Circulating Glutathione Peroxidase-3 in Elderly—Association with Renal Function, Cardiovascular Mortality, and Impact of Selenium and Coenzyme Q10 Supplementation
Abstract
1. Background
2. Materials and Methods
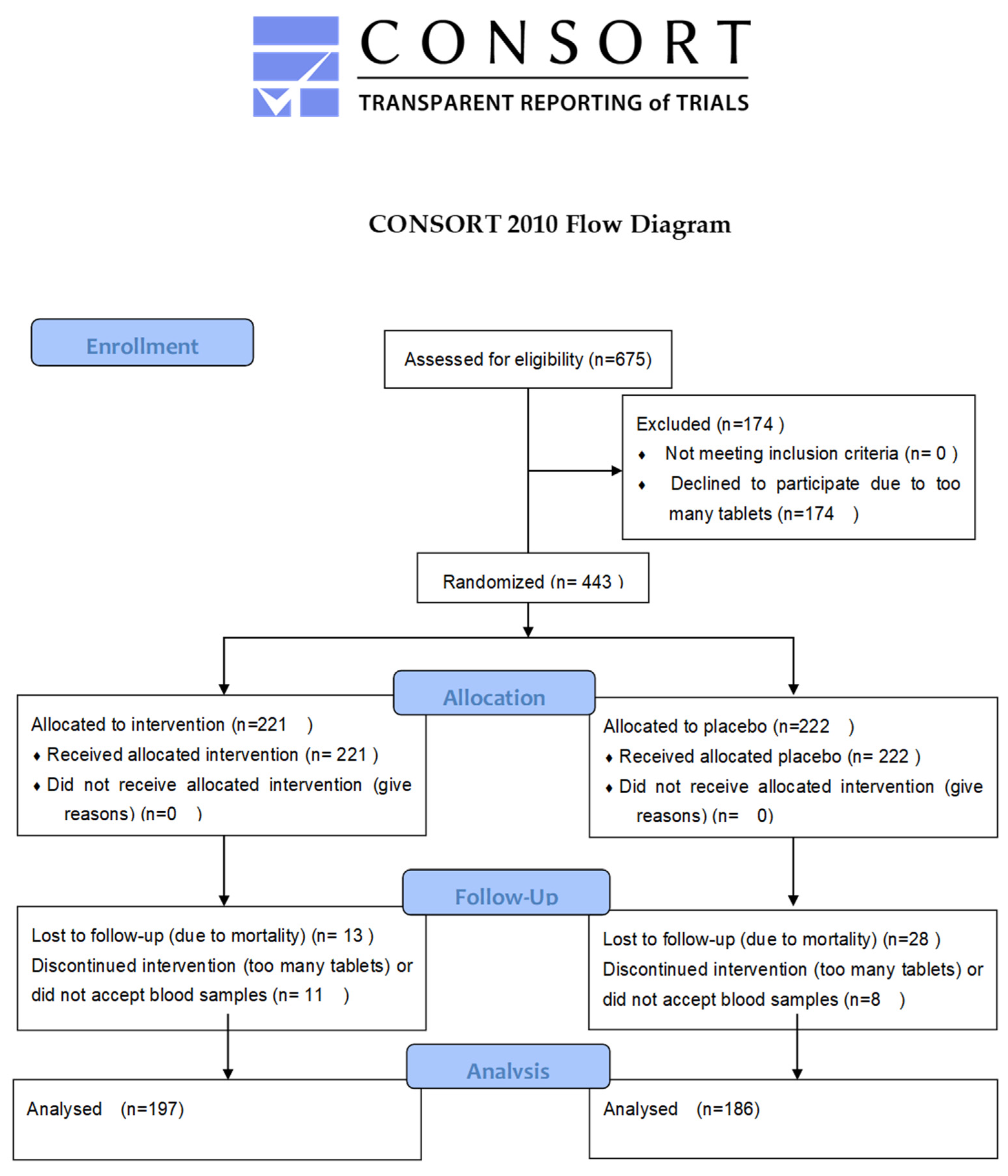
2.1. Study Participants and Clinical Follow-Up
2.2. Ethics Approval and Consent to Participate
2.3. Blood Sampling
2.4. Determination of Selenium
2.5. Determination of Selenoprotein P
2.6. Determination of GPx3
2.7. Renal Function
2.7.1. Creatinine and Cystatin-C
2.7.2. Assessment of Renal Function
2.8. Determination of Biomarkers
2.9. NT-proBNP and Copeptin Analyses
2.10. Determination of MR-proADM
2.11. Determination of P-Selectin
2.12. Determination of ICAM-1 and Hepatocyte Growth Factor HGF
2.13. Determination of D-Dimer
2.14. Statistical Methods
3. Results
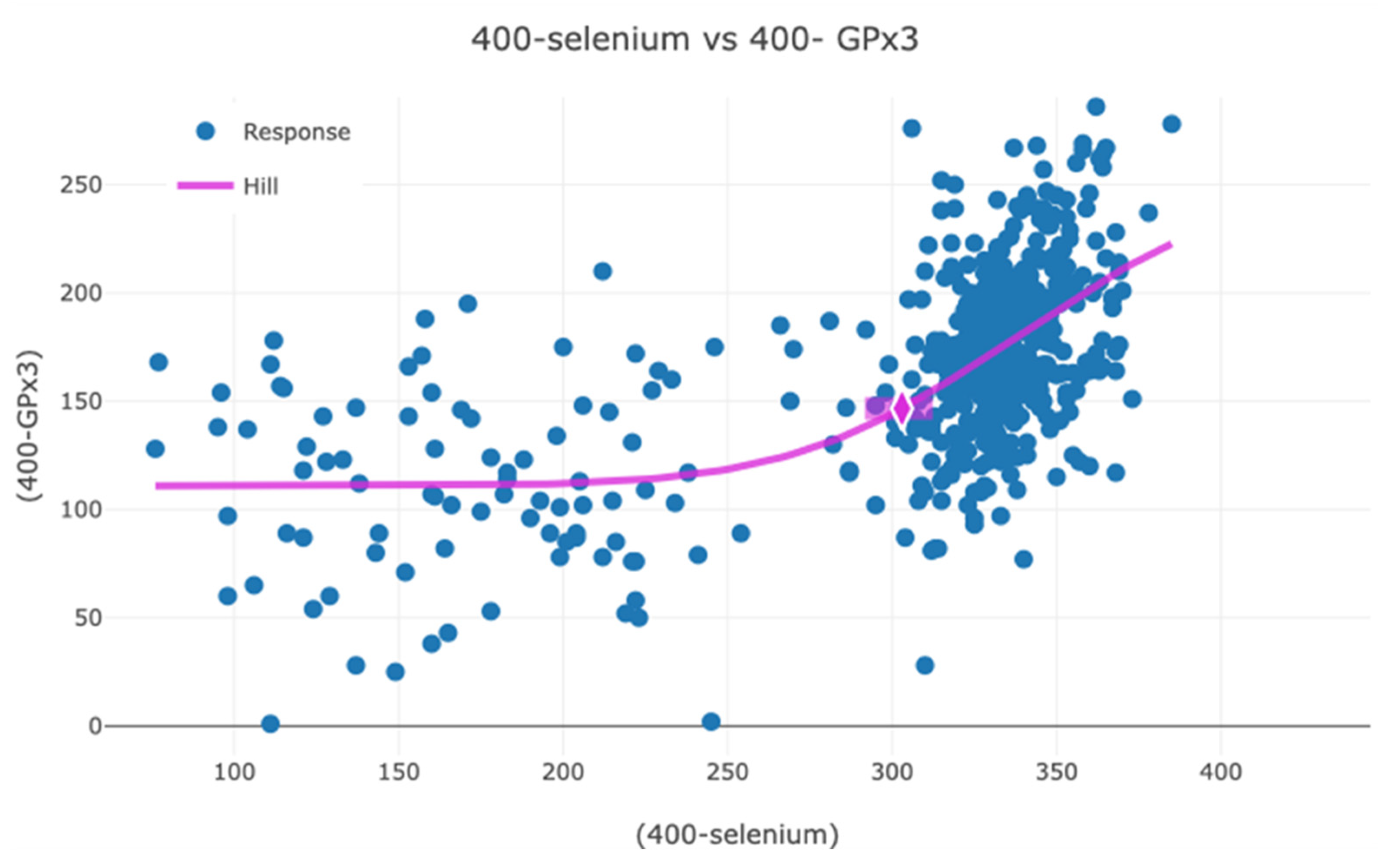
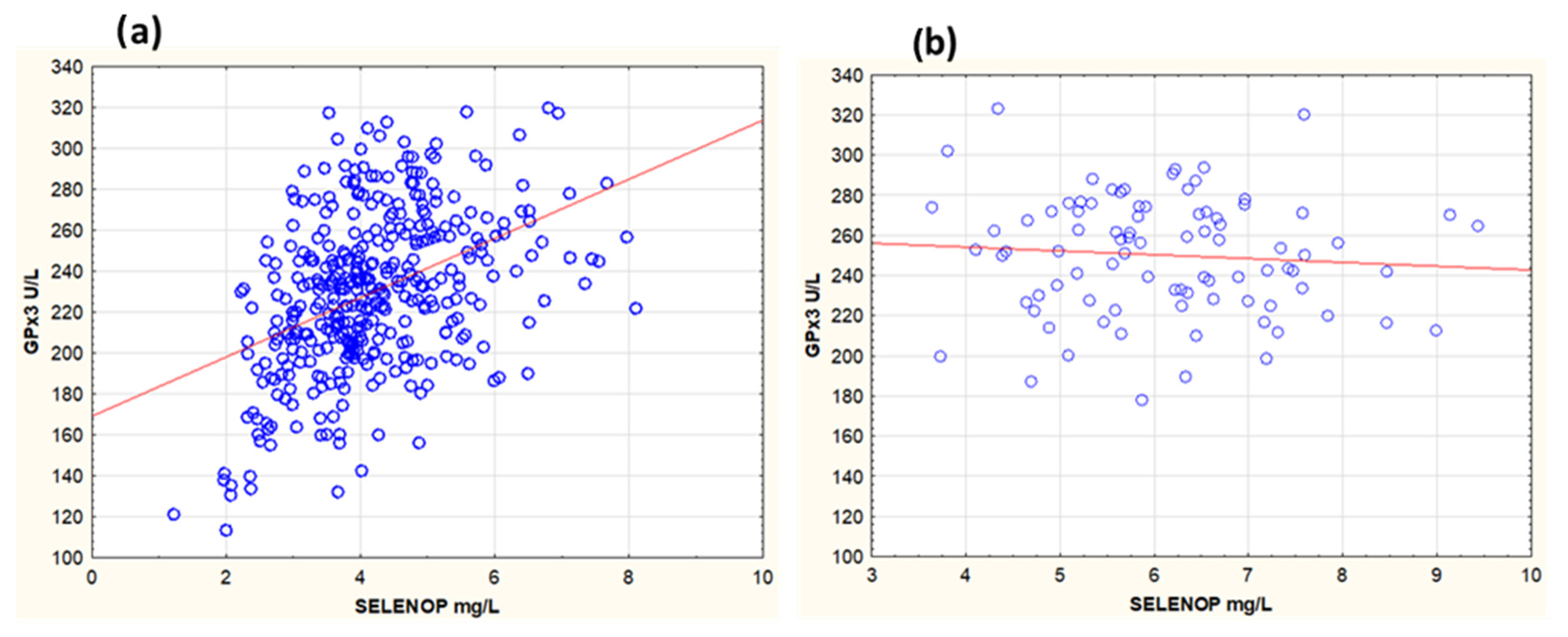
3.1. Association Between GPx3, Selenium, and SELENOP in Serum and Effect of Selenium and Coenzyme Q10 Supplementation
3.2. Association Between GPx3 and Biomarkers of Oxidative Stress, Inflammation, Endothelial Function, and Thromboembolism
3.3. Association with Biomarker of Myocardial Wall Tension
3.4. Association Between GPx3 and Renal Function
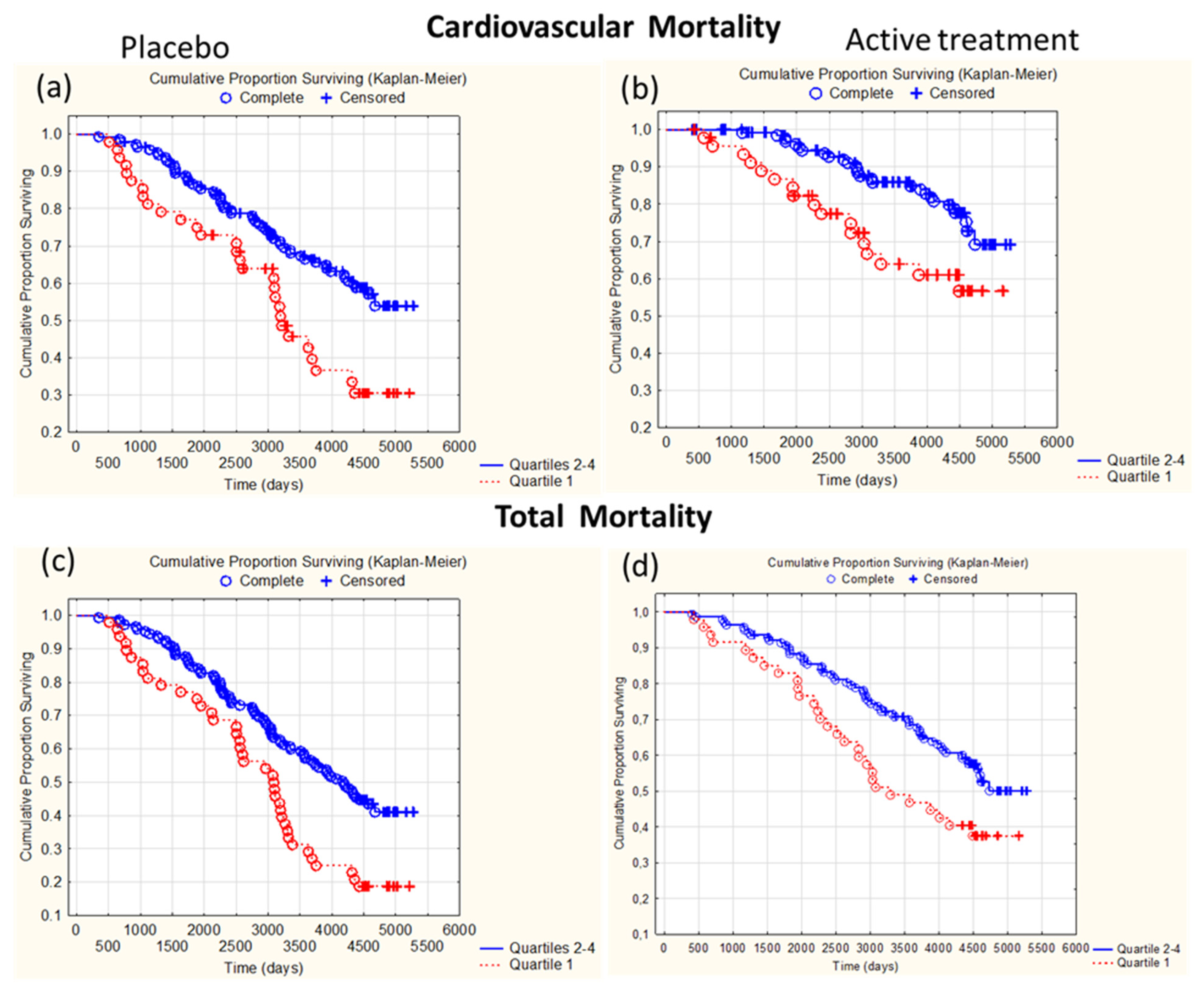
3.5. Association Between GPx3 Activity at Inclusion and Mortality
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Alexander, J.; Olsen, A.K. Selenium—A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67, 10320. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J. Selenium. In Handbook on the Toxicology of Metals; Nordberg, G.F., Costa, M., Eds.; Academic Press: Washington, DC, USA, 2022; Volume II, pp. 730–771. [Google Scholar]
- Tsuji, P.A.; Santesmasses, D.; Lee, B.J.; Gladyshev, V.N.; Hatfield, D.L. Historical Roles of Selenium and Selenoproteins in Health and Development: The Good, the Bad and the Ugly. Int. J. Mol. Sci. 2021, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Arner, E.S. Selenoproteins-What unique properties can arise with selenocysteine in place of cysteine? Exp. Cell Res. 2010, 316, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef]
- Johnson, C.C.; Fordyce, F.M.; Rayman, M.P. Symposium on ‘Geographical and geological influences on nutrition’: Factors controlling the distribution of selenium in the environment and their impact on health and nutrition. Proc. Nutr. Soc. 2010, 69, 119–132. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef]
- Dunning, B.J.; Bourgonje, A.R.; Bulthuis, M.L.C.; Alexander, J.; Aaseth, J.O.; Larsson, A.; van Goor, H.; Alehagen, U. Selenium and coenzyme Q(10) improve the systemic redox status while reducing cardiovascular mortality in elderly population-based individuals. Free Radic. Biol. Med. 2023, 204, 207–214. [Google Scholar] [CrossRef]
- Alehagen, U.; Alexander, J.; Aaseth, J.O.; Larsson, A.; Opstad, T.B. Supplementation with selenium and coenzyme Q(10) in an elderly Swedish population low in selenium—Positive effects on thyroid hormones, cardiovascular mortality, and quality of life. BMC Med. 2024, 22, 191. [Google Scholar] [CrossRef]
