Effects of Spherical and Rod-like Gold Nanoparticles on the Reactivity of Human Peripheral Blood Leukocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Reagents
2.3. Synthesis of the Gold Nanoparticles
2.4. Physicochemical Characterization of the GNPs
2.5. Collections of the Peripheral Blood Samples
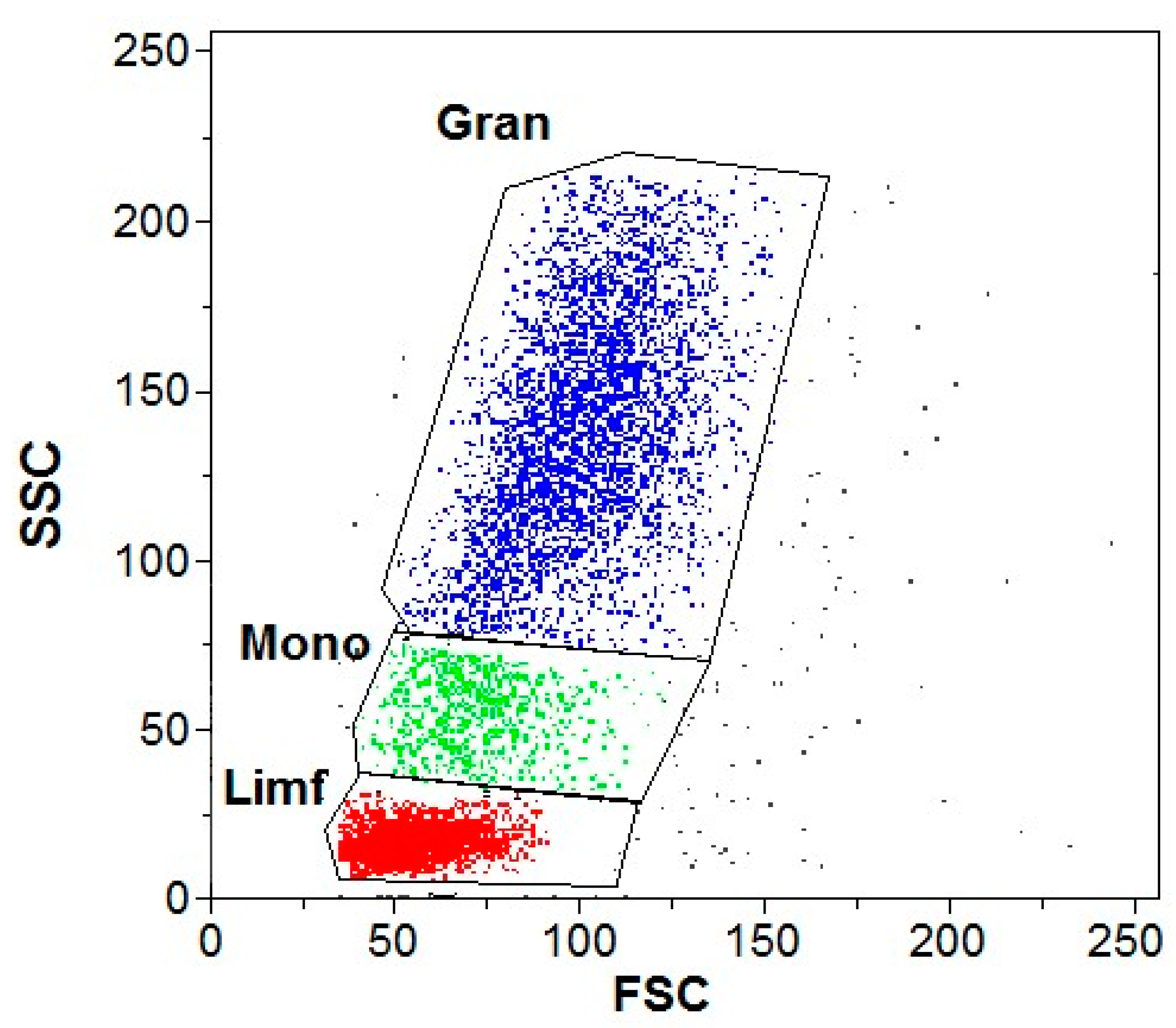
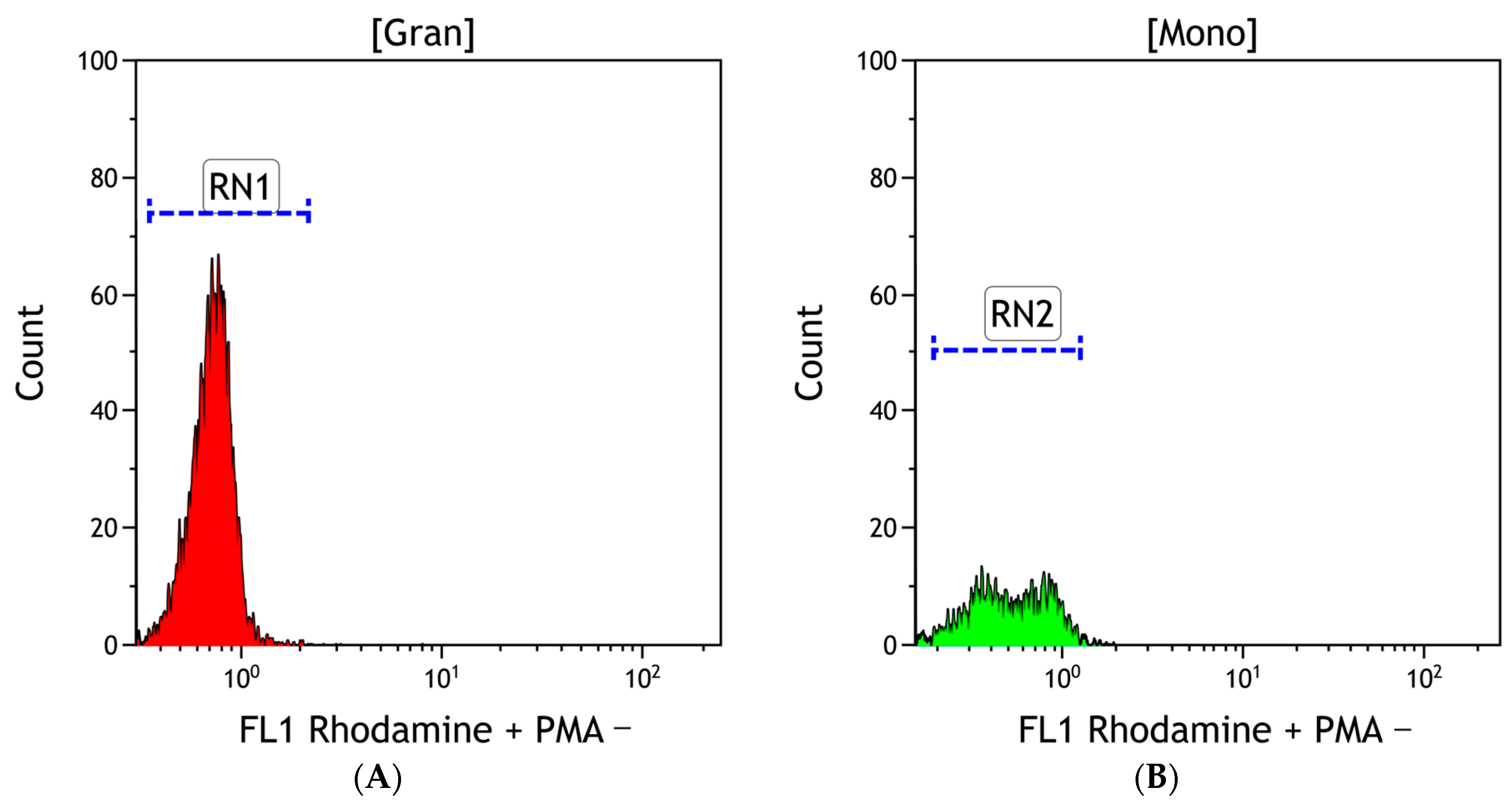
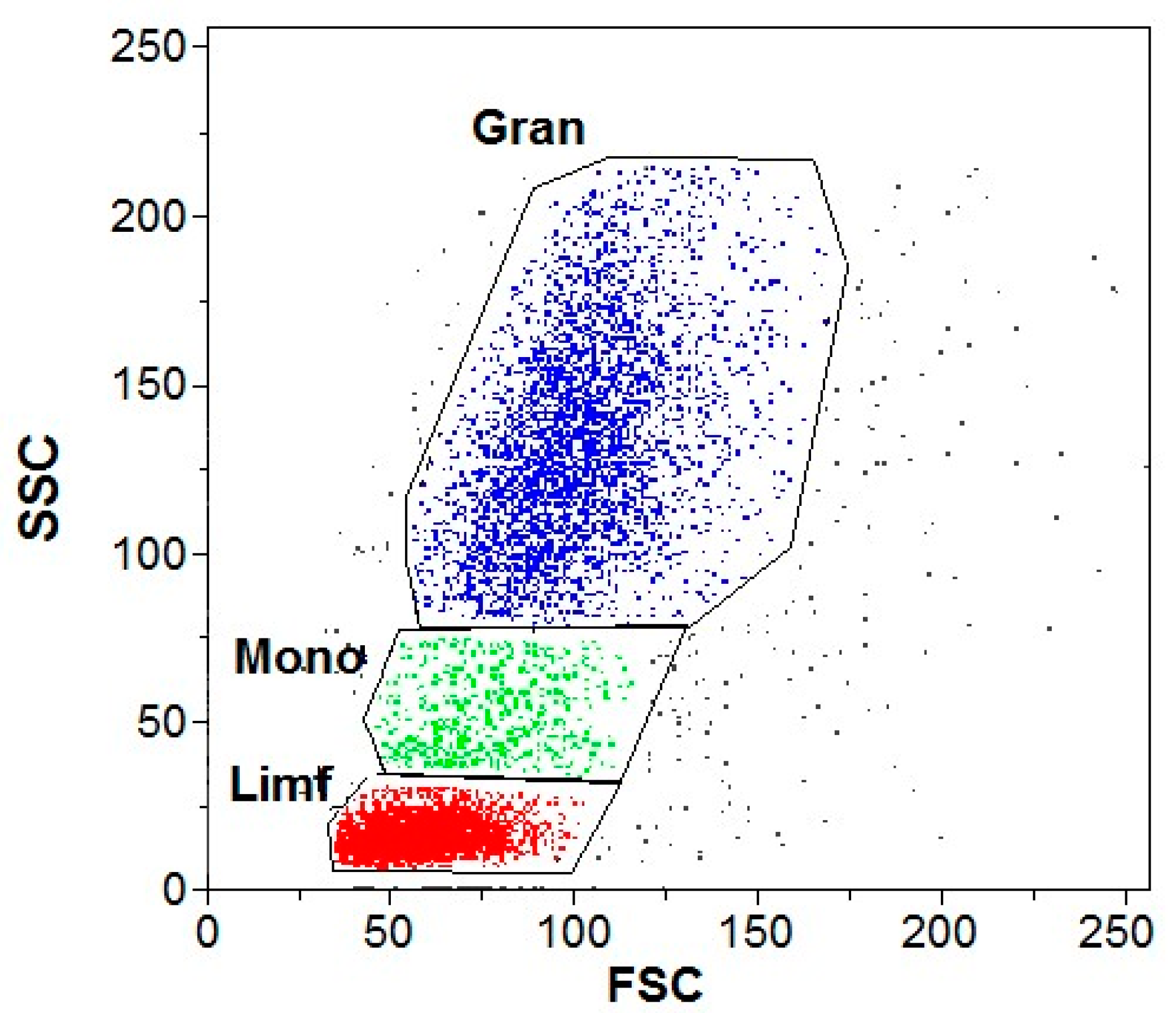
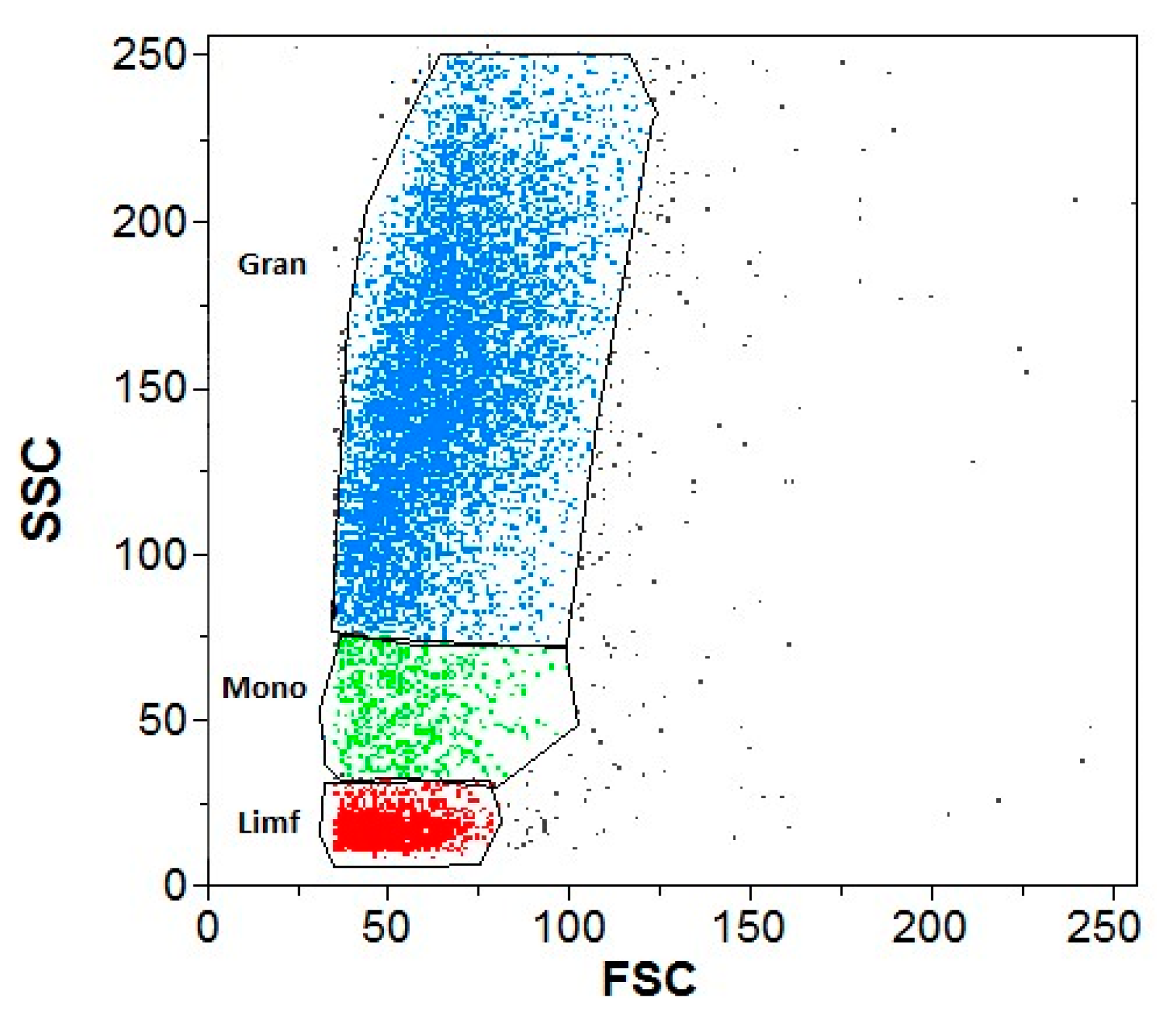
2.6. Flow Cytometry
2.7. Reactive Oxygen Species Production
2.8. Respiratory Burst
2.9. Pyroptosis Analysis
2.10. Enzyme-Linked Immunosorbent Assay (ELISA) Tests
2.11. Statistical Analyses
3. Results
3.1. Physicochemical Characterization of the GNPs
3.1.1. UV–Vis
3.1.2. HRTEM
3.1.3. Thermogravimetric Analysis
3.2. Flow Cytometry
3.2.1. ROS Production
A Comparison of Samples Incubated with GNPs and GNRs for Different Time Periods
A Comparison of Samples Incubated with GNPs or GNRs at Different Concentrations
A Comparison of Samples Incubated with Different Shapes of Gold Nanoparticles
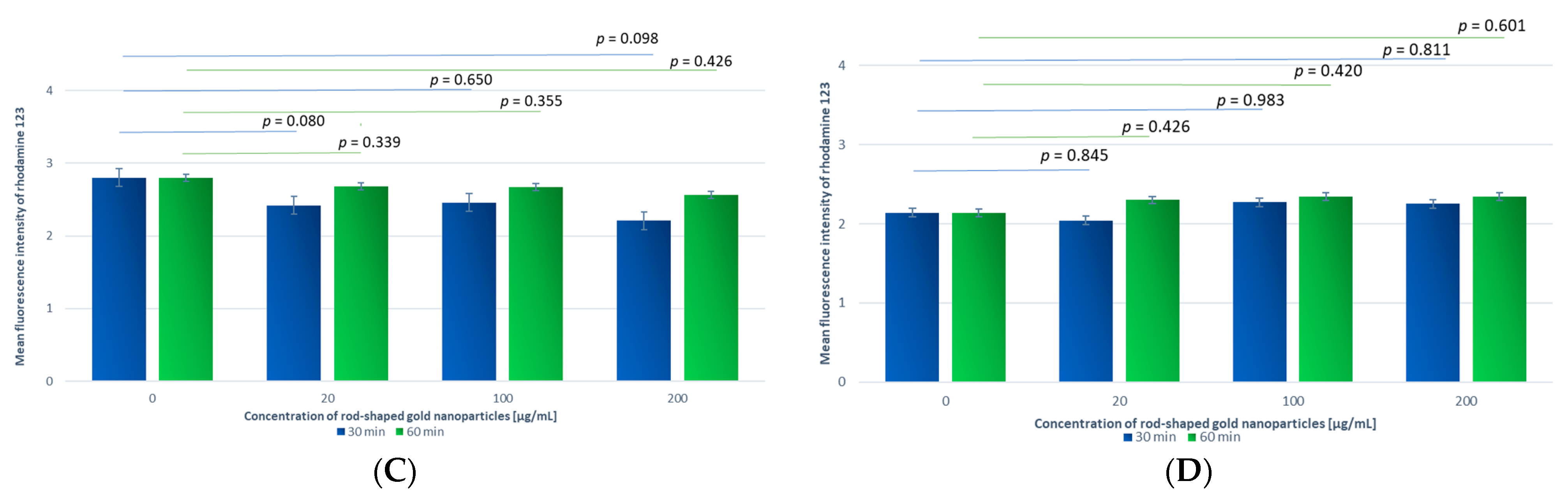
3.2.2. PMA-Addition-Stimulated Production of Reactive Oxygen Species
Comparison of Samples Incubated for 30 min with GNPs at Different Concentrations
Comparison of Samples Incubated with GNRs at the Same Concentration and Different Incubation Times
3.3. Pyroptosis
Comparison between Different GNP Shapes
3.4. ELISA Results on IL-1β Concentration
3.4.1. Comparison of Samples Incubated with GNPs at Different Concentrations
3.4.2. Comparison within Samples Incubated with Different Types of Gold Nanoparticles, GNPs or GNRs, at Different Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Description of the Synthesis of Spherical Gold Nanoparticles
Appendix B. Description of the Synthesis of Rod-Like Gold Nanoparticles
References
- McShan, D.; Ray, P.C.; Yu, H. Molecular Toxicity Mechanism of Nanosilver. J. Food Drug Anal. 2014, 22, 116–127. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Murugan, K.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. Parameters and Characteristics Governing Cellular Internalization and Trans-Barrier Trafficking of Nanostructures. Int. J. Nanomed. 2015, 10, 2191–2206. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A Repertoire of Biomedical Applications of Noble Metal Nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and Nanomedicines Currently on the Market: Challenges and Opportunities. Nanomedicine 2018, 14, 93–126. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Karsh, E.H.; Taqi, Z.J.; Jabir, M.S. Biocompatibility of Gold Nanoparticles: In-Vitro and In-Vivo Study. Mater. Today Proc. 2021, 42, 3041–3045. [Google Scholar] [CrossRef]
