Types of Membrane Transporters and the Mechanisms of Interaction between Them and Reactive Oxygen Species in Plants
Abstract
:1. Introduction
2. Types of Membrane Transporters in Plants
2.1. Ion Transporters
2.1.1. Na+ Transporters
2.1.2. K+ Transporters
2.1.3. Ca2+ Transporters
2.1.4. H+ Transporters
2.1.5. Anion Transporters
2.1.6. Other Ion Transporters
2.2. Sugar Transporters
2.3. Amino Acid Transporters
2.4. Other Compound Transporters
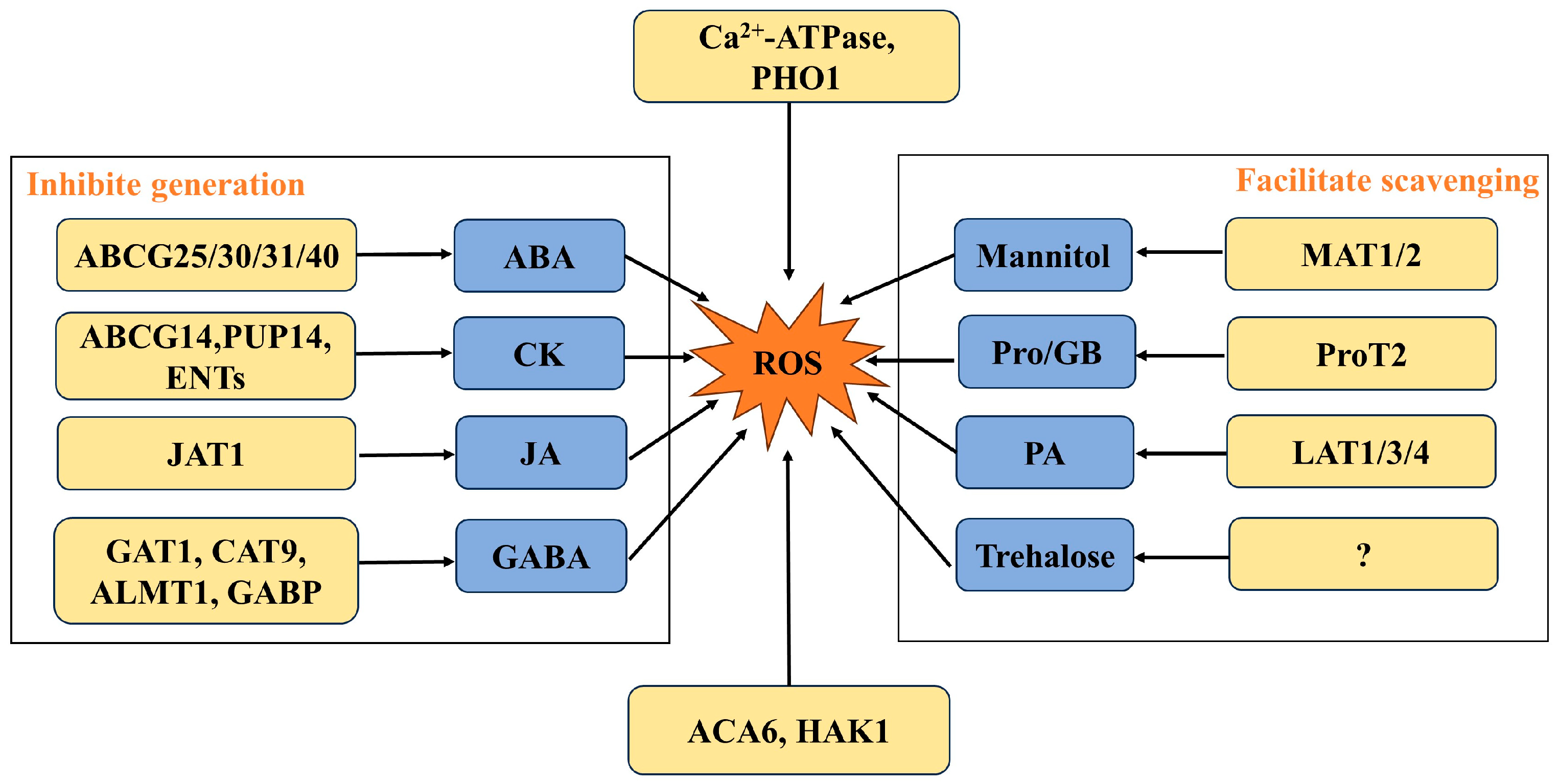
3. Membrane Transporters Regulate the Generation and Scavenging of ROS
3.1. Membrane Transporters Involved in the Generation of ROS
3.1.1. Membrane Transporters Directly Inhibit ROS Generation
3.1.2. Membrane Transporters Inhibit ROS Generation by Transporting ABA
3.1.3. Membrane Transporters Inhibit ROS Generation by Transporting GABA
3.1.4. Membrane Transporters Inhibit ROS Generation by Transporting Cytokinins (CKs)
3.1.5. Membrane Transporters Inhibit ROS Generation by Transporting Jasmonic Acid (JA)
3.2. Membrane Transporters Involved in the Scavenging of ROS
3.2.1. Membrane Transporters Directly Scavenge ROS
3.2.2. Membrane Transporters Scavenge ROS by Transporting Proline
3.2.3. Membrane Transporters Scavenge ROS by Transporting Mannitol
3.2.4. Membrane Transporters Scavenge ROS by Transporting Polyamines (PAs)
| Name | Species | Description | Localization | Family |
|---|---|---|---|---|
| ACA6 [154] | Arabidopsis, Rice | A Ca2+-ATPase responsible for the efflux of Ca2+ in the cytosol, reducing the concentration of Ca2+in the cytosol, thereby reducing the stimulation of RBOH by Ca2+ and reducing the generation of ROS. | Plasma membrane and endomembranes | P-type ATPase |
| PHO1 [124] | Arabidopsis | In Arabidopsis, it controls the efflux of phosphatidic acid and inhibits its activation of RBOH. | Plasma membrane | PHO |
| ABCG25/31 [126] | Arabidopsis | A G-type ABC transporter responsible for ABA efflux and involved in inhibiting plant ROS production. | Plasma membrane | ABC |
| ABCG30/40 [126] | Arabidopsis | A G-type ABC transporter responsible for ABA efflux and involved in inhibiting plant ROS production. | Plasma membrane | ABC |
| ABCG14 [140] | Arabidopsis | A G-type ABC transporter that controls the influx of CK into the cytosol and is also responsible for the transport of CK from roots to leaves. | Plasma membrane | ABC |
| CAT9 [97] | Tomato | A cationic amino acid transporter responsible for the bidirectional transport of GABA and other amino acids between the cytosol and vacuoles, which involves in inhibiting ROS generation and promoting ROS scavenging. | Vacuolar membrane | APC |
| GABP [99] | Arabidopsis | A bidirectional amino acid transporter responsible for the transport of GABA between the cytosol and mitochondria, which involves inhibiting ROS generation and promoting ROS scavenging. | Mitochondrial membrane | APC |
| ProT2 [160] | Arabidopsis | In Arabidopsis, it controls the influx of proline across the plasma membrane and promotes the scavenging of ROS. | Plasma membrane | AAAP |
| ALMT1 [75] | Arabidopsis, Wheat | An aluminum-activated malate transporter responsible for the transport GABA and Cl− across plasma membranes to inhibit ROS generation and promote ROS scavenging. | Plasma membrane and vacuolar membrane | ALMT |
| GAT1 [101] | Arabidopsis | A high-affinity GABA transporter that can transport GABA from the apoplast to the cytosol, which involves inhibiting ROS generation and promoting ROS scavenging. | Plasma membrane | AAAP |
| PUP14 [139] | Arabidopsis | In Arabidopsis, it is responsible for transporting apoplast free radicals or nucleosides of CK to the cytosol, which inhibits the generation of ROS. | Plasma membrane | PUP |
| ENTs [139] | Arabidopsis | In Arabidopsis, it is responsible for the transport of free radicals or nucleosides from the apoplast and vacuoles into the cytosol, which inhibits ROS production. | Plasma membrane and vacuolar membrane | ENT |
| JAT1 [146] | Arabidopsis | In Arabidopsis, it is responsible for the transport of JA in cells, which is involved in inhibiting ROS production. | Vacuolar membrane | MATE |
| HAK1 [157] | Arabidopsis | A high-affinity K+ transporter; the overexpression of HAK1 can significantly increase the activity of POD, CAT, and other antioxidant enzymes to scavenge ROS. | Plasma membrane | APC |
| LHT1 [105] | Arabidopsis | A lysine–histidine transporter that controls the flow of proline and scavenges ROS. | Plasma membrane | AAAP |
| MaT1 [171] | Arabidopsis, Apium graveolens | A phloem mannitol membrane transporter that can scavenge ROS through the transport of mannitol. | Plasma membrane | ND |
| MaT2 [170] | Arabidopsis, Apium graveolens | An H+/mannitol cotransporter that transports mannitol to scavenge ROS. | Plasma membrane | ND |
| LAT1 [177] | Arabidopsis | In Arabidopsis, it can control PA influx and scavenge ROS. | Plasma membrane | APC |
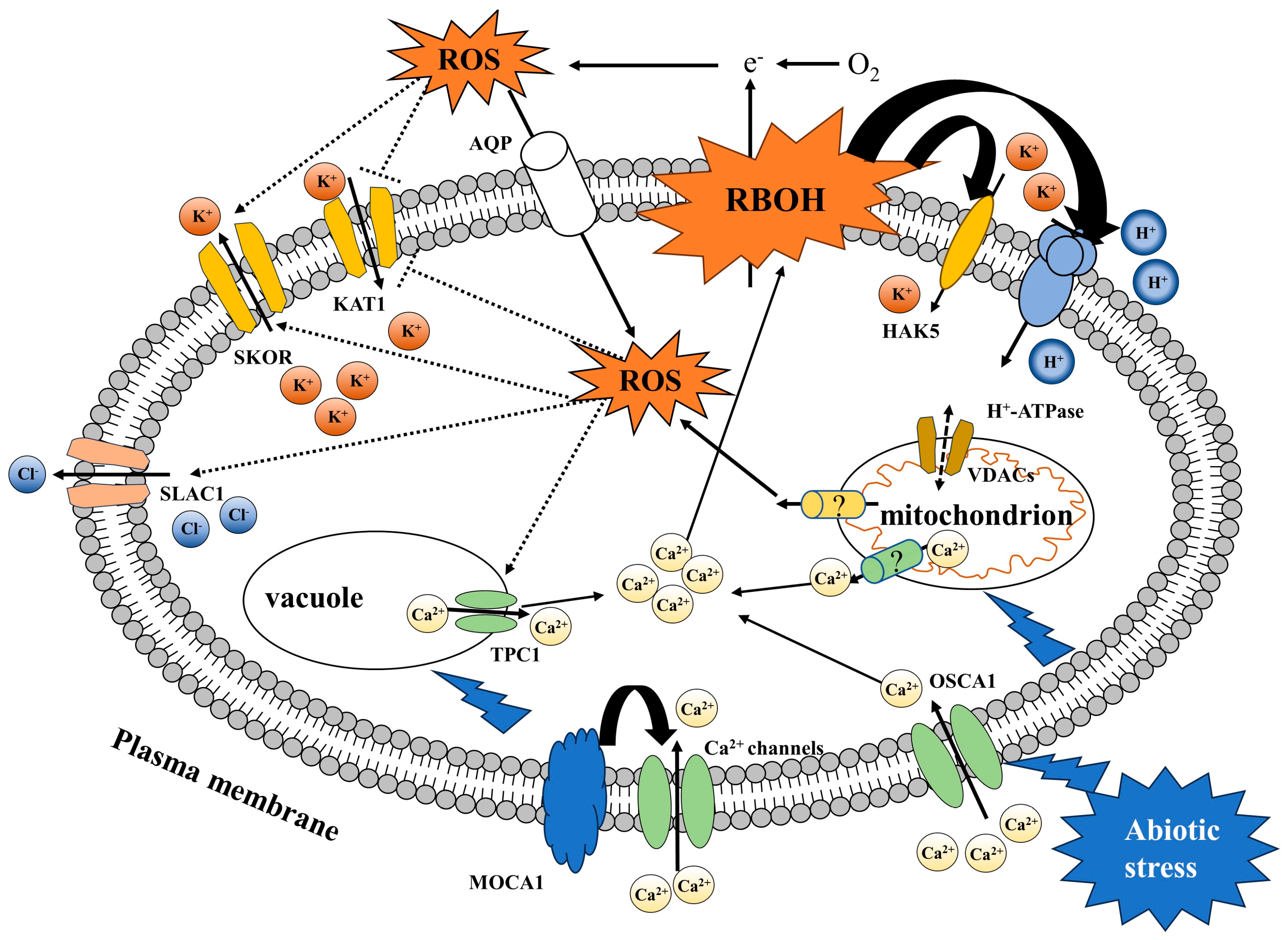
4. ROS Signaling Is Involved in the Regulation of Membrane Transporters under Abiotic Stress
4.1. ROS Signaling Regulates Ca2+ Transporters
4.2. ROS Signaling Regulates K+ Transporters
4.3. ROS Signaling Regulates Other Transporters
| Name | Species | Description | Localization | Family |
|---|---|---|---|---|
| TPC1 [182] | Arabidopsis, Rice, Wheat | As a voltage-dependent K+ channel, it plays a role in ROS-associated Ca2+ wave conduction and can also mediate the distribution of Ca2+ and Mg2+ in cells, with specificity for Ca2+. | Vacuolar membrane | TPC |
| OSCA1 [53] | Arabidopsis | As a mechanosensitive channel, it senses osmotic stress and is activated by mechanical tension on the membrane, playing a role in ROS-associated Ca2+ wave conduction and controlling the transport of Ca2+ from the apoplast to cytosol. | Plasma membrane | OSCA |
| SLAC1 [69] | Arabidopsis | A slow anion channel that controls the distribution of Cl− and NO3− in the xylem. GHR1 perceives ROS signals and activates SLAC by interacting with CPK3. | Plasma membrane | SLAC |
| KAT1 [35] | Arabidopsis | An inward-rectifying K+ channel belonging to the voltage-gated K+ channels that controls the influx of K+. Apoplast ROS activate the Ca2+ channel of guard cells and inhibit the activity of KAT1. | Plasma membrane | AKT |
| SKOR [185] | Arabidopsis | An outgoing K+ channel belonging to voltage-gated K+ channels that controls the efflux of K+. ROS can be perceived by a cysteine residue on this channel, activating SKOR. | Plasma membrane | SKOR |
