Sustained Systemic Antioxidative Effects of Intermittent Theta Burst Stimulation beyond Neurodegeneration: Implications in Therapy in 6-Hydroxydopamine Model of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing Conditions
2.2. Unilateral 6-Hydroxydopamine Lesion of the Right Substantia Nigra Pars Compacta
2.3. Rotarod Performance Test
2.4. Theta Burst Stimulation Protocol
2.5. Blood Serum and Brain Tissue Collection
2.6. Malondialdehyde Determination
2.7. Superoxide Anion Radical Determination
2.8. Nitric Oxide Determination
2.9. SOD Assay
2.10. Catalase Assay
2.11. GSH Content Determination
2.12. Total Sulfhydryl Groups (SH) Determination
2.13. Statistical Analyses
3. Results
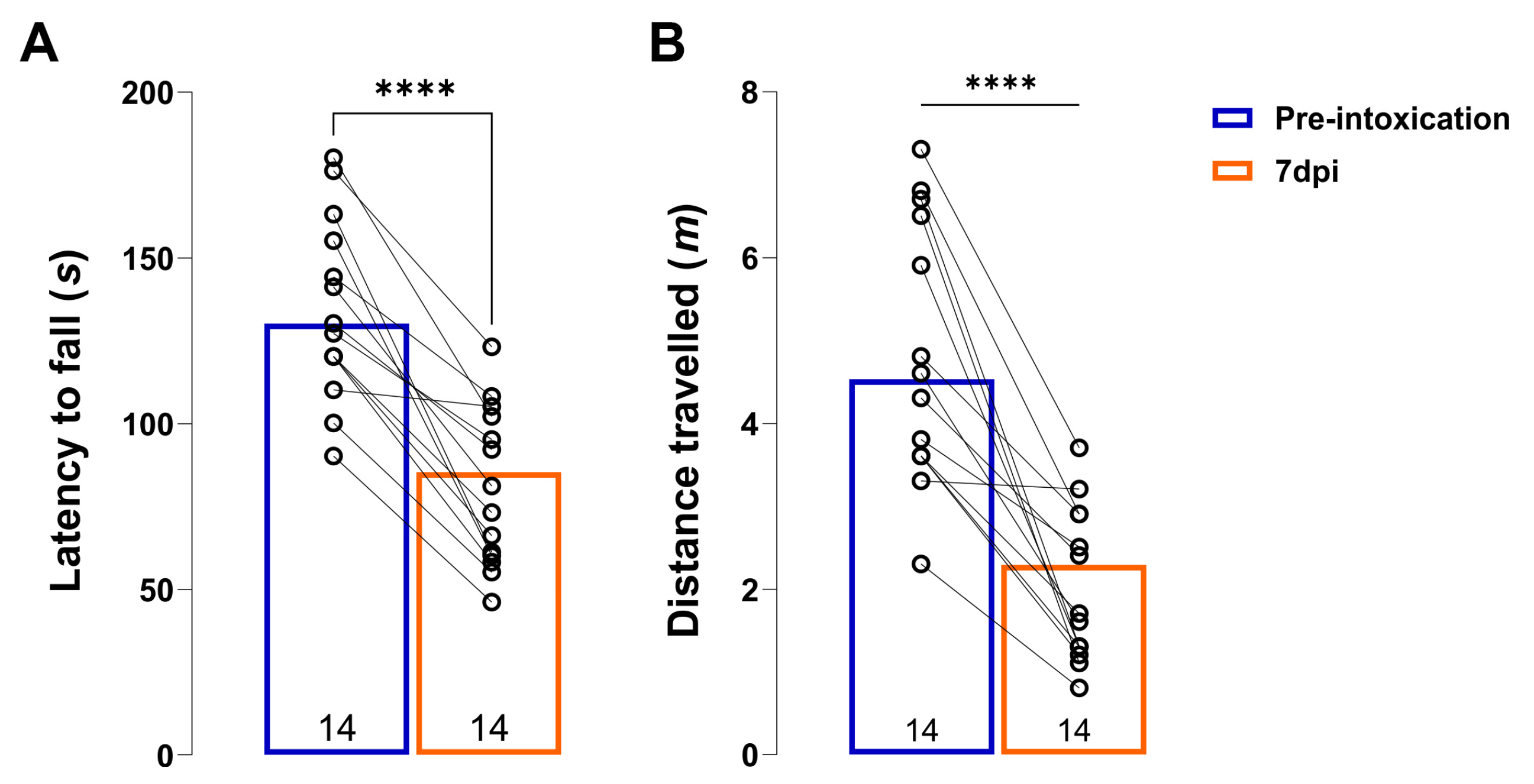
3.1. Behavioral Outcomes after Unilateral 6-OHDA Injection
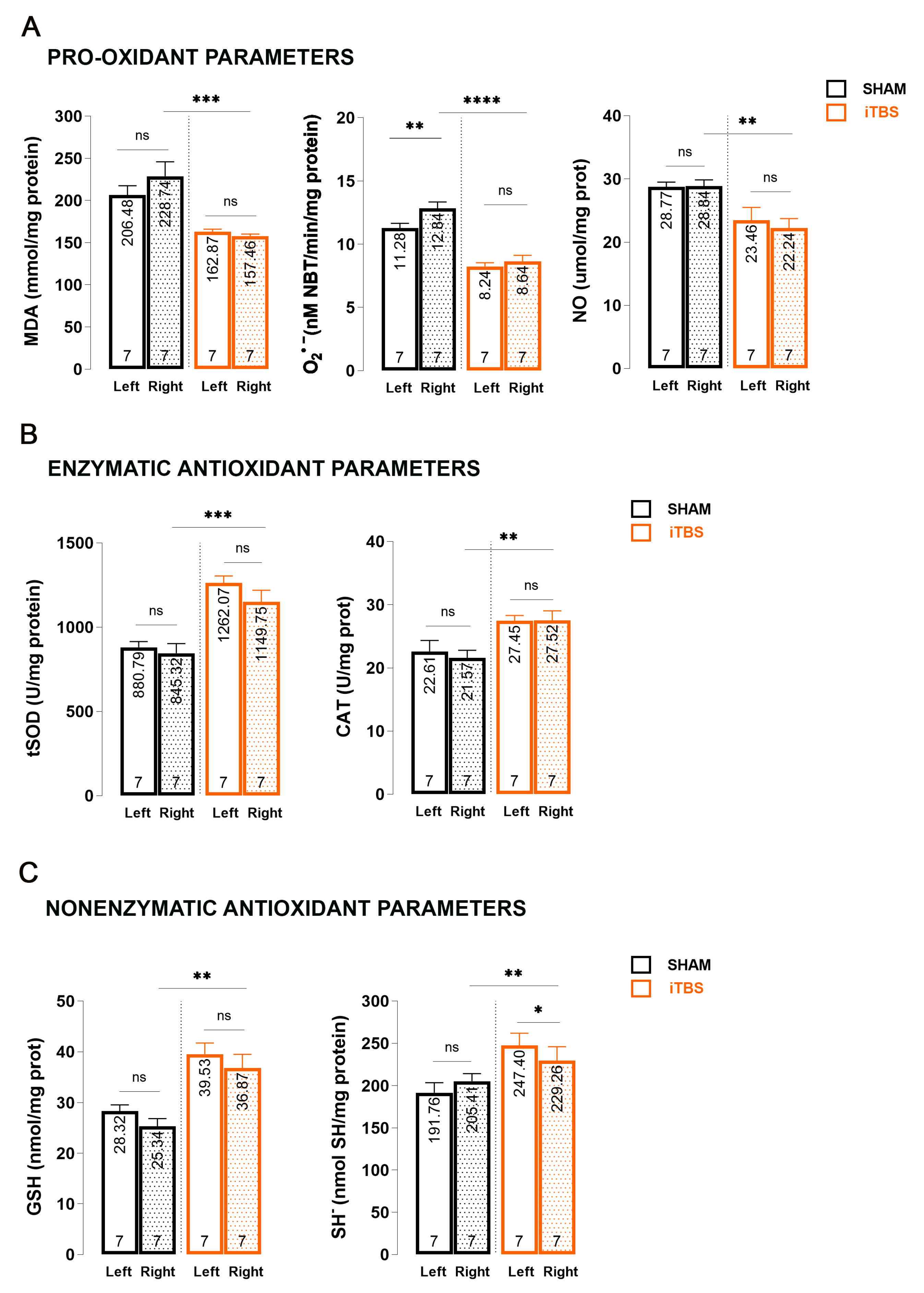
3.2. Effects of Prolonged iTBS Treatment on Oxidative Balance in the Caudoputamen of 6-OHDA-Induced Model of PD
3.3. Effects of Prolonged iTBS Treatment on Oxidative Balance in the Substantia Nigra Pars Compacta of 6-OHDA-Induced Model of PD
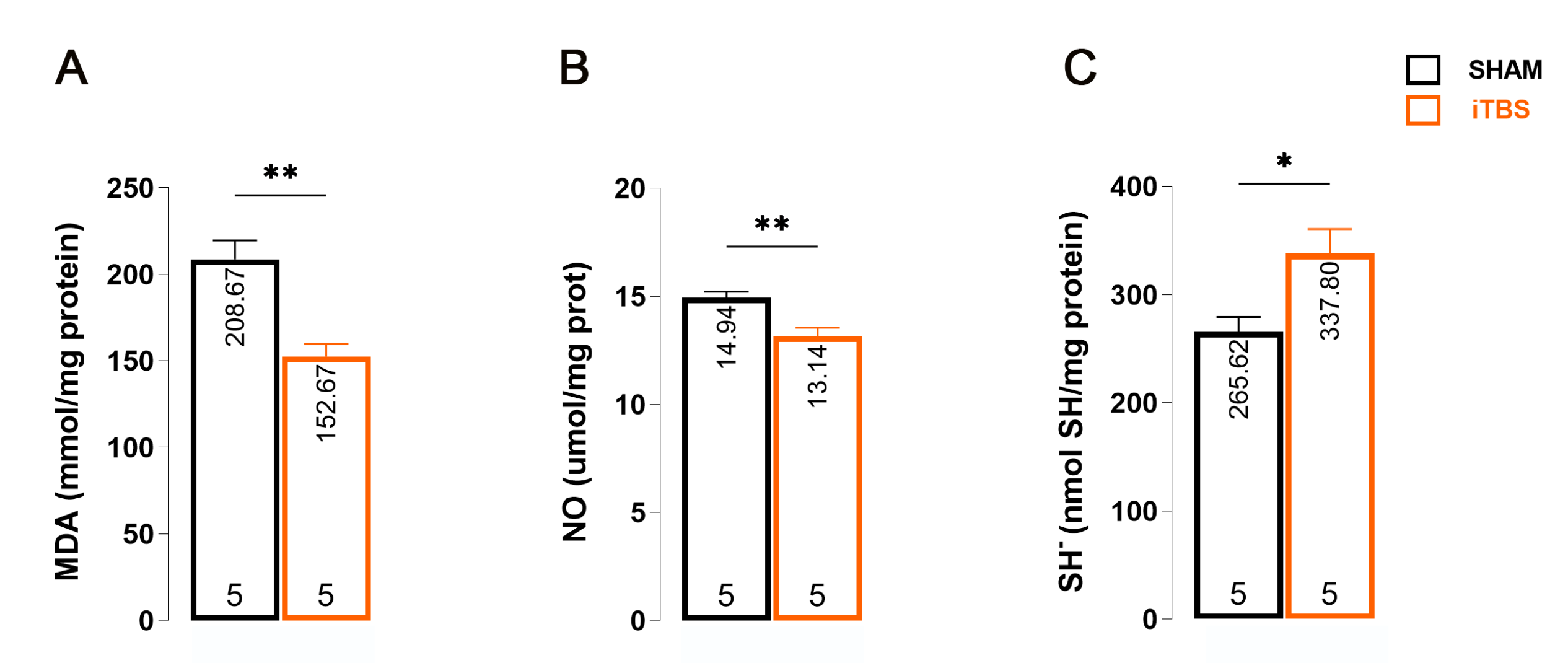
3.4. Effects of Prolonged iTBS Treatment on Oxidative Balance in the Serum of 6-OHDA-Induced Model of PD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson;s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Nakabeppu, Y.; Tsuchimoto, D.; Yamaguchi, H.; Sakumi, K. Oxidative damage in nucleic acids and Parkinson’s disease. J. Neurosci. Res. 2007, 85, 919–934. [Google Scholar] [CrossRef]
- Zeevalk, G.D.; Razmpour, R.; Bernard, L.P. Glutathione and Parkinson’s disease: Is this the elephant in the room? Biomed. Pharmacother. 2008, 62, 236–249. [Google Scholar] [CrossRef]
- Hernandez-Baltazar, D.; Zavala-Flores, L.M.; Villanueva-Olivo, A. The 6-hydroxydopamine model and parkinsonian pathophysiology: Novel findings in an older model. El modelo de 6-hidroxidopamina y la fisiopatología parkinsoniana: Nuevos hallazgos en un viejo modelo. Neurologia 2017, 32, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Masini, D.; Plewnia, C.; Bertho, M.; Scalbert, N.; Caggiano, V.; Fisone, G. A Guide to the Generation of a 6-Hydroxydopamine Mouse Model of Parkinson’s Disease for the Study of Non-Motor Symptoms. Biomedicines 2021, 9, 598. [Google Scholar] [CrossRef]
