Plant-Based Antioxidants in Gluten-Free Bread Production: Sources, Technological and Sensory Aspects, Enhancing Strategies and Constraints
Abstract
1. Introduction
- To match and/or further enhance the nutritional properties of GFB compared to regular wheat bread;
- To improve the overall quality of the GF diet;
- To assist with healing of the damaged mucosa in celiac patients;
- Contribution to broader health effects.
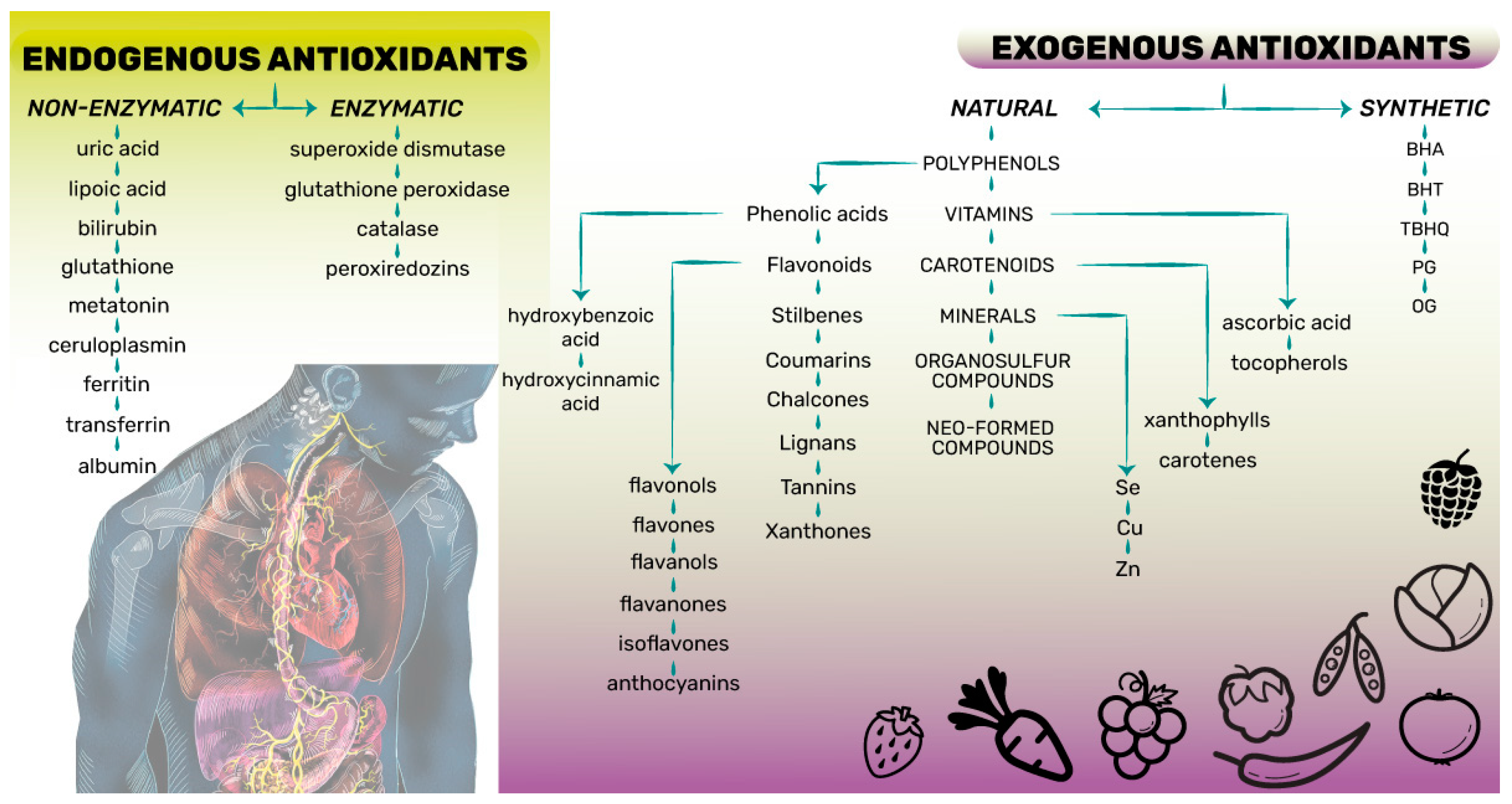
2. Definition, Classification and Health-Related Effects of Antioxidant Compounds
- The first preventive line of antioxidants suppresses or hinders free radicals’ or reactive species’ creation in cells by preventing the occurrence of reactions in which they are formed. The antioxidants involved in these reactions are predominantly endogenous enzymatic antioxidants such as superoxide dismutase, catalase, glutathione reductase, and the minerals Se, Cu, and Zn (Figure 1);
- The second repairing line of antioxidants neutralizes or scavenges free radicals or reactive species by donating an electron to them and interrupting radical chain reactions. Both endogenous non-enzymatic and natural exogenous antioxidants such as glutathione, albumin, vitamins C and E, carotenoids, and flavonoids are included in these reactions (Figure 1);
- The third line of antioxidants acts towards restoring and the reconstitution of the biomolecules and cell membranes damaged by free radicals or reactive species. These antioxidants include a complex group of enzymes (de novo enzymes) such as polymerases, glycosylases, nucleases, proteinases, proteases, and peptidases.
- Must be approved by regulatory bodies and have GRAS status (generally recognized as safe);
- Food product color, odor, or flavor should not be negatively affected by antioxidant addition;
- Should be effective when added at low concentrations (0.001–0.01%);
- Should be easily applicable and compatible with foods they are used in;
- Should be steady during processing and storage;
- Should be economical.
3. Methods of Quantification and Identification of Antioxidant Compounds Used in Gluten-Free Bread
- Defined mechanism of reaction and end point in a particular food matrix with a measurable content;
- Utilization of biologically relevant free radicals to closely reflect in vivo action;
- Simple performance, and accessible chemicals and equipment;
- Reliable within samples and between-day reproducibility;
- Adaptable for measurement of both lipophilic and hydrophilic antioxidants as well as for the use of other radical sources;
- Adaptable to high-throughput analysis for routine quality control analyses.
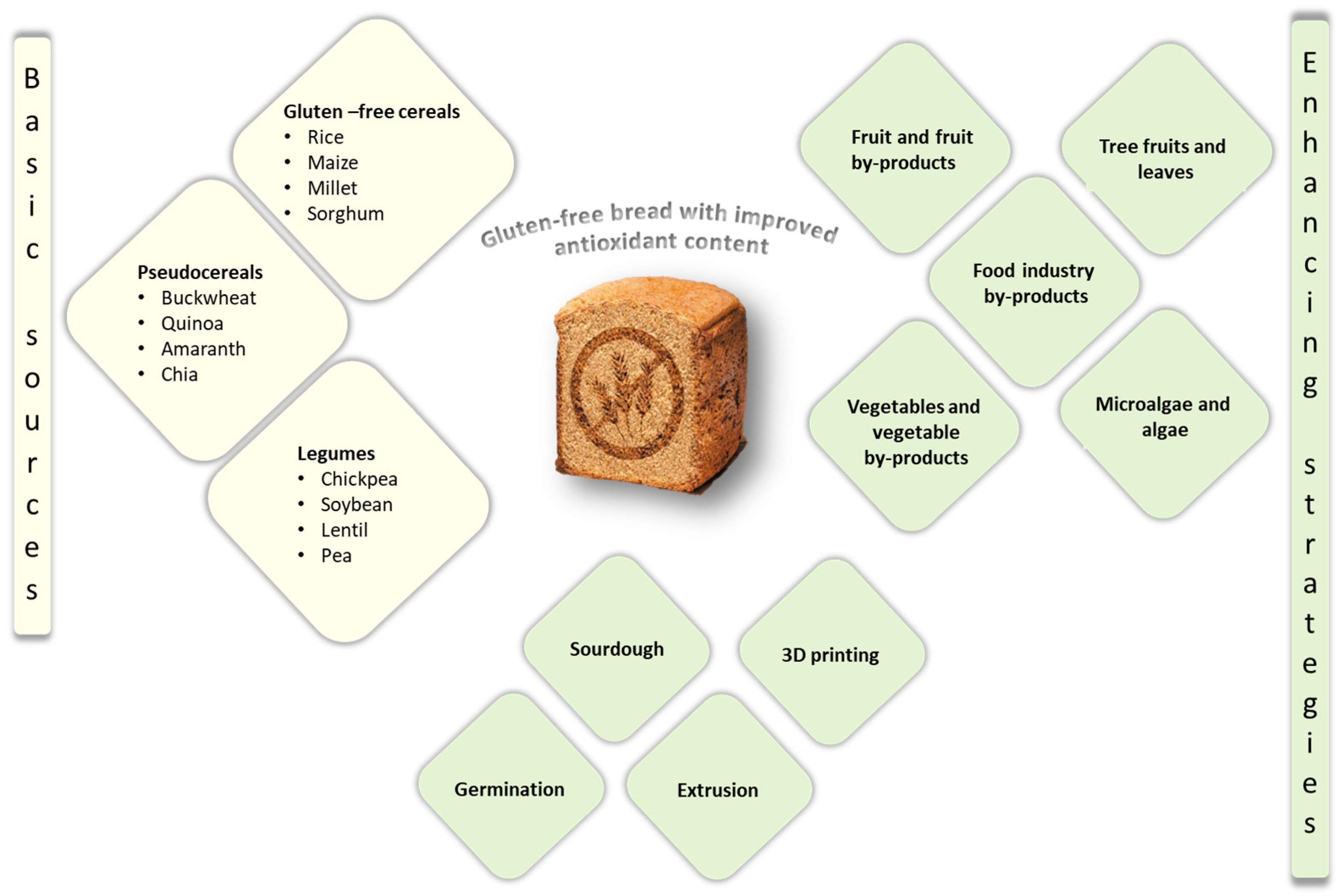
4. Basic Sources of Antioxidant Compounds in the Gluten-Free Bread Production
4.1. Gluten-Free Cereals as Basic Sources of Antioxidant Compounds in Gluten-Free Bread Production
4.1.1. Rice
4.1.2. Maize
4.1.3. Millet
4.1.4. Sorghum
| Raw Material | Variety | Total Phenolic Content [mg GAE/g d.b.] | Total Flavonoid Content [See Units in Footnote] | DPPH [See Units in Footnote] | FRAP [See Units in Footnote] | ABTS [See Units in Footnote] | ORAC [See Units in Footnote] | Anthocyanins [See Units in Footnote] | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Gluten-free cereals | |||||||||
| Rice | 0.104 | / | 0.66 ± 8.16 i | / | 8.20 ± 138.39 i | / | / | [41] | |
| 1.39 ± 0.13 | / | 3.52 ± 1.02 j | / | / | / | [56] | |||
| 0.063–0.069 | 0.01–0.06 c | / | / | 0.013–0.017 w | / | / | [108] | ||
| white | 0.20 | / | / | / | / | / | / | [90] | |
| brown | 0.72 | / | / | / | / | / | / | [90] | |
| red | 1.11 ± 0.22 | / | / | / | 0.012 ± 0.24 x | / | / | [93] | |
| ermes | 0.68 ± 1.6 | / | / | 2.00 ± 7.5 s | / | 14.44 ± 8.9 z | / | [89] | |
| nerone | 3.41 ± 9.9 | / | / | 12.85 ± 29.4 s | / | 72.28 ± 68.1 z | 1.22 ± 1.3 ** | [89] | |
| orange | 0.76 ± 0.7 | / | / | 2.57 ± 26.4 s | / | 25.51 ± 79.6 z | 0.006 ± 0.2 ** | [89] | |
| wild | 0.82 ± 1.4 | / | / | 1.96 ± 11.3 s | / | 31.82 ± 35.7 z | 0.014 ± 0.1 ** | [89] | |
| violet | 5.00 ± 23.7 | / | / | 20.90 ± 47.1 s | / | 117.84 ± 63.2 z | 2.48 ± 5.9 ** | [89] | |
| black | 2.40 ± 3.6 | / | / | 9.51 ± 29.4 s | / | 39.59 ± 90.7 z | 0.66 ± 1.3 ** | [89] | |
| Maize | 0.295 | / | 4.89 ± 153.80 i | / | 13.41 ± 210.15 i | / | / | [41] | |
| white | 6.75 ± 0.67 a | / | 34.41 ± 3.34 j | 18.05 ± 1.16 t | / | / | 0.45 ± 0.16 t | [99] | |
| white | 5.23 ± 0.33 | 0.25 ± 0.004 d | / | / | / | / | n.d. | [98] | |
| lemon yellow | 5.78 ± 0.037 | 0.28 ± 0.02 d | / | / | / | / | n.d. | [98] | |
| yellow | 5.4 ± 0.01 | 0.281 ± 0.002 d | / | / | / | / | n.d. | [98] | |
| yellow | 2.01 | 0.75 e | 3.81 k | 5.18 k | 3.98 k | / | / | [63] | |
| yellow | 11.25 ± 0.94 a | / | 46.73 ± 4.55 j | 32.11 ± 1.85 t | / | / | 0.75 ± 0.21 t | [99] | |
| orange | 5.81 ± 0.14 | 0.29 ± 0.002 d | / | / | / | / | n.d. | [98] | |
| red-yellow | 6.01 ± 0.23 | 0.268 ± 0.006 d | / | / | / | / | 0.0025 ± 0.06 ** | [98] | |
| red | 6.04 ± 0.20 | 0.267 ± 0.003 d | / | / | / | / | 0.015 ± 0.002 ** | [98] | |
| red | 16.45 ± 1.76 a | / | 67.57 ± 1.94 j | 49.02 ± 1.66 t | / | / | 9.35 ± 0.93 t | [99] | |