- Duntas, L.H. The Role of Iodine and Selenium in Autoimmune Thyroiditis. Horm. Metab. Res. 2015, 47, 721–726. [Google Scholar] [CrossRef]
- Fraczek-Jucha, M.; Kabat, M.; Szlosarczyk, B.; Czubek, U.; Nessler, J.; Gackowski, A. Selenium deficiency and the dynamics of changes of thyroid profile in patients with acute myocardial infarction and chronic heart failure. Kardiol. Pol. 2019, 77, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Rayman, M.P. Multiple Nutritional Factors and the Risk of Hashimoto’s Thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Kohrle, J. Selenium in Endocrinology-Selenoprotein-Related Diseases, Population Studies, and Epidemiological Evidence. Endocrinology 2021, 162, bqaa228. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Alehagen, U.; Johansson, P.; Svensson, E.; Aaseth, J.; Alexander, J. Improved cardiovascular health by supplementation with selenium and coenzyme Q10: Applying structural equation modelling (SEM) to clinical outcomes and biomarkers to explore underlying mechanisms in a prospective randomized double-blind placebo-controlled intervention project in Sweden. Eur. J. Nutr. 2022, 61, 3135–3148. [Google Scholar] [CrossRef]
- Ju, W.; Li, X.; Li, Z.; Wu, G.R.; Fu, X.F.; Yang, X.M.; Zhang, X.Q.; Gao, X.B. The effect of selenium supplementation on coronary heart disease: A systematic review and meta-analysis of randomized controlled trials. J. Trace Elem. Med. Biol. 2017, 44, 8–16. [Google Scholar] [CrossRef]
- Iglesias, P.; Selgas, R.; Romero, S.; Diez, J.J. Selenium and kidney disease. J. Nephrol. 2013, 26, 266–272. [Google Scholar] [CrossRef]
- Alehagen, U.; Aaseth, J.; Alexander, J.; Brismar, K.; Larsson, A. Selenium and Coenzyme Q10 Supplementation Improves Renal Function in Elderly Deficient in Selenium: Observational Results and Results from a Subgroup Analysis of a Prospective Randomised Double-Blind Placebo-Controlled Trial. Nutrients 2020, 12, 3780. [Google Scholar] [CrossRef]
- Wang, Y.; Lilienfeldt, N.; Hekimi, S. Understanding coenzyme Q. Physiol. Rev. 2024, 104, 1533–1610. [Google Scholar] [CrossRef]
- Kalen, A.; Appelkvist, E.L.; Dallner, G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989, 24, 579–584. [Google Scholar] [CrossRef]
- Xia, L.; Nordman, T.; Olsson, J.M.; Damdimopoulos, A.; Bjorkhem-Bergman, L.; Nalvarte, I.; Eriksson, L.C.; Arner, E.S.; Spyrou, G.; Bjornstedt, M. The mammalian cytosolic selenoenzyme thioredoxin reductase reduces ubiquinone. A novel mechanism for defense against oxidative stress. J. Biol. Chem. 2003, 278, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Park, S.J.; Lange, M.; Tseyang, T.; Doshi, M.B.; Kim, T.Y.; Song, Y.; Kim, D.I.; Greer, P.L.; Olzmann, J.A.; et al. Selenium reduction of ubiquinone via SQOR suppresses ferroptosis. Nat. Metab. 2024, 6, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R.; Flohe, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Liu, Y.; Greiner, C.D.; Holtzman, J.L. Physiologic concentrations of homocysteine inhibit the human plasma GSH peroxidase that reduces organic hydroperoxides. J. Lab. Clin. Med. 2000, 136, 58–65. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Takahashi, K. Glutathione peroxidase isolated from plasma reduces phospholipid hydroperoxides. Arch. Biochem. Biophys. 1993, 305, 541–545. [Google Scholar] [CrossRef]
