Oxidative Stress, Inflammation and Altered Glucose Metabolism Contribute to the Retinal Phenotype in the Choroideremia Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry
2.2. Transcriptomic Signature Analysis
2.3. Data Availability
2.4. PFKM Overexpression in Zebrafish Models
2.5. Zebrafish Characterisation
2.6. 2-NBDG Glucose Uptake Assay
2.7. RT-qPCR
2.8. Statistical Analysis
3. Results
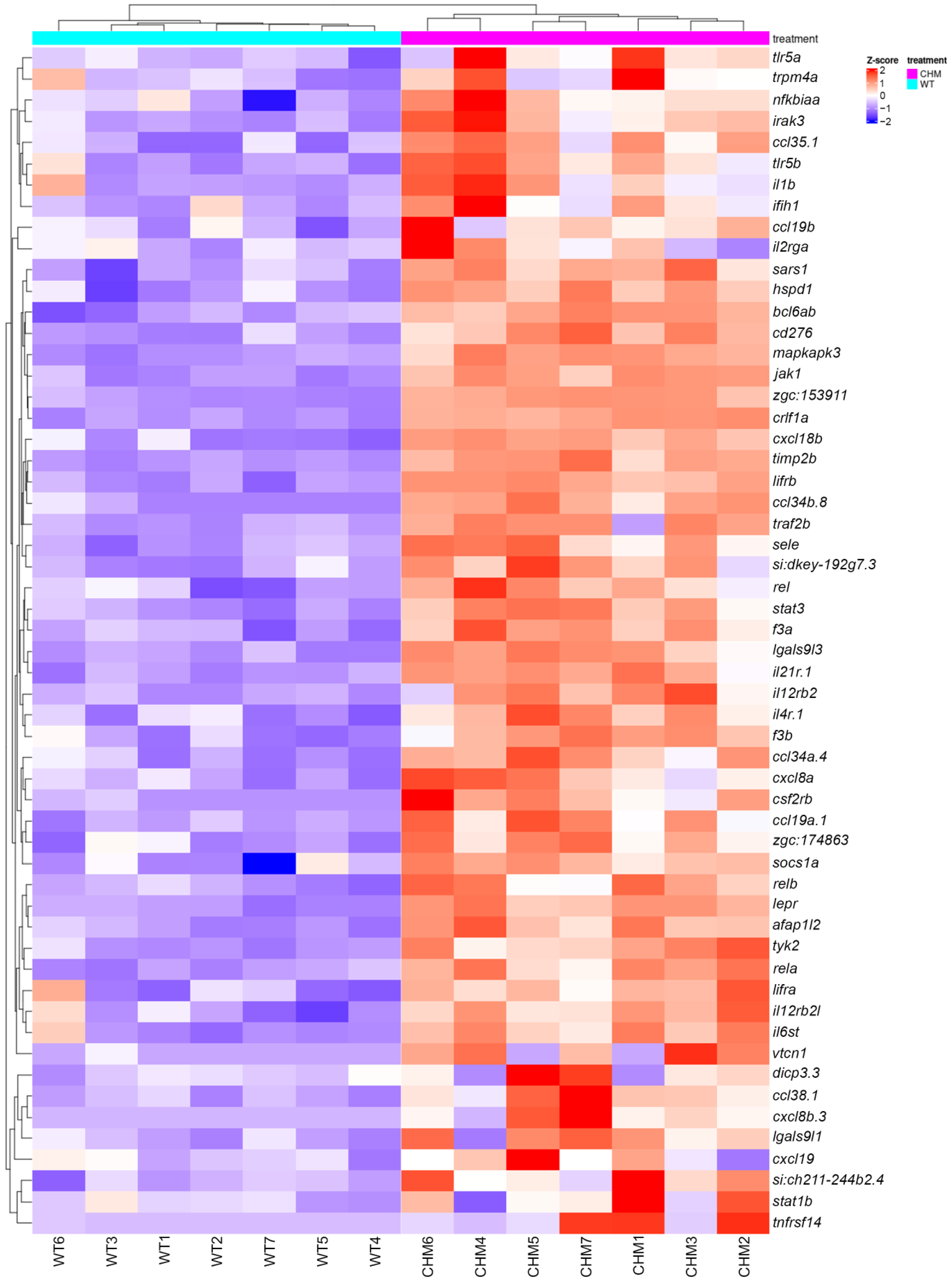
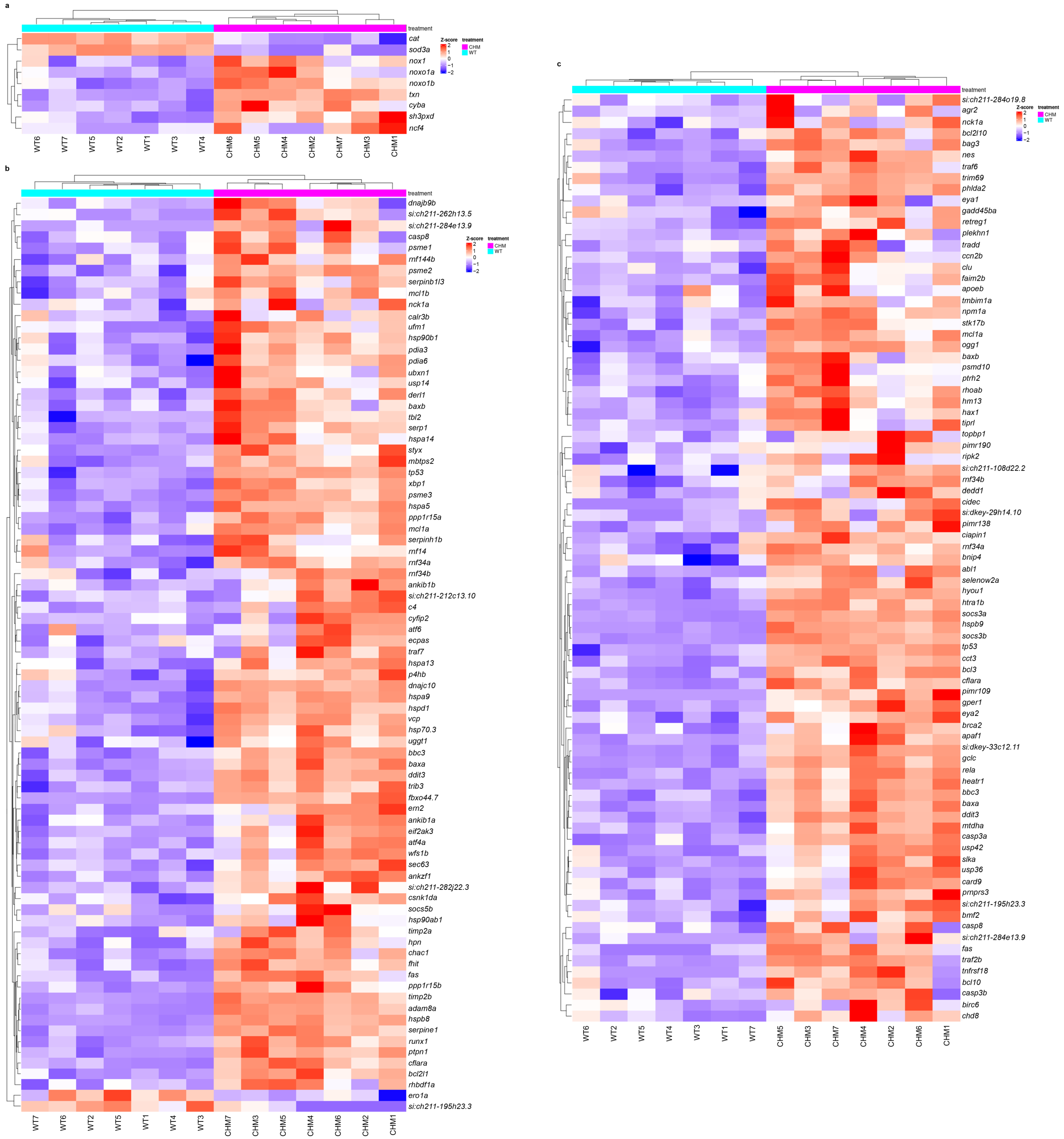
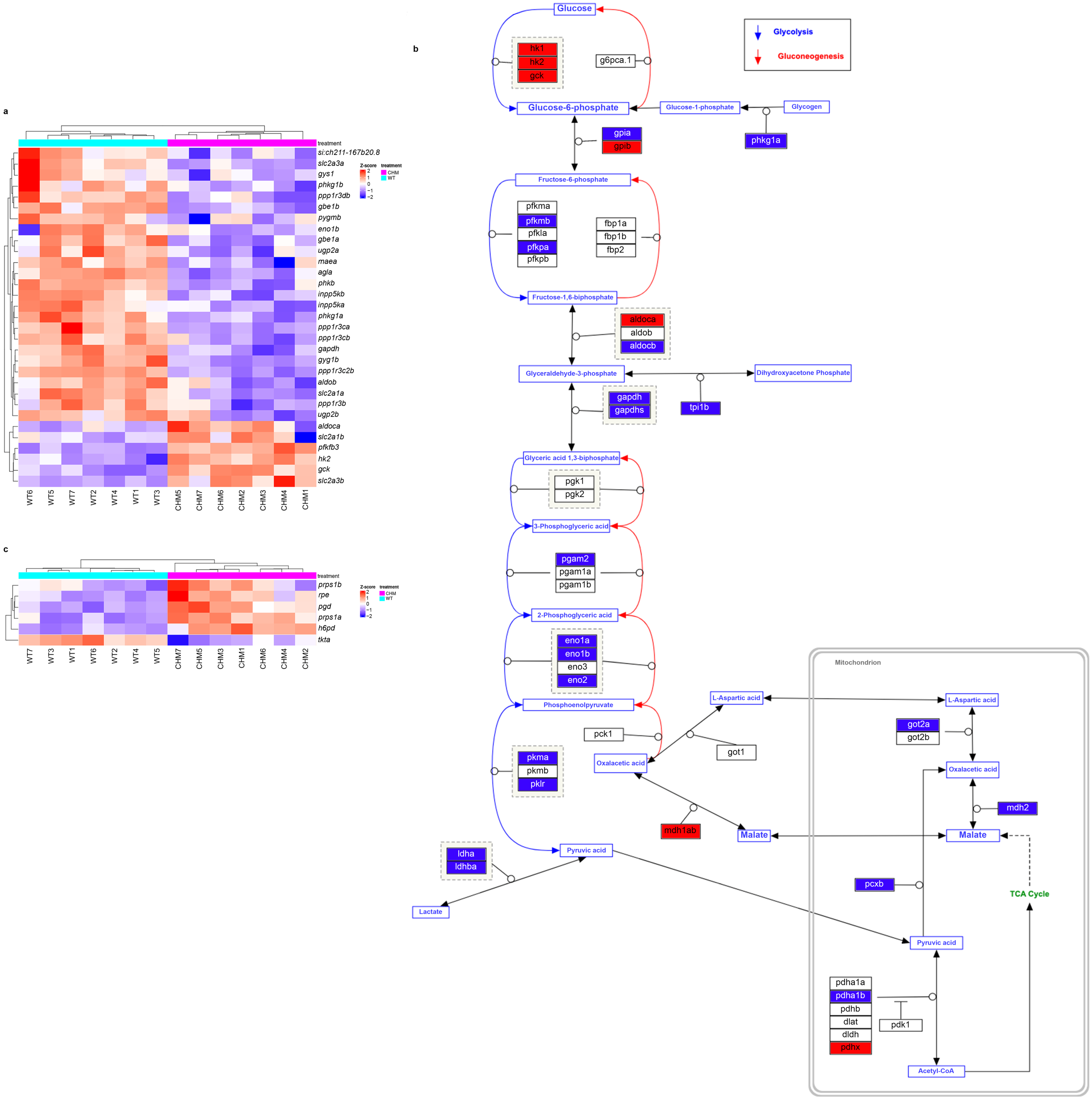
3.1. RNAseq Analysis Reveals Disrupted Glucose Metabolism in chmru848 Fish, Associated with Increased Inflammatory Responses
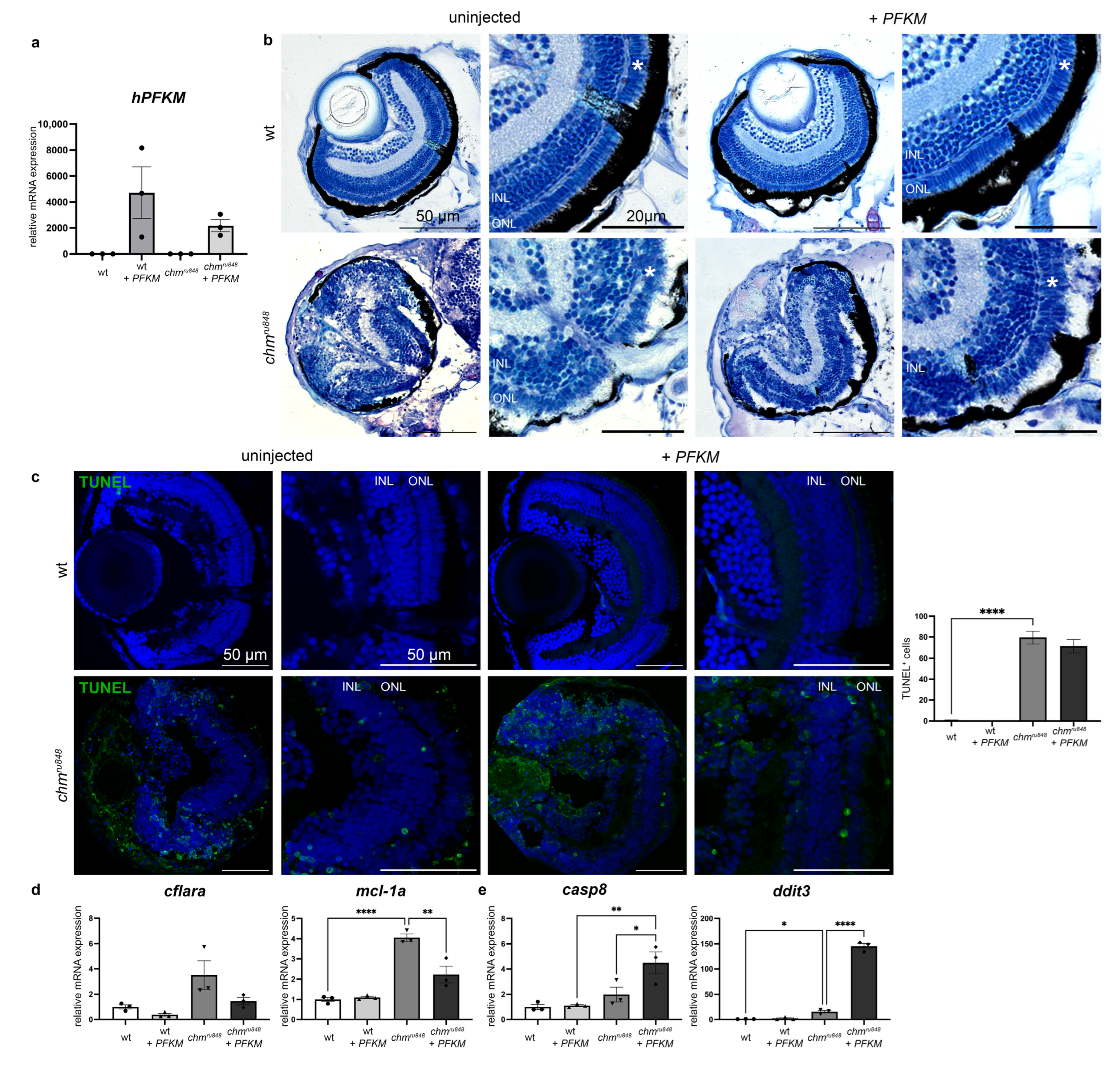
3.2. Human PFKM Overexpression Improves Retinal Phenotype
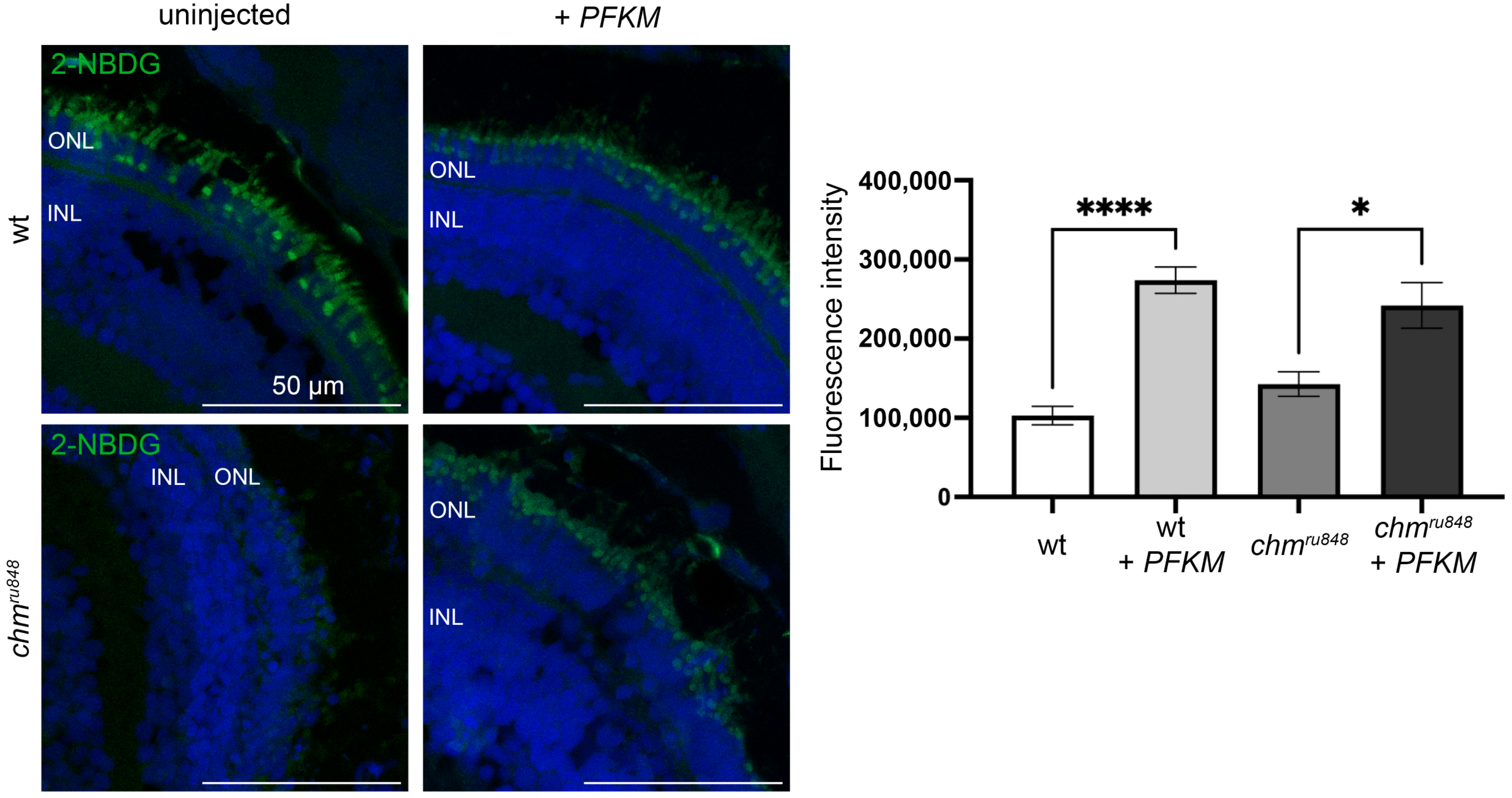
3.3. PFKM Increases Glucose Uptake in chmru848 Zebrafish
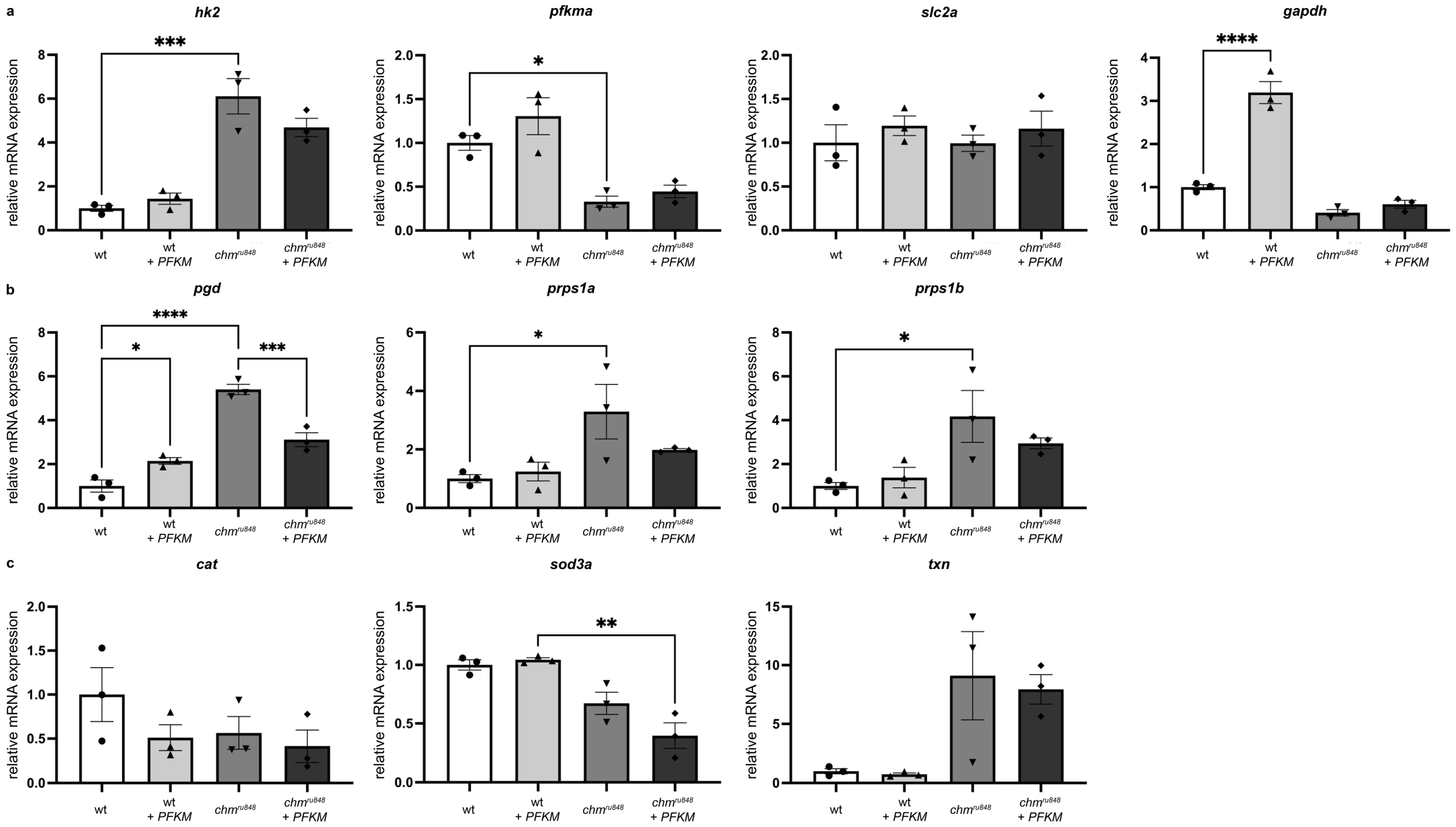
3.4. PFKM Overexpression Does Not Improve Activity of the Glycolysis Pathway or Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okawa, H.; Sampath, A.P.; Laughlin, S.B.; Fain, G.L. ATP Consumption by Mammalian Rod Photoreceptors