Revitalizing Photoaging Skin through Eugenol in UVB-Exposed Hairless Mice: Mechanistic Insights from Integrated Multi-Omics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culturing and UVB Irradiation
2.3. Cell Viability Assay
2.4. Determination of the ROS
2.5. Animal Experiments
2.6. Assessment of Wrinkle Formation
2.7. Determination of Skin Hydration
2.8. Analysis of the EU Accumulation in the Skin
2.9. Determination of MDA, SOD, MMP-1, PIP, and HA Levels
2.10. Hematoxylin and Eosin (HE) and Masson Staining
2.11. Immunohistochemistry Staining of p21 and p53
2.12. Real-Time Quantitative PCR (RT-qPCR)
2.13. Skin Transcriptome
2.14. Differential Gene Expression and Functional Enrichment Analysis
2.15. Fecal DNA Extraction and 16S rRNA Gene Sequencing
2.16. Microbiota Data Analysis
2.17. Statistical Analysis
3. Results
3.1. EU Alleviates UVB-Induced Photoaging in HaCaT Epidermal Keratinocytes and Hs68 Dermal Fibroblasts
3.1.1. EU Alleviates UVB-Induced Oxidative Damage in HaCaT Cells
3.1.2. EU Inhibits the UVB-Induced Increase in MMP Secretion in HaCaT Cells
3.1.3. EU Attenuates the UVB-Induced Decrease of ECM in Hs68 Cells
3.2. Dietary EU Mitigates the Wrinkle Formation Induced by Chronic UVB Exposure in the Skin of SKH-1 Hairless Mice
3.3. The EU Distribution in the Skin of Chronically UVB-Exposed Mice
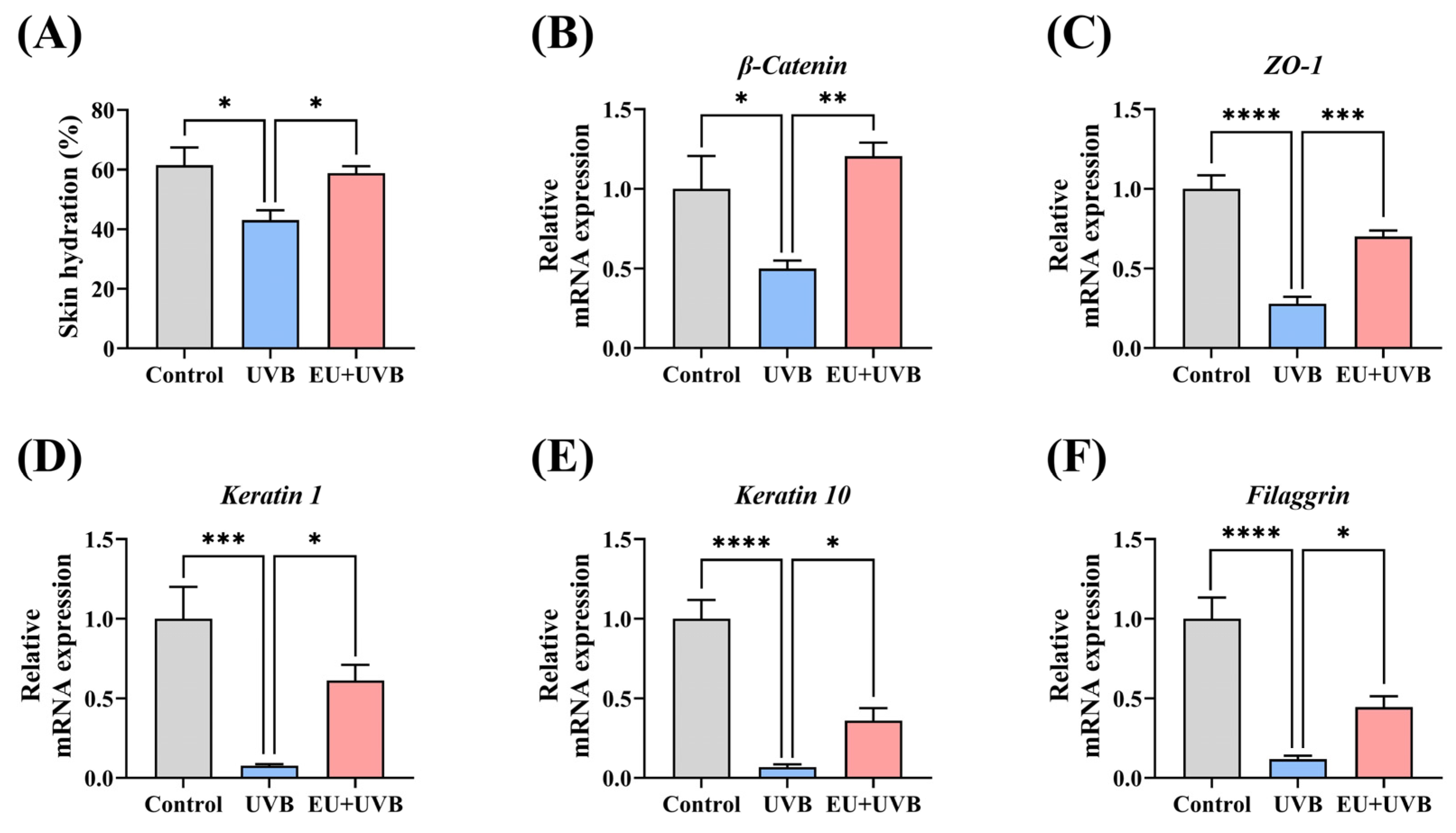
3.4. EU Promotes Skin Barrier Repair in Chronically UVB-Exposed Mice
3.5. EU Promotes Skin Tissue Regeneration in Chronically UVB-Exposed Mice
3.5.1. EU Mitigates the Chronic UVB-Induced Increase in Epidermal Thickness in Mice
3.5.2. EU Promotes ECM Synthesis in Photodamaged Mouse Skin
3.5.3. EU Promotes Skin Cell Proliferation in Photoaged Mice
3.6. EU Modulates the Skin Microenvironment in Chronically UVB-Exposed Mice
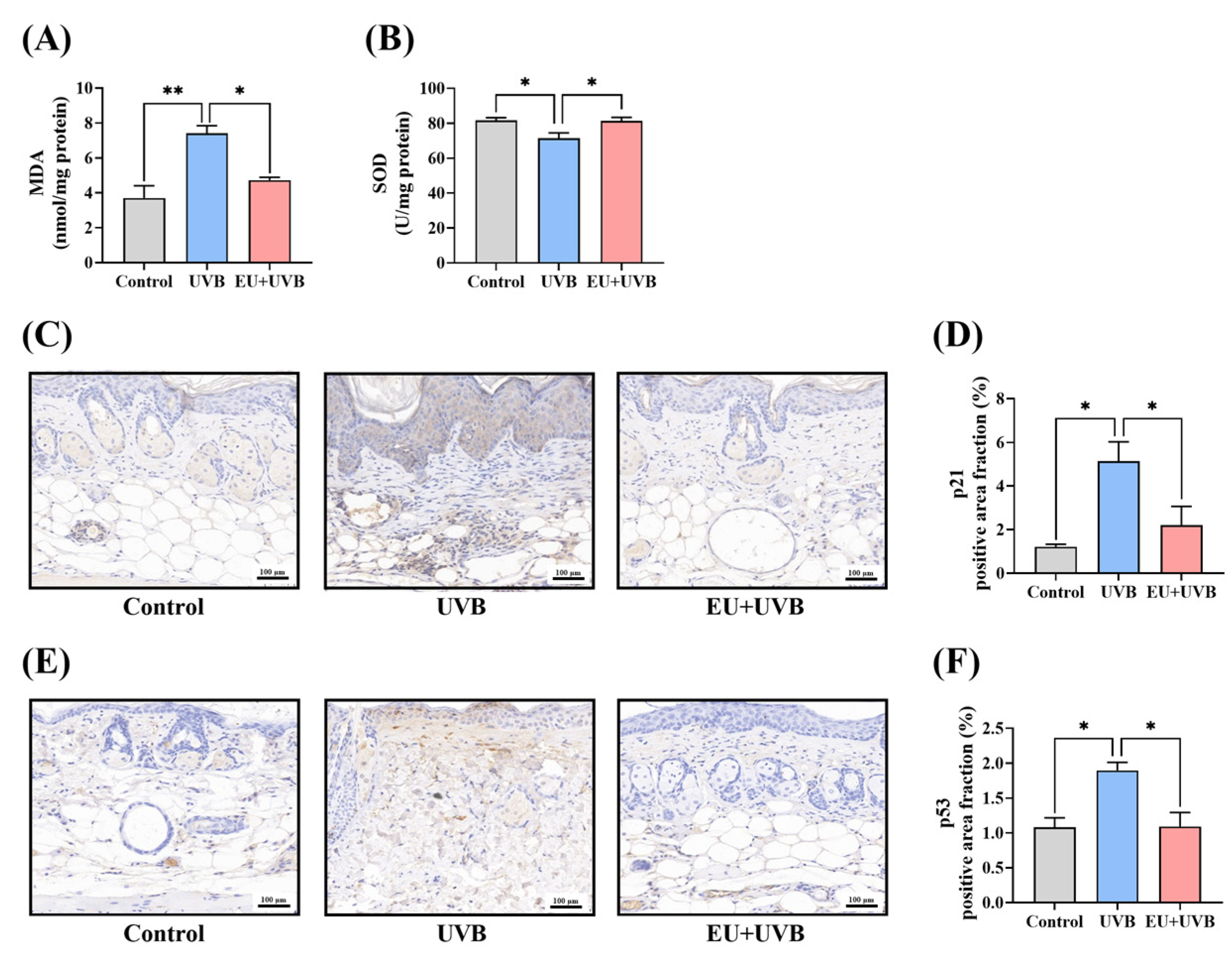
3.6.1. EU Alleviates Oxidative Stress in Photoaged Mouse Skin
3.6.2. EU Reduces the Expression of the p21 Senescence Marker and p53 DNA Damage Marker in the Skin of Mice Exposed to Chronic UVB Irradiation
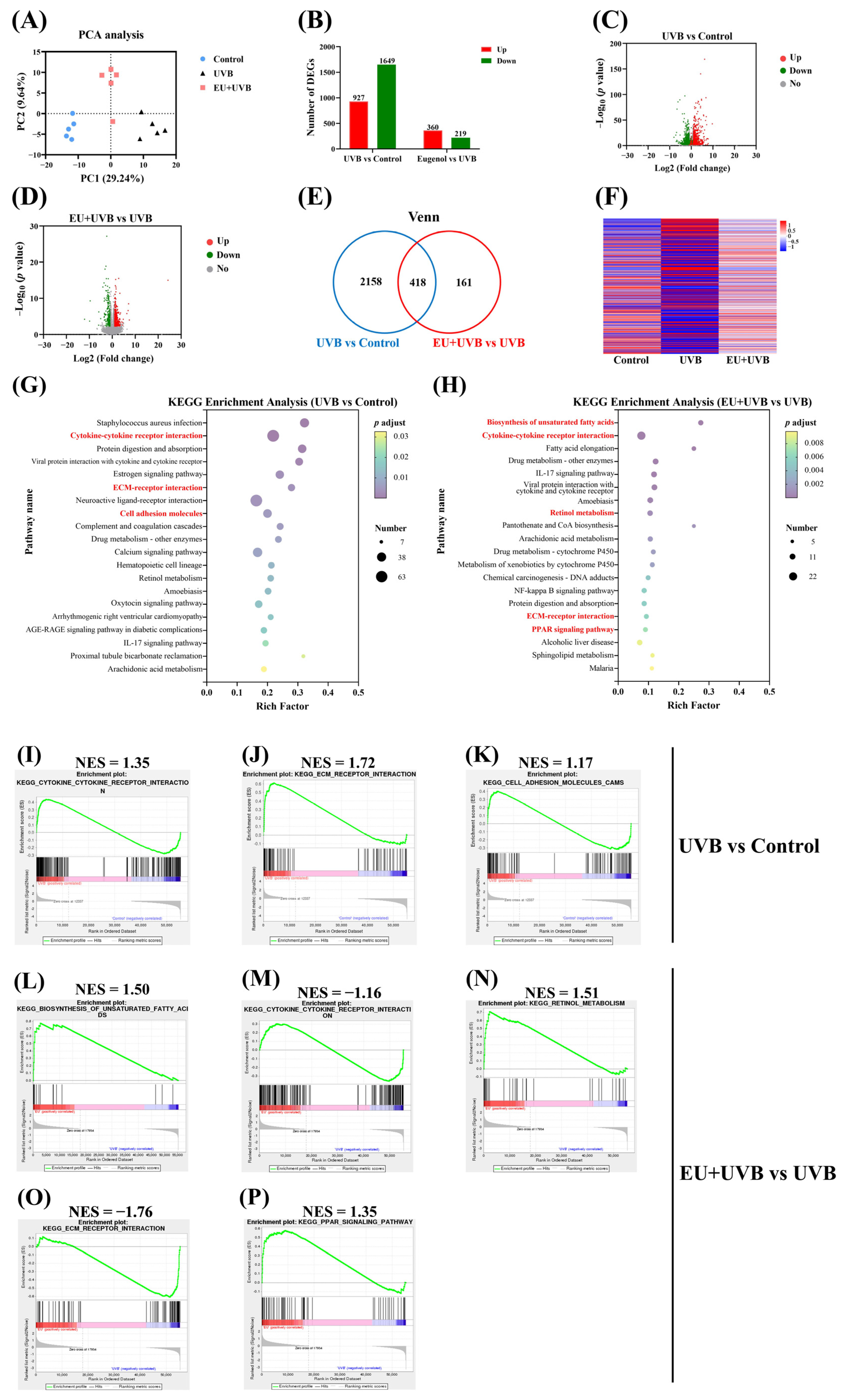
3.7. EU Modifies the Gene Expression Profiles in the Skin