- Takebe, G.; Yarimizu, J.; Saito, Y.; Hayashi, T.; Nakamura, H.; Yodoi, J.; Nagasawa, S.; Takahashi, K. A comparative study on the hydroperoxide and thiol specificity of the glutathione peroxidase family and selenoprotein P. J. Biol. Chem. 2002, 277, 41254–41258. [Google Scholar] [CrossRef]
- Bjornstedt, M.; Xue, J.; Huang, W.; Akesson, B.; Holmgren, A. The thioredoxin and glutaredoxin systems are efficient electron donors to human plasma glutathione peroxidase. J. Biol. Chem. 1994, 269, 29382–29384. [Google Scholar] [CrossRef]
- Bierl, C.; Voetsch, B.; Jin, R.C.; Handy, D.E.; Loscalzo, J. Determinants of human plasma glutathione peroxidase (GPx-3) expression. J. Biol. Chem. 2004, 279, 26839–26845. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef]
- Seale, L.A.; Ha, H.Y.; Hashimoto, A.C.; Berry, M.J. Relationship between selenoprotein P and selenocysteine lyase: Insights into selenium metabolism. Free Radic. Biol. Med. 2018, 127, 182–189. [Google Scholar] [CrossRef]
- Schomburg, L. Selenoprotein P—Selenium transport protein, enzyme and biomarker of selenium status. Free Radic. Biol. Med. 2022, 191, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Drutel, A.; Archambeaud, F.; Caron, P. Selenium and the thyroid gland: More good news for clinicians. Clin. Endocrinol. 2013, 78, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Zachara, B.A.; Pawluk, H.; Bloch-Boguslawska, E.; Sliwka, K.M.; Korenkiewicz, J.; Skok, Z.; Ryc, K. Tissue level, distribution, and total body selenium content in healthy and diseased humans in Poland. Arch. Environ. Health 2001, 56, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.E.; Winfrey, V.P.; Hill, K.E.; Burk, R.F. Megalin mediates selenoprotein P uptake by kidney proximal tubule epithelial cells. J. Biol. Chem. 2008, 283, 6854–6860. [Google Scholar] [CrossRef]
- Burk, R.F.; Olson, G.E.; Winfrey, V.P.; Hill, K.E.; Yin, D. Glutathione peroxidase-3 produced by the kidney binds to a population of basement membranes in the gastrointestinal tract and in other tissues. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G32–G38. [Google Scholar] [CrossRef]
- Whitin, J.C.; Bhamre, S.; Tham, D.M.; Cohen, H.J. Extracellular glutathione peroxidase is secreted basolaterally by human renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 2002, 283, F20–F28. [Google Scholar] [CrossRef]
- Chang, C.; Worley, B.L.; Phaeton, R.; Hempel, N. Extracellular Glutathione Peroxidase GPx3 and Its Role in Cancer. Cancers 2020, 12, 2197. [Google Scholar] [CrossRef]
- Willnow, T.E.; Christ, A. Endocytic receptor LRP2/megalin-of holoprosencephaly and renal Fanconi syndrome. Pflugers Arch. 2017, 469, 907–916. [Google Scholar] [CrossRef]