| VDACs [188] | Arabidopsis | A voltage-dependent anion channel related to the homeostasis of ROS in plant cells. | Plasma membrane and mitochondrial membrane | VDAC |
| HAK5 [42] | Arabidopsis, Rice, Mesembrya nthemumcry stallinum | A high-affinity K+ transporter that transports K+ into the cytosol at low K+ concentrations; its activity is controlled by RBOHD. | Plasma membrane | APC |
| AHA1 [186] | Arabidopsis | An H+-ATPase that can regulate membrane repolarization and JA synthesis; its activity is controlled by RBOHD. | Plasma membrane | P-type ATPase |
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gill, R.A.; Ahmar, S.; Ali, B.; Saleem, M.H.; Khan, M.U.; Zhou, W.; Liu, S. The Role of Membrane Transporters in Plant Growth and Development, and Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 12792. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Lal, S.K.; Lakra, N.; Mehta, S.; Shabek, N.; Narayan, O.P. Plant mineral transport systems and the potential for crop improvement. Planta 2021, 253, 45. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant 2021, 171, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Misra, V.; Mall, A.K. Plant sugar transporters and their role in abiotic stress. In Transporters and Plant Osmotic Stress; Academic Press: Cambridge, MA, USA, 2021; pp. 101–112. [Google Scholar]
- Tang, Z.; Zhao, F.-J. The roles of membrane transporters in arsenic uptake, translocation and detoxification in plants. Crit. Rev. Environ. Sci. Technol. 2020, 51, 2449–2484. [Google Scholar] [CrossRef]
- Li, H.; Testerink, C.; Zhang, Y. How roots and shoots communicate through stressful times. Trends Plant Sci. 2021, 26, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Kronzucker, H.J.; Britto, D.T. Sodium transport in plants: A critical review. New Phytol. 2011, 189, 54–81. [Google Scholar] [CrossRef]
- Gautam, T.; Dutta, M.; Jaiswal, V.; Zinta, G.; Gahlaut, V.; Kumar, S. Emerging Roles of SWEET Sugar Transporters in Plant Development and Abiotic Stress Responses. Cells 2022, 11, 1303. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Dynowski, M.; Schaaf, G.; Loque, D.; Moran, O.; Ludewig, U. Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem. J. 2008, 414, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.; Gechev, T. ROS and Abiotic Stress in Plants 2.0. Int. J. Mol. Sci. 2023, 24, 11917. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Xia, X.J.; Zhou, Y.H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Czarnocka, W.; Karpinski, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Conde, A.; Chaves, M.M.; Geros, H. Membrane transport, sensing and signaling in plant adaptation to environmental stress. Plant Cell Physiol. 2011, 52, 1583–1602. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Delhaize, E.; Frommer, W.B.; Guerinot, M.L.; Harrison, M.J.; Herrera-Estrella, L.; Horie, T.; Kochian, L.V.; Munns, R.; Nishizawa, N.K.; et al. Using membrane transporters to improve crops for sustainable food production. Nature 2013, 497, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Mishra, M.; Patil, G.; Mulkey, S.; Ramawat, N.; Pratap Singh, V.; Deshmukh, R.; Kumar Tripathi, D.; Nguyen, H.T.; Sharma, S. Avenues of the membrane transport system in adaptation of plants to abiotic stresses. Crit. Rev. Biotechnol. 2019, 39, 861–883. [Google Scholar] [CrossRef]
- Banik, S.; Dutta, D. Membrane Proteins in Plant Salinity Stress Perception, Sensing, and Response. J. Membr. Biol. 2023, 256, 109–124. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Xu, B.; Athman, A.; Conn, S.J.; Jordans, C.; Byrt, C.S.; Hare, R.A.; Tyerman, S.D.; Tester, M.; et al. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat. Biotechnol. 2012, 30, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Raddatz, N.; Pardo, J.M.; Yun, D.J. HKT sodium and potassium transporters in Arabidopsis thaliana and related halophyte species. Physiol. Plant. 2021, 171, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Spielmeyer, W.; Lagudah, E.S.; James, R.A.; Platten, J.D.; Dennis, E.S.; Munns, R. A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat. Plant Physiol. 2006, 142, 1718–1727. [Google Scholar] [CrossRef]
- Maser, P.; Eckelman, B.; Vaidyanathan, R.; Horie, T.; Fairbairn, D.J.; Kubo, M.; Yamagami, M.; Yamaguchi, K.; Nishimura, M.; Uozumi, N.; et al. Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Lett. 2002, 531, 157–161. [Google Scholar] [CrossRef]
- Rubio, F.; Gassmann, W.; Schroeder, J.I. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 1995, 270, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Rus, A.; Yokoi, S.; Sharkhuu, A.; Reddy, M.; Lee, B.H.; Matsumoto, T.K.; Koiwa, H.; Zhu, J.K.; Bressan, R.A.; Hasegawa, P.M. AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc. Natl. Acad. Sci. USA 2001, 98, 14150–14155. [Google Scholar] [CrossRef]
- Hauser, F.; Horie, T. A conserved primary salt tolerance mechanism mediated by HKT transporters: A mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ. 2010, 33, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Lee, C.E.; Lin, Y.S.; Lee, M.H.; Chen, P.Y.; Chang, H.C.; Chang, I.F. The Glutamate Receptor-Like Protein GLR3.7 Interacts With 14-3-3omega and Participates in Salt Stress Response in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Jing, W.; Zhang, Q.; Zhang, W. Cyclic nucleotide gated channel 10 negatively regulates salt tolerance by mediating Na+ transport in Arabidopsis. J. Plant Res. 2015, 128, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Jarratt-Barnham, E.; Wang, L.; Ning, Y.; Davies, J.M. The Complex Story of Plant Cyclic Nucleotide-Gated Channels. Int. J. Mol. Sci. 2021, 22, 874. [Google Scholar] [CrossRef]
- Qiu, Q.S.; Guo, Y.; Dietrich, M.A.; Schumaker, K.S.; Zhu, J.K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar] [CrossRef]
- Qiu, Q.S.; Barkla, B.J.; Vera-Estrella, R.; Zhu, J.K.; Schumaker, K.S. Na+/H+ exchange activity in the plasma membrane of Arabidopsis. Plant Physiol. 2003, 132, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Lhamo, D.; Wang, C.; Gao, Q.; Luan, S. Recent Advances in Genome-wide Analyses of Plant Potassium Transporter Families. Curr. Genom. 2021, 22, 164–180. [Google Scholar] [CrossRef]
- Dreyer, I.; Uozumi, N. Potassium channels in plant cells. FEBS J. 2011, 278, 4293–4303. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Schroeder, J.I. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 1994, 370, 655–658. [Google Scholar] [CrossRef]