- Kovacevic, M.; Balaz, I.; Marson, D.; Laurini, E.; Jovic, B. Mixed-Monolayer Functionalized Gold Nanoparticles for Cancer Treatment: Atomistic Molecular Dynamics Simulations Study. Biosystems 2021, 202, 104354. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Wang, J.-Q.; Ashby, C.R.; Zeng, L.; Fan, Y.-F.; Chen, Z.-S. Gold Nanoparticles: Synthesis, Physiochemical Properties and Therapeutic Applications in Cancer. Drug Discov. Today 2021, 26, 1284–1292. [Google Scholar] [CrossRef]
- Ujica, M.A.; Paltinean, G.A.; Mocanu, A.; Tomoaia-Cotisel, M. Silver and Gold Nanoparticles: Challenges and Perspectives. Acad. Rom. Sci. Ann.—Ser. Biol. Sci. 2020, 9, 97–139. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Hamed, M.T.; Bakr, B.A.; Shahin, Y.H.; Abu-Serie, M.M.; Awaad, A.K.; El-Kady, H.; Elwakil, B.H. In Vivo Bio-Distribution and Acute Toxicity Evaluation of Greenly Synthesized Ultra-Small Gold Nanoparticles with Different Biological Activities. Sci. Rep. 2022, 12, 6269. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, S.; Karimi, E.; Khajeh, K.; Hosseinkhani, S.; Javan, M. Peptide Mediated Targeted Delivery of Gold Nanoparticles into the Demyelination Site Ameliorates Myelin Impairment and Gliosis. Nanomed. Nanotechnol. Biol. Med. 2023, 47, 102609. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, S.; Wang, R.; Cheol Hwang, K.; Kim, H.; Kim, Y.-J. Enhanced Skin anti-Inflammatory and Moisturizing Action of Gold Nanoparticles Produced Utilizing Diospyros Kaki Fruit Extracts. Arab. J. Chem. 2023, 16, 104551. [Google Scholar] [CrossRef]
- Want, M.Y.; Yadav, P.; Khan, R.; Chouhan, G.; Islamuddin, M.; Aloyouni, S.Y.; Chattopadhyay, A.P.; AlOmar, S.Y.; Afrin, F. Critical Antileishmanial in vitro Effects of Highly Examined Gold Nanoparticles. Int. J. Nanomed. 2021, 16, 7285–7295. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, X.; Chen, L.; Gong, X.; Yang, H.; Duan, X.; Zhu, Y. Multifunctional Gold Nanoparticles in Cancer Diagnosis and Treatment. Int. J. Nanomed. 2022, 17, 2041–2067. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, B.; Hojjat-Farsangi, M.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and Targeted Drug Delivery in Cancer Therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Murphy, C.J. Toxicity and Cellular Uptake of Gold Nanoparticles: What We Have Learned so Far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef]
- Milan, J.; Niemczyk, K.; Kus-Liśkiewicz, M. Treasure on the Earth—Gold Nanoparticles and Their Biomedical Applications. Materials 2022, 15, 3355. [Google Scholar] [CrossRef]
- Nguyen, N.H.A.; Falagan-Lotsch, P. Mechanistic Insights into the Biological Effects of Engineered Nanomaterials: A Focus on Gold Nanoparticles. Int. J. Mol. Sci. 2023, 24, 4109. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold Nanoparticles Are Taken up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Goddard, Z.R.; Marín, M.J.; Russell, D.A.; Searcey, M. Active Targeting of Gold Nanoparticles as Cancer Therapeutics. Chem. Soc. Rev. 2020, 49, 8774–8789. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Lim, Z.-Z.J.; Li, J.-E.J.; Ng, C.-T.; Yung, L.-Y.L.; Bay, B.-H. Gold Nanoparticles in Cancer Therapy. Acta Pharmacol. Sin. 2011, 32, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold Nanoparticles of Diameter 1.4 nm Trigger Necrosis by Oxidative Stress and Mitochondrial Damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef]