in Darkness and in Light. Curr. Biol. 2008, 18, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Narayan, D.S.; Chidlow, G.; Wood, J.P.; Casson, R.J. Glucose Metabolism in Mammalian Photoreceptor Inner and Outer Segments. Clin. Exp. Ophthalmol. 2017, 45, 730–741. [Google Scholar] [CrossRef]
- Jaroszynska, N.; Harding, P.; Moosajee, M. Metabolism in the Zebrafish Retina. J. Dev. Biol. 2021, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.W.; Wubben, T.J.; Besirli, C.G. Photoreceptor Metabolic Reprogramming: Current Understanding and Therapeutic Implications. Commun. Biol. 2021, 4, 245. [Google Scholar] [CrossRef]
- Hurley, J.B.; Lindsay, K.J.; Du, J. Glucose, Lactate, and Shuttling of Metabolites in Vertebrate Retinas. J. Neurosci. Res. 2015, 93, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, C.; Onoe, S.; Niemeyer, G. Changes in Glucose Level Affect Rod Function More than Cone Function in the Isolated, Perfused Cat Eye. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2798–2808. [Google Scholar]
- Swarup, A.; Samuels, I.S.; Bell, B.A.; Han, J.Y.S.; Du, J.; Massenzio, E.; Abel, E.D.; Boesze-Battaglia, K.; Peachey, N.S.; Philp, N.J. Modulating GLUT1 Expression in Retinal Pigment Epithelium Decreases Glucose Levels in the Retina: Impact on Photoreceptors and Müller Glial Cells. Am. J. Physiol. Cell Physiol. 2019, 316, C121–C133. [Google Scholar] [CrossRef]
- Weh, E.; Goswami, M.; Chaudhury, S.; Fernando, R.; Miller, N.; Hager, H.; Sheskey, S.; Sharma, V.; Wubben, T.J.; Besirli, C.G. Metabolic Alterations Caused by Simultaneous Loss of HK2 and PKM2 Leads to Photoreceptor Dysfunction and Degeneration. Cells 2023, 12, 2043. [Google Scholar] [CrossRef]
- Punzo, C.; Xiong, W.; Cepko, C.L. Loss of Daylight Vision in Retinal Degeneration: Are Oxidative Stress and Metabolic Dysregulation to Blame? J. Biol. Chem. 2012, 287, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Rajala, R.V.S. Aerobic Glycolysis in the Retina: Functional Roles of Pyruvate Kinase Isoforms. Front. Cell Dev. Biol. 2020, 8, 266. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Mitsios, A.; Dubis, A.M.; Moosajee, M. Choroideremia: From Genetic and Clinical Phenotyping to Gene Therapy and Future Treatments. Ther. Adv. Ophthalmol. 2018, 10, 2515841418817490. [Google Scholar] [CrossRef]