3.8. EU Reverses Cytokine–Cytokine Receptor Interaction and ECM–Receptor Interaction in the Skin of Chronically UVB-Exposed Mice
3.9. PPI and TF Target Enrichment Analysis of the DEGs in the Key Pathways Influenced by EU Intervention
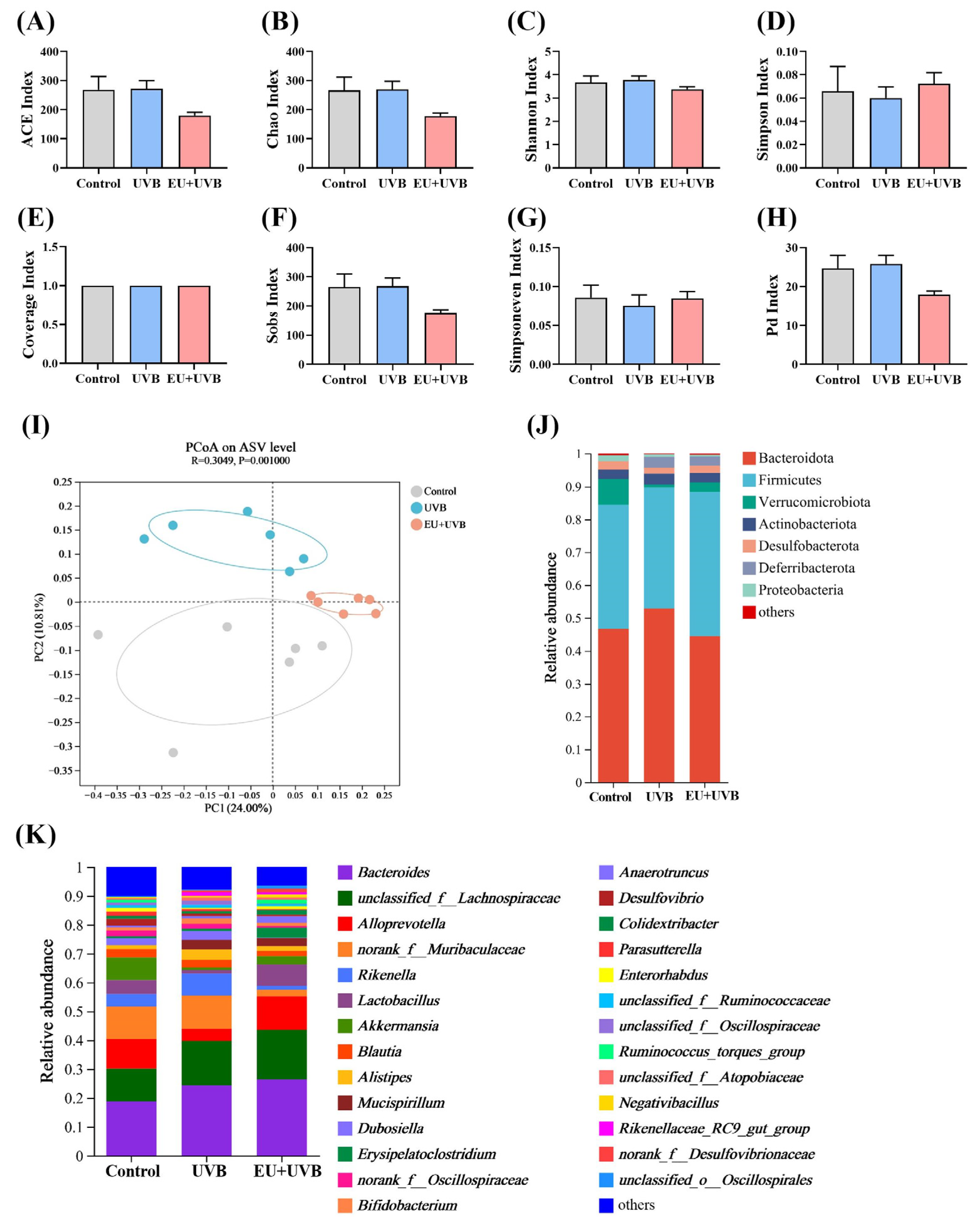
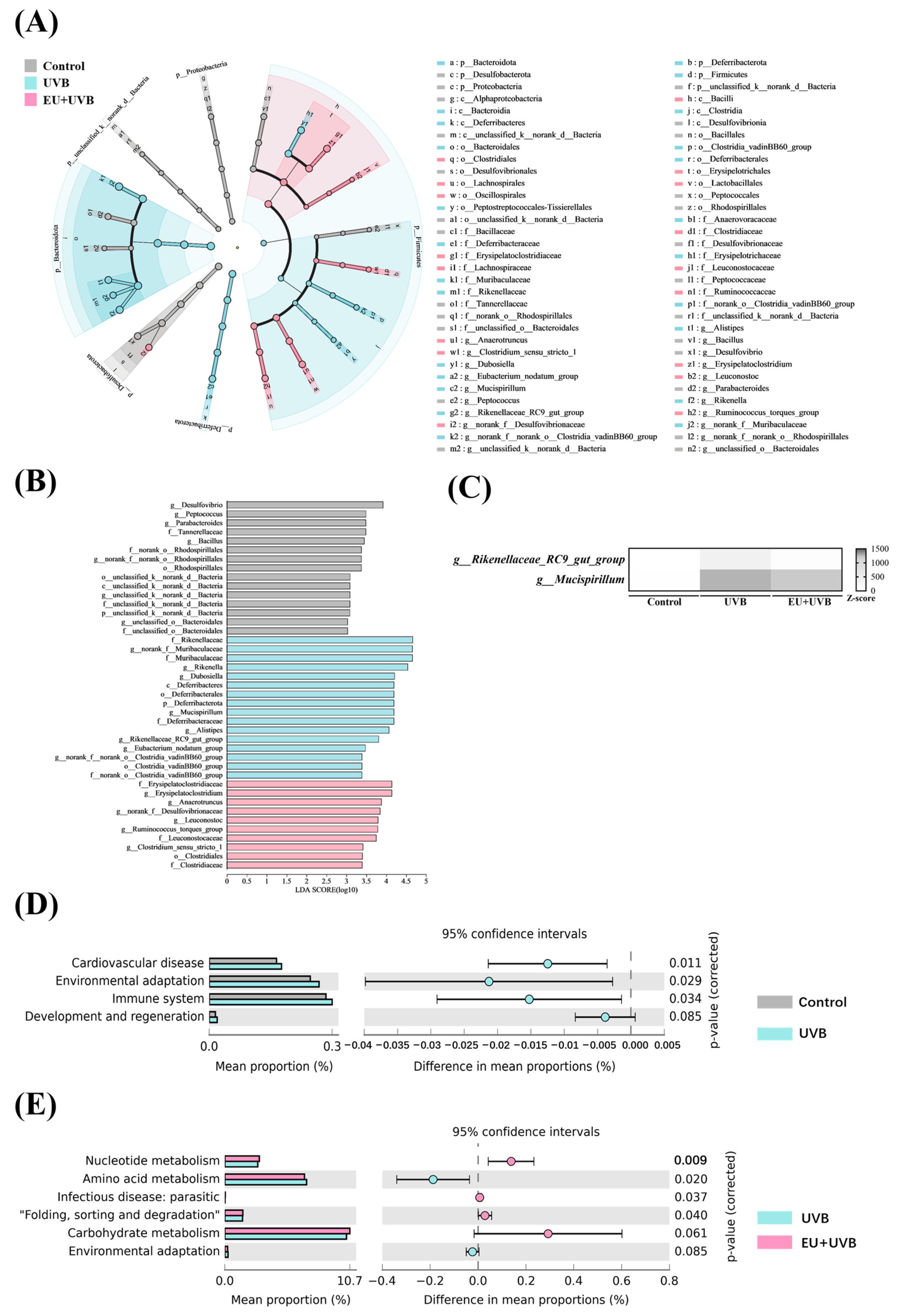
3.10. EU Modulates the Diversity and Composition of Gut Microbiota in Photoaged Mice
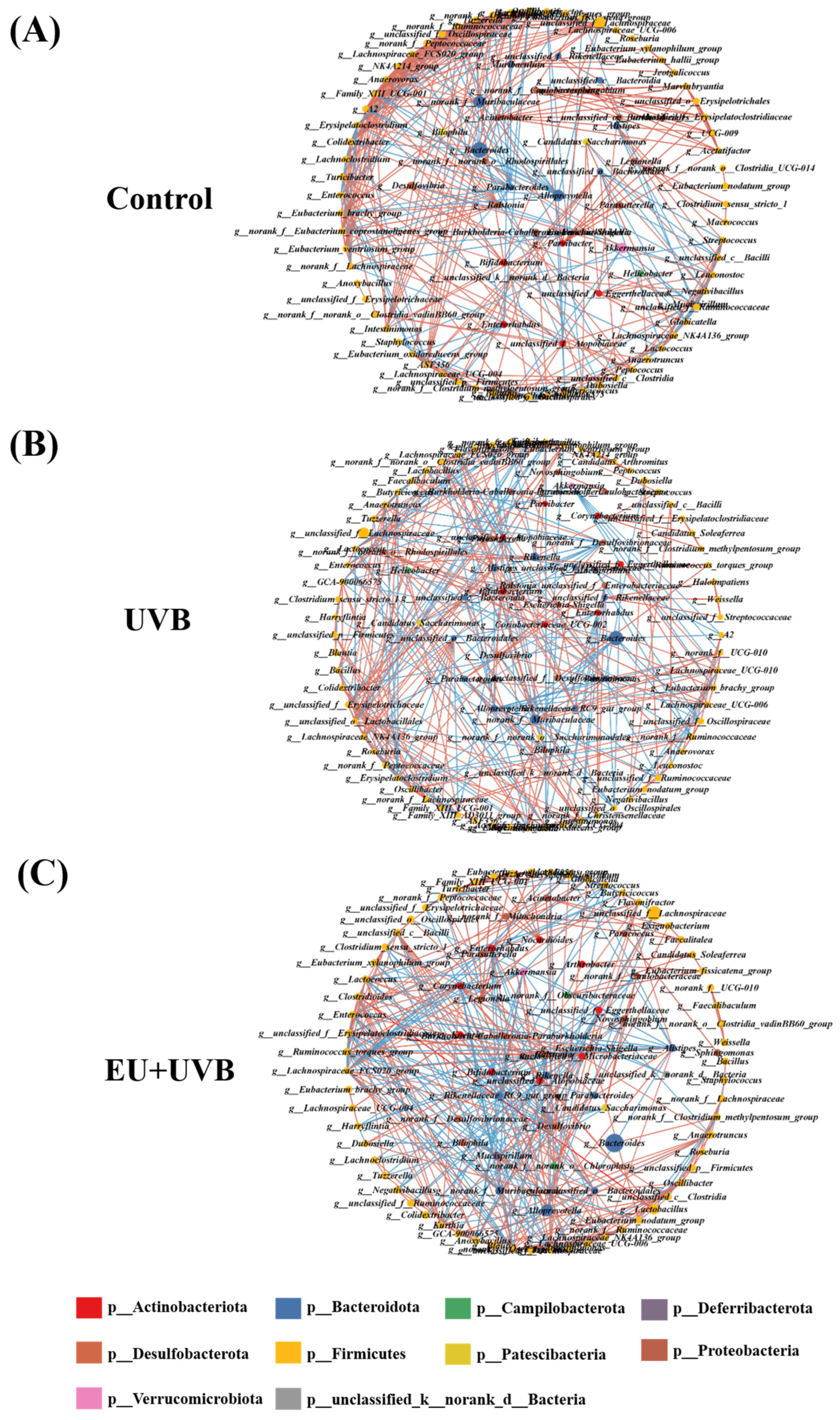
3.11. Characteristic Bacterial, Functional Prediction, and Co-Occurrence Network Analyses of the Gut Microbiota in the Photoaged Mice after EU Intervention
3.12. Analysis of the Correlation between the Skin Photoaging-Related Indexes, Hub Genes, and Relative Gut Microbial Abundance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geng, R.; Wang, Y.; Fang, J.; Zhao, Y.; Li, M.; Kang, S.G.; Huang, K.; Tong, T. Ectopic odorant receptors responding to flavor compounds in skin health and disease: Current insights and future perspectives. Crit. Rev. Food Sci. 2022, 21, 9392–9408. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin senescence: Mechanisms and impact on whole-body aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-induced (extrinsic) skin aging: Exposomal factors and underlying mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Leduc, C.; Verbeke, A.; Toussaint, O. UV, stress and aging. Derm.-Endocrinol. 2012, 4, 236–240. [Google Scholar] [CrossRef]
- Huang, A.H.; Chien, A.L. Photoaging: A review of current literature. Curr. Dermatol. Rep. 2020, 9, 22–29. [Google Scholar] [CrossRef]
- Bulbiankova, D.; Diaz-Puertas, R.; Alvarez-Martinez, F.J.; Herranz-Lopez, M.; Barrajon-Catalan, E.; Micol, V. Hallmarks and biomarkers of skin senescence: An updated review of skin senotherapeutics. Antioxidants 2023, 12, 444. [Google Scholar] [CrossRef]
- Geng, R.; Kang, S.; Huang, K.; Tong, T. Boosting the photoaged skin: The potential role of dietary components. Nutrients 2021, 13, 1691. [Google Scholar] [CrossRef]