| dark red | 6.11 ± 0.16 | 0.27 ± 0.003 d | / | / | / | / | 0.696 ± 0.003 ** | [98] | |
| purple | 34.25 ± 1.26 a | / | 78.32 ± 2.27 j | 54.96 ± 1.46 t | / | / | 12.45 ± 1.07 t | [99] | |
| light blue | 10.53 ± 0.06 | 0.34 ± 0.013 d | / | / | / | / | 0.378 ± 0.005 ** | [98] | |
| blue | 10.39–13.31 | / | / | 1.52–2.03 n | / | / | 0.65–1.05 ** | [101] | |
| dark blue | 7.35 ± 0.5 | 0.31 ± 0.017 d | / | / | / | / | 0.60 ± 0.007 ** | [98] | |
| multicolored | 4.49 ± 0.29 | 0.20 ± 0.013 d | / | / | / | / | 0.14 ± 0.002 ** | [98] | |
| black | / | / | / | 10.96 u | / | / | 5.375 u | [100] | |
| Millet | pearl | / | / | 0.73 ± 0.00 l | / | / | / | / | [32] |
| 1.39 ± 13.3 | / | 23.83 ± 0.67 m | / | 21.4 ± 0.43 y | / | / | [104] | ||
| Sorghum | 4.13 ± 9.3 | / | 195.8± 8.82 m | / | 51.7 ± 0.57 y | / | / | [104] | |
| white | 0.52 ± 0.2 | / | / | 1.46 ± 7.5 s | / | 22.36 ± 63.1 z | / | [89] | |
| red | 1.08 ± 5.1 | / | / | 3.24 ± 9.9 s | / | 47.35 ± 59.5 z | / | [89] | |
| Pseudocereals | |||||||||
| Buckwheat | 3.23 ± 14.1 | / | 6.20 ± 28.1 n | 4.36 ± 12.8 n | / | / | / | [39] | |
| 4.98 ± 0.11 | / | 53.08 ± 0.82 j | / | / | / | / | [56] | ||
| 7.25 ± 0.2 | 153 ± 12 f | 8.80 ± 0.52 o | 2.15 ± 3.5 n | / | / | / | [109] | ||
| light | 3.32 ± 4.76 | / | 1.36 ± 0.01 p | / | / | / | / | [46] | |
| wholegrain | 4.15 ± 13.8 | / | 1.26 ± 0.09 p | / | / | / | / | [46] | |
| Common | 29.3 ± 0.5 | 1.0 ± 0.2 g | / | 9.1 ± 1.6 v | / | 139.3 ± 33.4 * | / | [37] | |
| Tartary | 72.8 ± 0.5 | 22.2 ± 0.3 g | / | 40.5 ± 4.4 v | / | 450.3 ± 57.7 * | / | [37] | |
| Quinoa | 0.717 ± 5.5 | / | 0.577 ± 1.7 n | 0.921 ± 1.7 n | / | / | / | [39] | |
| 2.8 ± 0.1 | 92 ± 9 f | 6.22 ± 0.2 o | 0.59 ± 1.5 n | / | / | / | [109] | ||
| 5.22 ± 0.17 | / | 60.14 ± 0.76 j | / | / | / | / | [56] | ||
| 2.26 | 0.43 e | 5.67 k | 5.49 k | 2.02 k | / | / | [63] | ||
| white Spanish quinoa | / | / | 4.56 ± 0.03 q | 3.65 ± 0.30 q | 4.57 ± 0.28 q | / | / | [110] | |
| white Bolivian Real quinoa | / | / | 3.43 ± 0.14 q | 3.36 ± 0.11 q | 4.01 ± 0.25 q | / | / | [110] | |
| white Peruvian quinoa | / | / | 1.94 ± 0.11 q | 2.37 ± 0.28 q | 3.88 ± 0.19 q | / | / | [110] | |
| red Bolivian Real quinoa | / | / | 5.01 ± 0.04 q | 4.57 ± 0.17 q | 7.76 ± 0.17 q | / | / | [110] | |
| black Bolivian Real quinoa | / | / | 4.77 ± 0.02 q | 4.22 ± 0.00 q | 5.72 ± 0.34 q | / | / | [110] | |
| Amaranth | 21.2 ± 2.3 | 28.4 ± 1.3 n | 55.3 ± 1.6 n | [39] | |||||
| 2.55 ± 0.20 | 18.46 ± 0.93 j | [56] | |||||||
| 2.71 ± 0.1 | 65 ± 8 f | 3.60 ± 0.34 o | 0.39 ± 1.2 n | [109] | |||||
| Chia | 16.4 ± 0.9 | 1.1 ± 0.2 g | / | 11.0 ± 1.4 v | / | 131.0 ± 13.3 * | / | [37] | |
| Legumes | |||||||||
| Chickpea | 0.24–0.42 | 0.17–0.40 c | / | / | 0.03–0.04 w | / | / | [108] | |
| 1.22–1.67 b | 0.021–0.1 h | / | / | / | / | 0.04–0.066 ** | [111] | ||
| 0.93–10.84 | / | / | 0.73–1.13 v | / | 8.74–52.2 z | / | [112] | ||
| Soybean | 0.95–1.39 | 0.33–0.57 c | / | / | 0.07–0.08 w | / | / | [108] | |
| 0.98–2.62 | / | / | 1.24–1.96 v | / | 22.2–86.8 z | / | [112] | ||
| yellow | 13.35–14.64 | 0.39–0.50 g | 3.91–11.74 n | 6.43–10.86 n | 5.93–12.28 n | / | n.d. | [113] | |
| black | 16.46–21.49 | 0.74–0.90 g | 26.60–28.36 n | 35.87–55.02 n | 20.21–23.05 n | / | 0.58–1.03 ** | [113] | |
| Lentil | 2.22 | / | 21.00 ± 23.96 i | / | 188.45 ± 45.22 i | / | / | [40] | |
| red | 4.68 ± 0.3 | / | 64.26 ± 2.84 r | / | / | / | / | [114] | |
| Pea | 0.07–0.22 | 0.33–0.48 c | / | / | 0.01–0.02 w | / | / | [108] | |
4.2. Pseudocereals as Basic Sources of Antioxidant Compounds in Gluten-Free Bread Production
4.2.1. Buckwheat
4.2.2. Quinoa
4.2.3. Amaranth
4.2.4. Chia
4.3. Legumes as Basic Sources of Antioxidant Compounds in Gluten-Free Bread Production
4.3.1. Chickpea
4.3.2. Soybean
5. Strategies to Improve the Content of Antioxidant Compounds in Gluten-Free Bread
5.1. Effect of Bread-Making on Antioxidant Compounds Content in Gluten-Free Bread
- The presence of other antioxidant compounds beyond polyphenols such as vitamins with antioxidant activity not detectable by the applied methods [57];
5.2. Plant-Based Additives as Improvers of Antioxidant Compounds Content in Gluten-Free Bread
| Antioxidant Source | Addition Level (%) | Pre-Treatment/Technology | TPC [See Units in Footnote] | DPPH [See Units in Footnote] | ABTS [See Units in Footnote] | FRAP [See Units in Footnote] | TFC [See Units in Footnote] | Reference |
|---|---|---|---|---|---|---|---|---|
| Gluten-free cereals, pseudocereals and legumes | ||||||||
| Wholegrain rice flour | 100 | 0.70 ± 0.10 a | 10.50 ± 0.11 j | 6.97 ± 0.10 l | 0.98 ± 0.05 v | [55] | ||
| Maize flour | 10 | 0.16 ± 0.02 b | / | 3.4 ± 0.2 s | / | 0.067 ± 0.006 b | [57] | |
| Brown millet flour | 100 | 1.8 ± 0.10 a | 19.24 ± 0.10 j | 15.07 ± 0.10 l | 2.06 ± 0.07 v | / | [55] | |
| Wholegrain millet flour and Wholegrain millet extruded flour | 50 | Extrusion | 108.26 ± 0.001 c | 76.9 ± 1.02 j | / | 215.2 ± 1.9 w | / | [60] |
| Wholegrain sorghum flour | 100 | 3.87 ± 0.11 a | 35.01 ± 0.10 j | 54.51 ± 0.12 l | 3.67 ± 0.09 v | / | [55] | |
| White sorghum flour | 85 | 0.412 ± 0.021 d | / | 0.135 ± 0.018 o | 0.003 ± 0.02 p | / | [36] | |
| Amaranth | 50 | 0.138 ± 0.0 e | 0.103 ± 0.002 k | / | 0.61 ± 0.062 k | / | [39] | |