- Sung, J.H.; Ji, J.H.; Park, J.D.; Song, M.Y.; Song, K.S.; Ryu, H.R.; Yoon, J.U.; Jeon, K.S.; Jeong, J.; Han, B.S.; et al. Subchronic Inhalation Toxicity of Gold Nanoparticles. Part. Fibre Toxicol. 2011, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, L.; Mettenbrink, E.M.; DeAngelis, P.L.; Wilhelm, S. Nanoparticle Toxicology. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 269–289. [Google Scholar] [CrossRef]
- Miller, M.R.; Raftis, J.B.; Langrish, J.P.; McLean, S.G.; Samutrtai, P.; Connell, S.P.; Wilson, S.; Vesey, A.T.; Fokkens, P.H.B.; Boere, A.J.F.; et al. Inhaled Nanoparticles Accumulate at Sites of Vascular Disease. ACS Nano 2017, 11, 4542–4552. [Google Scholar] [CrossRef]
- Fernandes, R.; Smyth, N.R.; Muskens, O.L.; Nitti, S.; Heuer-Jungemann, A.; Ardern-Jones, M.R.; Kanaras, A.G. Interactions of Skin with Gold Nanoparticles of Different Surface Charge, Shape, and Functionality. Small 2015, 11, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xu, K.; Ji, L.; Tang, B. Effect of Gold Nanoparticles on Glutathione Depletion-Induced Hydrogen Peroxide Generation and Apoptosis in HL7702 Cells. Toxicol. Lett. 2011, 205, 86–95. [Google Scholar] [CrossRef]
- White, B.E.; White, M.K.; Nima Alsudani, Z.A.; Watanabe, F.; Biris, A.S.; Ali, N. Cellular Uptake of Gold Nanorods in Breast Cancer Cell Lines. Nanomaterials 2022, 12, 937. [Google Scholar] [CrossRef]
- Orlando, A.; Colombo, M.; Prosperi, D.; Corsi, F.; Panariti, A.; Rivolta, I.; Masserini, M.; Cazzaniga, E. Evaluation of Gold Nanoparticles Biocompatibility: A Multiparametric Study on Cultured Endothelial Cells and Macrophages. J. Nanopart. Res. 2016, 18, 58. [Google Scholar] [CrossRef]
- Carlander, U.; Midander, K.; Hedberg, Y.S.; Johanson, G.; Bottai, M.; Karlsson, H.L. Macrophage-Assisted Dissolution of Gold Nanoparticles. ACS Appl. Biol. Mater. 2019, 2, 1006–1016. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, X.; Kang, X.; Yang, H.; Guo, W.; Guan, L.; Wu, H.; Du, L. Surface Functionalization of Pegylated Gold Nanoparticles with Antioxidants Suppresses Nanoparticle-Induced Oxidative Stress and Neurotoxicity. Chem. Res. Toxicol. 2020, 33, 1195–1205. [Google Scholar] [CrossRef]
- Sumbayev, V.V.; Yasinska, I.M.; Garcia, C.P.; Gilliland, D.; Lall, G.S.; Gibbs, B.F.; Bonsall, D.R.; Varani, L.; Rossi, F.; Calzolai, L. Gold Nanoparticles Downregulate Interleukin-1β-Induced Pro-Inflammatory Responses. Small 2013, 9, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Błaszkiewicz, P.; Kotkowiak, M.; Dudkowiak, A. Fluorescence Quenching and Energy Transfer in a System of Hybrid Laser Dye and Functionalized Gold Nanoparticles. J. Lumin. 2017, 183, 303–310. [Google Scholar] [CrossRef]