- Tanveer, M.A.; Rashid, H.; Tasduq, S.A. Molecular basis of skin photoaging and therapeutic interventions by plant-derived natural product ingredients: A comprehensive review. Heliyon 2023, 9, e13580. [Google Scholar] [CrossRef]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Kumar, P.V.; Shams, R.; Singh, R.; Dar, A.H.; Pandiselvam, R.; Rusu, A.V.; Trif, M. A comprehensive review on clove (Caryophyllus aromaticus L.) essential oil and its significance in the formulation of edible coatings for potential food applications. Front. Nutr. 2022, 9, 987674. [Google Scholar] [CrossRef]
- Du, P.; Yuan, H.; Chen, Y.; Zhou, H.; Zhang, Y.; Huang, M.; Jiangfang, Y.; Su, R.; Chen, Q.; Lai, J.; et al. Identification of key aromatic compounds in basil (Ocimum L.) using sensory evaluation, metabolomics and volatilomics analysis. Metabolites 2023, 13, 85. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.W.; Im, G.J.; Chung, J.W.; Song, J.J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive staphylococcus aureus clinical strain biofilms. PLoS ONE 2015, 10, e119564. [Google Scholar] [CrossRef]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological properties and health benefits of eugenol: A comprehensive review. Oxidative Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef] [PubMed]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A natural compound with versatile pharmacological actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Y.; Wang, Y.; Geng, R.; Fang, J.; Kang, S.G.; Huang, K.; Tong, T. Eugenol, a major component of clove oil, attenuates adiposity, and modulates gut microbiota in high-fat diet-fed mice. Mol. Nutr. Food Res. 2022, 66, e2200387. [Google Scholar] [CrossRef]
- Ashjazadeh, M.A.; Jahandideh, A.; Abedi, G.; Akbarzadeh, A.; Hesaraki, S. Histopathology and histomorphological study of wound healing using clove extract nanofibers (eugenol) compared to zinc oxide nanofibers on the skin of rats. Arch. Razi Inst. 2019, 74, 267–277. [Google Scholar] [PubMed]
- De Araujo, L.A.; Da, F.F.; Rocha, T.M.; de Freitas, L.B.; Araujo, E.; Wong, D.; Lima, J.R.; Leal, L. Eugenol as a promising molecule for the treatment of dermatitis: Antioxidant and anti-inflammatory activities and its nanoformulation. Oxidative Med. Cell. Longev. 2018, 2018, 8194849. [Google Scholar]