- Kin, K.; Yasuhara, T.; Kameda, M.; Date, I. Animal Models for Parkinson’s Disease Research: Trends in the 2000s. Int. J. Mol. Sci. 2019, 20, 5402. [Google Scholar] [CrossRef] [PubMed]
- Soto-Otero, R.; Méndez-Alvarez, E.; Hermida-Ameijeiras, A.; Muñoz-Patiño, A.M.; Labandeira-Garcia, J.L. Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants: Potential implication in relation to the pathogenesis of Parkinson’s disease. J. Neurochem. 2000, 74, 1605–1612. [Google Scholar] [CrossRef]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis Int. J. Program. Cell Death 2007, 12, 913–922. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53 (Suppl. S3), S26–S38. [Google Scholar] [CrossRef]
- Hwang, O. Role of oxidative stress in Parkinson’s disease. Exp. Neurobiol. 2013, 22, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Somaa, F.A.; de Graaf, T.A.; Sack, A.T. Transcranial Magnetic Stimulation in the Treatment of Neurological Diseases. Front. Neurol. 2022, 13, 793253. [Google Scholar] [CrossRef] [PubMed]
- Chail, A.; Saini, R.K.; Bhat, P.S.; Srivastava, K.; Chauhan, V. Transcranial magnetic stimulation: A review of its evolution and current applications. Ind. Psychiatry J. 2018, 27, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Yulug, B.; Hanoglu, L.; Kilic, E.; Polat, B.; Schabitz, W.R. The Neuroprotective Role of Repetitive Transcranial Magnetic Stimulation (rTMS) for Neurodegenerative Diseases: A Short Review on Experimental Studies. Mini Rev. Med. Chem. 2016, 16, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Uzair, M.; Abualait, T.; Arshad, M.; Khallaf, R.A.; Niaz, A.; Thani, Z.; Yoo, W.K.; Túnez, I.; Demirtas-Tatlidede, A.; et al. Effects of transcranial magnetic stimulation on neurobiological changes in Alzheimer’s disease (Review). Mol. Med. Rep. 2022, 25, 109. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Rogasch, N.C.; Hoy, K.E.; Sullivan, C.M.; Cash, R.F.H.; Fitzgerald, P.B. Impact of different intensities of intermittent theta burst stimulation on the cortical properties during TMS-EEG and working memory performance. Hum. Brain Mapp. 2018, 39, 783–802. [Google Scholar] [CrossRef] [PubMed]