- Moosajee, M.; Tulloch, M.; Baron, R.A.; Gregory-Evans, C.Y.; Pereira-Leal, J.B.; Seabra, M.C. Single Choroideremia Gene in Nonmammalian Vertebrates Explains Early Embryonic Lethality of the Zebrafish Model of Choroideremia. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3009–3016. [Google Scholar] [CrossRef]
- Sarkar, H.; Lahne, M.; Nair, N.; Moosajee, M. Oxidative and Endoplasmic Reticulum Stress Represent Novel Therapeutic Targets for Choroideremia. Antioxidants 2023, 12, 1694. [Google Scholar] [CrossRef]
- Owen, N.; Toms, M.; Tian, Y.; Toualbi, L.; Richardson, R.; Young, R.; Tracey-White, D.; Dhami, P.; Beck, S.; Moosajee, M. Loss of the Crumbs Cell Polarity Complex Disrupts Epigenetic Transcriptional Control and Cell Cycle Progression in the Developing Retina. J. Pathol. 2023, 259, 441–454. [Google Scholar] [CrossRef]
- Krueger, F.; James, F.; Ewels, P.; Afyounian, E.; Weinstein, M.; Schuster-Boeckler, B.; Hulselmans, G. Sclamons FelixKrueger/TrimGalore: V0.6.10—Add Default Decompression Path 2023.