| Amaranth flour | 10 | 0.31 ± 0.03 b | / | 5.26 ± 0.15 s | / | 0.105 ± 0.007 b | [57] | |
| Amaranth, buckwheat and quinoa flour | 15, 30, 45 | Sourdough | 1.70–2.06 d | 6.81–14.59 j | / | / | / | [56] |
| Buckwheat flour | 10 | 0.64 ± 0.05 b | / | 3.4 ± 0.2 s | / | 0.192 ± 0.02 b | [57] | |
| Buckwheat | 50 | 0.65 ± 0.031 e | 0.59 ± 0.039 k | / | 1.48 ± 0.046 k | / | [39] | |
| Sprouted buckwheat | 100 | Germination | 1.16 ± 0.018 e | 0.77 ± 0.025 k | / | 2.64 ± 0.036 k | / | [39] |
| Wholegrain buckwheat flour | 30, 45 | 25.74–30.08 d | / | / | / | / | [43] | |
| Dehulled buckwheat flour | 10, 20, 30, 40 | 0.42–1.22 a | 0.76–2.56 l | 1.70–4.12 l | / | / | [42] | |
| Buckwheat hulls | 3, 6 | 0.006–0.18 e | 0.40–0.60 k | 1.91–2.61 k | 0.135–15.1 x | / | [45] | |
| Quinoa flour | 100 | 3.98 ± 0.15 a | 32.85 ± 0.11 j | 19.32 ± 0.12 l | 2.53 ± 0.09 v | / | [55] | |
| Quinoa | 50 | 0.307 ± 0.003 e | 0.168 ± 0.7 k | / | 0.714 ± 0.028 k | / | [39] | |
| Extruded lentil flour | 15 | Extrusion | 0.303 ± 0.013 e | 2.28 ± 28.80 l | 7.53 ± 1.18 l | 0.069 ± 0.009 x | / | [41] |
| Germinated sweet lupin and fenugreek mixtures | 5, 10, 15, 20 | Germination | / | 5.71–6.40 m | 2.45–3.17 m | 5.65–6.45 m | / | [63] |
| Fruit and fruit by-products | ||||||||
| Acerola fruit powder | 1, 2, 3, 4, 5 | 4.4–10.1 e | 67.8–231.9 n | 34.1–89.4 n | / | / | [65] | |
| Apple pomace | 5, 10, 15 | 0.036–0.22 e | / | 1.97–3.21 k | / | 0.08–0.22 y | [66] | |
| Defatted blackcurrant seeds | 5, 10, 15 | 0.10–0.12 f | / | 1.34–2.01 o | / | / | [67] | |
| Defatted strawberry seeds | 5, 10, 15 | 0.30–0.71 f | / | 3.16–5.60 o | / | / | [67] | |
| Extruded sour cherry pomace and rice flour | 10 | Extrusion | 59.4–308.7 g | / | 1.817–2.297 t | / | 11.7–97.3 z | [68] |
| Grape seed flour | 3, 6, 9 | 3.63–5.92 d | / | / | 5.75–9.75 q | / | [69] | |
| Pomegranate seed powder | 2.5, 5, 7.5, 10 | 1.29–2.47 e | 11.97–29.39 n | 5.16–6.22 n | / | / | [70] | |
| Rosehip powder, rosehip encapsulate | 7 | 3D printing | 0.46–0.81 d | 0.71–113.5 o | / | / | / | [52] |
| Vegetables and vegetable by-products | ||||||||
| Freeze-dried red and purple potatoes | 5 | 0.173–0.351 h | 1.995–2.113 k | 2.865–3.590 k | / | 0.080–0.147 y | [53] | |
| Red potatoes pulp | 5, 7.5, 10 | / | / | [51] | ||||
| Purple potatoes pulp | 5, 7.5, 10 | 0.14–0.39 h | / | 9.5–39.4 k | / | 0.019–0.057 * | [51] | |
| Broccoli leaf powder | 5 | 1.25 e | 0.95 l | 1.77 l | / | / | [48] | |
| Fried red onion | 5 | 1.5 ± 0.06 e | 0.85 ± 0.02 k | / | 0.96 ± 0.02 k | / | [35] | |
| Dried red onion | 5 | 1.87 ± 0.01 e | 1.23 ± 0.03 k | / | 1.43 ± 0.01 k | / | [35] | |
| Red onion peel | 5 | 5.28 ± 0.11 e | 4.70 ± 0.02 k | / | 6.36 ± 0.02 k | / | [35] | |
| Okara | 30 | 1.34 ± 0.024 d | 0.49 ± 8.58 o | 0.87 ± 0.025 k | 1.19 ± 0.043 q | / | [71] | |
| Herbs | ||||||||
| Hemp inflorescence | 1, 2, 3, 4, 5 | 0.30–0.65 e | 1.66–3.23 l | / | 1.60–3.00 l | 0.06–0.16 * | [38] | |
| Tree fruits and leaves | ||||||||
| Acorn flour | 23, 35 | Sourdough | 4.541–6.810 e | 0.055–0.076 p | 0.072–0.143 p | 0.046–0.069 p | / | [40] |
| Acorn flour | 23, 35 | 0.613–0.848 d | 0.037–0.043 p | 0.066–0.073 p | 0.041–0.064 p | 5.39–6.30 ** | [72] | |
| Carob fiber (commercial) | 1, 2, 3, 4, 5 | 7.5–9.1 e | / | 42.5–66.8 n | / | / | [47] | |
| Moringa oleifera leaves powder | 2.5, 5, 7.5, 10 | 2.03–2.39 e | 10.60–31.62 n | 4.72–7.51 n | / | / | [49] | |
| Microalgae and algae | ||||||||
| Microalgae Tetraselmis chuii | 4 | 0.24 d | 3.22 q | / | 0.47 q | / | [75] | |
| Microalgae biomass | 4 | 4.38–5.47 d | 2.59–3.16 r | / | / | / | [76] | |
| Ethanol-treated microalgae biomass | 4 | 1.93–3.92 d | 0.80–2.09 r | / | / | / | [76] | |
| Brown algae powder | 2, 4, 6, 8, 10 | 3.7–4.7 e | / | 157.6–248.7 n | 216.5–375.9 n | / | [77] | |
| Food industry by-products | ||||||||
| Coffee silverskin extract | 2.5 | 254.92 ± 7.73 i | / | 288.27 ± 3.57 u | / | / | [78] | |
| Coffee husk extract | 2.5 | 121.12 ± 6.12 i | / | 129.39 ± 1.80 u | / | / | [78] | |
| Ground green coffee parchment | 2 | 1.07 ± 1.16 d | 65.6 ± 1.6 j | / | / | / | [79] | |
| Flaxseed oil cake extract | 25, 50, 75, 100 | 0.203–0.234 e | 0.852–0.945 l | 0.890–1.128 l | 0.568–0.731 l | / | [34] | |
5.3. Germination as a Pre-Treatment to Improve the Antioxidant Compounds Content in Gluten-Free Bread
5.4. Technologies Applied to Improve the Antioxidant Compounds Content in Gluten-Free Bread
5.4.1. Sourdough
- Propagation of the selected LAB culture in DeMan, Rogosa, and Sharpe (MRS) broth and yeast on Yeast Extract Peptone Dextrose (YEPD) agar under defined conditions;
- Sterilization of the fermentation medium, i.e., flour blend and water (105 °C, 10 min);
- Inoculation with starter culture and fermentation, commonly for 24 h at 25 or 30 °C according to the culture’s affinity.
5.4.2. Extrusion
- Enzyme inactivation and microbial population reduction;
- Antinutrients content reduction;
- Increase in dietary fiber solubility;
- Reduction or increase in bioactive polyphenols content due to degradation or release from dietary fiber;
- Gelatinization and degradation of starch and protein aggregation.
