; Perrone, S. Oxidative Stress Biomarkers: Establishment of Reference Values for Isoprostanes, AOPP, and NPBI in Cord Blood. Mediators Inflamm. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Laschi, E.; Buonocore, G. Biomarkers of Oxidative Stress in the Fetus and in the Newborn. Free Radic. Biol. Med. 2019, 142, 23–31. [Google Scholar] [CrossRef]
- Pranty, A.I.; Shumka, S.; Adjaye, J. Bilirubin-Induced Neurological Damage: Current and Emerging IPSC-Derived Brain Organoid Models. Cells 2022, 11, 2647. [Google Scholar] [CrossRef]
- Jayanti, S.; Ghersi-Egea, J.-F.; Strazielle, N.; Tiribelli, C.; Gazzin, S. Severe Neonatal Hyperbilirubinemia and the Brain: The Old but Still Evolving Story. Pediatr. Med. 2021, 4, 37. [Google Scholar] [CrossRef]
- Teng, M.; Wu, T.-J.; Jing, X.; Day, B.W.; Pritchard, K.A.; Naylor, S.; Teng, R.-J. Temporal Dynamics of Oxidative Stress and Inflammation in Bronchopulmonary Dysplasia. Int. J. Mol. Sci. 2024, 25, 10145. [Google Scholar] [CrossRef]
- Martini, S.; Castellini, L.; Parladori, R.; Paoletti, V.; Aceti, A.; Corvaglia, L. Free Radicals and Neonatal Brain Injury: From Underlying Pathophysiology to Antioxidant Treatment Perspectives. Antioxidants 2021, 10, 2012. [Google Scholar] [CrossRef]
- Tenório, M.C.d.S.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.d.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Shahin, A.Y.; Hassanin, I.M.A.; Ismail, A.M.; Kruessel, J.S.; Hirchenhain, J. Effect of Oral N-Acetyl Cysteine on Recurrent Preterm Labor Following Treatment for Bacterial Vaginosis. Int. J. Gynecol. Obstet. 2009, 104, 44–48. [Google Scholar] [CrossRef]
- Buhimschi, C.S.; Bahtiyar, M.O.; Zhao, G.; Abdelghany, O.; Schneider, L.; Razeq, S.A.; Dulay, A.T.; Lipkind, H.S.; Mieth, S.; Rogers, L.; et al. Antenatal N-Acetylcysteine to Improve Outcomes of Premature Infants with Intra-Amniotic Infection and Inflammation (Triple I): Randomized Clinical Trial. Pediatr. Res. 2021, 89, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Joó, J.G.; Sulyok, E.; Bódis, J.; Kornya, L. Disrupted Balance of the Oxidant–Antioxidant System in the Pathophysiology of Female Reproduction: Oxidative Stress and Adverse Pregnancy Outcomes. Curr. Issues Mol. Biol. 2023, 45, 8091–8111. [Google Scholar] [CrossRef] [PubMed]
- DeFreitas, M.J.; Katsoufis, C.P.; Benny, M.; Young, K.; Kulandavelu, S.; Ahn, H.; Sfakianaki, A.; Abitbol, C.L. Educational Review: The Impact of Perinatal Oxidative Stress on the Developing Kidney. Front. Pediatr. 2022, 10, 853722. [Google Scholar] [CrossRef]
- Poston, L.; Briley, A.; Seed, P.; Kelly, F.; Shennan, A. Vitamin C and Vitamin E in Pregnant Women at Risk for Pre-Eclampsia (VIP Trial): Randomised Placebo-Controlled Trial. Lancet 2006, 367, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- McCance, D.R.; Holmes, V.A.; Maresh, M.J.; Patterson, C.C.; Walker, J.D.; Pearson, D.W.; Young, I.S. Vitamins C and E for Prevention of Pre-Eclampsia in Women with Type 1 Diabetes (DAPIT): A Randomised Placebo-Controlled Trial. Lancet 2010, 376, 259–266. [Google Scholar] [CrossRef]
- Nüsken, E.; Voggel, J.; Saschin, L.; Weber, L.T.; Dötsch, J.; Alcazar, M.A.A.; Nüsken, K.D. Kidney Lipid Metabolism: Impact on Pediatric Kidney Diseases and Modulation by Early-Life Nutrition. Pediatr. Nephrol. 2024. [Google Scholar] [CrossRef]
- Di Fabrizio, C.; Giorgione, V.; Khalil, A.; Murdoch, C.E. Antioxidants in Pregnancy: Do We Really Need More Trials? Antioxidants 2022, 11, 812. [Google Scholar] [CrossRef] [PubMed]
- Cottart, C.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J. Resveratrol Bioavailability and Toxicity in Humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Ding, J.; Kang, Y.; Fan, Y.; Chen, Q. Efficacy of Resveratrol to Supplement Oral Nifedipine Treatment in Pregnancy-Induced Preeclampsia. Endocr. Connect. 2017, 6, 595–600. [Google Scholar] [CrossRef]