- Zachara, B.A.; Trafikowska, U.; Adamowicz, A.; Nartowicz, E.; Manitius, J. Selenium, glutathione peroxidases, and some other antioxidant parameters in blood of patients with chronic renal failure. J. Trace Elem. Med. Biol. 2001, 15, 161–166. [Google Scholar] [CrossRef]
- Zou, Z.; Ren, T.; Li, Y.; Zeng, Q.; Wang, X.; Teng, J.; Xu, J.; Jia, P.; Ding, X. The Association Between Serum Glutathione Peroxidase-3 Concentration and Risk of Acute Kidney Injury After Cardiac Surgery: A Nested Case-Control Study. Am. J. Cardiol. 2023, 209, 29–35. [Google Scholar] [CrossRef]
- Pang, P.; Abbott, M.; Abdi, M.; Fucci, Q.A.; Chauhan, N.; Mistri, M.; Proctor, B.; Chin, M.; Wang, B.; Yin, W.; et al. Pre-clinical model of severe glutathione peroxidase-3 deficiency and chronic kidney disease results in coronary artery thrombosis and depressed left ventricular function. Nephrol. Dial. Transplant. 2018, 33, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Zachara, B.A. Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv. Clin. Chem. 2015, 68, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Hommos, M.S.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes in Human Kidneys with Healthy Aging. J. Am. Soc. Nephrol. 2017, 28, 2838–2844. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.C.; Mahoney, C.E.; Coleman Anderson, L.; Ottaviano, F.; Croce, K.; Leopold, J.A.; Zhang, Y.Y.; Tang, S.S.; Handy, D.E.; Loscalzo, J. Glutathione peroxidase-3 deficiency promotes platelet-dependent thrombosis in vivo. Circulation 2011, 123, 1963–1973. [Google Scholar] [CrossRef]
- Wolin, M.S. Plasma glutathione peroxidase activity is potentially a key regulator of vascular disease-associated thrombosis. Circulation 2011, 123, 1923–1924. [Google Scholar] [CrossRef]
- Demircan, K.; Bengtsson, Y.; Sun, Q.; Brange, A.; Vallon-Christersson, J.; Rijntjes, E.; Malmberg, M.; Saal, L.H.; Ryden, L.; Borg, A.; et al. Serum selenium, selenoprotein P and glutathione peroxidase 3 as predictors of mortality and recurrence following breast cancer diagnosis: A multicentre cohort study. Redox Biol. 2021, 47, 102145. [Google Scholar] [CrossRef]
- Saito, Y. Selenium Transport Mechanism via Selenoprotein P-Its Physiological Role and Related Diseases. Front. Nutr. 2021, 8, 685517. [Google Scholar] [CrossRef]
- Zachara, B.A.; Salak, A.; Koterska, D.; Manitius, J.; Wasowicz, W. Selenium and glutathione peroxidases in blood of patients with different stages of chronic renal failure. J. Trace Elem. Med. Biol. 2004, 17, 291–299. [Google Scholar] [CrossRef]
- Alehagen, U.; Johansson, P.; Bjornstedt, M.; Rosen, A.; Dahlstrom, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 2013, 167, 1860–1866. [Google Scholar] [CrossRef]
- Jensen-Urstad, K.; Bouvier, F.; Hojer, J.; Ruiz, H.; Hulting, J.; Samad, B.; Thorstrand, C.; Jensen-Urstad, M. Comparison of different echocardiographic methods with radionuclide imaging for measuring left ventricular ejection fraction during acute myocardial infarction treated by thrombolytic therapy. Am. J. Cardiol. 1998, 81, 538–544. [Google Scholar] [CrossRef]