5.4.3. 3D Printing
6. Role of Antioxidant Compounds in Gluten-Free Bread’s Technological Quality
6.1. Effect of Antioxidant Compounds Source and Addition on Crust and Crumb Color of Gluten-Free Bread
6.2. Effect of Antioxidant Compounds Source and Addition on the Specific Volume of Gluten-Free Bread
6.3. Effect of Antioxidant Compounds Source and Addition on the Crumb and Crust Texture of Gluten-Free Bread
6.4. Effect of Antioxidant Compounds Source and Addition on Gluten-Free Bread Storage and Shelf-Life
7. Sensory Properties of Antioxidant Compounds-Enriched Gluten-Free Bread
8. Bioaccessibility and Bioavailability of Antioxidant Compounds from Enriched Gluten-Free Bread
9. Summary and Conclusive Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benzie, I.F.F.; Choi, S.-W. Antioxidants in Food: Content, Measurement, Significance, Action, Cautions, Caveats, and Research Needs. Adv. Food Nutr. Res. 2014, 71, 1–53. [Google Scholar] [CrossRef]
- Capriles, V.; Arêas, J.A. Novel approaches in gluten-free breadmaking: Interface between food science, nutrition and health. Compr. Rev. Food Sci. Food Saf. 2014, 13, 871–890. [Google Scholar] [CrossRef]
- Capriles, V.D.; dos Santo, F.G.; Arêas, J.A.G. Gluten-free breadmaking: Improving nutritional and bioactive compounds. J. Cereal Sci. 2016, 67, 83–91. [Google Scholar] [CrossRef]
- Nanditha, B.; Prabhasankar, P. Antioxidants in Bakery Products: A Review. Crit. Rev. Food Sci. Nutr. 2008, 49, 1–27. [Google Scholar] [CrossRef]
- Torres, M.D.; Arufe, S.; Chenlo, F.; Moreira, R. Coeliacs cannot live by gluten-free bread alone—Every once in a while they need antioxidants. Int. J. Food Sci. Technol. 2017, 52, 81–90. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef]
- Finley, J.W.; Kong, A.N.; Hintze, K.J.; Jeffery, E.H.; Ji, L.L.; Lei, X.G. Antioxidants in Foods: State of the Science Important to the Food Industry. J. Agric. Food Chem. 2011, 59, 6837–6846. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential applications of antioxidants—A review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Stojiljković, V.; Todorović, A.; Pejić, S.; Kasapović, J.; Saičić, Z.S.; Radlović, N.; Pajović, S.B. Antioxidant Status and Lipid Peroxidation in Small Intestinal Mucosa of Children with Celiac Disease. Clin. Biochem. 2009, 42, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Maluf, S.W.; Wilhelm Filho, D.; Parisotto, E.B.; Medeiros, G.d.S.d.; Pereira, C.H.J.; Maraslis, F.T.; Dornelles Schoeller, C.C.; da Rosa, J.S.; Fröde, T.S. DNA Damage, Oxidative Stress, and Inflammation in Children with Celiac Disease. Genet. Mol. Biol. 2020, 43, e20180390. [Google Scholar] [CrossRef]
- Dugas, B.; Dugas, N.; Conti, M.; Calenda, A.; Pino, P.; Thomas, Y.; Mazier, D.; Vouldoukis, I. Wheat Gliadin Promotes the Interleukin-4-Induced IgE Production by Normal Human Peripheral Mononuclear Cells through a Redox-Dependent Mechanism. Cytokine 2003, 21, 270–280. [Google Scholar] [CrossRef]
- Stojiljković, V.; Pejić, S.; Kasapović, J.; Gavrilović, L.; Stojiljković, S.; Nikolić, D.; Pajovićet, S.B. Glutathione redox cycle in small intestinal mucosa and peripheral blood of pediatric celiac disease patients. An. Acad. Bras. Cienc. 2012, 84, 175–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murray, I.A.; Daniels, I.; Coupland, K.; Smith, J.A.; Long, R.G. Increased activity and expression of iNOS in human duodenal enterocytes from patients with celiac disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G319–G326. [Google Scholar] [CrossRef]
- Beckett, C.G.; Dell’Olio, D.; Shidrawi, R.G.; Rosen-Bronson, S.; Ciclitira, P.J. Gluten-induced nitric oxide and pro-inflammatory cytokine release by cultured coeliac small intestinal biopsies. Eur. J. Gastroenterol. Hepatol. 1999, 11, 529–535. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A review on the gluten-free diet: Technological and nutritional challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef]
- Kamiński, M.; Nowak, J.K.; Skonieczna-Żydecka, K.; Stachowska, E. Gluten-free diet yesterday, today and tomorrow: Forecasting using Google Trends data. Arab J. Gastroenterol. 2020, 21, 67–68. [Google Scholar] [CrossRef]
- Prada, M.; Godinho, C.; Rodrigues, D.L.; Lopes, C.; Garrido, M.V. The impact of a gluten-free claim on the perceived healthfulness, calories, level of processing and expected taste of food products. Food Qual. Prefer. 2019, 73, 284–287. [Google Scholar] [CrossRef]
- Alencar, N.M.M.; Araújo, V.A.; Faggian, L.; da Silveira Araújo, M.B.; Capriles, V.D. What about gluten-free products? An insight on celiac consumers’ opinions and expectations. J. Sens. Stud. 2021, 36, e12664. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef]
- Yang, C.S.; Ho, C.T.; Zhang, J.; Wan, X.; Zhang, K.; Lim, J. Antioxidants: Differing Meanings in Food Science and Health Science. J. Agric. Food Chem. 2018, 66, 3063–3068. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Pruteanu, L.L.; Bailey, D.S.; Grădinaru, A.C.; Jäntschi, L. The Biochemistry and Effectiveness of Antioxidants in Food, Fruits, and Marine Algae. Antioxidants 2023, 12, 860. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidant characterization; methodology and mechanism. Biochem. Pharmacol. 1995, 49, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Br. J. Clin. Pharmacol. 2013, 75, 637–644. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Arora, K.; Tlais, A.Z.A.; Augustin, G.; Grano, D.; Filannino, P.; Gobbetti, M.; Di Cagno, R. Physicochemical, nutritional, and functional characterization of gluten-free ingredients and their impact on the bread texture. LWT Food Sci. Technol. 2023, 177, 114566. [Google Scholar] [CrossRef]
- Gkountenoudi-Eskitzi, I.; Kotsiou, K.; Irakli, M.N.; Lazaridis, A.; Biliaderis, C.G.; Lazaridou, A. In vitro and in vivo glycemic responses and antioxidant potency of acorn and chickpea fortified gluten-free breads. Food Res. Int. 2023, 166, 112579. [Google Scholar] [CrossRef] [PubMed]
- Krupa-Kozak, U.; Bączek, N.; Capriles, V.D.; Łopusiewicz, Ł. Novel Gluten-Free Bread with an Extract from Flaxseed By-Product: The Relationship between Water Replacement Level and Nutritional Value, Antioxidant Properties, and Sensory Quality. Molecules 2022, 27, 2690. [Google Scholar] [CrossRef] [PubMed]
- Bedrníček, J.; Jirotková, D.; Kadlec, J.; Laknerová, I.; Vrchotová, N.; Tříska, J.; Samková, E.; Smetana, P. Thermal stability and bioavailability of bioactive compounds after baking of bread enriched with different onion by-products. Food Chem. 2020, 319, 126562. [Google Scholar] [CrossRef] [PubMed]
- Curti, M.I.; Palavecino, P.M.; Savio, M.; Baroni, M.V.; Ribotta, P.D. Sorghum (Sorghum bicolor L. Moench) Gluten-freeb: The effect of milling conditions on the technological properties and in vitro bioaccessibility of Polyphenols and Minerals. Foods 2023, 12, 3030. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Lukšič, L.; Molinari, R.; Kreft, I.; Bonafaccia, G.; Manzi, L.; Merendino, N. Development of gluten-free bread using tartary buckwheat and chia flour rich in flavonoids and omega-3 fatty acids as ingredients. Food Chem. 2014, 165, 232–240. [Google Scholar] [CrossRef]
- Pecyna, A.; Buczaj, A.; Różyło, R.; Kobus, Z. Physical and Antioxidant Properties of Innovative Gluten-Free Bread with the Addition of Hemp Inflorescence. Appl. Sci. 2023, 13, 4889. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Beltrão Martins, R.; Garzón, R.; Peres, J.A.; Barros, A.I.R.N.A.; Raymundo, A.; Rosell, C.M. Acorn flour and sourdough: An innovative combination to improve gluten free bread characteristics. Eur. Food Res. Technol. 2022, 248, 1691–1702. [Google Scholar] [CrossRef]
- Rico, D.; Cano, A.B.; Martín-Diana, A.B. Pulse-Cereal Blend Extrusion for Improving the Antioxidant Properties of a Gluten-Free Flour. Molecules 2021, 26, 5578. [Google Scholar] [CrossRef] [PubMed]
- Wronkowska, M.; Zielińska, D.; Szawara-Nowak, D.; Troszyńska, A.; Soral-Śmietana, M. Antioxidative and reducing capacity, macroelements content and sensorial properties of buckwheat-enhanced gluten-free bread: Buckwheat-enhanced gluten-free bread. Int. J. Food Sci. Technol. 2010, 45, 1993–2000. [Google Scholar] [CrossRef]
- Brites, L.T.G.F.; Rebellato, A.P.; Meinhart, A.D.; Godoy, H.T.; Steel, C.J. Antioxidant-enriched gluten-free bread made with buckwheat flour: Evaluation of technological and nutritional quality. Cereal Chem. 2022, 99, 995–1006. [Google Scholar] [CrossRef]
- Cornejo, F.; Caceres, P.J.; Martínez-Villaluenga, C.; Rosell, C.M.; Frias, J. Effects of germination on the nutritive value and bioactive compounds of brown rice breads. Food Chem. 2015, 173, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, Á.L.; Villanueva, M.; Rico, D.; Harasym, J.; Ronda, F.; Martín-Diana, A.B.; Caballero, P.A. Valorisation of Buckwheat By-Product as a Health-Promoting Ingredient Rich in Fibre for the Formulation of Gluten-Free Bread. Foods 2023, 12, 2781. [Google Scholar] [CrossRef] [PubMed]
- Sakač, M.; Torbica, A.; Sedej, I.; Hadnađev, M. Influence of breadmaking on antioxidant capacity of gluten free breads based on rice and buckwheat flours. Food Res. Int. 2011, 44, 2806–2813. [Google Scholar] [CrossRef]
- Różyło, R.; Dziki, D.; Gawlik-Dziki, U.; Biernacka, B.; Wójcik, M.; Ziemichód, A. Physical and antioxidant properties of gluten-free bread enriched with carob fibre. Int. Agrophys. 2017, 31, 411–418. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Bączek, N.; Šimková, K.; Starowicz, M.; Jeliński, T. Application of Broccoli Leaf Powder in Gluten-Free Bread: An Innovative Approach to Improve Its Bioactive Potential and Technological Quality. Foods 2021, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Bourekoua, H.; Różyło, R.; Gawlik-Dziki, U.; Benatallah, L.; Zidoune, M.N.; Dziki, D. Evaluation of physical, sensorial, and antioxidant properties of gluten-free bread enriched with Moringa oleifera leaf powder. Eur. Food Res. Technol. 2018, 244, 189–195. [Google Scholar] [CrossRef]