- Stekic, A.; Zeljkovic, M.; Zaric Kontic, M.; Mihajlovic, K.; Adzic, M.; Stevanovic, I.; Ninkovic, M.; Grkovic, I.; Ilic, T.V.; Nedeljkovic, N.; et al. Intermittent Theta Burst Stimulation Ameliorates Cognitive Deficit and Attenuates Neuroinflammation via PI3K/Akt/mTOR Signaling Pathway in Alzheimer’s-Like Disease Model. Front. Aging Neurosci. 2022, 14, 889983. [Google Scholar] [CrossRef]
- Dragic, M.; Zeljkovic, M.; Stevanovic, I.; Ilic, T.; Ilic, N.; Nedeljkovic, N.; Ninkovic, M. Theta burst stimulation ameliorates symptoms of experimental autoimmune encephalomyelitis and attenuates reactive gliosis. Brain Res. Bull. 2020, 162, 208–217. [Google Scholar] [CrossRef]
- Stanojevic, J.; Dragic, M.; Stevanovic, I.; Ilic, T.; Stojanovic, I.; Zeljkovic, M.; Ninkovic, M. Intermittent theta burst stimulation ameliorates cognitive impairment and hippocampal gliosis in the Streptozotocin-induced model of Alzheimer’s disease. Behav. Brain Res. 2022, 433, 113984. [Google Scholar] [CrossRef]
- Dragić, M.; Zeljković, M.; Stevanović, I.; Adžić, M.; Stekić, A.; Mihajlović, K.; Grković, I.; Ilić, N.; Ilić, T.V.; Nedeljković, N.; et al. Downregulation of CD73/A2AR-Mediated Adenosine Signaling as a Potential Mechanism of Neuroprotective Effects of Theta-Burst Transcranial Magnetic Stimulation in Acute Experimental Autoimmune Encephalomyelitis. Brain Sci. 2021, 11, 736. [Google Scholar] [CrossRef]
- Stanojevic, J.B.; Zeljkovic, M.; Dragic, M.; Stojanovic, I.R.; Ilic, T.V.; Stevanovic, I.D.; Ninkovic, M.B. Intermittent theta burst stimulation attenuates oxidative stress and reactive astrogliosis in the streptozotocin-induced model of Alzheimer’s disease-like pathology. Front. Aging Neurosci. 2023, 15, 1161678. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Z.; Zhang, Z.; Liu, D.; Manolios, E.N.; Chen, C.; Yan, X.; Zuo, W.; Chen, N. The extended application of The Rat Brain in Stereotaxic Coordinates in rats of various body weight. J. Neurosci. Methods 2018, 307, 60–69. [Google Scholar] [CrossRef]
- Zeljkovic Jovanovic, M.; Stanojevic, J.; Stevanovic, I.; Stekic, A.; Bolland, S.J.; Jasnic, N.; Ninkovic, M.; Zaric Kontic, M.; Ilic, T.V.; Rodger, J.; et al. Intermittent Theta Burst Stimulation Improves Motor and Behavioral Dysfunction through Modulation of NMDA Receptor Subunit Composition in Experimental Model of Parkinson’s Disease. Cells 2023, 12, 1525. [Google Scholar] [CrossRef]