- Pal, D.; Banerjee, S.; Mukherjee, S.; Roy, A.; Panda, C.K.; Das, S. Eugenol restricts DMBA croton oil induced skin carcinogenesis in mice: Downregulation of c-Myc and H-ras, and activation of p53 dependent apoptotic pathway. J. Dermatol. Sci. 2010, 59, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M. Eugenol—From the remote Maluku Islands to the international market place: A review of a remarkable and versatile molecule. Molecules 2012, 17, 6953–6981. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Lin, P.; Ngo, H.; Yi, T.H. Clove attenuates UVB-induced photodamage and repairs skin barrier function in hairless mice. Food Funct. 2018, 9, 4936–4947. [Google Scholar] [CrossRef]
- Yarosh, D.B.; Yee, V. SKH-1 hairless mice repair UV-induced pyrimidine dimers in epidermal DNA. J. Photochem. Photobiol. B Biol. 1990, 7, 173–179. [Google Scholar] [CrossRef]
- Kang, W.; Choi, D.; Park, T. Dietary suberic acid protects against UVB-induced skin photoaging in hairless mice. Nutrients 2019, 11, 2948. [Google Scholar] [CrossRef]
- Geng, R.; Kang, S.; Huang, K.; Tong, T. A-ionone protects against UVB-induced photoaging in epidermal keratinocytes. Chin. Herb. Med. 2022, 15, 132–138. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Nearing, J.T.; Douglas, G.M.; Hayes, M.G.; MacDonald, J.; Desai, D.K.; Allward, N.; Jones, C.; Wright, R.J.; Dhanani, A.S.; Comeau, A.M.; et al. Microbiome differential abundance methods produce different results across 38 datasets. Nat. Commun. 2022, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Park, N.J.; Bong, S.K.; Lee, S.; Jung, Y.; Jegal, H.; Kim, J.; Kim, S.K.; Kim, Y.K.; Kim, S.N. Compound K improves skin barrier function by increasing SPINK5 expression. J. Ginseng Res. 2020, 44, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Barrera, R.; Torres-Alvarez, B.; Castanedo-Cazares, J.P.; Oros-Ovalle, C.; Moncada, B. Solar elastosis and presence of mast cells as key features in the pathogenesis of melasma. Clin. Exp. Dermatol. 2008, 33, 305–308. [Google Scholar] [CrossRef]