- Hryhorenko, N.; Krupa-Kozak, U.; Bączek, N.; Rudnicka, B.; Wróblewska, B. Gluten-free bread enriched with whole-grain red sorghum flour gains favourable technological and functional properties and consumers acceptance. J. Cereal Sci. 2023, 110, 103646. [Google Scholar] [CrossRef]
- Gumul, D.; Korus, J.; Surma, M.; Ziobro, R. Pulp obtained after isolation of starch from red and purple potatoes (Solanum tuberosum L.) as an innovative ingredient in the production of gluten-free bread. PLoS ONE 2020, 15, e0229841. [Google Scholar] [CrossRef]
- Matas, A.; Igual, M.; García-Segovia, P.; Martínez-Monzó, J. Application of 3D Printing in the Design of Functional Gluten-Free Dough. Foods 2022, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Gumul, D.; Ziobro, R.; Ivanišová, E.; Korus, A.; Árvay, J.; Tóth, T. Gluten-free bread with an addition of freeze-dried red and purple potatoes as a source of phenolic compounds in gluten-free diet. Int. J. Food Sci. Nutr. 2017, 68, 43–51. [Google Scholar] [CrossRef]
- Olojede, A.O.; Sanni, A.I.; Banwo, K.; Adesulu-Dahunsi, A.T. Sensory and antioxidant properties and in-vitro digestibility of gluten-free sourdough made with selected starter cultures. LWT Food Sci. Technol. 2020, 129, 109576. [Google Scholar] [CrossRef]
- Banu, I.; Aprodu, I. Assessing the Performance of Different Grains in Gluten-Free Bread Applications. Appl. Sci. 2020, 10, 8772. [Google Scholar] [CrossRef]
- Yeşil, S.; Levent, H. The influence of fermented buckwheat, quinoa and amaranth flour on gluten-free bread quality. LWT Food Sci. Technol. 2022, 160, 113301. [Google Scholar] [CrossRef]
- Ziobro, R.; Gumul, D.; Korus, J.; Korus, A. Starch bread with a share of non-wheat flours as a source of bioactive compounds in gluten-free diet. J. Food Nutr. Res. 2016, 55, 11–21. [Google Scholar]
- Mildner-Szkudlarz, S.; Barbara Różańska, M.; Siger, A.; Zembrzuska, J.; Szwengiel, A. Formation of Maillard reaction products in a model bread system of different gluten-free flours. Food Chem. 2023, 429, 136994. [Google Scholar] [CrossRef]
- Dos Reis Gallo, L.R.; Reis, C.E.G.; Mendonça, M.A.; Da Silva, V.S.N.; Pacheco, M.T.B.; Botelho, R.B.A. Impact of Gluten-Free Sorghum Bread Genotypes on Glycemic and Antioxidant Responses in Healthy Adults. Foods 2021, 10, 2256. [Google Scholar] [CrossRef]
- Pessanha, K.L.F.; Menezes, J.P.D.; Silva, A.D.A.; Ferreira, M.V.D.S.; Takeiti, C.Y.; Carvalho, C.W.P. Impact of whole millet extruded flour on the physicochemical properties and antihyperglycemic activity of gluten free bread. LWT Food Sci. Technol. 2021, 147, 111495. [Google Scholar] [CrossRef]
- Coronel, E.B.; Guiotto, E.N.; Aspiroz, M.C.; Tomás, M.C.; Nolasco, S.M.; Capitani, M.I. Development of gluten-free premixes with buckwheat and chia flours: Application in a bread product. LWT Food Sci. Technol. 2021, 141, 110916. [Google Scholar] [CrossRef]
- Zdybel, B.; Różyło, R.; Sagan, A. Use of a waste product from the pressing of chia seed oil in wheat and gluten-free bread processing. J. Food Process. Preserv. 2019, 43, e14002. [Google Scholar] [CrossRef]
- Alshehry, G.; Algarni, E.; Aljumayi, H.; Algheshairy, R.M.; Alharbi, H.F. Development and Characterization of Multigrain Pan Bread Prepared Using Quinoa, Lupin, and Fenugreek Seeds with Yellow Maize as a Gluten-Free Diet. J. Food Qual. 2022, 2022, 4331353. [Google Scholar] [CrossRef]
- Drakula, S.; Novotni, D.; Čukelj Mustač, N.; Voučko, B.; Krpan, M.; Hruškar, M.; Ćurić, D. Alteration of phenolics and antioxidant capacity of gluten-free bread by yellow pea flour addition and sourdough fermentation. Food Biosci. 2021, 44, 101424. [Google Scholar] [CrossRef]
- Bourekoua, H.; Gawlik-Dziki, U.; Różyło, R.; Zidoune, M.N.; Dziki, D. Acerola fruit as a natural antioxidant ingredient for gluten-free bread: An approach to improve bread quality. Food Sci. Technol. Int. 2021, 27, 13–21. [Google Scholar] [CrossRef]
- Gumul, D.; Ziobro, R.; Korus, J.; Kruczek, M. Apple Pomace as a Source of Bioactive Polyphenol Compounds in Gluten-Free Breads. Antioxidants 2021, 10, 807. [Google Scholar] [CrossRef]
- Korus, J.; Juszczak, L.; Ziobro, R.; Witczak, M.; Grzelak, K.; Sójka, M. Defatted strawberry and blackcurrant seeds as functional ingredients of gluten-free bread: Defatted strawberry and blackcurrant seeds in gluten free bread. J. Texture Stud. 2012, 43, 29–39. [Google Scholar] [CrossRef]
- Gumul, D.; Korus, A.; Ziobro, R. Extruded Preparations with Sour Cherry Pomace Influence Quality and Increase the Level of Bioactive Components in Gluten-Free Breads. Int. J. Food Sci. 2020, 2020, 8024398. [Google Scholar] [CrossRef] [PubMed]
- Kapcsándi, V.; Hanczné Lakatos, E.; Sik, B.; Linka, L.Á.; Székelyhidi, R. Antioxidant and polyphenol content of different Vitis vinifera seed cultivars and two facilities of production of a functional bakery product. Chem. Pap. 2021, 75, 5711–5717. [Google Scholar] [CrossRef] [PubMed]
- Bourekoua, H.; Różyło, R.; Gawlik-Dziki, U.; Benatallah, L.; Zidoune, M.N.; Dziki, D. Pomegranate seed powder as a functional component of gluten-free bread (Physical, sensorial and antioxidant evaluation). Int. J. Food Sci. Technol. 2018, 53, 1906–1913. [Google Scholar] [CrossRef]
- Pešić, M.B.; Pešić, M.M.; Bezbradica, J.; Stanojević, A.B.; Ivković, P.; Milinčić, D.D.; Demin, M.; Kostić, A.Ž.; Dojčinović, B.; Stanojević, S.P. Okara-Enriched Gluten-Free Bread: Nutritional, Antioxidant and Sensory Properties. Molecules 2023, 28, 4098. [Google Scholar] [CrossRef] [PubMed]
- Beltrão Martins, R.; Gouvinhas, I.; Nunes, M.C.; Alcides Peres, J.; Raymundo, A.; Barros, A.I.R.N.A. Acorn Flour as a Source of Bioactive Compounds in Gluten-Free Bread. Molecules 2020, 25, 3568. [Google Scholar] [CrossRef] [PubMed]
- Paciulli, M.; Rinaldi, M.; Cirlini, M.; Scazzina, F.; Chiavaro, E. Chestnut flour addition in commercial gluten-free bread: A shelf-life study. LWT Food Sci. Technol. 2016, 70, 88–95. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Abduvakhidov, A.; Caputo, P.; Crupi, P.; Muraglia, M.; Oliviero Rossi, C.; Clodoveo, M.L.; Aiello, F.; Restuccia, D. Kefir Enriched with Carob (Ceratonia siliqua L.) Leaves Extract as a New Ingredient during a Gluten-Free Bread-Making Process. Fermentation 2022, 8, 305. [Google Scholar] [CrossRef]
- Nunes, M.C.; Fernandes, I.; Vasco, I.; Sousa, I.; Raymundo, A. Tetraselmis chuii as a Sustainable and Healthy Ingredient to Produce Gluten-Free Bread: Impact on Structure, Colour and Bioactivity. Foods 2020, 9, 579. [Google Scholar] [CrossRef]
- Qazi, M.W.; De Sousa, I.G.; Nunes, M.C.; Raymundo, A. Improving the Nutritional, Structural, and Sensory Properties of Gluten-Free Bread with Different Species of Microalgae. Foods 2022, 11, 397. [Google Scholar] [CrossRef]
- Różyło, R.; Hameed Hassoon, W.; Gawlik-Dziki, U.; Siastała, M.; Dziki, D. Study on the physical and antioxidant properties of gluten-free bread with brown algae. CyTA J. Food 2017, 15, 196–203. [Google Scholar] [CrossRef]
- Guglielmetti, A.; Fernandez-Gomez, B.; Zeppa, G.; Del Castillo, M.D. Nutritional Quality, Potential Health Promoting Properties and Sensory Perception of an Improved Gluten-Free Bread Formulation Containing Inulin, Rice Protein and Bioactive Compounds Extracted from Coffee Byproducts. Pol. J. Food Nutr. Sci. 2019, 69, 157–166. [Google Scholar] [CrossRef]
- Littardi, P.; Rinaldi, M.; Grimaldi, M.; Cavazza, A.; Chiavaro, E. Effect of Addition of Green Coffee Parchment on Structural, Qualitative and Chemical Properties of Gluten-Free Bread. Foods 2020, 10, 5. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Methods for the assessment of antioxidant activity in foods. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 287–333. [Google Scholar]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT Food Sci. Technol. 2021, 150, 111932. [Google Scholar] [CrossRef]
- Gökmen, V.; Serpen, A.; Fogliano, V. Direct measurement of the total antioxidant capacity of foods: The ‘QUENCHER’ approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J. Dietary fiber: Still alive. Food Chem. 2024, 439, 1380762024. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Larrauri, J.A. New approaches in the preparation of high dietary fibre powders from fruits by-products. Trends Food Sci. Technol. 1999, 10, 3–8. [Google Scholar] [CrossRef]
- Taylor, J.R.N. Millets: Their Unique Nutritional and Health-Promoting Attributes. In Gluten-Free Ancient Grains Cereals, Pseudocereals, and Legumes: Sustainable, Nutritious, and Health-Promoting Foods for the 21st Century; Taylor, J.R.N., Awika, J.M., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 55–103. [Google Scholar]
- Rosell, C.M.; Barro, F.; Sousa, C.; Mena, M.C. Cereals for developing gluten-free products and analytical tools for gluten detection. J. Cereal Sci. 2014, 59, 354–364. [Google Scholar] [CrossRef]
- Rocchetti, G.; Lucini, L.; Rodriguez, J.M.L.; Barba, F.J.; Giuberti, G. Gluten-free flours from cereals, pseudocereals and legumes: Phenolic fingerprints and in vitro antioxidant properties. Food Chem. 2019, 271, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Culetu, A.; Susman, I.E.; Duta, D.E.; Belc, N. Nutritional and Functional Properties of Gluten-Free Flours. Appl. Sci. 2021, 11, 6283. [Google Scholar] [CrossRef]
- Kim, G.R.; Jung, E.S.; Lee, S.; Lim, S.-H.; Ha, S.H.; Lee, C.H. Combined mass spectrometry-based metabolite profiling of different pigmented rice (Oryza sativa L.) seeds and correlation with antioxidant activities. Molecules 2014, 19, 15673–15686. [Google Scholar] [CrossRef] [PubMed]
- Anggraini, T.; Novelina, N.; Limber, U.; Amelia, R. Antioxidant activities of some red, black and white rice cultivar from West Sumatra, Indonesia. Pak. J. Nutr. 2015, 14, 112–117. [Google Scholar] [CrossRef][Green Version]