- Darby, J.R.T.; Mohd Dollah, M.H.B.; Regnault, T.R.H.; Williams, M.T.; Morrison, J.L. Systematic Review: Impact of Resveratrol Exposure during Pregnancy on Maternal and Fetal Outcomes in Animal Models of Human Pregnancy Complications—Are We Ready for the Clinic? Pharmacol. Res. 2019, 144, 264–278. [Google Scholar] [CrossRef]
- Ramli, I.; Posadino, A.M.; Giordo, R.; Fenu, G.; Fardoun, M.; Iratni, R.; Eid, A.H.; Zayed, H.; Pintus, G. Effect of Resveratrol on Pregnancy, Prenatal Complications and Pregnancy-Associated Structure Alterations. Antioxidants 2023, 12, 341. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Binder, N.K.; Beard, S.; Nguyen, T.-V.; Kaitu’u-Lino, T.J.; Tong, S. Melatonin Enhances Antioxidant Molecules in the Placenta, Reduces Secretion of Soluble Fms-like Tyrosine Kinase 1 (SFLT) from Primary Trophoblast but Does Not Rescue Endothelial Dysfunction: An Evaluation of Its Potential to Treat Preeclampsia. PLoS ONE 2018, 13, e0187082. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.G.; Hansell, J.A.; Raut, S.; Giussani, D.A. Melatonin Improves Placental Efficiency and Birth Weight and Increases the Placental Expression of Antioxidant Enzymes in Undernourished Pregnancy. J. Pineal Res. 2009, 46, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, R.; Hallmann, A.; Sokołowska, E.; Kaletha, K.; Klimek, J. Melatonin Enhances Antioxidant Action of α-Tocopherol and Ascorbate against NADPH- and Iron-Dependent Lipid Peroxidation in Human Placental Mitochondria. J. Pineal Res. 2010, 49, 149–155. [Google Scholar] [CrossRef]
- Fantasia, I.; Bussolaro, S.; Stampalija, T.; Rolnik, D.L. The Role of Melatonin in Pregnancies Complicated by Placental Insufficiency: A Systematic Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 278, 22–28. [Google Scholar] [CrossRef]
- Naemi, M.; Farahani, Z.; Norooznezhad, A.H.; Khodarahmi, R.; Hantoushzadeh, S.; Ahangari, R.; Shariat, M. Possible Potentials of Curcumin for Pregnancies Complicated by Intra-Uterine Growth Restriction: Role of Inflammation, Angiogenesis, and Oxidative Stress. Heliyon 2021, 7, e08034. [Google Scholar] [CrossRef]
- Filardi, T.; Varì, R.; Ferretti, E.; Zicari, A.; Morano, S.; Santangelo, C. Curcumin: Could This Compound Be Useful in Pregnancy and Pregnancy-Related Complications? Nutrients 2020, 12, 3179. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Giannubilo, S.R.; Marzioni, D. The Multifaced Actions of Curcumin in Pregnancy Outcome. Antioxidants 2021, 10, 126. [Google Scholar] [CrossRef]
- Epstein, J.; Sanderson, I.R.; MacDonald, T.T. Curcumin as a Therapeutic Agent: The Evidence from in Vitro, Animal and Human Studies. Br. J. Nutr. 2010, 103, 1545–1557. [Google Scholar] [CrossRef]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted Antioxidants. FASEB J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, P.; Zhu, F.; Liao, J.; Wu, Y.; Hu, M.; Fu, H.; Qiao, J.; Lin, L.; Huang, B.; et al. The Potent Antioxidant MitoQ Protects Against Preeclampsia During Late Gestation but Increases the Risk of Preeclampsia When Administered in Early Pregnancy. Antioxid. Redox Signal 2021, 34, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Feniouk, B.A.; Skulachev, V.P. Cellular and Molecular Mechanisms of Action of Mitochondria-Targeted Antioxidants. Curr. Aging Sci. 2017, 10, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yin, J.; Chen, J.; Ma, X.; Wu, M.; Liu, G.; Yao, K.; Tan, B.; Yin, Y. Mitochondria-Targeted Antioxidants: A Step towards Disease Treatment. Oxid. Med. Cell. Longev. 2020, 2020, 8837893. [Google Scholar] [CrossRef]