- Pyo, Y.J.; Gierth, M.; Schroeder, J.I.; Cho, M.H. High-affinity K+ transport in Arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and postgermination growth under low-potassium conditions. Plant Physiol. 2010, 153, 863–875. [Google Scholar] [CrossRef]
- Li, W.; Xu, G.; Alli, A.; Yu, L. Plant HAK/KUP/KT K+ transporters: Function and regulation. Semin. Cell Dev. Biol. 2018, 74, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, L.; Shen, Z.; Jing, W.; Ge, H.; Zhao, J.; Zhang, W. The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice. Plant Cell Environ. 2015, 38, 2766–2779. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Aleman, F.; Martinez, V.; Rubio, F. The Arabidopsis thaliana HAK5 K+ transporter is required for plant growth and K+ acquisition from low K+ solutions under saline conditions. Mol. Plant 2010, 3, 326–333. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Long-Tang, H.; Li-Na, Z.; Li-Wei, G.; Anne-Alienor, V.; Herve, S.; Yi-Dong, Z. Constitutive expression of CmSKOR, an outward K+ channel gene from melon, in Arabidopsis thaliana involved in saline tolerance. Plant Sci. 2018, 274, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Adem, G.D.; Chen, G.; Shabala, L.; Chen, Z.H.; Shabala, S. GORK Channel: A Master Switch of Plant Metabolism? Trends Plant Sci. 2020, 25, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Grefen, C.; Chen, Z.; Honsbein, A.; Donald, N.; Hills, A.; Blatt, M.R. A novel motif essential for SNARE interaction with the K+ channel KC1 and channel gating in Arabidopsis. Plant Cell 2010, 22, 3076–3092. [Google Scholar] [CrossRef] [PubMed]
- Honsbein, A.; Sokolovski, S.; Grefen, C.; Campanoni, P.; Pratelli, R.; Paneque, M.; Chen, Z.; Johansson, I.; Blatt, M.R. A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell 2009, 21, 2859–2877. [Google Scholar] [CrossRef] [PubMed]
- Cellier, F.; Conejero, G.; Ricaud, L.; Luu, D.T.; Lepetit, M.; Gosti, F.; Casse, F. Characterization of AtCHX17, a member of the cation/H+ exchangers, CHX family, from Arabidopsis thaliana suggests a role in K+ homeostasis. Plant J. 2004, 39, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, P.; Motes, C.M.; Park, S.; Hirschi, K.D. CHX14 is a plasma membrane K-efflux transporter that regulates K+ redistribution in Arabidopsis thaliana. Plant Cell Environ. 2015, 38, 2223–2238. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Kaandorp, J.A. Mathematical modeling of calcium homeostasis in yeast cells. Cell Calcium 2006, 39, 337–348. [Google Scholar] [CrossRef]
- Zhai, Y.; Wen, Z.; Han, Y.; Zhuo, W.; Wang, F.; Xi, C.; Liu, J.; Gao, P.; Zhao, H.; Wang, Y.; et al. Heterogeneous expression of plasma-membrane-localised OsOSCA1.4 complements osmotic sensing based on hyperosmolality and salt stress in Arabidopsis osca1 mutant. Cell Calcium 2020, 91, 102261. [Google Scholar] [CrossRef]
- Guo, K.M.; Babourina, O.; Christopher, D.A.; Borsics, T.; Rengel, Z. The cyclic nucleotide-gated channel, AtCNGC10, influences salt tolerance in Arabidopsis. Physiol. Plant 2008, 134, 499–507. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zeng, W.; Jiang, Y. Tuning the ion selectivity of two-pore channels. Proc. Natl. Acad. Sci. USA 2017, 114, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Anil, V.S.; Rajkumar, P.; Kumar, P.; Mathew, M.K. A plant Ca2+ pump, ACA2, relieves salt hypersensitivity in yeast. Modulation of cytosolic calcium signature and activation of adaptive Na+ homeostasis. J. Biol. Chem. 2008, 283, 3497–3506. [Google Scholar] [CrossRef]
- Limonta, M.; Romanowsky, S.; Olivari, C.; Bonza, M.C.; Luoni, L.; Rosenberg, A.; Harper, J.F.; De Michelis, M.I. ACA12 is a deregulated isoform of plasma membrane Ca2+-ATPase of Arabidopsis thaliana. Plant Mol. Biol. 2014, 84, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Frangne, N.; Gomès, E.; Martinoia, E.; Palmgren, M.G. The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 2000, 124, 1814–1827. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Shabala, S.; Isayenkov, S.; Cuin, T.A.; Pottosin, I. Calcium transport across plant membranes: Mechanisms and functions. New Phytol. 2018, 220, 49–69. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Wei, J.; Zhao, Z.; Sun, D.; Cui, S. A Na+/Ca2+ exchanger-like protein (AtNCL) involved in salt stress in Arabidopsis. J. Biol. Chem. 2012, 287, 44062–44070. [Google Scholar] [CrossRef] [PubMed]
- Conn, S.J.; Gilliham, M.; Athman, A.; Schreiber, A.W.; Baumann, U.; Moller, I.; Cheng, N.H.; Stancombe, M.A.; Hirschi, K.D.; Webb, A.A.; et al. Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell 2011, 23, 240–257. [Google Scholar] [CrossRef]
- Han, N.; Shao, Q.; Bao, H.; Wang, B. Cloning and Characterization of a Ca2+/H+ Antiporter from Halophyte Suaeda salsa L. Plant Mol. Biol. Rep. 2010, 29, 449–457. [Google Scholar] [CrossRef]
- Niu, X.; Zhu, J.K.; Narasimhan, M.L.; Bressan, R.A.; Hasegawa, P.M. Plasma-membrane H+-ATPase gene expression is regulated by NaCl in cells of the halophyte Atriplex nummularia L. Planta. 1993, 190, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Maeshima, M. Molecular cloning of vacuolar H+-pyrophosphatase and its developmental expression in growing hypocotyl of mung bean. Plant Physiol. 1998, 116, 589–597. [Google Scholar] [CrossRef]
- Queiros, F.; Fontes, N.; Silva, P.; Almeida, D.; Maeshima, M.; Geros, H.; Fidalgo, F. Activity of tonoplast proton pumps and Na+/H+ exchange in potato cell cultures is modulated by salt. J. Exp. Bot. 2009, 60, 1363–1374. [Google Scholar] [CrossRef]
- Martinoia, E.; Maeshima, M.; Neuhaus, H.E. Vacuolar transporters and their essential role in plant metabolism. J. Exp. Bot. 2007, 58, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Façanha, A.R.; Tavares, R.M.; Gerós, H. Role of Tonoplast Proton Pumps and Na+/H+ Antiport System in Salt Tolerance of Populus euphratica Oliv. J. Plant Growth Regul. 2009, 29, 23–34. [Google Scholar] [CrossRef]
- Gaxiola, R.A.; Li, J.; Undurraga, S.; Dang, L.M.; Allen, G.J.; Alper, S.L.; Fink, G.R. Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc. Natl. Acad. Sci. USA 2001, 98, 11444–11449. [Google Scholar] [CrossRef]
- Teakle, N.L.; Tyerman, S.D. Mechanisms of Cl− transport contributing to salt tolerance. Plant Cell Environ. 2010, 33, 566–589. [Google Scholar] [CrossRef]