- van Royen, N.; Jaffe, C.C.; Krumholz, H.M.; Johnson, K.M.; Lynch, P.J.; Natale, D.; Atkinson, P.; Deman, P.; Wackers, F.J. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am. J. Cardiol. 1996, 77, 843–850. [Google Scholar] [CrossRef]
- Alehagen, U.; Aaseth, J.; Schomburg, L.; Larsson, A.; Opstad, T.; Alexander, J. Selenoprotein P increases upon selenium and coenzyme Q(10) supplementation and is associated with telomere length, quality of life and reduced inflammation and mortality. Free Radic. Biol. Med. 2024, 222, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Schottker, B.; Holleczek, B.; Hybsier, S.; Kohrle, J.; Schomburg, L.; Brenner, H. Strong associations of serum selenoprotein P with all-cause mortality and mortality due to cancer, cardiovascular, respiratory and gastrointestinal diseases in older German adults. Eur. J. Epidemiol. 2024, 39, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin. Chem. 2006, 52, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Lindahl, T.L.; Aaseth, J.; Svensson, E.; Johansson, P. Levels of sP-selectin and hs-CRP Decrease with Dietary Intervention with Selenium and Coenzyme Q10 Combined: A Secondary Analysis of a Randomized Clinical Trial. PLoS ONE 2015, 10, e0137680. [Google Scholar] [CrossRef]
- Alehagen, U.; Aaseth, J.; Lindahl, T.L.; Larsson, A.; Alexander, J. Dietary Supplementation with Selenium and Coenzyme Q(10) Prevents Increase in Plasma D-Dimer While Lowering Cardiovascular Mortality in an Elderly Swedish Population. Nutrients 2021, 13, 1344. [Google Scholar] [CrossRef]
- Roxborough, H.E.; Mercer, C.; McMaster, D.; Maxwell, A.P.; Young, I.S. Plasma glutathione peroxidase activity is reduced in haemodialysis patients. Nephron 1999, 81, 278–283. [Google Scholar] [CrossRef]
- Wong, F.N.; Tan, J.A.; Keng, T.C.; Ng, K.P.; Chua, K.H.; Kuppusamy, U.R. Association between plasma soluble RAGE and renal function is unaffected by medication usage and enzymatic antioxidants in chronic kidney disease with type 2 diabetes. Clin. Chim. Acta 2016, 453, 56–61. [Google Scholar] [CrossRef]
- Fassett, R.G.; Robertson, I.K.; Ball, M.J.; Geraghty, D.P.; Coombes, J.S. Effects of atorvastatin on oxidative stress in chronic kidney disease. Nephrology 2015, 20, 697–705. [Google Scholar] [CrossRef]
- Perri, G.; Mathers, J.C.; Martin-Ruiz, C.; Parker, C.; Walsh, J.S.; Eastell, R.; Demircan, K.; Chillon, T.S.; Schomburg, L.; Robinson, L.; et al. Selenium status and its determinants in very old adults: Insights from the Newcastle 85+ Study. Br. J. Nutr. 2024, 131, 901–910. [Google Scholar] [CrossRef]
- Noronha, I.L.; Santa-Catharina, G.P.; Andrade, L.; Coelho, V.A.; Jacob-Filho, W.; Elias, R.M. Glomerular filtration in the aging population. Front. Med. 2022, 9, 769329. [Google Scholar] [CrossRef] [PubMed]