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ewels, P.A.; Peltzer, A.; Fillinger, S.; Patel, H.; Alneberg, J.; Wilm, A.; Garcia, M.U.; Di Tommaso, P.; Nahnsen, S. The Nf-Core Framework for Community-Curated Bioinformatics Pipelines. Nat. Biotechnol. 2020, 38, 276–278. [Google Scholar] [CrossRef]
- Harshil, P.; Phil, E.; Alexander, P.; Olga, B.; Jonathan, M.; Gregor, S.; Maxime, U.G.; Denis, M.; Pranathi, V.; Nf-Core Bot; et al. Nf-Core/Rnaseq: Nf-Core/Rnaseq v3.13.2—Cobalt Colt 2023. Available online: https://zenodo.org/doi/10.5281/zenodo.1400710 (accessed on 18 December 2024).
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R a Language and Environment for Statistical Computing: Reference Index; R Foundation for Statistical Computing: Vienna, Austria, 2008; ISBN 978-3-900051-07-0. [Google Scholar]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER Version 14: More Genomes, a New PANTHER GO-Slim and Improvements in Enrichment Analysis Tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef]
- Gu, Z. Complex Heatmap Visualization. iMeta 2022, 1, e43. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Moosajee, M.; Gregory-Evans, K.; Ellis, C.D.; Seabra, M.C.; Gregory-Evans, C.Y. Translational Bypass of Nonsense Mutations in Zebrafish Rep1, Pax2.1 and Lamb1 Highlights a Viable Therapeutic Option for Untreatable Genetic Eye Disease. Hum. Mol. Genet. 2008, 17, 3987–4000. [Google Scholar] [CrossRef]
- Agrawal, A.; Balcı, H.; Hanspers, K.; Coort, S.L.; Martens, M.; Slenter, D.N.; Ehrhart, F.; Digles, D.; Waagmeester, A.; Wassink, I.; et al. WikiPathways 2024: Next Generation Pathway Database. Nucleic Acids Res. 2023, 52, D679–D689. [Google Scholar] [CrossRef] [PubMed]
- Pico, A.; Wackers, L.; Willighagen, E.; Weitz, E. Glycolysis and Gluconeogenesis (WP1356). 2021. Available online: https://www.wikipathways.org/instance/WP1356 (accessed on 18 December 2024).
- Zou, C.; Wang, Y.; Shen, Z. 2-NBDG as a Fluorescent Indicator for Direct Glucose Uptake Measurement. J. Biochem. Biophys. Methods 2005, 64, 207–215. [Google Scholar] [CrossRef]
- Daniele, L.L.; Han, J.Y.S.; Samuels, I.S.; Komirisetty, R.; Mehta, N.; McCord, J.L.; Yu, M.; Wang, Y.; Boesze-Battaglia, K.; Bell, B.A.; et al. Glucose Uptake by GLUT1 in Photoreceptors Is Essential for Outer Segment Renewal and Rod Photoreceptor Survival. FASEB J. 2022, 36, e22428. [Google Scholar] [CrossRef]
- Joost, H.-G.; Bell, G.I.; Best, J.D.; Birnbaum, M.J.; Charron, M.J.; Chen, Y.T.; Doege, H.; James, D.E.; Lodish, H.F.; Moley, K.H.; et al. Nomenclature of the GLUT/SLC2A Family of Sugar/Polyol Transport Facilitators. Am. J. Physiol.-Endocrinol. Metab. 2002, 282, E974–E976. [Google Scholar] [CrossRef] [PubMed]
- Navale, A.M.; Paranjape, A.N. Glucose Transporters: Physiological and Pathological Roles. Biophys. Rev. 2016, 8, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Lechermeier, C.G.; Zimmer, F.; Lüffe, T.M.; Lesch, K.-P.; Romanos, M.; Lillesaar, C.; Drepper, C. Transcript Analysis of Zebrafish GLUT3 Genes, Slc2a3a and Slc2a3b, Define Overlapping as Well as Distinct Expression Domains in the Zebrafish (Danio rerio) Central Nervous System. Front. Mol. Neurosci. 2019, 12, 199. [Google Scholar] [CrossRef]
- Delcourt, N.; Quevedo, C.; Nonne, C.; Fons, P.; O’Brien, D.; Loyaux, D.; Diez, M.; Autelitano, F.; Guillemot, J.-C.; Ferrara, P.; et al. Targeted Identification of Sialoglycoproteins in Hypoxic Endothelial Cells and Validation in Zebrafish Reveal Roles for Proteins in Angiogenesis. J. Biol. Chem. 2015, 290, 3405–3417. [Google Scholar] [CrossRef]
- Facchinello, N.; Astone, M.; Audano, M.; Oberkersch, R.E.; Spizzotin, M.; Calura, E.; Marques, M.; Crisan, M.; Mitro, N.; Santoro, M.M. Oxidative Pentose Phosphate Pathway Controls Vascular Mural Cell Coverage by Regulating Extracellular Matrix Composition. Nat. Metab. 2022, 4, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, H.; Tracey-White, D.; Hagag, A.M.; Burgoyne, T.; Nair, N.; Jensen, L.D.; Edwards, M.M.; Moosajee, M. Loss of REP1 Impacts Choroidal Melanogenesis and Vasculogenesis in Choroideremia. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2024, 1870, 166963. [Google Scholar] [CrossRef]
- Raza, A.; Franklin, M.J.; Dudek, A.Z. Pericytes and Vessel Maturation during Tumor Angiogenesis and Metastasis. Am. J. Hematol. 2010, 85, 593–598. [Google Scholar] [CrossRef]
- Lloyd, P.G.; Hardin, C.D.; Sturek, M. Examining Glucose Transport in Single Vascular Smooth Muscle Cells with a Fluorescent Glucose Analog. Physiol. Res. 1999, 48, 401–410. [Google Scholar] [PubMed]
- Yoshioka, K.; Saito, M.; Oh, K.B.; Nemoto, Y.; Matsuoka, H.; Natsume, M.; Abe, H. Intracellular Fate of 2-NBDG, a Fluorescent Probe for Glucose Uptake Activity, in Escherichia Coli Cells. Biosci. Biotechnol. Biochem. 1996, 60, 1899–1901. [Google Scholar] [CrossRef]