- Qiu, J.; Henderson, S.W.; Tester, M.; Roy, S.J.; Gilliham, M. SLAH1, a homologue of the slow type anion channel SLAC1, modulates shoot Cl− accumulation and salt tolerance in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 4495–4505. [Google Scholar] [CrossRef]
- Cubero-Font, P.; Maierhofer, T.; Jaslan, J.; Rosales, M.A.; Espartero, J.; Diaz-Rueda, P.; Muller, H.M.; Hurter, A.L.; Al-Rasheid, K.A.; Marten, I.; et al. Silent S-Type Anion Channel Subunit SLAH1 Gates SLAH3 Open for Chloride Root-to-Shoot Translocation. Curr. Biol. 2016, 26, 2213–2220. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Agorio, A.; Jossier, M.; Depre, S.; Thomine, S.; Filleur, S. Characterization of the Chloride Channel-Like, AtCLCg, Involved in Chloride Tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2016, 57, 764–775. [Google Scholar] [CrossRef]
- Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004, 37, 645–653. [Google Scholar] [CrossRef]
- Ligaba, A.; Katsuhara, M.; Ryan, P.R.; Shibasaka, M.; Matsumoto, H. The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiol. 2006, 142, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Mumm, P.; Imes, D.; Endler, A.; Weder, B.; Al-Rasheid, K.A.; Geiger, D.; Marten, I.; Martinoia, E.; Hedrich, R. AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 2010, 63, 1054–1062. [Google Scholar] [CrossRef]
- Motoda, H.; Sasaki, T.; Kano, Y.; Ryan, P.R.; Delhaize, E.; Matsumoto, H.; Yamamoto, Y. The Membrane Topology of ALMT1, an Aluminum-Activated Malate Transport Protein in Wheat (Triticum aestivum). Plant Signal Behav. 2007, 2, 467–472. [Google Scholar] [CrossRef]
- Liu, R.; Cui, B.; Lu, X.; Song, J. The positive effect of salinity on nitrate uptake in Suaeda salsa. Plant Physiol. Biochem. 2021, 166, 958–963. [Google Scholar] [CrossRef]
- Hall, J.L.; Williams, L.E. Transition metal transporters in plants. J. Exp. Bot. 2003, 54, 2601–2613. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Masuda, H.; Bashir, K.; Inoue, H.; Tsukamoto, T.; Takahashi, M.; Nakanishi, H.; Aoki, N.; Hirose, T.; Ohsugi, R.; et al. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 2010, 62, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, M.; Kobae, Y.; Mimura, T.; Maeshima, M. Deletion of a histidine-rich loop of AtMTP1, a vacuolar Zn2+/H+ antiporter of Arabidopsis thaliana, stimulates the transport activity. J. Biol. Chem. 2008, 283, 8374–8383. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Punshon, T.; Lanzirotti, A.; Li, L.; Alonso, J.M.; Ecker, J.R.; Kaplan, J.; Guerinot, M.L. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 2006, 314, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Peiter, E.; Montanini, B.; Gobert, A.; Pedas, P.; Husted, S.; Maathuis, F.J.; Blaudez, D.; Chalot, M.; Sanders, D. A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 8532–8537. [Google Scholar] [CrossRef]
- Huang, D.; Dai, W. Two iron-regulated transporter (IRT) genes showed differential expression in poplar trees under iron or zinc deficiency. J. Plant Physiol. 2015, 186–187, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lanquar, V.; Lelievre, F.; Bolte, S.; Hames, C.; Alcon, C.; Neumann, D.; Vansuyt, G.; Curie, C.; Schroder, A.; Kramer, U.; et al. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005, 24, 4041–4051. [Google Scholar] [CrossRef] [PubMed]
- Voith von Voithenberg, L.; Park, J.; Stube, R.; Lux, C.; Lee, Y.; Philippar, K. A Novel Prokaryote-Type ECF/ABC Transporter Module in Chloroplast Metal Homeostasis. Front. Plant Sci. 2019, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Rea, P.A.; Li, Z.S.; Lu, Y.P.; Drozdowicz, Y.M.; Martinoia, E. From vacuolar gs-x pumps to multispecific abc transporters. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 727–760. [Google Scholar] [CrossRef]
- Chen, L.Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tang, Z.; Zhang, Y.; Niu, L.; Yang, F.; Zhang, D.; Hu, Y. Rice SUT and SWEET Transporters. Int. J. Mol. Sci. 2021, 22, 11198. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yao, L.; Hao, X.; Li, N.; Qian, W.; Yue, C.; Ding, C.; Zeng, J.; Yang, Y.; Wang, X. Tea plant SWEET transporters: Expression profiling, sugar transport, and the involvement of CsSWEET16 in modifying cold tolerance in Arabidopsis. Plant Mol. Biol. 2018, 96, 577–592. [Google Scholar] [CrossRef]
- Zhou, A.; Ma, H.; Feng, S.; Gong, S.; Wang, J. A Novel Sugar Transporter from Dianthus spiculifolius, DsSWEET12, Affects Sugar Metabolism and Confers Osmotic and Oxidative Stress Tolerance in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 497. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Qu, X.Q.; Hou, B.H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef]
- Kong, W.; An, B.; Zhang, Y.; Yang, J.; Li, S.; Sun, T.; Li, Y. Sugar Transporter Proteins (STPs) in Gramineae Crops: Comparative Analysis, Phylogeny, Evolution, and Expression Profiling. Cells 2019, 8, 560. [Google Scholar] [CrossRef]
- Schneider, S.; Schneidereit, A.; Udvardi, P.; Hammes, U.; Gramann, M.; Dietrich, P.; Sauer, N. Arabidopsis inositol transporter2 mediates H+ symport of different inositol epimers and derivatives across the plasma membrane. Plant Physiol. 2007, 145, 1395–1407. [Google Scholar] [CrossRef]
- Klemens, P.A.W.; Patzke, K.; Trentmann, O.; Poschet, G.; Buttner, M.; Schulz, A.; Marten, I.; Hedrich, R.; Neuhaus, H.E. Overexpression of a proton-coupled vacuolar glucose exporter impairs freezing tolerance and seed germination. New Phytol. 2014, 202, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Wormit, A.; Trentmann, O.; Feifer, I.; Lohr, C.; Tjaden, J.; Meyer, S.; Schmidt, U.; Martinoia, E.; Neuhaus, H.E. Molecular identification and physiological characterization of a novel monosaccharide transporter from Arabidopsis involved in vacuolar sugar transport. Plant Cell 2006, 18, 3476–3490. [Google Scholar] [CrossRef] [PubMed]
- Aluri, S.; Büttner, M. Identification and functional expression of the Arabidopsis thaliana vacuolar glucose transporter 1 and its role in seed germination and flowering. Proc. Natl. Acad. Sci. USA 2007, 104, 2537–2542. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Cao, X.; Shi, S.; Li, S.; Gao, J.; Ma, Y.; Zhao, Q.; Chen, Q. Genome-wide survey and expression analysis of the amino acid transporter superfamily in potato (Solanum tuberosum L.). Plant Physiol. Biochem. 2016, 107, 164–177. [Google Scholar] [CrossRef]