- Carpenter, E.L.; Le, M.N.; Miranda, C.L.; Reed, R.L.; Stevens, J.F.; Indra, A.K.; Ganguli-Indra, G. Photoprotective properties of isothiocyanate and nitrile glucosinolate derivatives from meadowfoam (Limnanthes alba) against UVB irradiation in human skin equivalent. Front. Pharmacol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Yang, Z.; Dong, L.; Jin, S.; Han, X.; Li, F. Comparison of microfat, nanofat, and extracellular matrix/stromal vascular fraction gel for skin rejuvenation: Basic animal research. Aesthet. Surg. J. 2023, 43, 573–586. [Google Scholar] [CrossRef]
- National-Toxicology-Program. Carcinogenesis studies of eugenol (CAS No. 97-53-0) in F344/N rats and B6C3F1 mice (feed studies). Natl. Toxicol. Program Tech. Rep. Ser. 1983, 223, 1–159. [Google Scholar]
- Api, A.M.; Belsito, D.; Bhatia, S.; Bruze, M.; Calow, P.; Dagli, M.L.; Dekant, W.; Fryer, A.D.; Kromidas, L.; La Cava, S.; et al. RIFM fragrance ingredient safety assessment, eugenol, CAS registry number 97-53-0. Food Chem. Toxicol. 2016, 97S, S25–S37. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.J.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; Dekant, W.; et al. Update to RIFM fragrance ingredient safety assessment, eugenol, CAS registry number 97-53-0. Food Chem. Toxicol. 2022, 163 (Suppl. S1), 113027. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Fischer, I.U.; von Unruh, G.E.; Dengler, H.J. The metabolism of eugenol in man. Xenobiotica 1990, 20, 209. [Google Scholar] [CrossRef]
- Sutton, J.D.; Sangster, S.A.; Caldwell, J. Dose-dependent variation in the disposition of eugenol in the rat. Biochem. Pharmacol. 1985, 34, 465–466. [Google Scholar] [CrossRef]
- LópezOtín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2022, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Gollapalli, K.; Mangiola, S.; Schranner, D.; Yusuf, M.A.; Chamoli, M.; Shi, S.L.; Lopes, B.B.; Nair, T.; Riermeier, A.; et al. Taurine deficiency as a driver of aging. Science 2023, 380, n9257. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Chang, K.W.; Liu, C.J.; Tseng, Y.H.; Lu, H.H.; Lee, S.Y.; Lin, S.C. Ripe areca nut extract induces G1 phase arrests and senescence-associated phenotypes in normal human oral keratinocyte. Carcinogenesis 2006, 27, 1273–1284. [Google Scholar] [CrossRef]