- Gunaratne, A.; Wu, K.; Li, D.; Bentota, A.; Corke, H.; Cai, Y.-Z. Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem. 2013, 138, 1153–1161. [Google Scholar] [CrossRef]
- Rocchetti, G.; Giuberti, G.; Lucini, L. Gluten-free cereal-based food products: The potential of metabolomics to investigate changes in phenolics profile and their in vitro bioaccessibility. Curr. Opin. Food Sci. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Conte, P.; Fadda, C.; Drabińska, N.; Krupa-Kozak, U. Technological and Nutritional Challenges, and Novelty in Gluten-Free Breadmaking: A Review. Pol. J. Food Nutr. Sci. 2019, 69, 5–21. [Google Scholar] [CrossRef]
- Djordjević, M.; Šoronja-Simović, D.; Nikolić, I.; Djordjević, M.; Šereš, Z.; Milašinović-Šeremešić, M. Sugar beet and apple fibres coupled with hydroxypropylmethylcellulose as functional ingredients in gluten-free formulations: Rheological, technological and sensory aspects. Food Chem. 2019, 295, 189–197. [Google Scholar] [CrossRef] [PubMed]
- de la Hera, E.; Talegón, M.; Caballero, P.; Gómez, M. Influence of maize flour particle size on gluten-free breadmaking. J. Sci. Food Agric. 2013, 93, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Žilić, S.; Serpen, A.; Akıllıoğlu, G.; Gökmen, V.; Vančetović, J. Phenolic Compounds, Carotenoids, Anthocyanins, and Antioxidant Capacity of Colored Maize (Zea mays L.) Kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Yang, K.; Zhang, Y.; Li, Z. The effects of temperature on the germination behavior of white, yellow, red and purple maize plant seeds. Acta Physiol. Plant. 2015, 37, 174. [Google Scholar] [CrossRef]
- Rodríguez, V.M.; Soengas, P.; Landa, A.; Ordás, A.; Revilla, P. Effects of selection for color intensity on antioxidant capacity in maize (Zea mays L.). Euphytica 2013, 193, 339–345. [Google Scholar] [CrossRef][Green Version]
- Urias-Lugo, D.A.; Heredia, J.B.; Serna-Saldívar, S.O.; Muy-Rangel, M.D.; Valdez-Torres, J.B. Total phenolics, total anthocyanins and antioxidant capacity of native and elite blue maize hybrids (Zea mays L.). CyTA J. Food 2015, 13, 336–339. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W. Sorghum and millet phenols and antioxidants. J. Cereal Sci. 2006, 44, 236–251. [Google Scholar] [CrossRef]
- Shahidi, F.; Chandrasekara, A. Millet grain phenolics and their role in disease risk reduction and health promotion: A review. J. Funct. Food. 2013, 5, 570–581. [Google Scholar] [CrossRef]
- Ragaee, S.; Abdelaal, E.; Noaman, M. Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006, 98, 32–38. [Google Scholar] [CrossRef]
- Tanwar, R.; Panghal, A.; Chaudhary, G.; Kumari, A.; Chhikara, N. Nutritional, Phytochemical and Functional Potential of Sorghum: A Review. Food Chem. Adv. 2023, 3, 100501. [Google Scholar] [CrossRef]
- Awika, J.M. Sorghum: Its Unique Nutritional and Health-Promoting Attributes. In Gluten-Free Ancient Grains Cereals, Pseudocereals, and Legumes: Sustainable, Nutritious, and Health-Promoting Foods for the 21st Century; Taylor, J.R.N., Awika, J.M., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 21–54. [Google Scholar]
- Moraes, É.A.; Marineli, R.D.S.; Lenquiste, S.A.; Steel, C.J.; de Menezes, C.B.; Queiroz, V.A.V.; Júnior, M.R.M. Sorghum flour fractions: Correlations among polysaccharides, phenolic compounds, antioxidant activity and glycemic index. Food Chem. 2015, 180, 116–123. [Google Scholar] [CrossRef]
- Vivar-Quintana, A.M.; Absi, Y.; Hernández-Jiménez, M.; Revilla, I. Nutritional Value, Mineral Composition, Fatty Acid Profile and Bioactive Compounds of Commercial Plant-Based Gluten-Free Flours. Appl. Sci. 2023, 13, 2309. [Google Scholar] [CrossRef]
- Chlopicka, J.; Pasko, P.; Gorinstein, S.; Jedryas, A.; Zagrodzki, P. Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT Food Sci. Technol. 2012, 46, 548–555. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, L.; Zhang, X.; Wang, M.; Liu, H.; Zhu, Y. Nutritional components, volatile constituents and antioxidant activities of 6 chickpea species. Food Biosci. 2021, 41, 100964. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Favero, B.T.; Morzelle, M.C.; Franchin, M.; Alvarez-Parrilla, E.; de la Rosa, L.A.; Geraldi, M.V.; Maróstica Júnior, M.R.; Shahidi, F.; Schwember, A.R. Is Chickpea a Potential Substitute for Soybean? Phenolic Bioactives and Potential Health Benefits. Int. J. Mol. Sci. 2019, 20, 2644. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-L.; Zhang, H.-S.; Zhao, X.-S.; Xue, H.-H.; Xue, J.; Sun, Y.-H. Composition, Distribution, and Antioxidant Activity of Phenolic Compounds in 18 Soybean Cultivars. J. AOAC Int. 2018, 101, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Cebadera-Miranda, L.; Cámara, R.M.; Reis, F.S.; Barros, L.; Berrios, J.D.J.; Ferreira, I.C.F.R.; Cámara, M. Lentil flour formulations to develop new snack-type products by extrusion processing: Phytochemicals and antioxidant capacity. J. Funct. Foods 2015, 19, 537–544. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef]
- Tömösközi, S.; Langó, B. Buckwheat: Its Unique Nutritional and Health-Promoting Attributes. In Gluten-Free Ancient Grains Cereals, Pseudocereals, and Legumes: Sustainable, Nutritious, and Health-Promoting Foods for the 21st Century; Taylor, J.R.N., Awika, J.M., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 161–177. [Google Scholar]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Li, S.Q.; Zhang, Q.H. Advances in the development of functional food from buckwheat. Crit. Rev. Food Sci. Nutr. 2001, 41, 451–464. [Google Scholar] [CrossRef]
- Boots, A.W.; Drent, M.; De Boer, V.C.; Bast, A.; Haenen, G.R.M.M. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin. Nutr. 2011, 30, 506–512. [Google Scholar] [CrossRef]
- Vogrinčič, M.; Timoracka, M.; Melichacova, S.; Vollmannova, A.; Kreft, I. Degradation of rutin and polyphenols during preparation of tartary buckwheat bread. J. Agric. Food Chem. 2010, 58, 4883–4887. [Google Scholar] [CrossRef]
- Henrion, M.; Labat, E.; Lamothe, L. Pseudocereals as Healthy Grains: An Overview. In Innovative Processing Technologies for Healthy Grains, 1st ed.; Pojić, M., Tiwari, U., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 37–60. [Google Scholar]
- Xu, X.; Luo, Z.; Yang, Q.; Xiao, Z.; Lu, X. Effect of quinoa flour on baking performance, antioxidant properties and digestibility of wheat bread. Food Chem. 2019, 294, 87–95. [Google Scholar] [CrossRef]
- Escribano, J.; Cabanes, J.; Jiménez-Atiénzar, M.; Ibañez-Tremolada, M.; Gómez-Pando, L.R.; García-Carmona, F.; Gandía-Herrero, F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Schoenlechner, R. Quinoa: Its Unique Nutritional and Health-Promoting Attributes. In Gluten-Free Ancient Grains Cereals, Pseudocereals, and Legumes: Sustainable, Nutritious, and Health-Promoting Foods for the 21st Century; Taylor, J.R.N., Awika, J.M., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 105–129. [Google Scholar]
- Mota, C.; Santos, M.; Mauro, R.; Samman, N.; Matos, A.S.; Torres, D.; Castanheira, I. Protein content and amino acids profile of pseudocereals. Food Chem. 2016, 193, 55–61. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, S.; Schoenlechner, R. Amaranth: Its Unique Nutritional and Health-Promoting Attributes. In Gluten-Free Ancient Grains Cereals, Pseudocereals, and Legumes: Sustainable, Nutritious, and Health-Promoting Foods for the 21st Century; Taylor, J.R.N., Awika, J.M., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 131–159. [Google Scholar]
- Venskutonis, P.R.; Kraujalis, P. Nutritional components of amaranth seeds and vegetables: A review on composition, properties, and uses. Compr. Rev. Food Sci. Food Saf. 2013, 12, 381–412. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Czaplicki, S.; Zadernowski, R.; Mattila, P.; Hellström, J.; Naczk, M. Phenolic acids in seeds and products obtained from Amaranthus cruentus. J. Food Nutr. Res. 2012, 51, 96–101. [Google Scholar]
- Steffensen, S.K.; Rinnan, A.; Mortensen, A.G.; Laursen, B.; de Troiani, R.M.; Noellemeyer, E.J.; Janovska, D.; Dusek, K.; Délano-Frier, J.; Taberner, A.; et al. Variations in the polyphenol content of seeds of field grown Amaranthus genotypes. Food Chem. 2011, 129, 131–138. [Google Scholar] [CrossRef]
- Paśko, P.; Sajewicz, M.; Gorinstein, S.; Zachwieja, Z. Analysis of selected phenolic acids and flavonoids in Amaranthus cruentus and Chenopodium quinoa seeds and sprouts by HPLC. Acta Chromatogr. 2008, 20, 661–672. [Google Scholar] [CrossRef]
- Vollmanová, A.; Margitanová, E.; Tóth, T.; Timoracká, M.; Urminská, D.; Bojňanská, T.; Čičová, I. Cultivar influence on total polyphenol and rutin contents and total antioxidant capacity in buckwheat, amaranth, and quinoa seeds. Czech J. Food Sci. 2013, 31, 589–595. [Google Scholar] [CrossRef]
- EU (European Union). Commission implementing decision of January 22, 2013. C 2013–123. In Official Journal of EU; European Union: Brussels, Belgium, 2013; pp. L21–L34. [Google Scholar]
- Sandoval-Oliveros, M.R.; Peredes-Lopez, O. Isolation and characterization of proteins from chia seeds (Salvia hispanica L.). J. Agric. Food Chem. 2013, 61, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cruz, O.; Paredes-López, O. Phytochemical profile and nutraceutical potential of chia seeds (Salvia hispanica L.) by ultra high performance liquid chromatography. J. Chromatogr. A 2014, 1346, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Caudillo, E.; Tecante, A.; Valdivia-López, M.A. Dietary fiber and antioxidant activity of phenolic compounds present in Mexican Chia (Salvia hispanica L.) seeds. Food Chem. 2008, 107, 656–663. [Google Scholar] [CrossRef]
- USDA. USDA Food Composition Database. 2016. Available online: https://ndb.nal.usda.gov/ndb/ (accessed on 28 December 2017).