- Snowden, C.J.; Thomas, B.; Baxter, C.J.; Smith, J.A.; Sweetlove, L.J. A tonoplast Glu/Asp/GABA exchanger that affects tomato fruit amino acid composition. Plant J. 2015, 81, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Shinozaki, K. Identification of polyamine transporters in plants: Paraquat transport provides crucial clues. Plant Cell Physiol. 2014, 55, 855–861. [Google Scholar] [CrossRef]
- Michaeli, S.; Fait, A.; Lagor, K.; Nunes-Nesi, A.; Grillich, N.; Yellin, A.; Bar, D.; Khan, M.; Fernie, A.R.; Turano, F.J.; et al. A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J. 2011, 67, 485–498. [Google Scholar] [CrossRef]
- Dundar, E.; Bush, D.R. BAT1, a bidirectional amino acid transporter in Arabidopsis. Planta 2009, 229, 1047–1056. [Google Scholar] [CrossRef]
- Meyer, A.; Eskandari, S.; Grallath, S.; Rentsch, D. AtGAT1, a high affinity transporter for gamma-aminobutyric acid in Arabidopsis thaliana. J. Biol. Chem. 2006, 281, 7197–7204. [Google Scholar] [CrossRef]
- Duan, Y.; Zhu, X.; Shen, J.; Xing, H.; Zou, Z.; Ma, Y.; Wang, Y.; Fang, W. Genome-wide identification, characterization and expression analysis of the amino acid permease gene family in tea plants (Camellia sinensis). Genomics 2020, 112, 2866–2874. [Google Scholar] [CrossRef]
- Dinkeloo, K.; Boyd, S.; Pilot, G. Update on amino acid transporter functions and on possible amino acid sensing mechanisms in plants. Semin. Cell Dev. Biol. 2018, 74, 105–113. [Google Scholar] [CrossRef]
- Batushansky, A.; Kirma, M.; Grillich, N.; Pham, P.A.; Rentsch, D.; Galili, G.; Fernie, A.R.; Fait, A. The transporter GAT1 plays an important role in GABA-mediated carbon-nitrogen interactions in Arabidopsis. Front. Plant Sci. 2015, 6, 785. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Shi, M.; Hao, D.; Wei, Q.; Wang, Z.; Fu, S.; Su, Y.; Xia, J. Disruption of an amino acid transporter LHT1 leads to growth inhibition and low yields in rice. BMC Plant Biol. 2019, 19, 268. [Google Scholar] [CrossRef]
- Gani, U.; Vishwakarma, R.A.; Misra, P. Membrane transporters: The key drivers of transport of secondary metabolites in plants. Plant Cell Rep. 2021, 40, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Hwang, J.U.; Lee, M.; Kim, Y.Y.; Assmann, S.M.; Martinoia, E.; Lee, Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA 2010, 107, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Kuromori, T.; Miyaji, T.; Yabuuchi, H.; Shimizu, H.; Sugimoto, E.; Kamiya, A.; Moriyama, Y.; Shinozaki, K. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc. Natl. Acad. Sci. USA 2010, 107, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Shitan, N.; Yazaki, K. Accumulation and membrane transport of plant alkaloids. Curr. Pharm. Biotechnol. 2007, 8, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Shimizu, T.; Miyakawa, S.; Kanno, Y.; Koshiba, T.; Kamiya, Y.; Seo, M. Identification of Arabidopsis thaliana NRT1/PTR family (NPF) proteins capable of transporting plant hormones. J. Plant Res. 2015, 128, 679–686. [Google Scholar] [CrossRef] [PubMed]
- David, L.C.; Berquin, P.; Kanno, Y.; Seo, M.; Daniel-Vedele, F.; Ferrario-Mery, S. N availability modulates the role of NPF3.1, a gibberellin transporter, in GA-mediated phenotypes in Arabidopsis. Planta 2016, 244, 1315–1328. [Google Scholar] [CrossRef]
- Tal, I.; Zhang, Y.; Jorgensen, M.E.; Pisanty, O.; Barbosa, I.C.; Zourelidou, M.; Regnault, T.; Crocoll, C.; Olsen, C.E.; Weinstain, R.; et al. The Arabidopsis NPF3 protein is a GA transporter. Nat. Commun. 2016, 7, 11486. [Google Scholar] [CrossRef]
- Komarova, N.Y.; Thor, K.; Gubler, A.; Meier, S.; Dietrich, D.; Weichert, A.; Suter Grotemeyer, M.; Tegeder, M.; Rentsch, D. AtPTR1 and AtPTR5 transport dipeptides in planta. Plant Physiol. 2008, 148, 856–869. [Google Scholar] [CrossRef]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K.; et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef]
- Liu, P.; Wu, X.; Gong, B.; Lu, G.; Li, J.; Gao, H. Review of the Mechanisms by Which Transcription Factors and Exogenous Substances Regulate ROS Metabolism under Abiotic Stress. Antioxidants 2022, 11, 2106. [Google Scholar] [CrossRef]
- Shabala, S.; Shabala, L.; Barcelo, J.; Poschenrieder, C. Membrane transporters mediating root signalling and adaptive responses to oxygen deprivation and soil flooding. Plant Cell Environ. 2014, 37, 2216–2233. [Google Scholar] [CrossRef]
- Kurusu, T.; Kuchitsu, K.; Tada, Y. Plant signaling networks involving Ca2+ and Rboh/Nox-mediated ROS production under salinity stress. Front. Plant Sci. 2015, 6, 427. [Google Scholar] [CrossRef]
- Asai, S.; Ichikawa, T.; Nomura, H.; Kobayashi, M.; Kamiyoshihara, Y.; Mori, H.; Kadota, Y.; Zipfel, C.; Jones, J.D.G.; Yoshioka, H. The variable domain of a plant calcium-dependent protein kinase (CDPK) confers subcellular localization and substrate recognition for NADPH oxidase. J. Biol. Chem. 2013, 288, 14332–14340. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ohura, I.; Kawakita, K.; Yokota, N.; Fujiwara, M.; Shimamoto, K.; Doke, N.; Yoshioka, H. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 2007, 19, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Kadota, Y.; Shirasu, K.; Zipfel, C. Regulation of the NADPH Oxidase RBOHD During Plant Immunity. Plant Cell Physiol. 2015, 56, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, M.; Galganska, H.; Nowak, W.; Sadowski, J. Exogenously induced expression of ethylene biosynthesis, ethylene perception, phospholipase D, and Rboh-oxidase genes in broccoli seedlings. J. Exp. Bot. 2010, 61, 3475–3491. [Google Scholar] [CrossRef]
- Park, J.; Gu, Y.; Lee, Y.; Yang, Z.; Lee, Y. Phosphatidic acid induces leaf cell death in Arabidopsis by activating the Rho-related small G protein GTPase-mediated pathway of reactive oxygen species generation. Plant Physiol. 2004, 134, 129–136. [Google Scholar] [CrossRef]
- Liu, T.Y.; Huang, T.K.; Tseng, C.Y.; Lai, Y.S.; Lin, S.I.; Lin, W.Y.; Chen, J.W.; Chiou, T.J. PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis. Plant Cell 2012, 24, 2168–2183. [Google Scholar] [CrossRef]
- Hamburger, D.; Rezzonico, E.; MacDonald-Comber Petétot, J.; Somerville, C.; Poirier, Y. Identification and Characterization of the Arabidopsis PHO1 Gene Involved in Phosphate Loading to the Xylem. Plant Cell 2002, 14, 889–902. [Google Scholar] [CrossRef]
- Lee, M.; Lee, K.; Lee, J.; Noh, E.W.; Lee, Y. AtPDR12 contributes to lead resistance in Arabidopsis. Plant Physiol. 2005, 138, 827–836. [Google Scholar] [CrossRef]