- Rahme, K.; Chen, L.; Hobbs, R.G.; Morris, M.A.; O’Driscoll, C.; Holmes, J.D. PEGylated Gold Nanoparticles: Polymer Quantification as a Function of PEG Lengths and Nanoparticle Dimensions. RSC Adv. 2013, 3, 6085–6094. [Google Scholar] [CrossRef]
- Błaszkiewicz, P.; Kotkowiak, M.; Coy, E.; Dudkowiak, A. Tailoring Fluorescence and Singlet Oxygen Generation of a Chlorophyll Derivative and Gold Nanorods via a Silica Shell. J. Phys. Chem. C 2020, 124, 2088–2095. [Google Scholar] [CrossRef]
- Błaszkiewicz, P.; Kotkowiak, M.; Coy, E.; Dudkowiak, A. Laser-Induced Optoacoustic Spectroscopy Studies of Inorganic Functionalized Metallic Nanorods. J. Phys. Chem. C 2019, 123, 27181–27186. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Scarabelli, L.; Sánchez-Iglesias, A.; Pérez-Juste, J.; Liz-Marzán, L.M. A “Tips and Tricks” Practical Guide to the Synthesis of Gold Nanorods. J. Phys. Chem. Lett. 2015, 6, 4270–4279. [Google Scholar] [CrossRef]
- Yazdani, M. Concerns in the Application of Fluorescent Probes DCDHF-DA, DHR 123 and DHE to Measure Reactive Oxygen Species in vitro. Toxicol. Vitr. 2015, 30, 578–582. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence Probes Used for Detection of Reactive Oxygen Species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Koss-Mikołajczyk, I.; Baranowska, M.; Namieśnik, J.; Bartoszek, A. Determination of Antioxidantactivity of Phytochemicals in Cellular Models by Fluorescence/Luminescence Methods. Adv. Hyg. Exp. Med. 2017, 71, 602–617. [Google Scholar] [CrossRef]
- Freebern, W.J.; Bigwarfe, T.J.; Price, K.D.; Haggerty, H.G. Methods: Implementation of in vitro and Ex Vivo Phagocytosis and Respiratory Burst Function Assessments in Safety Testing. J. Immunotoxicol. 2013, 10, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Przyjemska, M.; Olejnik, A.; Grajek, W. The Influence of Beetroot and Chokeberry Juice on Oxygen Metabolism and Apoptosis of Polymorphonuclear Granulocytes in Vitro. Food Sci. Technol. Qual. 2007, 2, 174–186. [Google Scholar]
- Barasinski, M.; Hilbig, J.; Neumann, S.; Rafaja, D.; Garnweitner, G. Simple Model of the Electrophoretic Migration of Spherical and Rod-Shaped Au Nanoparticles in Gels with Varied Mesh Sizes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129716. [Google Scholar] [CrossRef]
- Woźniak, A.; Malankowska, A.; Nowaczyk, G.; Grześkowiak, B.F.; Tuśnio, K.; Słomski, R.; Zaleska-Medynska, A.; Jurga, S. Size and Shape-Dependent Cytotoxicity Profile of Gold Nanoparticles for Biomedical Applications. J. Mater. Sci. Mater. Med. 2017, 28, 92. [Google Scholar] [CrossRef]
- Natarajan, P.; Sukthankar, P.; Changstrom, J.; Holland, C.S.; Barry, S.; Hunter, W.B.; Sorensen, C.M.; Tomich, J.M. Synthesis and Characterization of Multifunctional Branched Amphiphilic Peptide Bilayer Conjugated Gold Nanoparticles. ACS Omega 2018, 3, 11071–11083. [Google Scholar] [CrossRef]