- Zachara, B.A.; Adamowicz, A.; Trafikowska, U.; Trafikowska, A.; Manitius, J.; Nartowicz, E. Selenium and glutathione levels, and glutathione peroxidase activities in blood components of uremic patients on hemodialysis supplemented with selenium and treated with erythropoietin. J. Trace Elem. Med. Biol. 2001, 15, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Saint-Georges, M.D.; Bonnefont, D.J.; Bourely, B.A.; Jaudon, M.C.; Cereze, P.; Chaumeil, P.; Gard, C.; D’Auzac, C.L. Correction of selenium deficiency in hemodialyzed patients. Kidney Int. Suppl. 1989, 27, S274–S277. [Google Scholar] [PubMed]
- Badri, S.; Vahdat, S.; Pourfarzam, M.; Assarzadeh, S.; Seirafian, S.; Ataei, S. Potential Benefits of Selenium Supplementation in Patients with Kidney Disease. J. Res. Pharm. Pract. 2021, 10, 149–158. [Google Scholar] [CrossRef]
- Zachara, B.A.; Gromadzinska, J.; Zbrog, Z.; Swiech, R.; Wasowicz, W.; Twardowska, E.; Jablonska, E.; Sobala, W. Selenium supplementation to chronic kidney disease patients on hemodialysis does not induce the synthesis of plasma glutathione peroxidase. Acta Biochim. Pol. 2009, 56, 183–187. [Google Scholar] [CrossRef]
- Berthelot, E.; Eliahou, L.; Jagu, A.; Damy, T.; Hanon, O.; Hulot, J.S.; Meune, C.; Roig, C.; Roubille, F.; Sabouret, P.; et al. [Natriuretic peptides in the diagnosis and monitoring of heart failure]. Rev. Prat. 2024, 74, 185–193. [Google Scholar]
- Wu, X.; Tang, S.; Dai, Q.; Yi, B.; Yang, S.; Sun, J.; Zhong, Y.; Lin, W.; Liu, J.; Liu, Y.; et al. Vitamin D-vitamin D receptor alleviates oxidative stress in ischemic acute kidney injury via upregulating glutathione peroxidase 3. FASEB J. 2023, 37, e22738. [Google Scholar] [CrossRef]
- Pei, J.; Tian, X.; Yu, C.; Luo, J.; Zhang, J.; Hua, Y.; Wei, G. GPX3 and GSTT1 as biomarkers related to oxidative stress during renal ischemia reperfusion injuries and their relationship with immune infiltration. Front. Immunol. 2023, 14, 1136146. [Google Scholar] [CrossRef]
- Decharatchakul, N.; Settasatian, C.; Settasatian, N.; Komanasin, N.; Kukongviriyapan, U.; Intharapetch, P.; Senthong, V.; Sawanyawisuth, K. Association of combined genetic variations in SOD3, GPX3, PON1, and GSTT1 with hypertension and severity of coronary artery disease. Heart Vessel. 2020, 35, 918–929. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Q.; Zhang, K.; Sun, W.; Jia, X.; Yang, Y.; Yin, J.; Tang, C.; Zhang, J. Se deficiency induces renal pathological changes by regulating selenoprotein expression, disrupting redox balance, and activating inflammation. Metallomics 2020, 12, 1576–1584. [Google Scholar] [CrossRef]
- Loscalzo, J. Redox Dysregulation in Vascular Pathobiology. Free Radic. Biol. Med. 2014, 75 (Suppl. S1), S2. [Google Scholar] [CrossRef] [PubMed]
- Poggio, P.; Songia, P.; Moschetta, D.; Valerio, V.; Myasoedova, V.; Perrucci, G.L.; Pompilio, G. MiRNA profiling revealed enhanced susceptibility to oxidative stress of endothelial cells from bicuspid aortic valve. J. Mol. Cell. Cardiol. 