- Kang, J.; Yim, S.; Choi, H.; Kim, A.; Lee, K.P.; Lopez-Molina, L.; Martinoia, E.; Lee, Y. Abscisic acid transporters cooperate to control seed germination. Nat. Commun. 2015, 6, 8113. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Zhang, Q.; Cui, M.; Zhao, M.; Li, N.; Wang, S.; Wu, R.; Zhang, L.; Cao, Y.; et al. The interaction of ABA and ROS in plant growth and stress resistances. Front. Plant Sci. 2022, 13, 1050132. [Google Scholar] [CrossRef] [PubMed]
- Maslenkova, L.T.; Zanev, Y.; Popova, L.P. Effect of abscisic acid on the photosynthetic oxygen evolution in barley chloroplasts. Photosynth. Res. 1989, 21, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Liu, R.; Yan, L.; Liu, Z.Q.; Jiang, S.C.; Shen, Y.Y.; Wang, X.F.; Zhang, D.P. Light-harvesting chlorophyll a/b-binding proteins are required for stomatal response to abscisic acid in Arabidopsis. J. Exp. Bot. 2012, 63, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in Stomatal Defense against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Wang, Z.; Liu, X.; Yao, J.; Kong, X.; Shi, H.; Zhu, J.K. Two Chloroplast Proteins Negatively Regulate Plant Drought Resistance Through Separate Pathways. Plant Physiol. 2020, 182, 1007–1021. [Google Scholar] [CrossRef]
- Cho, M.; Cho, H.T. The function of ABCB transporters in auxin transport. Plant Signal Behav. 2013, 8, e22990. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Bielach, A.; Hrtyan, M. Redox regulation at the site of primary growth: Auxin, cytokinin and ROS crosstalk. Plant Cell Environ. 2017, 40, 2586–2605. [Google Scholar] [CrossRef]
- Chen, C.; Letnik, I.; Hacham, Y.; Dobrev, P.; Ben-Daniel, B.H.; Vankova, R.; Amir, R.; Miller, G. Ascorbate peroxidase6 protects Arabidopsis desiccating and germinating seeds from stress and mediates cross talk between reactive oxygen species, abscisic acid, and auxin. Plant Physiol. 2014, 166, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Lecourieux, D.; Mazars, C.; Pauly, N.; Ranjeva, R.; Pugin, A. Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 2002, 14, 2627–2641. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Shabala, L.; Zhou, M.; Zhang, G.; Shabala, S. Barley responses to combined waterlogging and salinity stress: Separating effects of oxygen deprivation and elemental toxicity. Front. Plant Sci. 2013, 4, 313. [Google Scholar] [CrossRef] [PubMed]
- Davenport, R. Glutamate receptors in plants. Ann. Bot. 2002, 90, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Lancien, M.; Roberts, M.R. Regulation of Arabidopsis thaliana 14-3-3 gene expression by gamma-aminobutyric acid. Plant Cell Environ. 2006, 29, 1430–1436. [Google Scholar] [CrossRef]
- Liu, C.J.; Zhao, Y.; Zhang, K. Cytokinin Transporters: Multisite Players in Cytokinin Homeostasis and Signal Distribution. Front. Plant Sci. 2019, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Kang, J.; Kiba, T.; Park, J.; Kojima, M.; Do, J.; Kim, K.Y.; Kwon, M.; Endler, A.; Song, W.Y.; et al. Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc. Natl. Acad. Sci. USA 2014, 111, 7150–7155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Novak, O.; Wei, Z.; Gou, M.; Zhang, X.; Yu, Y.; Yang, H.; Cai, Y.; Strnad, M.; Liu, C.J. Arabidopsis ABCG14 protein controls the acropetal translocation of root-synthesized cytokinins. Nat. Commun. 2014, 5, 3274. [Google Scholar] [CrossRef]
- Poitout, A.; Crabos, A.; Petrik, I.; Novak, O.; Krouk, G.; Lacombe, B.; Ruffel, S. Responses to Systemic Nitrogen Signaling in Arabidopsis Roots Involve trans-Zeatin in Shoots. Plant Cell 2018, 30, 1243–1257. [Google Scholar] [CrossRef]
- Zurcher, E.; Liu, J.; di Donato, M.; Geisler, M.; Muller, B. Plant development regulated by cytokinin sinks. Science 2016, 353, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, W.; Chan, Z.; Wu, Y. Endogenous Cytokinin Overproduction Modulates ROS Homeostasis and Decreases Salt Stress Resistance in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 1004. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Burgess, P.; Zhang, X.; Huang, B. Enhancing cytokinin synthesis by overexpressing ipt alleviated drought inhibition of root growth through activating ROS-scavenging systems in Agrostis stolonifera. J. Exp. Bot. 2016, 67, 1979–1992. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, G.; Cao, C.; Liu, P. Metabolism, signaling, and transport of jasmonates. Plant Commun. 2021, 2, 100231. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. MYC2: The master in action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Maruta, T.; Inoue, T.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Arabidopsis NADPH oxidases, AtrbohD and AtrbohF, are essential for jasmonic acid-induced expression of genes regulated by MYC2 transcription factor. Plant Sci. 2011, 180, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Zhang, M.; Zhang, H.; Xiong, H.; Liu, P.; Ali, J.; Li, J.; Li, Z. OsMIOX, a myo-inositol oxygenase gene, improves drought tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.). Plant Sci. 2012, 196, 143–151. [Google Scholar] [CrossRef]
- Yin, X.M.; Huang, L.F.; Zhang, X.; Wang, M.L.; Xu, G.Y.; Xia, X.J. OsCML4 improves drought tolerance through scavenging of reactive oxygen species in rice. J. Plant Biol. 2015, 58, 68–73. [Google Scholar] [CrossRef]
- Ward, J.M.; Maser, P.; Schroeder, J.I. Plant ion channels: Gene families, physiology, and functional genomics analyses. Annu. Rev. Physiol. 2009, 71, 59–82. [Google Scholar] [CrossRef]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.; Pereira, A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 2010, 154, 1254–1271. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Huda, K.M.; Banu, M.S.; Garg, B.; Tula, S.; Tuteja, R.; Tuteja, N. OsACA6, a P-type IIB Ca2+ ATPase promotes salinity and drought stress tolerance in tobacco by ROS scavenging and enhancing the expression of stress-responsive genes. Plant J. 2013, 76, 997–1015. [Google Scholar] [CrossRef]