- Zaric, M.; Drakulic, D.; Dragic, M.; Gusevac Stojanovic, I.; Mitrovic, N.; Grkovic, I.; Martinovic, J. Molecular Alterations and Effects of Acute Dehydroepiandrosterone Treatment Following Brief Bilateral Common Carotid Artery Occlusion: Relevance to Transient Ischemic Attack. Neuroscience 2019, 410, 128–139. [Google Scholar] [CrossRef]
- Girotti, M.J.; Khan, N.; McLellan, B.A. Early measurement of systemic lipid peroxidation products in the plasma of major blunt trauma patients. J. Trauma 1991, 31, 32–35. [Google Scholar] [CrossRef]
- Kono, Y.; Kobayashi, K.; Tagawa, S.; Adachi, K.; Ueda, A.; Sawa, Y.; Shibata, H. Antioxidant activity of polyphenolics in diets. Rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochim. Et Biophys. Acta 1997, 1335, 335–342. [Google Scholar] [CrossRef]
- Navarro-Gonzálvez, J.A.; García-Benayas, C.; Arenas, J. Semiautomated measurement of nitrate in biological fluids. Clin. Chem. 1998, 44, 679–681. [Google Scholar] [CrossRef]
- Salatić, I.; Dragović, T.; Stevanović, I.; Pavlović, B.D.; Ninković, M. Is the insulin necessary for the struggle against oxidative stress in diabetes mellitus type 2–a pilot study. Vojnosanit. Pregl. 2022, 79, 433–440. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, V.; Daković, D.; Stevanović, I. Oxidative/antioxidative effects of colloidal silver ions and chlorhexidine in saliva and gingival fluid of periodontal patients. Vojnosanit. Pregl. 2022, 79, 441–447. [Google Scholar] [CrossRef]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta; Int. J. Clin. Chem. 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Stohs, S.J.; Lawson, T.A.; Anderson, L.; Bueding, E. Effects of oltipraz, BHA, ADT and cabbage on glutathione metabolism, DNA damage and lipid peroxidation in old mice. Mech. Ageing Dev. 1986, 37, 137–145. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Latchoumycandane, C.; Anantharam, V.; Jin, H.; Kanthasamy, A.; Kanthasamy, A. Dopaminergic neurotoxicant 6-OHDA induces oxidative damage through proteolytic activation of PKCδ in cell culture and animal models of Parkinson’s disease. Toxicol. Appl. Pharmacol. 2011, 256, 314–323. [Google Scholar] [CrossRef]