- Ozcan, S.; Alessio, N.; Acar, M.B.; Mert, E.; Omerli, F.; Peluso, G.; Galderisi, U. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging 2016, 8, 1316–1329. [Google Scholar] [CrossRef]
- Freitas-Rodriguez, S.; Folgueras, A.R.; Lopez-Otin, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Q.; Wufuer, H.; Li, Z.; Sun, R.; Jiang, Z.; Dou, X.; Fu, Q.; Campisi, J.; Sun, Y. Rutin is a potent senomorphic agent to target senescent cells and can improve chemotherapeutic efficacy. Aging Cell 2023, 23, e13921. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; D’Adda, D.F.F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, J.; Song, M.J.; Park, C.H.; Lee, D.H.; Lee, S.H.; Chung, J.H. Inhibition of matrix metalloproteinase expression by selective clearing of senescent dermal fibroblasts attenuates ultraviolet-induced photoaging. Biomed. Pharmacother. 2022, 150, 113034. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, L.L.; Yan, L.; Zhang, F.; Yin, N.B.; Lin, H.B.; Huang, C.Y.; Wang, L.; Yu, J.; Wang, D.M.; et al. Transcriptome analysis of skin photoaging in chinese females reveals the involvement of skin homeostasis and metabolic changes. PLoS ONE 2013, 8, e61946. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Wang, X.J. TGFbeta signaling in photoaging and UV-induced skin cancer. J. Investig. Dermatol. 2021, 141, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Alafiatayo, A.A.; Lai, K.S.; Ahmad, S.; Mahmood, M.; Shaharuddin, N.A. RNA-Seq analysis revealed genes associated with UV-induced cell necrosis through MAPK/TNF-alpha pathways in human dermal fibroblast cells as an inducer of premature photoaging. Genomics 2020, 112, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Boyajian, J.L.; Ghebretatios, M.; Schaly, S.; Islam, P.; Prakash, S. Microbiome and human aging: Probiotic and prebiotic potentials in longevity, skin health and cellular senescence. Nutrients 2021, 13, 4550. [Google Scholar] [CrossRef] [PubMed]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2018, 23, 570. [Google Scholar] [CrossRef]
- Vaiserman, A.; Romanenko, M.; Piven, L.; Moseiko, V.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Koliada, A. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol. 2020, 20, 221. [Google Scholar] [CrossRef]