2019, 131, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.X.; Wei, B.; Jiang, L.Y.; Ying, Y.Y.; Li, K.; Chen, T.X.; Huang, R.F.; Shi, M.J.; Xu, H. Intercellular mitochondrial transfer as a means of revitalizing injured glomerular endothelial cells. World J. Stem Cells 2022, 14, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Voetsch, B.; Jin, R.C.; Bierl, C.; Benke, K.S.; Kenet, G.; Simioni, P.; Ottaviano, F.; Damasceno, B.P.; Annichino-Bizacchi, J.M.; Handy, D.E.; et al. Promoter polymorphisms in the plasma glutathione peroxidase (GPx-3) gene: A novel risk factor for arterial ischemic stroke among young adults and children. Stroke 2007, 38, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Buijsse, B.; Lee, D.H.; Steffen, L.; Erickson, R.R.; Luepker, R.V.; Jacobs, D.R., Jr.; Holtzman, J.L. Low serum glutathione peroxidase activity is associated with increased cardiovascular mortality in individuals with low HDLc’s. PLoS ONE 2012, 7, e38901. [Google Scholar] [CrossRef]
- Zamora-Ginez, I.; Baez-Duarte, B.G.; Nieva-Vazquez, A.; Garcia-Aragon, K.H.; Monjaraz-Guzman, E.; Mendoza-Carrera, F.; Meneses-Zamora, P.; Flores-Blanco, C.V.; Jesus, K.L. Relationship of the low-density lipoprotein (LDL)/high-density lipoprotein (HDL) index with antioxidant enzymes and with the oxLDL/HDL index. Gac. Med. Mex. 2019, 155, 453–457. [Google Scholar] [CrossRef]

| Active | Placebo | p-Value | |
|---|---|---|---|
| n | 197 | 186 | |
| Age Years, (SD) | 76.9 (3.4) | 77.1 (3.0) | |
| Males/Females n | 100/97 | 101/85 | |
| History | |||
| Smokers (Present), n (%) | 14 (7.1) | 16 (8.6) | 0.59 |
| BMI, kg/m2 (SD) | 26.8 (3.9) | 27.1 (4.1) | 0.68 |
| Diabetes, n (%) | 41 (20.8) | 42 (20.7) | 0.67 |
| Hypertension, n (%) | 140 (71.1) | 143 (76.9) | 0.20 |
| IHD, n (%) | 41 (20.8) | 43 (23.1) | 0.59 |
| Medications | |||
| ACEI, n (%) | 33 (16.8) | 41 (22.0) | 0.19 |
| ARB, n (%) | 10 (5.0) | 13 (6.4) | 0.43 |
| Betablockers, n (%) | 69 (35.0) | 62 (33.3) | 0.73 |
| Anticoagulants, n (%) | 23 (11.5) | 24 (12.7) | 0.71 |
| Diuretics, n (%) | 61 (30.5) | 74 (39.8) | 0.71 |
| Statins, n (%) | 40 (20.3) | 39 (21.0) | 0.87 |
| Examinations | |||
| EF < 40%, n (%) | 10 (5.1) | 12 (6.5) | 0.56 |
| s-selenium, µg/L, (SD) | 65.5 (15.9) | 65.7 (18.0) | 0.97 |
| SELENOP, mg/L, (SD) | 4.14 (1.10) | 4.21 (1.22) | 0.59 |
| GPx3, U/L, (SD) | 232.0 (37.7) | 227.2 (39.2) | 0.23 |
| Creatinine, µmol/L (SD) | 92.3 (26.8) | 91.2 (30.6) | 0.80 |
| Cystatin C, mg/L (SD) | 1.23 (0.31) | 1.23 (0.34) | 0.99 |
| CKD-EPI, mL/min/1.73 m2 (SD) | 61.4 (16.0) | 64.7 (18.1) | 0.16 |
| Effect | Sum of Squares | Degrees of Freedom | Mean Squares | F | p |
|---|---|---|---|---|---|
| Intercept | 695 | 1 | 695 | 0.87 | 0.35 |
| GPx3 incl. | 22,124 | 1 | 22,124 | 27.56 | <0.0001 |
| CKD-EPI creat. incl. | 2875 | 1 | 2875 | 3.58 | 0.06 |
| Age | 4011 | 1 | 4011 | 5.00 | 0.03 |
| Selenium incl. | 703 | 1 | 703 | 0.88 | 0.35 |
| Smoking | 998 | 1 | 998 | 1.24 | 0.27 |
| Active treatment | 21,220 | 1 | 21,220 | 26.43 | <0.0001 |
| Corr. Hypertension | 29 | 1 | 29 | 0.04 | 0.85 |
| Corr. Diabetes mellitus | 5 | 1 | 5 | 0.01 | 0.93 |
| IHD | 180 | 1 | 180 | 0.22 | 0.64 |
| EF < 40%, incl. | 516 | 1 | 516 | 0.64 | 0.42 |
| Hb < 120 g/L | 27 | 1 | 27 | 0.03 | 0.85 |
| Error | 97,945 | 122 | 802 |
| Group | Variable | Inclusion | 48 Months | p-Value |
|---|---|---|---|---|
| Active treatment | ||||