- Tieu, K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2011, 1, a009316. [Google Scholar] [CrossRef]
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.; Benabid, A.L.; Sadoul, R.; Verna, J.M. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001, 65, 135–172. [Google Scholar] [CrossRef]
- Dexter, D.T.; Carter, C.J.; Wells, F.R.; Javoy-Agid, F.; Agid, Y.; Lees, A.; Jenner, P.; Marsden, C.D. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J. Neurochem. 1989, 52, 381–389. [Google Scholar] [CrossRef]
- Sriraksa, N.; Wattanathorn, J.; Muchimapura, S.; Tiamkao, S.; Brown, K.; Chaisiwamongkol, K. Cognitive-enhancing effect of quercetin in a rat model of Parkinson’s disease induced by 6-hydroxydopamine. Evid.-Based Complement. Altern. Med. Ecam 2012, 2012, 823206. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.P.; Cass, W.A. Oxidative stress and dopamine depletion in an intrastriatal 6-hydroxydopamine model of Parkinson’s disease. Neuroscience 2007, 144, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Siew, C.J.; Ponnusamy, K. Effect of quercetin and desferrioxamine on 6-hydroxydopamine (6-OHDA) induced neurotoxicity in striatum of rats. J. Toxicol. Sci. 2013, 38, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Sian, J.; Dexter, D.T.; Lees, A.J.; Daniel, S.; Agid, Y.; Javoy-Agid, F.; Jenner, P.; Marsden, C.D. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994, 36, 348–355. [Google Scholar] [CrossRef]
- Ahmad, M.; Saleem, S.; Ahmad, A.S.; Yousuf, S.; Ansari, M.A.; Khan, M.B.; Ishrat, T.; Chaturvedi, R.K.; Agrawal, A.K.; Islam, F. Ginkgo biloba affords dose-dependent protection against 6-hydroxydopamine-induced parkinsonism in rats: Neurobehavioural, neurochemical and immunohistochemical evidences. J. Neurochem. 2005, 93, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, J.C.; Neves, K.R.; Leal, L.K.; do Carmo, M.R.; Brito, G.A.; Naffah-Mazzacoratti, M.d.G.; Cavalheiro, É.A.; Viana, G.S. Valproic Acid Neuroprotection in the 6-OHDA Model of Parkinson’s Disease Is Possibly Related to Its Anti-Inflammatory and HDAC Inhibitory Properties. J. Neurodegener. Dis. 2015, 2015, 313702. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Ali, S.A. Oxidative stress-related biomarkers in Parkinson’s disease: A systematic review and meta-analysis. Iran. J. Neurol. 