- Ratanapokasatit, Y.; Laisuan, W.; Rattananukrom, T.; Petchlorlian, A.; Thaipisuttikul, I.; Sompornrattanaphan, M. How microbiomes affect skin aging: The updated evidence and current perspectives. Life 2022, 12, 936. [Google Scholar] [CrossRef]
- Ghaly, S.; Kaakoush, N.O.; Lloyd, F.; Gordon, L.; Forest, C.; Lawrance, I.C.; Hart, P.H. Ultraviolet irradiation of skin alters the faecal microbiome independently of vitamin d in mice. Nutrients 2018, 10, 1069. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.; Fang, J.; Kang, S.G.; Huang, K.; Tong, T. Chronic exposure to UVB induces nephritis and gut microbiota dysbiosis in mice based on the integration of renal transcriptome profiles and 16S rRNA sequencing data. Environ. Pollut. 2023, 333, 122035. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Z.; Ma, Y.; Miao, L.; Zhao, K.; Wang, D.; Wang, M.; Ruan, H.; Xu, F.; Zhou, Q.; et al. Fecal microbiota transplantation affects the recovery of AD-skin lesions and enhances gut microbiota homeostasis. Int. Immunopharmacol. 2023, 118, 110005. [Google Scholar] [CrossRef]
- Kim, W.K.; Jang, Y.J.; Han, D.H.; Seo, B.; Park, S.; Lee, C.H.; Ko, G. Administration of lactobacillus fermentum KBL375 causes taxonomic and functional changes in gut microbiota leading to improvement of atopic dermatitis. Front. Mol. Biosci. 2019, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; An, M.; Tian, W.; Wang, K.; Du, B.; Li, P. Sacha inchi albumin delays skin-aging by alleviating inflammation, oxidative stress and regulating gut microbiota in d-galactose induced-aging mice. J. Sci. Food Agric. 2023, 103, 4470–4480. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Ma, W.; Pan, Z.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Chen, W.; Cui, S. Ameliorative effect of Lactobacillus plantarum CCFM8661 on oleic acid-induced acne: Integrated gut microbiota link to acne pathogenesis. J. Sci. Food Agric. 2023, 8, 13. [Google Scholar]
- Skopelja-Gardner, S.; Tai, J.; Sun, X.; Tanaka, L.; Kuchenbecker, J.A.; Snyder, J.M.; Kubes, P.; Mustelin, T.; Elkon, K.B. Acute skin exposure to ultraviolet light triggers neutrophil-mediated kidney inflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2019097118. [Google Scholar] [CrossRef]