- Barletta, M.A.; Marino, G.; Spagnolo, B.; Bianchi, F.P.; Falappone, P.C.F.; Spagnolo, L.; Gatti, P. Coenzyme Q10 + Alpha Lipoic Acid for Chronic COVID Syndrome. Clin. Exp. Med. 2022, 23, 667–678. [Google Scholar] [CrossRef]
- Mathys, L.; Balzarini, J. The Role of Cellular Oxidoreductases in Viral Entry and Virus Infection-Associated Oxidative Stress: Potential Therapeutic Applications. Expert. Opin. Ther. Targets 2016, 20, 123–143. [Google Scholar] [CrossRef]
- Daskou, M.; Fotooh Abadi, L.; Gain, C.; Wong, M.; Sharma, E.; Kombe Kombe, A.J.; Nanduri, R.; Kelesidis, T. The Role of the NRF2 Pathway in the Pathogenesis of Viral Respiratory Infections. Pathogens 2023, 13, 39. [Google Scholar] [CrossRef]
- Kesic, M.J.; Simmons, S.O.; Bauer, R.; Jaspers, I. Nrf2 Expression Modifies Influenza A Entry and Replication in Nasal Epithelial Cells. Free Radic. Biol. Med. 2011, 51, 444–453. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Hybertson, B.M.; Cota-Gomez, A.; Geraci, K.P.; Gao, B. Nrf2 Activator PB125® as a Potential Therapeutic Agent against COVID-19. Antioxidants 2020, 9, 518. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, J.; Zhao, H. Impacts of Maternal Preeclampsia Exposure on Offspring Neuronal Development: Recent Insights and Interventional Approaches. Int. J. Mol. Sci. 2024, 25, 11062. [Google Scholar] [CrossRef]
- Meral, G.; Aslan, E.S.; Burkay, N.; Alper Acar, E.G.; Karagöz, M.F.; Özkaya, M.; Sahin, E.; Alp, M.Y. Importance of Using Epigenetic Nutrition and Supplements Based on Nutrigenetic Tests in Personalized Medicine. Cureus 2024, 16, e66959. [Google Scholar] [CrossRef] [PubMed]
- Sakowicz, A.; Bralewska, M.; Rybak-Krzyszkowska, M.; Grzesiak, M.; Pietrucha, T. New Ideas for the Prevention and Treatment of Preeclampsia and Their Molecular Inspirations. Int. J. Mol. Sci. 2023, 24, 12100. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Paz, M.; Morán, L.; López-Alcántara, N.; Freixo, C.; Andrade, R.J.; Lucena, M.I.; Cubero, F.J. Oxidative Stress in Drug-Induced Liver Injury (DILI): From Mechanisms to Biomarkers for Use in Clinical Practice. Antioxidants 2021, 10, 390. [Google Scholar] [CrossRef]
- Battista, C.; Howell, B.A.; Siler, S.Q.; Watkins, P.B. An Introduction to DILIsym® Software, a Mechanistic Mathematical Representation of Drug-Induced Liver Injury. In Drug-Induced Liver Toxicity; Humana: New York, NY, USA, 2018; pp. 101–121. [Google Scholar]
- Donato, M.T.; Tolosa, L. High-Content Screening for the Detection of Drug-Induced Oxidative Stress in Liver Cells. Antioxidants 2021, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.L.; Silvola, R.M.; Haas, D.M.; Quinney, S.K. Physiologically Based Pharmacokinetic Modelling in Pregnancy: Model Reproducibility and External Validation. Br. J. Clin. Pharmacol. 2022, 88, 1441–1451. [Google Scholar] [CrossRef]
- Thépaut, E.; Brochot, C.; Chardon, K.; Personne, S.; Zeman, F.A. Pregnancy-PBPK Models: How Are Biochemical and Physiological Processes Integrated? Comput. Toxicol. 2023, 27, 100282. [Google Scholar] [CrossRef]
- Dmitriev, A.V.; Rudik, A.V.; Karasev, D.A.; Pogodin, P.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. In Silico Prediction of Drug–Drug Interactions Mediated by Cytochrome P450 Isoforms. Pharmaceutics 2021, 13, 538. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, Y.; Li, L.; Shen, X.; Chen, G.; Wang, X.; Liang, X.; Tan, M.; Huang, Z. Computational Approaches in Preclinical Studies on Drug Discovery and Development. Front. Chem. 2020, 8, 726. [Google Scholar] [CrossRef]

| Challenges in Assessing Oxidative Stress with Antivirals | Details | |
|---|---|---|