2018, 17, 137–144. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Mariani, L.L.; Mangone, G.; Le Febvre de Nailly, D.; Charbonnier-Beaupel, F.; Corvol, J.C. Molecular basis of dopamine replacement therapy and its side effects in Parkinson’s disease. Cell Tissue Res. 2018, 373, 111–135. [Google Scholar] [CrossRef]
- Medina-Fernández, F.J.; Escribano, B.M.; Padilla-Del-Campo, C.; Drucker-Colín, R.; Pascual-Leone, Á.; Túnez, I. Transcranial magnetic stimulation as an antioxidant. Free Radic. Res. 2018, 52, 381–389. [Google Scholar] [CrossRef]
- Cullen, C.L.; Young, K.M. How Does Transcranial Magnetic Stimulation Influence Glial Cells in the Central Nervous System? Front. Neural Circuits 2016, 10, 26. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.X.; Dai, S.X.; Guo, Y.C.; Han, F.F.; Zheng, J.J.; Li, G.H.; Huang, J.F. Meta-Analysis of Parkinson’s Disease and Alzheimer’s Disease Revealed Commonly Impaired Pathways and Dysregulation of NRF2-Dependent Genes. J. Alzheimer’s Dis. JAD 2017, 56, 1525–1539. [Google Scholar] [CrossRef]

| Parameters | SNpc | CPu |
|---|---|---|
| MDA | Interaction: F(1, 21) = 1.22; p = 0.2814 | Interaction: F(1, 22) = 3.44; p = 0.0769 |
| Hemisphere: F(1, 21) = 2.13; p = 0.1593 | Hemisphere: F(1, 22) = 3.30; p = 0.0826 | |
| Treatment: F(1, 21) = 42.51; p < 0.0001 | Treatment: F(1, 22) = 211.0; p < 0.0001 | |
| O2•− | Interaction: F(1, 20) = 4.02; p = 0.0585 | Interaction: F(1, 18) = 0.83; p = 0.3730 |
| Hemisphere: F(1, 20) = 13.62; p = 0.0015 | Hemisphere: F(1, 18) = 0.83; p = 0.3729 | |
| Treatment: F(1, 20) = 83.32; p < 0.0001 | Treatment: F(1, 18) = 220.1; p < 0.0001 | |
| NO | Interaction: F(1, 20) = 0.66; p = 0.4235 | Interaction: F(1, 24) = 0.79; p = 0.3805 |
| Hemisphere: F(1, 20) = 0.003; p = 0.9563 | Hemisphere: F(1, 24) = 0.79; p = 0.3805 | |
| Treatment: F(1, 20) = 22.00; p = 0.0001 | Treatment: F(1, 24) = 39.77; p < 0.0001 | |
| tSOD | Interaction: F(1, 21) = 0.66; p = 0.8945 | Interaction: F(1, 18) = 0.83; p = 0.3730 |
| Hemisphere: F(1, 21) = 0.003; p = 0.1376 | Hemisphere: F(1, 18) = 0.83; p = 0.3729 | |
| Treatment: F(1, 21) = 22.00; p < 0.0001 | Treatment: F(1, 18) = 220.1; p < 0.0001 | |
| CAT | Interaction: F(1, 20) = 2.27; p = 0.1471 | Interaction: F(1, 21) = 1.42; p = 0.2463 |