- Yoshioka, K.; Oh, K.B.; Saito, M.; Nemoto, Y.; Matsuoka, H. Evaluation of 2-[N-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-Yl)Amino]-2-Deoxy-D-Glucose, a New Fluorescent Derivative of Glucose, for Viability Assessment of Yeast Candida Albicans. Appl. Microbiol. Biotechnol. 1996, 46, 400–404. [Google Scholar] [CrossRef]
- Konadu, B.; Cox, C.K.; Garrett, M.R.; Gibert, Y. Excess Glucose or Fat Differentially Affects Metabolism and Appetite-Related Gene Expression during Zebrafish Embryogenesis. iScience 2023, 26, 107063. [Google Scholar] [CrossRef]
- Li, Q.; Gui, X.; Zhang, H.; Zhu, W.; Zhang, R.; Shen, W.; Song, H. Role of Glucose Metabolism in Ocular Angiogenesis (Review). Mol. Med. Rep. 2022, 26, 363. [Google Scholar] [CrossRef] [PubMed]
- Roosing, S.; Collin, R.W.J.; Den Hollander, A.I.; Cremers, F.P.M.; Siemiatkowska, A.M. Prenylation Defects in Inherited Retinal Diseases. J. Med. Genet. 2014, 51, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Köhnke, M.; Delon, C.; Hastie, M.L.; Nguyen, U.T.T.; Wu, Y.-W.; Waldmann, H.; Goody, R.S.; Gorman, J.J.; Alexandrov, K. Rab GTPase Prenylation Hierarchy and Its Potential Role in Choroideremia Disease. PLoS ONE 2013, 8, e81758. [Google Scholar] [CrossRef] [PubMed]
- Erol, Ö.D.; Şenocak, Ş.; Aerts-Kaya, F. The Role of Rab GTPases in the Development of Genetic and Malignant Diseases. Mol. Cell Biochem. 2023, 479, 255–281. [Google Scholar] [CrossRef] [PubMed]
- Kwok, M.C.M.; Holopainen, J.M.; Molday, L.L.; Foster, L.J.; Molday, R.S. Proteomics of Photoreceptor Outer Segments Identifies a Subset of SNARE and Rab Proteins Implicated in Membrane Vesicle Trafficking and Fusion. Mol. Cell Proteom. 2008, 7, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Keeling, E.; Lotery, A.; Tumbarello, D.; Ratnayaka, J. Impaired Cargo Clearance in the Retinal Pigment Epithelium (RPE) Underlies Irreversible Blinding Diseases. Cells 2018, 7, 16. [Google Scholar] [CrossRef]
- Prashar, A.; Schnettger, L.; Bernard, E.M.; Gutierrez, M.G. Rab GTPases in Immunity and Inflammation. Front. Cell. Infect. Microbiol. 2017, 7, 435. [Google Scholar] [CrossRef]
- Gordiyenko, N.V.; Fariss, R.N.; Zhi, C.; MacDonald, I.M. Silencing of the CHM Gene Alters Phagocytic and Secretory Pathways in the Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Strunnikova, N.V.; Barb, J.; Sergeev, Y.V.; Thiagarajasubramanian, A.; Silvin, C.; Munson, P.J.; Macdonald, I.M. Loss-of-Function Mutations in Rab Escort Protein 1 (REP-1) Affect Intracellular Transport in Fibroblasts and Monocytes of Choroideremia Patients. PLoS ONE 2009, 4, e8402. [Google Scholar] [CrossRef]
- Futter, C.E.; Ramalho, J.S.; Jaissle, G.B.; Seeliger, M.W.; Seabra, M.C. The Role of Rab27a in the Regulation of Melanosome Distribution within Retinal Pigment Epithelial Cells. MBoC 2004, 15, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal 2014, 21, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.L.; Richardson, R.; Tracey-White, D.; Abbouda, A.; Mitsios, A.; Horneffer-van der Sluis, V.; Takis, P.; Owen, N.; Skinner, J.; Welch, A.A.; et al. REP1 Deficiency Causes Systemic Dysfunction of Lipid Metabolism and Oxidative Stress in Choroideremia. JCI Insight 2021, 6, e146934. [Google Scholar] [CrossRef] [PubMed]
- Bukke, V.N.; Villani, R.; Archana, M.; Wawrzyniak, A.; Balawender, K.; Orkisz, S.; Ferraro, L.; Serviddio, G.; Cassano, T. The Glucose Metabolic Pathway as A Potential Target for Therapeutics: Crucial Role of Glycosylation in Alzheimer’s Disease. IJMS 2020, 21, 7739. [Google Scholar] [CrossRef] [PubMed]
- Gorbatyuk, M.S.; Starr, C.R.; Gorbatyuk, O.S. Endoplasmic Reticulum Stress: New Insights into the Pathogenesis and Treatment of Retinal Degenerative Diseases. Prog. Retin. Eye Res. 2020, 79, 100860. [Google Scholar] [CrossRef]
- Sun, J.; Luan, Y.; Xiang, D.; Tan, X.; Chen, H.; Deng, Q.; Zhang, J.; Chen, M.; Huang, H.; Wang, W.; et al. The 11S Proteasome Subunit PSME3 Is a Positive Feedforward Regulator of NF-κB and Important for Host Defense against Bacterial Pathogens. Cell Rep. 2016, 14, 737–749. [Google Scholar] [CrossRef] [PubMed]
- B Domènech, E.; Marfany, G. The Relevance of Oxidative Stress in the Pathogenesis and Therapy of Retinal Dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.M.; Russell, L.; Chan, C.-C. Choroideremia: New Findings from Ocular Pathology and Review of Recent Literature. Surv. Ophthalmol. 2009, 54, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Roubeix, C.; Sennlaub, F.; Saban, D.R. Microglia versus Monocytes: Distinct Roles in Degenerative Diseases of the Retina. Trends Neurosci. 2020, 43, 433–449. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhang, B.; Zhao, L.; Lyubasyuk, V.; Wang, K.; Xu, M.; Li, Y.; Wu, F.; Wen, C.; et al. A Missense Mutation in HK1 Leads to Autosomal Dominant Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7159–7164. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.S.; Koboldt, D.C.; Bowne, S.J.; Lang, S.; Blanton, S.H.; Cadena, E.; Avery, C.E.; Lewis, R.A.; Webb-Jones, K.; Wheaton, D.H.; et al. A Dominant Mutation in Hexokinase 1 (HK1) Causes Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7147–7158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, Z.; Zhao, P.; Huang, L.; Xu, M.; Yang, Y.; Chen, X.; Lu, F.; Zhang, X.; Wang, H.; et al. Whole-Exome Sequencing Revealed HKDC1 as a Candidate Gene Associated with Autosomal-Recessive Retinitis Pigmentosa. Hum. Mol. Genet. 2018, 27, 4157–4168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ekström, P. Pyruvate Kinase 2, an Energy Metabolism Related Enzyme, May Have a Neuroprotective Function in Retinal Degeneration. ASN Neuro 2023, 15, 17590914231151534. [Google Scholar] [CrossRef]
- Aït-Ali, N.; Fridlich, R.; Millet-Puel, G.; Clérin, E.; Delalande, F.; Jaillard, C.; Blond, F.; Perrocheau, L.; Reichman, S.; Byrne, L.C.; et al. Rod-Derived Cone Viability Factor Promotes Cone Survival by Stimulating Aerobic Glycolysis. Cell 2015, 161, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, H.; Toms, M.; Moosajee, M. Involvement of Oxidative and Endoplasmic Reticulum Stress in RDH12-Related Retinopathies. Int. J. Mol. Sci. 2021, 22, 8863. [Google Scholar] [CrossRef]
- Santhanam, A.; Shihabeddin, E.; Wei, H.; Wu, J.; O’Brien, J. Molecular Basis of Retinal Remodeling in a Zebrafish Model of Retinitis Pigmentosa. Cell Mol. Life Sci. 2023, 80, 362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sheng, Y.; Pan, M.; Tu, J.; Zhao, X.; Ge, Q.; Lu, Z. Spatial Transcriptomic Analysis Reveals Regional Transcript Changes in Early and Late Stages of Rd1 Model Mice with Retinitis Pigmentosa. IJMS 2023, 24, 14869. [Google Scholar] [CrossRef] [PubMed]
- Olivares-González, L.; Velasco, S.; Campillo, I.; Salom, D.; González-García, E.; Soriano Del Castillo, J.M.; Rodrigo, R. Nutraceutical Supplementation Ameliorates Visual Function, Retinal Degeneration, and Redox Status in Rd10 Mice. Antioxidants 2021, 10, 1033. [Google Scholar] [CrossRef]
- Appelbaum, T.; Santana, E.; Aguirre, G.D. Strong Upregulation of Inflammatory Genes Accompanies Photoreceptor Demise in Canine Models of Retinal Degeneration. PLoS ONE 2017, 12, e0177224. [Google Scholar] [CrossRef]
- Xu, H.; Chen, M. Immune Response in Retinal Degenerative Diseases—Time to Rethink? Prog. Neurobiol. 2022, 219, 102350. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, J. Senescence in the Pathogenesis of Age-Related Macular Degeneration. Cell Mol. Life Sci. 2020, 77, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Toualbi, L.; Toms, M.; Almeida, P.V.; Harbottle, R.; Moosajee, M. Gene Augmentation of CHM Using Non-Viral Episomal Vectors in Models of Choroideremia. IJMS 2023, 24, 15225. [Google Scholar] [CrossRef] [PubMed]
- Moosajee, M.; Tracey-White, D.; Smart, M.; Weetall, M.; Torriano, S.; Kalatzis, V.; Da Cruz, L.; Coffey, P.; Webster, A.R.; Welch, E. Functional Rescue of REP1 Following Treatment with PTC124 and Novel Derivative PTC-414 in Human Choroideremia Fibroblasts and the Nonsense-Mediated Zebrafish Model. Hum. Mol. Genet. 2016, 25, 3416–3431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hou, C.; Yan, N. Neuroinflammation in Retinitis Pigmentosa: Therapies Targeting the Innate Immune System. Front. Immunol. 2022, 13, 1059947. [Google Scholar] [CrossRef]
- Lee, S.Y.; Usui, S.; Zafar, A.; Oveson, B.C.; Jo, Y.-J.; Lu, L.; Masoudi, S.; Campochiaro, P.A. N-Acetylcysteine Promotes Long-Term Survival of Cones in a Model of Retinitis Pigmentosa. J. Cell. Physiol. 2011, 226, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Iftikhar, M.; Hafiz, G.; Akhlaq, A.; Tsai, G.; Wehling, D.; Lu, L.; Wall, G.M.; Singh, M.S.; Kong, X. Oral N-Acetylcysteine Improves Cone Function in Retinitis Pigmentosa Patients in Phase I Trial. J. Clin. Investig. 2020, 130, 1527–1541. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wang, S.K.; Rana, P.; West, E.R.; Hong, C.M.; Feng, H.; Wu, D.M.; Cepko, C.L. AAV-Txnip Prolongs Cone Survival and Vision in Mouse Models of Retinitis Pigmentosa. Elife 2021, 10, e66240. [Google Scholar] [CrossRef] [PubMed]
- Punzo, C.; Kornacker, K.; Cepko, C.L. Stimulation of the Insulin/mTOR Pathway Delays Cone Death in a Mouse Model of Retinitis Pigmentosa. Nat. Neurosci. 2009, 12, 44–52. [Google Scholar] [CrossRef]
- Venkatesh, A.; Ma, S.; Le, Y.Z.; Hall, M.N.; Rüegg, M.A.; Punzo, C. Activated mTORC1 Promotes Long-Term Cone Survival in Retinitis Pigmentosa Mice. J. Clin. Investig. 2015, 125, 1446–1458. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Cepko, C.L. Gene Therapies for Retinitis Pigmentosa That Target Glucose Metabolism. Cold Spring Harb. Perspect. Med. 2023, 14, a041289. [Google Scholar] [CrossRef]