- Locardi, F.; Canepa, E.; Villa, S.; Nelli, I.; Lambruschini, C.; Ferretti, M.; Canepa, F. Thermogravimetry and Evolved Gas Analysis for the Investigation of Ligand-Exchange Reaction in Thiol-Functionalized Gold Nanoparticles. J. Anal. Appl. Pyrolysis 2018, 132, 11–18. [Google Scholar] [CrossRef]
- Mahato, K.; Nagpal, S.; Shah, M.A.; Srivastava, A.; Maurya, P.K.; Roy, S.; Jaiswal, A.; Singh, R.; Chandra, P. Gold Nanoparticle Surface Engineering Strategies and Their Applications in Biomedicine and Diagnostics. 3 Biotech 2019, 9, 57. [Google Scholar] [CrossRef]
- Bajaj, M.; Wangoo, N.; Jain, D.V.S.; Sharma, R.K. Quantification of Adsorbed and Dangling Citrate Ions on Gold Nanoparticle Surface Using Thermogravimetric Analysis. Sci. Rep. 2020, 10, 8213. [Google Scholar] [CrossRef]
- Fajstavr, D.; Karasová, A.; Michalcová, A.; Ulbrich, P.; Slepičková Kasálková, N.; Siegel, J.; Švorčík, V.; Slepička, P. PEGylated Gold Nanoparticles Grafted with N-Acetyl-L-Cysteine for Polymer Modification. Nanomaterials 2021, 11, 1434. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.R.; Zheng, J.; Tang, X.; Goering, P.L. Silver Nanoparticle-Induced Autophagic-Lysosomal Disruption and NLRP3-Inflammasome Activation in HepG2 Cells Is Size-Dependent. Toxicol. Sci. 2016, 150, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.K.; Deda, D.K.; de Rodrigues, S.F.P.; Drewes, C.C.; Bolonheis, S.M.; Kiyohara, P.K.; de Toledo, S.P.; Colli, W.; Araki, K.; Farsky, S.H.P. In Vivo and In Vitro Toxicity and anti-Inflammatory Properties of Gold Nanoparticle Bioconjugates to the Vascular System. Toxicol. Sci. 2014, 142, 497–507. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Zoulikha, M.; He, W. Targeted Drug Delivery for Chronic Lymphocytic Leukemia. Pharm. Res. 2022, 39, 441–461. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dief, A.M.; Alsehli, M.; Awaad, A. A Higher Dose of PEGylated Gold Nanoparticles Reduces the Accelerated Blood Clearance Phenomenon Effect and Induces Spleen B Lymphocytes in Albino Mice. Histochem. Cell Biol. 2022, 157, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Dheyab, M.A.; Aziz, A.A.; Moradi Khaniabadi, P.; Jameel, M.S.; Oladzadabbasabadi, N.; Mohammed, S.A.; Abdullah, R.S.; Mehrdel, B. Monodisperse Gold Nanoparticles: A Review on Synthesis and Their Application in Modern Medicine. Int. J. Mol. Sci. 2022, 23, 7400. [Google Scholar] [CrossRef]
- Harris, J.M.; Martin, N.E.; Modi, M. Pegylation: A Novel Process for Modifying Pharmacokinetics. Clin. Pharmacokinet. 2001, 40, 539–551. [Google Scholar] [CrossRef]
- He, Z.; Li, C.; Zhang, X.; Zhong, R.; Wang, H.; Liu, J.; Du, L. The Effects of Gold Nanoparticles on the Human Blood Functions. Artif. Cells Nanomed. Biotechnol. 2018, 46, 720–726. [Google Scholar] [CrossRef]
- Kah, J.C.Y.; Wong, K.Y.; Neoh, K.G.; Song, J.H.; Fu, J.W.P.; Mhaisalkar, S.; Olivo, M.; Sheppard, C.J.R. Critical Parameters in the Pegylation of Gold Nanoshells for Biomedical Applications: An in Vitro Macrophage Study. Null 2009, 17, 181–193. [Google Scholar] [CrossRef]