- Reeve, V.E.; Allanson, M.; Domanski, D.; Painter, N. Gender differences in UV-induced inflammation and immunosuppression in mice reveal male unresponsiveness to UVA radiation. Photoch. Photobio. Sci. 2012, 11, 173–179. [Google Scholar] [CrossRef]
- Zhong, Q.Y.; Lin, B.; Chen, Y.T.; Huang, Y.P.; Feng, W.P.; Wu, Y.; Long, G.H.; Zou, Y.N.; Liu, Y.; Lin, B.Q.; et al. Gender differences in UV-induced skin inflammation, skin carcinogenesis and systemic damage. Environ. Toxicol. Pharmacol. 2021, 81, 103512. [Google Scholar] [CrossRef]
- Simon, G.; Dana, B.; Jacqueline, C.; Michael, W.C.; Anderson, P.J.; Stephanie, T.; Allan, G.K.; Rachel, E.N.; Prue, H.H. Vitamin D C3-epimer levels are proportionally higher with oral vitamin D supplementation compared to ultraviolet irradiation of skin in mice but not humans. J. Steroid Biochem. 2018, 186, 110–116. [Google Scholar]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV light touches the brain and endocrine system through skin, and why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, T.; Geng, R.; Kang, S.-G.; Li, X.; Huang, K. Revitalizing Photoaging Skin through Eugenol in UVB-Exposed Hairless Mice: Mechanistic Insights from Integrated Multi-Omics. Antioxidants 2024, 13, 168. https://doi.org/10.3390/antiox13020168
Tong T, Geng R, Kang S-G, Li X, Huang K. Revitalizing Photoaging Skin through Eugenol in UVB-Exposed Hairless Mice: Mechanistic Insights from Integrated Multi-Omics. Antioxidants. 2024; 13(2):168. https://doi.org/10.3390/antiox13020168
Chicago/Turabian StyleTong, Tao, Ruixuan Geng, Seong-Gook Kang, Xiaomin Li, and Kunlun Huang. 2024. "Revitalizing Photoaging Skin through Eugenol in UVB-Exposed Hairless Mice: Mechanistic Insights from Integrated Multi-Omics" Antioxidants 13, no. 2: 168. https://doi.org/10.3390/antiox13020168
APA StyleTong, T., Geng, R., Kang, S.-G., Li, X., & Huang, K. (2024). Revitalizing Photoaging Skin through Eugenol in UVB-Exposed Hairless Mice: Mechanistic Insights from Integrated Multi-Omics. Antioxidants, 13(2), 168. https://doi.org/10.3390/antiox13020168