- Mekky, R.H.; Contreras, M.M.; El-Gindi, M.R.; Abdel-Monem, A.R.; Adbel-Sattar, E.; Segura-Carretero, A. Profiling of phenolic and other compounds from Egyptian cultivars of chickpea (Cicer arietinum L.) and antioxidant activity: A comparative study. RSC Adv. 2015, 5, 17751–17767. [Google Scholar] [CrossRef]
- Alajaji, S.A.; El-Adawy, T.A. Nutritional composition of chickpea (Cicerarietinum L.) as affected by microwave cooking and other traditional cooking methods. J. Food Compos. Anal. 2006, 19, 806–812. [Google Scholar] [CrossRef]
- Mohammadi, K. Nutritional composition of Iranian desi and kabuli chickpea (Cicerarietinum L.) cultivars in autumn sowing. Int. J. Agric. Biol. Eng. 2015, 9, 514–517. [Google Scholar]
- Sreerama, Y.N.; Sashikala, V.B.; Pratape, V.M. Variability in the Distribution of Phenolic Compounds in Milled Fractions of Chickpea and Horse Gram: Evaluation of Their Antioxidant Properties. J. Agric. Food Chem. 2010, 58, 8322–8330. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.R.; Morgan, T.; Terry, J.G. A randomized trial comparing the effect of casein with that of soya protein containing varying amounts of isoflavones on plasma concentrations of lipids and lipoproteins. Arch. Intern. Med. 1999, 159, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Król-Grzymała, A.; Amarowicz, R. Phenolic Compounds of Soybean Seeds from Two European Countries and Their Antioxidant Properties. Molecules 2020, 25, 2075. [Google Scholar] [CrossRef] [PubMed]
- Seo, A.; Morr, C.V. Improved high performance liquid chromatographic analysis of phenolic acids and isoflavonoids from soybean protein products. J. Agric. Food Chem. 1984, 32, 530–533. [Google Scholar] [CrossRef]
- Wu, Q.; Li, S.; Li, X.; Fu, X.; Sui, Y.; Guo, T.; Xie, B.; Sun, Z. A significant inhibitory effect on advanced glycation end product formation by catechin as the major metabolite of Lotus Seedpod oligomeric procyanidins. Nutrients 2014, 6, 3230–3244. [Google Scholar] [CrossRef]
- Zielinski, H.; Michalska, A.; Amigo-Benavent, M.; del Castillo, M.; Dolores Piskula, M.K. Changes in protein quality and antioxidant properties of buckwheat seeds and groats induced by roasting. J. Agric. Food Chem. 2009, 57, 4771–4776. [Google Scholar] [CrossRef]
- Rupasinghe, H.; Wang, L.; Huber, G.; Pitts, N. Effect of Baking on Dietary Fibre and Phenolics of Muffins Incorporated with Apple Skin Powder. Food Chem. 2008, 107, 1217–1224. [Google Scholar] [CrossRef]
- Sensoy, I.; Rosen, R.T.; Ho, C.; Karwe, M.V. Effect of processing on buckwheat phenolics and antioxidant activity. Food Chem. 2006, 99, 388–393. [Google Scholar] [CrossRef]
- Mesías, M.; Delgado-Andrade, C. Melanoidins as a potential functional food ingredient. Curr. Opin. Food Sci. 2017, 14, 37–42. [Google Scholar] [CrossRef]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef]
- Djordjević, M.; Djordjević, M.; Maravić, N.; Teofilović, V.; Šoronja-Simović, D.; Šereš, Z. Processing of alfalfa seeds by convective hot air drying, vacuum drying and germination: Proximate composition, techno-functional, thermal and structural properties evaluation. Food Chem. 2023, 402, 134300. [Google Scholar] [CrossRef]
- Lancetti, R.; Salvucci, E.; Moiraghi, M.; Pérez, G.T.; Sciarini, L.S. Gluten-free flour fermented with autochthonous starters for sourdough production: Effect of the fermentation process. Food Biosci. 2022, 47, 101723. [Google Scholar] [CrossRef]
- Păcularu-Burada, B.; Turturică, M.; Rocha, J.M.; Bahrim, G.-E. Statistical Approach to Potentially Enhance the Postbiotication of Gluten-Free Sourdough. Appl. Sci. 2021, 11, 5306. [Google Scholar] [CrossRef]
- Păcularu-Burada, B.; Ceoromila (Cantaragiu), A.-M.; Vasile, M.A.; Bahrim, G.-E. Novel insights into different kefir grains usefulness as valuable multiple starter cultures to achieve bioactive gluten-free sourdoughs. LWT Food Sci. Technol. 2022, 165, 113670. [Google Scholar] [CrossRef]
- Radoš, K.; Benković, M.; Čukelj Mustač, N.; Habuš, M.; Voučko, B.; Vukušić Pavičić, T.; Ćurić, D.; Ježek, D.; Novotni, D. Powder properties, rheology and 3D printing quality of gluten-free blends. J. Food Eng. 2023, 338, 111251. [Google Scholar] [CrossRef]
- Souza Guedes, J.; Sousa Bitencourt, B.; Esteves Duarte Augusto, P. Modification of maize starch by dry heating treatment (DHT) and its use as gelling ingredients in fruit-based 3D-printed food for dysphagic people. Food Biosci. 2023, 56, 103310. [Google Scholar] [CrossRef]
- Ricci, I.; Derossi, A.; Severini, C. 3D printed food from fruits and vegetables. In Fundamentals of 3D Printing and Applications; Godoi, F.C., Bhandari, B.R., Prakash, S., Zhang, M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 117–149. [Google Scholar]
- Lisovska, T.; Harasym, J. 3D Printing Progress in Gluten-Free Food-Clustering Analysis of Advantages and Obstacles. Appl. Sci. 2023, 13, 12362. [Google Scholar] [CrossRef]
- Matos, M.E.; Rosell, C.M. Understanding gluten-free dough for reaching breads with physical quality and nutritional balance. J. Sci. Food Agric. 2015, 95, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Kittisuban, P.; Ritthiruangdej, P.; Suphantharik, M. Optimization of hydroxypropylmethylcellulose, yeast b-glucan, and whey protein levels based on physical properties of gluten-free rice bread using response surface methodology. LWT Food Sci. Technol. 2014, 57, 738–748. [Google Scholar] [CrossRef]
- Aoki, N.; Kataoka, T.; Nishiba, Y. Crucial role of amylose in the rising of gluten- and additive-free rice bread. J. Cereal Sci. 2020, 92, 102905. [Google Scholar] [CrossRef]
- Djordjević, M.; Djordjević, M.; Šoronja-Simović, D.; Nikolić, I.; Šereš, Z. Delving into the Role of Dietary Fiber in Gluten-Free Bread Formulations: Integrating Fundamental Rheological, Technological, Sensory and Nutritional Aspects. Polysaccharides 2022, 3, 59–82. [Google Scholar] [CrossRef]
- Ahlborn, G.J.; Pike, O.A.; Hendrix, S.B.; Hess, W.M.; Huber, C.S. Sensory, mechanical, and microscopic evaluation of staling in low-protein and gluten- free breads. Cereal Chem. 2005, 82, 328–335. [Google Scholar] [CrossRef]
- Caruso, M.C.; Galgano, F.; Colangelo, M.A.; Condelli, N.; Scarpa, T.; Tolve, R.; Favati, F. Evaluation of the oxidative stability of bakery products by OXITEST method and sensory analysis. Eur. Food Res. Technol. 2017, 243, 1183–1191. [Google Scholar] [CrossRef]
- Basso, F.M.; Mangolim, C.S.; Aguiar, M.F.A.; Monteiro, A.R.G.; Peralta, R.M.; Matioli, G. Potential use of cyclodextrin-glycosyltransferase enzyme in bread-making and the development of gluten-free breads with pinion and corn flours. Int. J Food Sci. Nutr. 2015, 66, 275–281. [Google Scholar] [CrossRef]
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.B.; Marques, M.C.; Hacke, A.; Loubet Filho, P.S.; Cazarin, C.B.B.; Mariutti, L.R.B. Trust your gut: Bioavailability and bioaccessibility of dietary compounds. Curr. Res. Food Sci. 2022, 5, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Sẃieca, M.; Dziki, D.; Baraniak, B.; Tomiło, J.; Czyż, J. Quality and antioxidant properties of breads enriched with dry onion (Allium cepa L.) skin. Food Chem. 2013, 138, 1621–1628. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Oracz, J. Bioavailability and Bioactivity of Plant Antioxidants. Antioxidants 2022, 11, 2336. [Google Scholar] [CrossRef]
- Pavlatou, M.; Papastamataki, M.; Apostolakou, F.; Papassotiriou, I.; Tentolouris, N. FORT and FORD: Two simple and rapid assays in the evaluation of oxidative stress in patients with type 2 diabetes mellitus. Metabolism 2009, 58, 1657–1662. [Google Scholar] [CrossRef]
| Basic Flour | Antioxidant Source | Addition Level (%) | Quantification Methods | Profiling | Reference |
|---|---|---|---|---|---|
| Gluten-free cereals, pseudocereals and legumes | |||||
| Rice flour, potato starch | Amaranth, quinoa, buckwheat, sprouted buckwheat | 50 100 | TPC, DPPH, FRAP | Simple polyphenols Flavonoids HPLC–DAD | [39] |
| / | Soaked and germinated brown rice flour | 100 | TPC, ORAC | / | [44] |
| / | Quinoa flour, wholegrain sorghum flour, brown millet flour, wholegrain rice flour | 100 | TPC, DPPH, ABTS, FRAP | / | [55] |
| Rice flour | Buckwheat hulls | 3.61, 3.83 7.23, 7.66 | TPC, DPPH, ABTS, ORAC, FRAP, QUENCHER | / | [45] |
| Refined buckwheat flour | Wholegrain buckwheat flour | 30, 45 | TPC, ORAC | Rutin and quercetin HPLC–DAD | [43] |
| Rice flour | Buckwheat flour (light and wholegrain) | 10, 20, 30 | TPC, antioxidant activity with β carotene, RED, DPPH, CHEL | Rutin and quercetin HPLC–DAD | [46] |
| Maize starch | Dehulled buckwheat flour | 10, 20, 30, 40 | TPC, DPPH, ABTS, reducing capacity by voltammetry method | / | [42] |
| Rice flour and maize starch | Buckwheat, quinoa and amaranth flour | 15, 30, 45 | TPC, DPPH | / | [56] |
| Maize starch and potato starch | Maize flour, buckwheat flour and amaranth flour | 10 | TPC, TFC, ABTS, phenolic acids, flavonols, anthocyanins, tannins | / | [57] |