- Huang, H.; Feng, W.; Chen, Y.; Shi, J. Inorganic Nanoparticles in Clinical Trials and Translations. Nano Today 2020, 35, 100972. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.-H. Surface Chemistry of Gold Nanoparticles Mediates Their Exocytosis in Macrophages. ACS Nano 2014, 8, 6232–6241. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.; Kushwaha, P.; Bhutia, Y.C.; Flora, S. Oxidative Stress Following Exposure to Silver and Gold Nanoparticles in Mice. Toxicol. Ind. Health 2016, 32, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold Nanoparticles: Distribution, Bioaccumulation and Toxicity. In Vitro and in Vivo Studies. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, N.; Zhu, Y.; Lu, Y.; Chen, Q.; Fan, S.; Huang, Q.; Chen, X.; Xia, L.; Wei, Y.; et al. Gold Nanoparticles Synergize with Bacterial Lipopolysaccharide to Enhance Class A Scavenger Receptor Dependent Particle Uptake in Neutrophils and Augment Neutrophil Extracellular Traps Formation. Ecotoxicol. Environ. Saf. 2021, 211, 111900. [Google Scholar] [CrossRef]
- Coimbra, J.L.; de PFDantas, G.; de Andrade, L.M.; Brener, M.R.; Viana, P.I.; Lopes, R.A.; Gontijo, D.O.; Ervilha, L.O.; Assis, M.Q.; Barcelos, L.S.; et al. Gold Nanoparticle Intratesticular Injections as a Potential Animal Sterilization Tool: Long-Term Reproductive and Toxicological Implications. Toxicology 2023, 492, 153543. [Google Scholar] [CrossRef]
- Yogo, K.; Misawa, M.; Shimizu, M.; Shimizu, H.; Kitagawa, T.; Hirayama, R.; Ishiyama, H.; Furukawa, T.; Yasuda, H. Effect of Gold Nanoparticle Radiosensitization on Plasmid DNA Damage Induced by High-Dose-Rate Brachytherapy. Int. J. Nanomed. 2021, 16, 359–370. [Google Scholar] [CrossRef]
- Pradhan, A.; Bepari, M.; Maity, P.; Roy, S.S.; Roy, S.; Choudhury, S.M. Gold Nanoparticles from Indole-3-Carbinol Exhibit Cytotoxic, Genotoxic and Antineoplastic Effects through the Induction of Apoptosis. RSC Adv. 2016, 6, 56435–56449. [Google Scholar] [CrossRef]
- Martínez-Torres, A.C.; Lorenzo-Anota, H.Y.; García-Juárez, M.G.; Zarate-Triviño, D.G.; Rodríguez-Padilla, C. Chitosan Gold Nanoparticles Induce Different ROS-Dependent Cell Death Modalities in Leukemic Cells. Int. J. Nanomed. 2019, 14, 7173–7190. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Haupenthal, D.P.; Mendes, C.; de Bem Silveira, G.; Zaccaron, R.P.; Corrêa, M.E.A.B.; Nesi, R.T.; Pinho, R.A.; da Silva Paula, M.M.; Silveira, P.C.L. Effects of Treatment with Gold Nanoparticles in a Model of Acute Pulmonary Inflammation Induced by Lipopolysaccharide. J. Biomed. Mater. Res. 2020, 108, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally Relevant Concentrations of Resveratrol and Hydroxytyrosol Mitigate Oxidative Burst of Human Granulocytes and Monocytes and the Production of Pro-Inflammatory Mediators in LPS-Stimulated RAW 264.7 Macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef]