| Hemisphere: F(1, 20) = 1.31; p = 0.2656 | Hemisphere: F(1, 21) = 0.68; p = 0.4158 | |
| Treatment: F(1, 20) = 22.32; p = 0.0001 | Treatment: F(1, 21) = 49.55; p < 0.0001 | |
| GSH | Interaction: F(1, 20) = 0.21; p = 0.6476 | Interaction: F(1, 18) = 10.02; p = 0.0054 |
| Hemisphere: F(1, 20) = 3.41; p = 0.0795 | Hemisphere: F(1, 18) = 0.04; p = 0.8333 | |
| Treatment: F(1, 20) = 35.64; p = 0.0001 | Treatment: F(1, 18) = 60.78; p < 0.0001 | |
| SH− | Interaction: F(1, 21) = 5.80; p = 0.0252 | Interaction: F(1, 22) = 0.39; p = 0.5342 |
| Hemisphere: F(1, 21) = 7.35; p = 0.0130 | Hemisphere: F(1, 22) = 2.80; p = 0.1081 | |
| Treatment: F(1, 21) = 13.32; p = 0.0015 | Treatment: F(1, 22) = 27.97; p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeljkovic Jovanovic, M.; Stanojevic, J.; Stevanovic, I.; Ninkovic, M.; Nedeljkovic, N.; Dragic, M. Sustained Systemic Antioxidative Effects of Intermittent Theta Burst Stimulation beyond Neurodegeneration: Implications in Therapy in 6-Hydroxydopamine Model of Parkinson’s Disease. Antioxidants 2024, 13, 218. https://doi.org/10.3390/antiox13020218
Zeljkovic Jovanovic M, Stanojevic J, Stevanovic I, Ninkovic M, Nedeljkovic N, Dragic M. Sustained Systemic Antioxidative Effects of Intermittent Theta Burst Stimulation beyond Neurodegeneration: Implications in Therapy in 6-Hydroxydopamine Model of Parkinson’s Disease. Antioxidants. 2024; 13(2):218. https://doi.org/10.3390/antiox13020218
Chicago/Turabian StyleZeljkovic Jovanovic, Milica, Jelena Stanojevic, Ivana Stevanovic, Milica Ninkovic, Nadezda Nedeljkovic, and Milorad Dragic. 2024. "Sustained Systemic Antioxidative Effects of Intermittent Theta Burst Stimulation beyond Neurodegeneration: Implications in Therapy in 6-Hydroxydopamine Model of Parkinson’s Disease" Antioxidants 13, no. 2: 218. https://doi.org/10.3390/antiox13020218
APA StyleZeljkovic Jovanovic, M., Stanojevic, J., Stevanovic, I., Ninkovic, M., Nedeljkovic, N., & Dragic, M. (2024). Sustained Systemic Antioxidative Effects of Intermittent Theta Burst Stimulation beyond Neurodegeneration: Implications in Therapy in 6-Hydroxydopamine Model of Parkinson’s Disease. Antioxidants, 13(2), 218. https://doi.org/10.3390/antiox13020218