| / | Rice flour (white, brown, black, red and wild) Corn flour (purple and yellow) | 100 | / | Anthocyanin Flavonoids Phenolic acids RP-UHPLC-ESI-MS | [58] |
| Maize starch and potato starch | Wholegrain red sorghum flour | 10, 20, 30, 40 | TPC DPPH, ABTS, FRAP, CUPRAC | / | [50] |
| Sorghum flour and maize starch | Germinated white sorghum flour | 20 | TPC, TFC, DPPH tannins | / | [54] |
| Potato starch and cassava flour | Sorghum (white, brown and bronze) | 22.26 | ORAC | / | [59] |
| Rice flour and cassava flour | White sorghum flour | 85 | TPC, ABTS, FRAP | Phenolic acids Flavonoids HPLC-DAD-ESI-MS/MS | [36] |
| / | Wholegrain millet and wholegrain millet extruded flour | 100, 50 | TPC, DPPH, FRAP | / | [60] |
| Buckwheat flour (light and wholegrain) | Chia flour | 9.8, 10 | DPPH | / | [61] |
| Buckwheat flour (common and Tartary) | Chia flour | 10 | TPC, TFC, FRAP, ORAC | / | [37] |
| Maize and rice flour | Chia and chia seed residues | 5 | TPC, DPPH | / | [62] |
| Maize and quinoa flour | Germinated sweet lupin and fenugreek mixtures | 5, 10, 15, 20 | DPPH, ABTS, FRAP TFC, total phenolic acids | / | [63] |
| Maize flour | Extruded lentil flour | 15 | TPC, DPPH, ORAC, FRAP, ABTS, QUENCHER | / | [41] |
| Wholegrain rice, wholegrain millet flour, corn starch, corn extrudate | Yellow pea flour | 25 | TPC, DPPH, FRAP | Phenolic acids HPLC–DAD | [64] |
| Fruit and fruit by-products | |||||
| White rice | Acerola fruit powder | 1, 2, 3, 4, 5 | TPC, DPPH, ABTS, RED | / | [65] |
| Maize starch and potato starch | Apple pomace | 5, 10, 15 | TPC, TFC ABTS | Flavonols Phenolic acids Flavon-3-ols Dihydrochalcones UPLC-PDA-MS/MS | [66] |
| Maize starch and potato starch | Defatted blackcurrant and strawberry seeds | 5, 10, 15 | TPC, ABTS | / | [67] |
| Rice flour, maize starch and potato starch | Extruded sour cherry pomace and rice flour | 10 | TPC, TFC, ABTS, phenolic acids, anthocyanins | / | [68] |
| Gluten-free flour mixture | Grape seed flour | 3, 6, 9 | TPC, FRAP | / | [69] |
| Rice and field bean semolina | Pomegranate seed powder | 2.5, 5, 7.5, 10 | TPC, DPPH, ABTS, RED, •OH | / | [70] |
| Gluten-free flour | Rosehip powder, rosehip encapsulate | 7 | TPC, DPPH, total carotenoids | / | [52] |
| Vegetables and vegetable by-products | |||||
| Maize starch and potato starch | Freeze-dried red and purple potatoes | 5 | TPC, TFC, DPPH, ABTS, FOMO flavonols, phenolic acid, anthocyanins, carotenoids | Chlorogenic acid Neo-chlorogenic acid Rutin Quercetin HPLC | [53] |
| Maize starch and potato starch | Red and purple potato pulp | 5, 7.5, 10 | TPC, TFC, flavonols, anthocyanins ABTS | / | [51] |
| Maize starch and potato starch | Broccoli leaf powder | 5 | TPC ABTS, DPPH, PCL | / | [48] |
| Unhusked white buckwheat flour, corn flour, rice flour, linseed flour | Fried red onion, dried red onion and red onion peel | 5 | TPC, DPPH, FRAP FORD, FORT (in vivo) | Quercetin Quercetin-3,4′-O-diglucoside Quercetin-4′-O-glucoside Rutin HPLC-MS/MS | [35] |
| Buckwheat flour, rice flour and millet flour | Okara | 10, 20, 30 | TPC, DPPH, FRAP, ABTS | / | [71] |
| Herbs | |||||
| Rice flour | Hemp inflorescence | 1, 2, 3, 4, 5 | TPC, TFC, DPPH, FRAP | / | [38] |
| Tree fruits and leaves | |||||
| Rice flour | Acorn flour | 23, 35 | TPC, DPPH, FRAP, ABTS | / | [40] |
| Buckwheat flour, rice flour and potato starch | Acorn flour | 23, 35 | TPC, TFC, ODC, DPPH, FRAP, ABTS | Phenolic acids Flavonoids RP-HPLC-DAD | [72] |
| Rice flour and maize starch | Acorn and chickpea flour mixtures | 30 | TPC, TFC, DPPH, ABTS, FRAP | Phenolic acids HPLC-DAD Tocopherols and carotenoids HPLC-FLD | [33] |
| Maize starch and potato starch | Chestnut flour | 10, 20 | DPPH | / | [73] |
| White rice, maize, and buckwheat flour | Carob fiber (commercial) | 1, 2, 3, 4, 5 | TPC, ABTS, CHEL, FRAP | / | [47] |
| Rice flour | Kefir beverage with carob leaves | 155 mL water replaced with 150 mL kefir | DPPH, ABTS | / | [74] |
| Rice and field bean semolina | Moringa oleifera leaves powder | 2.5, 5, 7.5, 10 | TPC, DPPH, ABTS, RED, •OH | / | [49] |
| Microalgae and algae | |||||
| Buckwheat flour, rice flour and potato starch | Microalgae Tetraselmis chuii | 1, 2, 4 | TPC, DPPH, FRAP | / | [75] |
| Buckwheat flour, rice flour, potato starch | Microalgae biomass and ethanol-treated microalgae biomass | 4 | TPC, DPPH, carotenoids, chlorophyll a and chlorophyll b | / | [76] |
| White rice flour, maize flour and millet flour | Brown algae powder | 2, 4, 6, 8, 10 | TPC, ABTS, CHEL, FRAP, •OH | / | [77] |
| Food industry by-products | |||||
| Maize starch, inulin and rice protein | Coffee silverskin and coffee husk extract | 2.5 | TPC, ABTS, QUENCHER | / | [78] |
| Maize starch and rice flour | Ground green coffee parchment | 2 | TPC, DPPH | / | [79] |
| Maize starch and potato starch | Flaxseed oil cake extract | 25, 50, 75, 100 | TPC ABTS, DPPH, FRAP, PCL | / | [34] |
| Antioxidant Source | Addition Level (g/100 g%) | Volume (cm3) | Specific Volume (cm3/g) | Crumb Hardness (N) | Reference |
|---|---|---|---|---|---|
| Gluten-free cereals, pseudocereals and and legumes | |||||
| Pregelatinized rice | 100 | / | 0.6 ± 0.1 * | / | [32] |
| Wholegrain rice flour | 100 | / | 1.81 | 13.74 ± 0.23 | [55] |
| Maize flour | 10 | 548.6 ± 10.5 ** | 2.87 ± 0.02 | ~0.5 | [57] |
| Broccoli leaf powder | 5 | / | 3.08 ± 0.16 | 13.80 ± 0.07 | [48] |
| Millet | 100 | / | 1.3 ± 0.0 * | / | [32] |
| Brown millet flour | 100 | / | 1.64 | 25.70 ± 0.15 | [55] |
| Wholegrain millet and wholegrain millet extruded flour | 100, 50 | 0.59–0.93 | / | 623.6–3957 | [60] |
| Wholegrain sorghum flour | 100 | / | 1.52 | 21.47 ± 0.32 | [55] |
| Wholegrain red sorghum flour | 10, 20, 30, 40 | / | 2.41–3.21 | 6.90–13.92 | [50] |
| White sorghum flour | 85 | / | 4.68–4.78 | 14.87–25.87 | [36] |
| Quinoa flour | 100 | / | 1.92 | 10.81 ± 0.14 | [55] |
| Buckwheat | 100 | / | 2.0 ± 0.1 * | / | [32] |
| Buckwheat hulls | 3,6 | 396–470 ** | 2.5–2.8 | 2.2–3.2 | [45] |
| Wholegrain buckwheat flour | 30, 45 | / | 2.88–2.93 | 30.34–33.87 | [43] |
| Buckwheat flour | 10 | 35.5 ± 6.1 ** | 3.12 ± 0.01 | ~0.3 | [57] |
| Amaranth flour | 10 | 563.3 ± 3.7 ** | 2.78 ± 0.03 | ~0.2 | [57] |
| Chia flour | 9.8, 10 | / | 1.73–2.04 | 8.54–12.65 | [61] |
| 10 | 397–428 | 1.34–1.49 | / | [37] | |
| Chia and chia seed waste | 5 | ~115–130 | / | ~37–41 | [62] |
| Fruit and fruit by-products | |||||
| Acerola fruit powder | 1, 2, 3, 4, 5 | 502–571.6 | / | 9.9–11.5 | [65] |
| Defatted blackcurrant seeds | 5, 10, 15 | 485–527 | / | ~1.9–3.7 | [67] |
| Defatted strawberry seeds | 5, 10, 15 | 575–608 | / | ~1.7–2.1 | [67] |
| Extruded sour cherry pomace and rice flour | 10 | 521–562 ** | / | ~0.6–0.9 | [68] |
| Pomegranate seed powder | 2.5, 5, 7.5, 10 | / | ~2.65–2.85 | 20.03–24.40 | [70] |
| Rosehip powder, rosehip encapsulate | 7 | / | / | 0.42–1.85 | [52] |
| Herbs | |||||
| Hemp inflorescence | 1, 2, 3, 4, 5 | ~0.98–1.08 | 13.11–15.76 | [38] | |
| Tree fruits and leaves | |||||
| Acorn flour | 23, 35 | / | / | 4.24–5.07 | [40] |
| 965.56–1255 | / | 22.74–28.90 | [72] | ||
| Acorn and chickpea flour mixtures | 30 | / | 1.85–3.27 | 1.80–7.10 | [33] |
| Chestnut flour | 10, 20 | / | 2.6–3.1 | 1.42–4.04 | [73] |
| Carob fiber (commercial) | 1, 2, 3, 4, 5 | ~157–165 | / | ~25–28.5 | [47] |
| Moringa oleifera leaves powder | 2.5, 5, 7.5, 10 | / | ~2.37–2.48 | 22.40–26.04 | [49] |
| Microalgae and algae | |||||
| Microalgae Tetraselmis chuii | 1, 2, 4 | 612–642 | / | 3.74–6.28 | [75] |
| Microalgae biomass and ethanol-treated microalgae biomass | 4 | / | 1.95–2.96 | 1.68–4.85 | [76] |
| Brown algae powder | 2, 4, 6, 8, 10 | ~136–140.5 | / | ~32–46 | [77] |
| Food industry by-products | |||||
| Ground green coffee parchment | 2 | / | ~3.65 | 1.79 ± 0.35 | [79] |
| Flaxseed oil cake extract | 25, 50, 75, 100 | / | 2.39–3.06 | / | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djordjević, M.; Djordjević, M.; Starowicz, M.; Krupa-Kozak, U. Plant-Based Antioxidants in Gluten-Free Bread Production: Sources, Technological and Sensory Aspects, Enhancing Strategies and Constraints. Antioxidants 2024, 13, 142. https://doi.org/10.3390/antiox13020142
Djordjević M, Djordjević M, Starowicz M, Krupa-Kozak U. Plant-Based Antioxidants in Gluten-Free Bread Production: Sources, Technological and Sensory Aspects, Enhancing Strategies and Constraints. Antioxidants. 2024; 13(2):142. https://doi.org/10.3390/antiox13020142
Chicago/Turabian StyleDjordjević, Marijana, Miljana Djordjević, Małgorzata Starowicz, and Urszula Krupa-Kozak. 2024. "Plant-Based Antioxidants in Gluten-Free Bread Production: Sources, Technological and Sensory Aspects, Enhancing Strategies and Constraints" Antioxidants 13, no. 2: 142. https://doi.org/10.3390/antiox13020142
APA StyleDjordjević, M., Djordjević, M., Starowicz, M., & Krupa-Kozak, U. (2024). Plant-Based Antioxidants in Gluten-Free Bread Production: Sources, Technological and Sensory Aspects, Enhancing Strategies and Constraints. Antioxidants, 13(2), 142. https://doi.org/10.3390/antiox13020142