- Haase, H.; Fahmi, A.; Mahltig, B. Impact of Silver Nanoparticles and Silver Ions on Innate Immune Cells. J. Biomed. Nanotechnol. 2014, 10, 1146–1156. [Google Scholar] [CrossRef]
- Blüml, S.; Rosc, B.; Lorincz, A.; Seyerl, M.; Kirchberger, S.; Oskolkova, O.; Bochkov, V.N.; Majdic, O.; Ligeti, E.; Stöckl, J. The Oxidation State of Phospholipids Controls the Oxidative Burst in Neutrophil Granulocytes. J. Immunol. 2008, 181, 4347–4353. [Google Scholar] [CrossRef]
- Chen, Y.; Junger, W.G. Measurement of Oxidative Burst in Neutrophils. Methods Mol. Biol. 2012, 844, 115–124. [Google Scholar] [CrossRef]
- Lun, A.; Schmitt, M.; Renz, H. Phagocytosis and Oxidative Burst: Reference Values for Flow Cytometric Assays Independent of Age. Clin. Chem. 2000, 46, 1836–1839. [Google Scholar] [CrossRef]
- Prietl, B.; Meindl, C.; Roblegg, E.; Pieber, T.R.; Lanzer, G.; Fröhlich, E. Nano-Sized and Micro-Sized Polystyrene Particles Affect Phagocyte Function. Cell Biol. Toxicol. 2014, 30, 1–16. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, X.; Boudreau, M.D.; Feng, G.; Miao, Y.; Dong, S.; Wu, H.; Zeng, M.; Yin, J.-J. Orally Administered Gold Nanoparticles Protect against Colitis by Attenuating Toll-like Receptor 4- and Reactive Oxygen/Nitrogen Species-Mediated Inflammatory Responses but Could Induce Gut Dysbiosis in Mice. J. Nanobiotechnol. 2018, 16, 86. [Google Scholar] [CrossRef]
- Kreuzaler, P.A.; Staniszewska, A.D.; Li, W.; Omidvar, N.; Kedjouar, B.; Turkson, J.; Poli, V.; Flavell, R.A.; Clarkson, R.W.E.; Watson, C.J. Stat3 Controls Lysosomal-Mediated Cell Death in Vivo. Nat. Cell Biol. 2011, 13, 303–309. [Google Scholar] [CrossRef]
- Miao, E.A.; Rajan, J.V.; Aderem, A. Caspase-1 Induced Pyroptotic Cell Death. Immunol. Rev. 2011, 243, 206–214. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and Diseases. Sig. Transduct. Target Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Simard, J.-C.; Vallieres, F.; de Liz, R.; Lavastre, V.; Girard, D. Silver Nanoparticles Induce Degradation of the Endoplasmic Reticulum Stress Sensor Activating Transcription Factor-6 Leading to Activation of the NLRP-3 Inflammasome. J. Biol. Chem. 2015, 290, 5926–5939. [Google Scholar] [CrossRef]
- Mamun, A.A.; Wu, Y.; Nasrin, F.; Akter, A.; Taniya, M.A.; Munir, F.; Jia, C.; Xiao, J. Role of Pyroptosis in Diabetes and Its Therapeutic Implications. J. Inflamm. Res. 2021, 14, 2187–2206. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold Nanoparticles (GNPs) in Biomedical and Clinical Applications: A Review. Nano Sel. 2022, 3, 792–828. [Google Scholar] [CrossRef]
- Wang, X.-M.; Wu, X.-W.; Zhao, X.-Y.; Wang, C.-W.; Zhou, J.-N. Exposure-Time-Dependent Subcellular Staging of Gold Nanoparticles Deposition and Vesicle Destruction in Mice Livers. Nanomed. Nanotechnol. Biol. Med. 2021, 34, 102393. [Google Scholar] [CrossRef] [PubMed]