| CKD-EPI, mL/min/1.73 m2 (SD) | 60.7 (16.4) | 75.9 (21.4) | <0.0001 | |
| CKD-EPI Cys C, mL/min/1.73 m2 (SD) | 57.4 (16.4) | 72.6 (21.1) | <0.0001 | |
| eGFR < 60 mL/min/1.73 m2 (%) | 46/91 (50.5) | 20/91 (22.0) | 0.0001 | |
| Placebo | ||||
| CKD-EPI, mL/min/1.73 m2 (SD) | 63.6 (17.6) | 65.4 (15.9) | 0.50 | |
| CKD-EPI Cys C, mL/min/1.73 m2 (SD) | 60.9 (18.0) | 65.4 (20.5) | 0.13 | |
| eGFR < 60 mL/min/1.73 m2 (%) | 36/82 (43.9) | 28/82 (34.1) | 0.20 |
| (a) Placebo Group | |||||
| Variable | β | β95% CI | HR | HR 95% CI | p-Value |
| GPx3 incl. Q1 < 204 | 0.67 | 0.17–1.16 | 1.95 | 1.19–3.19 | 0.008 |
| Hyperlipidaemia | 0.19 | −0.32–0.69 | 1.21 | 0.73–1.99 | 0.47 |
| Hb < 120 g/L | 0.22 | −0.36–0.80 | 1.25 | 0.70–2.23 | 0.45 |
| Diabetes mellitus | 0.52 | 0.022–1.02 | 1.69 | 1.02–2.78 | 0.04 |
| Hypertension | 0.07 | −0.42–0.56 | 1.07 | 0.66–1.75 | 0.78 |
| hsCRP incl. | 0.004 | −0.010–0.017 | 1.00 | 0.99–1.02 | 0.61 |
| CKD-EPI Creat. incl. | 0.005 | −0.013–0.023 | 1.01 | 0.99–1.02 | 0.59 |
| BMI | 0.007 | −0.039–0.054 | 1.01 | 0.96–1.06 | 0.74 |
| (b) Active Treatment Group | |||||
| Variable | β | β95% CI | HR | HR 95% CI | p-Value |
| GPx3 incl. Q1 < 205.9 U/L | 0.73 | 0.090–1.37 | 2.07 | 1.09–3.93 | 0.025 |
| Hyperlipidaemia | 0.10 | −0.62–0.81 | 1.10 | 0.54–2.25 | 0.79 |
| Hb < 120 g/L | 0.18 | −1.15–0.79 | 0.83 | 0.31–2.19 | 0.71 |
| Diabetes | 0.34 | −0.34–1.02 | 1.41 | 0.71–2.78 | 0.32 |
| Hypertension | 0.44 | −0.27–1.14 | 1.55 | 0.76–3.14 | 0.23 |
| hsCRP incl. | 0.011 | −0.013–0.034 | 1.01 | 0.99–1.03 | 0.37 |
| CKD-EPI Creat. incl. | 0.009 | −0.033–0.015 | 0.99 | 0.97–1.01 | 0.46 |
| BMI | −0.06 | −0.14–0.025 | 0.94 | 0.87–1.03 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexander, J.; Aaseth, J.O.; Schomburg, L.; Chillon, T.S.; Larsson, A.; Alehagen, U. Circulating Glutathione Peroxidase-3 in Elderly—Association with Renal Function, Cardiovascular Mortality, and Impact of Selenium and Coenzyme Q10 Supplementation. Antioxidants 2024, 13, 1566. https://doi.org/10.3390/antiox13121566
Alexander J, Aaseth JO, Schomburg L, Chillon TS, Larsson A, Alehagen U. Circulating Glutathione Peroxidase-3 in Elderly—Association with Renal Function, Cardiovascular Mortality, and Impact of Selenium and Coenzyme Q10 Supplementation. Antioxidants. 2024; 13(12):1566. https://doi.org/10.3390/antiox13121566
Chicago/Turabian StyleAlexander, Jan, Jan Olav Aaseth, Lutz Schomburg, Thilo Samson Chillon, Anders Larsson, and Urban Alehagen. 2024. "Circulating Glutathione Peroxidase-3 in Elderly—Association with Renal Function, Cardiovascular Mortality, and Impact of Selenium and Coenzyme Q10 Supplementation" Antioxidants 13, no. 12: 1566. https://doi.org/10.3390/antiox13121566
APA StyleAlexander, J., Aaseth, J. O., Schomburg, L., Chillon, T. S., Larsson, A., & Alehagen, U. (2024). Circulating Glutathione Peroxidase-3 in Elderly—Association with Renal Function, Cardiovascular Mortality, and Impact of Selenium and Coenzyme Q10 Supplementation. Antioxidants, 13(12), 1566. https://doi.org/10.3390/antiox13121566