- Li, J.Y.; Fu, Y.L.; Pike, S.M.; Bao, J.; Tian, W.; Zhang, Y.; Chen, C.Z.; Zhang, Y.; Li, H.M.; Huang, J.; et al. The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell 2010, 22, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K. ABC transporters involved in the transport of plant secondary metabolites. FEBS Lett. 2006, 580, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, C.; Gao, Z.; Zhang, Y.; Jiang, H.; Zhu, L.; Ren, D.; Yu, L.; Xu, G.; Qian, Q. OsHAK1, a High-Affinity Potassium Transporter, Positively Regulates Responses to Drought Stress in Rice. Front. Plant Sci. 2017, 8, 1885. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Zhang, Q.; Liu, D.; Wang, H.; Yin, J.; Wang, R.; He, M.; Cui, M.; Shang, Z.; Wang, D.; et al. A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J. Exp. Bot. 2015, 66, 5853–5866. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.M.; Noh, E.K.; Kim, H.G.; Jeon, B.W.; Bae, K.; Hu, H.C.; Kwak, J.M.; Park, O.K. Arabidopsis annexins AnnAt1 and AnnAt4 interact with each other and regulate drought and salt stress responses. Plant Cell Physiol. 2010, 51, 1499–1514. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Funck, D.; Szabados, L.; Rentsch, D. Proline metabolism and transport in plant development. Amino Acids 2010, 39, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Okumoto, S.; Schmidt, R.; Tegeder, M.; Fischer, W.N.; Rentsch, D.; Frommer, W.B.; Koch, W. High affinity amino acid transporters specifically expressed in xylem parenchyma and developing seeds of Arabidopsis. J. Biol. Chem. 2002, 277, 45338–45346. [Google Scholar] [CrossRef]
- Lee, Y.H.; Tegeder, M. Selective expression of a novel high-affinity transport system for acidic and neutral amino acids in the tapetum cells of Arabidopsis flowers. Plant J. 2004, 40, 60–74. [Google Scholar] [CrossRef]
- Ben Rejeb, K.; Abdelly, C.; Savoure, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Alia; Mohanty, P.; Matysik, J. Effect of proline on the production of singlet oxygen. Amino Acids. 2001, 21, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.; Coitino, E.L.; Borsani, O.; Monza, J. Molecular mechanisms for the reaction between ˙OH radicals and proline: Insights on the role as reactive oxygen species scavenger in plant stress. J. Phys. Chem. B 2014, 118, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Hasanuzzaman, M.; Fujita, M. Coordinate induction of antioxidant defense and glyoxalase system by exogenous proline and glycinebetaine is correlated with salt tolerance in mung bean. Front. Agric. China 2011, 5, 1–14. [Google Scholar] [CrossRef]
- Hoque, M.A.; Okuma, E.; Banu, M.N.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J. Plant Physiol. 2007, 164, 553–561. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, K.; de Campos, M.K.; Domingues, D.S.; Pereira, L.F.; Vieira, L.G. The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol. Biol. Rep. 2013, 40, 3269–3279. [Google Scholar] [CrossRef]
- Juchaux-Cachau, M.; Landouar-Arsivaud, L.; Pichaut, J.P.; Campion, C.; Porcheron, B.; Jeauffre, J.; Noiraud-Romy, N.; Simoneau, P.; Maurousset, L.; Lemoine, R. Characterization of AgMaT2, a plasma membrane mannitol transporter from celery, expressed in phloem cells, including phloem parenchyma cells. Plant Physiol. 2007, 145, 62–74. [Google Scholar] [CrossRef]
- Noiraud, N.; Maurousset, L.; Lemoine, R. Identification of a mannitol transporter, AgMaT1, in celery phloem. Plant Cell 2001, 13, 695–705. [Google Scholar] [CrossRef]
- Chutipaijit, S. Changes in physiological and antioxidant activity of indica rice seedlings in response to mannitol-induced osmotic stress. Chil. J. Agric. Res. 2016, 76, 455–462. [Google Scholar] [CrossRef]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; Van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. 2014, 79, 544–567. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ma, H.; Yu, L.; Wang, X.; Zhao, J. Genome-wide survey and expression analysis of amino acid transporter gene family in rice (Oryza sativa L.). PLoS ONE 2012, 7, e49210. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mu, J.; Bai, J.; Fu, F.; Zou, T.; An, F.; Zhang, J.; Jing, H.; Wang, Q.; Li, Z.; et al. Paraquat Resistant1, a Golgi-localized putative transporter protein, is involved in intracellular transport of paraquat. Plant Physiol. 2013, 162, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Drolet, G.; Dumbroff, E.B.; Legge, R.L.; Thompson, J.E. Radical scavenging properties of polyamines. Phytochemistry 1986, 25, 367–371. [Google Scholar] [CrossRef]
- Chai, H.; Guo, J.; Zhong, Y.; Hsu, C.C.; Zou, C.; Wang, P.; Zhu, J.K.; Shi, H. The plasma-membrane polyamine transporter PUT3 is regulated by the Na+/H+ antiporter SOS1 and protein kinase SOS2. New Phytol. 2020, 226, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Larher, F. Chances in polyamine titers associated with the proline response and osmotic adjustment of rape leaf discs submitted to osmotic stresses. Plant Sci. Int. J. Exp. Plant Biol. 1995, 112, 175–186. [Google Scholar]
- Shen, W.; Nada, K.; Tachibana, S. Involvement of polyamines in the chilling tolerance of cucumber cultivars. Plant Physiol. 2000, 124, 431–439. [Google Scholar] [CrossRef]
- Demidchik, V.; Shabala, S.N.; Davies, J.M. Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J. 2007, 49, 377–386. [Google Scholar] [CrossRef]
- Kiselyov, K.; Muallem, S. ROS and intracellular ion channels. Cell Calcium 2016, 60, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Choi, W.G.; Gilroy, S.; Morris, R.J. A ROS-Assisted Calcium Wave Dependent on the AtRBOHD NADPH Oxidase and TPC1 Cation Channel Propagates the Systemic Response to Salt Stress. Plant Physiol. 2016, 171, 1771–1784. [Google Scholar] [CrossRef]
- Mittler, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.P. ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol. 2014, 202, 35–49. [Google Scholar] [CrossRef]
- Garcia-Mata, C.; Wang, J.; Gajdanowicz, P.; Gonzalez, W.; Hills, A.; Donald, N.; Riedelsberger, J.; Amtmann, A.; Dreyer, I.; Blatt, M.R. A minimal cysteine motif required to activate the SKOR K+ channel of Arabidopsis by the reactive oxygen species H2O2. J. Biol. Chem. 2010, 285, 29286–29294. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, H.; Yang, L.; Chen, C.; Shabala, L.; Xiong, M.; Niu, M.; Liu, J.; Zheng, Z.; Zhou, L.; et al. Tissue-specific respiratory burst oxidase homolog-dependent H2O2 signaling to the plasma membrane H+-ATPase confers potassium uptake and salinity tolerance in Cucurbitaceae. J. Exp. Bot. 2019, 70, 5879–5893. [Google Scholar] [CrossRef]
- Sierla, M.; Horak, H.; Overmyer, K.; Waszczak, C.; Yarmolinsky, D.; Maierhofer, T.; Vainonen, J.P.; Salojarvi, J.; Denessiouk, K.; Laanemets, K.; et al. The Receptor-like Pseudokinase GHR1 Is Required for Stomatal Closure. Plant Cell 2018, 30, 2813–2837. [Google Scholar] [CrossRef] [PubMed]