- Karan, K.R.; Trumpff, C.; McGill, M.A.; Thomas, J.E.; Sturm, G.; Lauriola, V.; Sloan, R.P.; Rohleder, N.; Kaufman, B.A.; Marsland, A.L.; et al. Mitochondrial Respiratory Capacity Modulates LPS-Induced Inflammatory Signatures in Human Blood. Brain Behav. Immun. Health 2020, 5, 100080. [Google Scholar] [CrossRef] [PubMed]
- Strahler, J.; Rohleder, N.; Wolf, J.M. Acute Psychosocial Stress Induces Differential Short-Term Changes in Catecholamine Sensitivity of Stimulated Inflammatory Cytokine Production. Brain Behav. Immun. 2015, 43, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Tim, B.; Błaszkiewicz, P.; Nowicka, A.B.; Kotkowiak, M. Optimizing SERS Performance through Aggregation of Gold Nanorods in Langmuir-Blodgett Films. Appl. Surf. Sci. 2022, 573, 151518. [Google Scholar] [CrossRef]
- Szustakiewicz, P.; Kołsut, N.; Leniart, A.; Lewandowski, W. Universal Method for Producing Reduced Graphene Oxide/Gold Nanoparticles Composites with Controlled Density of Grafting and Long-Term Stability. Nanomaterials 2019, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.; Kumar, D.; Meenan, B.J.; Dixon, D. Polyethylene Glycol Functionalized Gold Nanoparticles: The Influence of Capping Density on Stability in Various Media. Gold Bull. 2011, 44, 99–105. [Google Scholar] [CrossRef]






| Sex | Number of Cases | Age (Mean ± Standard Deviation) | Blood Type | Rh Antigen | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | A | B | AB | + | − | |||
| Female | 6 | 33 ± 5 | 3 | 1 | 2 | 0 | 5 | 1 |
| Male | 16 | 38 ± 7 | 5 | 5 | 6 | 0 | 14 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talarska, P.; Błaszkiewicz, P.; Kostrzewa, A.; Wirstlein, P.; Cegłowski, M.; Nowaczyk, G.; Dudkowiak, A.; Grabarek, B.O.; Głowacka-Stalmach, P.; Szarpak, A.; et al. Effects of Spherical and Rod-like Gold Nanoparticles on the Reactivity of Human Peripheral Blood Leukocytes. Antioxidants 2024, 13, 157. https://doi.org/10.3390/antiox13020157
Talarska P, Błaszkiewicz P, Kostrzewa A, Wirstlein P, Cegłowski M, Nowaczyk G, Dudkowiak A, Grabarek BO, Głowacka-Stalmach P, Szarpak A, et al. Effects of Spherical and Rod-like Gold Nanoparticles on the Reactivity of Human Peripheral Blood Leukocytes. Antioxidants. 2024; 13(2):157. https://doi.org/10.3390/antiox13020157
Chicago/Turabian StyleTalarska, Patrycja, Paulina Błaszkiewicz, Artur Kostrzewa, Przemysław Wirstlein, Michał Cegłowski, Grzegorz Nowaczyk, Alina Dudkowiak, Beniamin Oskar Grabarek, Paulina Głowacka-Stalmach, Agnieszka Szarpak, and et al. 2024. "Effects of Spherical and Rod-like Gold Nanoparticles on the Reactivity of Human Peripheral Blood Leukocytes" Antioxidants 13, no. 2: 157. https://doi.org/10.3390/antiox13020157
APA StyleTalarska, P., Błaszkiewicz, P., Kostrzewa, A., Wirstlein, P., Cegłowski, M., Nowaczyk, G., Dudkowiak, A., Grabarek, B. O., Głowacka-Stalmach, P., Szarpak, A., & Żurawski, J. (2024). Effects of Spherical and Rod-like Gold Nanoparticles on the Reactivity of Human Peripheral Blood Leukocytes. Antioxidants, 13(2), 157. https://doi.org/10.3390/antiox13020157