- Byrne, L.C.; Dalkara, D.; Luna, G.; Fisher, S.K.; Clérin, E.; Sahel, J.-A.; Léveillard, T.; Flannery, J.G. Viral-Mediated RdCVF and RdCVFL Expression Protects Cone and Rod Photoreceptors in Retinal Degeneration. J. Clin. Investig. 2015, 125, 105–116. [Google Scholar] [CrossRef]
- Cho, E.S.; Cha, Y.H.; Kim, H.S.; Kim, N.H.; Yook, J.I. The Pentose Phosphate Pathway as a Potential Target for Cancer Therapy. Biomol. Ther. 2018, 26, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-L.; Hsu, Y.-C.; Lee, J.-J.; Chen, M.-J.; Lin, C.-H.; Huang, S.-Y.; Cheng, S.-P. Targeting the Pentose Phosphate Pathway Increases Reactive Oxygen Species and Induces Apoptosis in Thyroid Cancer Cells. Mol. Cell. Endocrinol. 2020, 499, 110595. [Google Scholar] [CrossRef] [PubMed]
- Vulliamy, T.J.; D’Urso, M.; Battistuzzi, G.; Estrada, M.; Foulkes, N.S.; Martini, G.; Calabro, V.; Poggi, V.; Giordano, R.; Town, M. Diverse Point Mutations in the Human Glucose-6-Phosphate Dehydrogenase Gene Cause Enzyme Deficiency and Mild or Severe Hemolytic Anemia. Proc. Natl. Acad. Sci. USA 1988, 85, 5171–5175. [Google Scholar] [CrossRef]
- Hanczko, R.; Fernandez, D.R.; Doherty, E.; Qian, Y.; Vas, G.; Niland, B.; Telarico, T.; Garba, A.; Banerjee, S.; Middleton, F.A.; et al. Prevention of Hepatocarcinogenesis and Increased Susceptibility to Acetaminophen-Induced Liver Failure in Transaldolase-Deficient Mice by N-Acetylcysteine. J. Clin. Investig. 2009, 119, 1546–1557. [Google Scholar] [CrossRef]
- Tylki-Szymańska, A.; Stradomska, T.J.; Wamelink, M.M.C.; Salomons, G.S.; Taybert, J.; Pawłowska, J.; Jakobs, C. Transaldolase Deficiency in Two New Patients with a Relative Mild Phenotype. Mol. Genet. Metab. 2009, 97, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.R.; McCool, B.A.; Singleton, C.K. Molecular Genetics of Transketolase in the Pathogenesis of the Wernicke-Korsakoff Syndrome. Metab. Brain Dis. 1995, 10, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.; Hanczko, R.; Telarico, T.; Oaks, Z.; Landas, S. Oxidative Stress, Inflammation and Carcinogenesis Are Controlled through the Pentose Phosphate Pathway by Transaldolase. Trends Mol. Med. 2011, 17, 395–403. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| hPFKM NM_000289.6 | ATGACCCATGAAGAGCACCA | GCACCGGTGAAGATACCAAC |
| cflara NM_001313772.1 | TGAAAGGACATGAGAGAAATGTGC | ATGGAGTGGTTTGTGTTGTGTTG |
| mcl-1a NM_131599.1 | GCGATACTCGGCAGCTCTTA | GAAGACGCTGGATCATTCCTTT |
| casp8 NM_131510.2 | CAGAGACCAGGAACAAGGAGG | TAATTGTGCCAGCCGAAGAGT |
| ddit3 NM_001082825.1 | CAGCTGAACAATGGTTAACATGA | AATCAAGTTTGAATGTGAGTTGTTG |
| hk2 NM_213066.1 | AAACCACCCAGAGTTTGCTC | AGACGCAGTGTGTCCAGAAC |
| pfkma NM_001004575.2 | AATACCATCACCACGACCTGT | GGTAACCGCAGTATCCTCCC |
| slc2a1b XM_002662528.6 | TGATGGAAGGCGGAAAGCAAT | ACAGACAGAGACCACAGGG |
| gapdh NM_001115114.1 | CGTCTTGAGAAACCTGCCAAG | AACCTGGTGCTCCGTGTATC |
| pgd NM_213453.2 | GAGTTCGGCTGGTCTCTGAA | ATCTCGTGTCTGTACCCGTC |
| prps1a NM_001359894.1 | CCGGTGGAGCAAAGAGAGTG | CACTCTGTCCTTCACGTCCC |
| prps1b NM_001076568.2 | AGGAGCCAAGAGGGTTACCT | TCTCCAACCAGAACCATGCG |
| cat NM_130912.2 | ACGATGACAACGTGACCCAA | CCATCAGGTTTTGCACCATGC |
| sod3a NM_001099236.1 | TCAAGTGCGTGCCATCCATA | CCGCCGGATAAGTCCTTGTT |
| txnb NM_001002461.1 | GACCATCGGGCCGTACTTTA | CATAAAGCGGCCACATCCTGT |
| b-actin NM_181601.5 | CGAGCTGTCTTCCCATCCA | TCACCAACGTAGCTGTCTTTCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méjécase, C.; Nair, N.; Sarkar, H.; Soro-Barrio, P.; Toms, M.; Halliday, S.; Linkens, K.; Jaroszynska, N.; Maurer, C.; Owen, N.; et al. Oxidative Stress, Inflammation and Altered Glucose Metabolism Contribute to the Retinal Phenotype in the Choroideremia Zebrafish. Antioxidants 2024, 13, 1587. https://doi.org/10.3390/antiox13121587
Méjécase C, Nair N, Sarkar H, Soro-Barrio P, Toms M, Halliday S, Linkens K, Jaroszynska N, Maurer C, Owen N, et al. Oxidative Stress, Inflammation and Altered Glucose Metabolism Contribute to the Retinal Phenotype in the Choroideremia Zebrafish. Antioxidants. 2024; 13(12):1587. https://doi.org/10.3390/antiox13121587
Chicago/Turabian StyleMéjécase, Cécile, Neelima Nair, Hajrah Sarkar, Pablo Soro-Barrio, Maria Toms, Sophia Halliday, Katy Linkens, Natalia Jaroszynska, Constance Maurer, Nicholas Owen, and et al. 2024. "Oxidative Stress, Inflammation and Altered Glucose Metabolism Contribute to the Retinal Phenotype in the Choroideremia Zebrafish" Antioxidants 13, no. 12: 1587. https://doi.org/10.3390/antiox13121587
APA StyleMéjécase, C., Nair, N., Sarkar, H., Soro-Barrio, P., Toms, M., Halliday, S., Linkens, K., Jaroszynska, N., Maurer, C., Owen, N., & Moosajee, M. (2024). Oxidative Stress, Inflammation and Altered Glucose Metabolism Contribute to the Retinal Phenotype in the Choroideremia Zebrafish. Antioxidants, 13(12), 1587. https://doi.org/10.3390/antiox13121587