- Robert, N.; d’Erfurth, I.; Marmagne, A.; Erhardt, M.; Allot, M.; Boivin, K.; Gissot, L.; Monachello, D.; Michaud, M.; Duchene, A.M.; et al. Voltage-dependent-anion-channels (VDACs) in Arabidopsis have a dual localization in the cell but show a distinct role in mitochondria. Plant Mol. Biol. 2012, 78, 431–446. [Google Scholar] [CrossRef]
- Sanyal, S.K.; Kanwar, P.; Fernandes, J.L.; Mahiwal, S.; Yadav, A.K.; Samtani, H.; Srivastava, A.K.; Suprasanna, P.; Pandey, G.K. Arabidopsis Mitochondrial Voltage-Dependent Anion Channels Are Involved in Maintaining Reactive Oxygen Species Homeostasis, Oxidative and Salt Stress Tolerance in Yeast. Front. Plant Sci. 2020, 11, 50. [Google Scholar] [CrossRef]
- Analin, B.; Bakka, K.; Challabathula, D. Exacerbation of Drought-Induced Physiological and Biochemical Changes in Leaves of Pisum sativum upon Restriction of COX and AOX Pathways of Mitochondrial Oxidative Electron Transport. J. Biosci. 2023, 49, 5. [Google Scholar] [CrossRef]
- Cai, J.; Longo, A.; Dickstein, R. Expression and Mutagenesis Studies in the Medicago Truncatula Iron Transporter MtVTL8 Confirm its Role in Symbiotic Nitrogen Fixation and Reveal Amino Acids Essential for Transport. Front. Plant Sci. 2023, 14, 1306491. [Google Scholar] [CrossRef]
- do Carmo Santos, M.L.; Santos, T.A.; Dos Santos Lopes, N.; Macedo Ferreira, M.; Martins Alves, A.M.; Pirovani, C.P.; Micheli, F. The Selenium-Independent Phospholipid Hydroperoxide Glutathione Peroxidase from Theobroma cacao (TcPHGPX) Protects Plant Cells Against Damages and Cell Death. Plant Physiol. Biochem. 2024, 207, 108332. [Google Scholar] [CrossRef]
- Dong, Z.; Li, L.; Du, G.; Zhang, Y.; Wang, X.; Li, S.; Xiang, W. A Previously Unidentified Sugar Transporter for Engineering of High-Yield Streptomyces. Appl. Microbiol. Biotechnol. 2024, 108, 72. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Yan, P.; Feng, B.; Mamat, A.; Wang, J. The Apple Ca2+/H+ Exchanger MdCAX2L-2 Functions Positively in Modulation of Ba2+ Tolerance. Plant Physiol. Biochem. 2023, 207, 108314. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.D.; Makita, Y.; Miyazaki, T.; Matsui, M.; Shin, R. Arabidopsis Transcriptomic Analysis Reveals Cesium Inhibition of Root Growth Involves Abscisic Acid Signaling. Planta 2024, 259, 36. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, P.; Li, T.; Tian, Z.; Xu, R. GhCNGC13 and 32 Act as Critical Links between Growth and Immunity in Cotton. Int. J. Mol. Sci. 2023, 25, 1. [Google Scholar] [CrossRef] [PubMed]
- Sanden, N.C.H.; Kanstrup, C.; Crocoll, C.; Schulz, A.; Nour-Eldin, H.H.; Halkier, B.A.; Xu, D. An UMAMIT-GTR Transporter Cascade Controls Glucosinolate Seed Loading in Arabidopsis. Nat. Plants 2024, 10, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Sigalas, P.P.; Buchner, P.; Kroper, A.; Hawkesford, M.J. The Functional Diversity of the High-Affinity Nitrate Transporter Gene Family in Hexaploid Wheat: Insights from Distinct Expression Profiles. Int. J. Mol. Sci. 2023, 25, 509. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, S.; Li, Y.; Zhou, X.; Liu, X.; Yang, W.; Chen, R. Identification and Characterization of Yellow Stripe-Like Genes in Maize Suggest their Roles in the Uptake and Transport of Zinc and Iron. BMC Plant Biol. 2024, 24, 3. [Google Scholar] [CrossRef]
- Suslov, M.; Daminova, A.; Egorov, J. Real-Time Dynamics of Water Transport in the Roots of Intact Maize Plants in Response to Water Stress: The Role of Aquaporins and the Contribution of Different Water Transport Pathways. Cells 2024, 13, 154. [Google Scholar] [CrossRef]
- Tian, J.; Chang, K.; Lei, Y.; Li, S.; Wang, J.; Huang, C.; Zhong, F. Genome-Wide Identification of Proline Transporter Gene Family in Non-Heading Chinese Cabbage and Functional Analysis of BchProT1 under Heat Stress. Int. J. Mol. Sci. 2023, 25, 99. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Deng, W.; Su, Y.; Ma, L.; Yang, H.; Yao, F.; Lin, W. Transcriptome Analysis Reveals Novel Insights into the Hyperaccumulator Phytolacca acinosa Roxb. Responses to Cadmium Stress. Plants 2024, 13, 297. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.K.; Alavi, M.S.; Roohbakhsh, A. The Role of ATP-binding Cassette Transporter G1 (ABCG1) in Alzheimer’s Disease: A Review of the Mechanisms. Basic Clin. Pharmacol. 2024, 38275217. [Google Scholar] [CrossRef] [PubMed]
- Danielewski, M.; Rapak, A.; Kruszynska, A.; Malodobra-Mazur, M.; Oleszkiewicz, P.; Dzimira, S.; Kucharska, A.Z.; Slupski, W.; Matuszewska, A.; Nowak, B.; et al. Cornelian Cherry (Cornus mas L.) Fruit Extract Lowers SREBP-1c and C/EBPalpha in Liver and Alters Various PPAR-alpha, PPAR-gamma, LXR-alpha Target Genes in Cholesterol-Rich Diet Rabbit Model. Int. J. Mol. Sci. 2024, 25, 1199. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Yan, Y.; Niu, T.; Zhang, M.; Fan, C.; Liang, W.; Shu, Y.; Guo, C.; Guo, D.; et al. GmABCG5, An ATP-Binding Cassette G Transporter Gene, is Involved in the Iron Deficiency Response in Soybean. Front. Plant Sci. 2023, 14, 1289801. [Google Scholar] [CrossRef]
- Meena, V.; Kaur, G.; Joon, R.; Shukla, V.; Choudhary, P.; Roy, J.K.; Singh, B.; Pandey, A.K. Transcriptome and Biochemical Analysis in Hexaploid Wheat with Contrasting Tolerance to Iron Deficiency Pinpoints Multi-Layered Molecular Process. Plant Physiol. Biochem. 2024, 207, 108336. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, D.; Wu, X.; Jiang, X.; Gong, B.; Gao, H. Types of Membrane Transporters and the Mechanisms of Interaction between Them and Reactive Oxygen Species in Plants. Antioxidants 2024, 13, 221. https://doi.org/10.3390/antiox13020221
Yuan D, Wu X, Jiang X, Gong B, Gao H. Types of Membrane Transporters and the Mechanisms of Interaction between Them and Reactive Oxygen Species in Plants. Antioxidants. 2024; 13(2):221. https://doi.org/10.3390/antiox13020221
Chicago/Turabian StyleYuan, Ding, Xiaolei Wu, Xiangqun Jiang, Binbin Gong, and Hongbo Gao. 2024. "Types of Membrane Transporters and the Mechanisms of Interaction between Them and Reactive Oxygen Species in Plants" Antioxidants 13, no. 2: 221. https://doi.org/10.3390/antiox13020221
APA StyleYuan, D., Wu, X., Jiang, X., Gong, B., & Gao, H. (2024). Types of Membrane Transporters and the Mechanisms of Interaction between Them and Reactive Oxygen Species in Plants. Antioxidants, 13(2), 221. https://doi.org/10.3390/antiox13020221