| Complexity of Oxidative Stress Mechanisms | Multiple ROS Sources | Antivirals can induce ROS production through different mechanisms (e.g., mitochondrial dysfunction, ER stress), making it challenging to pinpoint the exact source. |
| Antioxidant System Variability | Individual differences in antioxidant defense systems can affect the overall oxidative stress response to antivirals. | |
| Temporal Dynamics | Acute vs. Chronic Effects | Short-term oxidative stress may differ significantly from long-term effects, requiring longitudinal studies to capture the full impact of antiviral therapy. |
| Adaptive Responses | The body may adapt to oxidative stress over time, potentially masking the true extent of damage in long-term assessments. | |
| Measurement Challenges (Biomarker limitations) | Specificity | Many oxidative stress biomarkers lack specificity to particular ROS or antioxidants, making it difficult to attribute changes to specific antiviral drugs. |
| Stability | Some oxidative stress markers are unstable and can be affected by sample handling and storage conditions. | |
| Methodological Issues | Invasiveness: Direct measurement of ROS often requires invasive procedures, limiting their applicability in clinical settings. | |
| Indirect Measurements | Many assessments rely on indirect markers of oxidative damage (e.g., lipid peroxidation products), which may not always accurately reflect the current oxidative state. | |
| Confounding Factors | Underlying Disease State | The viral infection itself can induce oxidative stress, making it challenging to distinguish drug-induced effects from disease-related oxidative stress. |
| Lifestyle Factors | Diet, exercise, and other lifestyle factors can significantly influence oxidative stress levels, potentially confounding the assessment of antiviral-induced effects. | |
| Variability in Drug Responses | Pharmacogenomics | Genetic variations can affect how individuals metabolize antivirals, leading to differences in drug-induced oxidative stress. |
| Drug Interactions | Many patients receive multiple medications, which can interact and affect oxidative stress levels in unpredictable ways. | |
| Tissue-Specific Effects | Localized vs. Systemic Effects | Antivirals may induce oxidative stress in specific tissues or organs, which may not be reflected in systemic measurements |
| Accessibility | Some tissues affected by oxidative stress may not be easily accessible for direct measurement. | |
| Technological Limitations | In vivo vs. In vitro Discrepancies | Results from cell culture studies may not accurately reflect the complex in vivo environment. |
| Real-Time Monitoring | Current technologies often lack the ability to provide real-time, continuous monitoring of oxidative stress in clinical settings. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, B.; Gouveia, M.J.; Vale, N. Oxidative Stress Induced by Antivirals: Implications for Adverse Outcomes During Pregnancy and in Newborns. Antioxidants 2024, 13, 1518. https://doi.org/10.3390/antiox13121518
Costa B, Gouveia MJ, Vale N. Oxidative Stress Induced by Antivirals: Implications for Adverse Outcomes During Pregnancy and in Newborns. Antioxidants. 2024; 13(12):1518. https://doi.org/10.3390/antiox13121518
Chicago/Turabian StyleCosta, Bárbara, Maria João Gouveia, and Nuno Vale. 2024. "Oxidative Stress Induced by Antivirals: Implications for Adverse Outcomes During Pregnancy and in Newborns" Antioxidants 13, no. 12: 1518. https://doi.org/10.3390/antiox13121518
APA StyleCosta, B., Gouveia, M. J., & Vale, N. (2024). Oxidative Stress Induced by Antivirals: Implications for Adverse Outcomes During Pregnancy and in Newborns. Antioxidants, 13(12), 1518. https://doi.org/10.3390/antiox13121518









