Variations in Proline Content, Polyamine Profiles, and Antioxidant Capacities among Different Provenances of European Beech (Fagus sylvatica L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Provenance Experiment Setup
2.2. Measurements of Osmolyte Accumulation
2.2.1. Quantification of Free Proline (PRO)
2.2.2. HPLC Quantification of Polyamines (PAs)
2.3. Evaluation of Antioxidant Potential and Quantification of Total Phenolic and Flavonoid Contents
2.3.1. DPPH and ABTS Assays
2.3.2. NO Assay
2.3.3. FRAP Assay
2.3.4. The Total Phenolic Content (TPC)
2.3.5. The Total Flavonoid Content (TFC)
2.4. Statistical Analysis
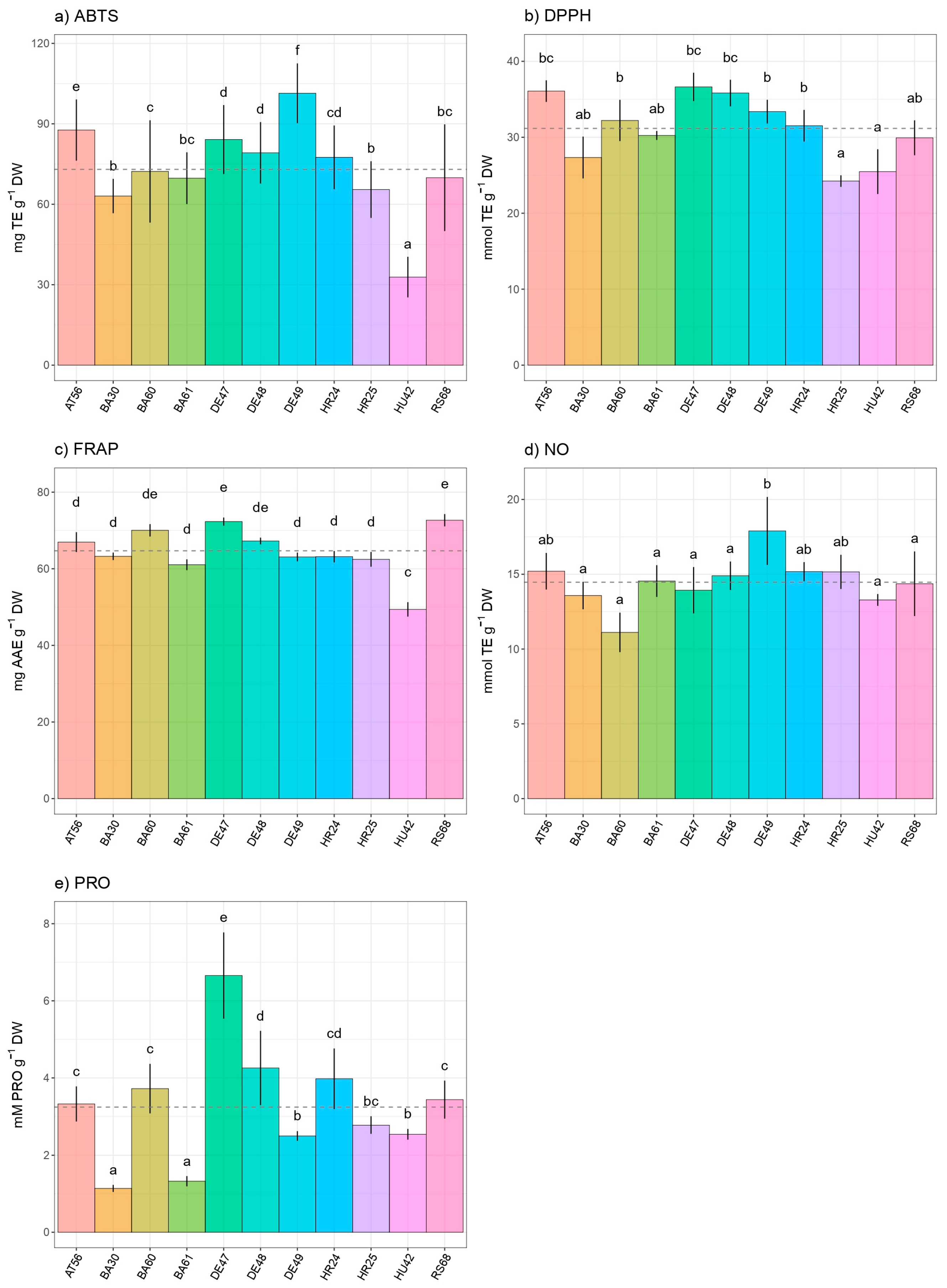
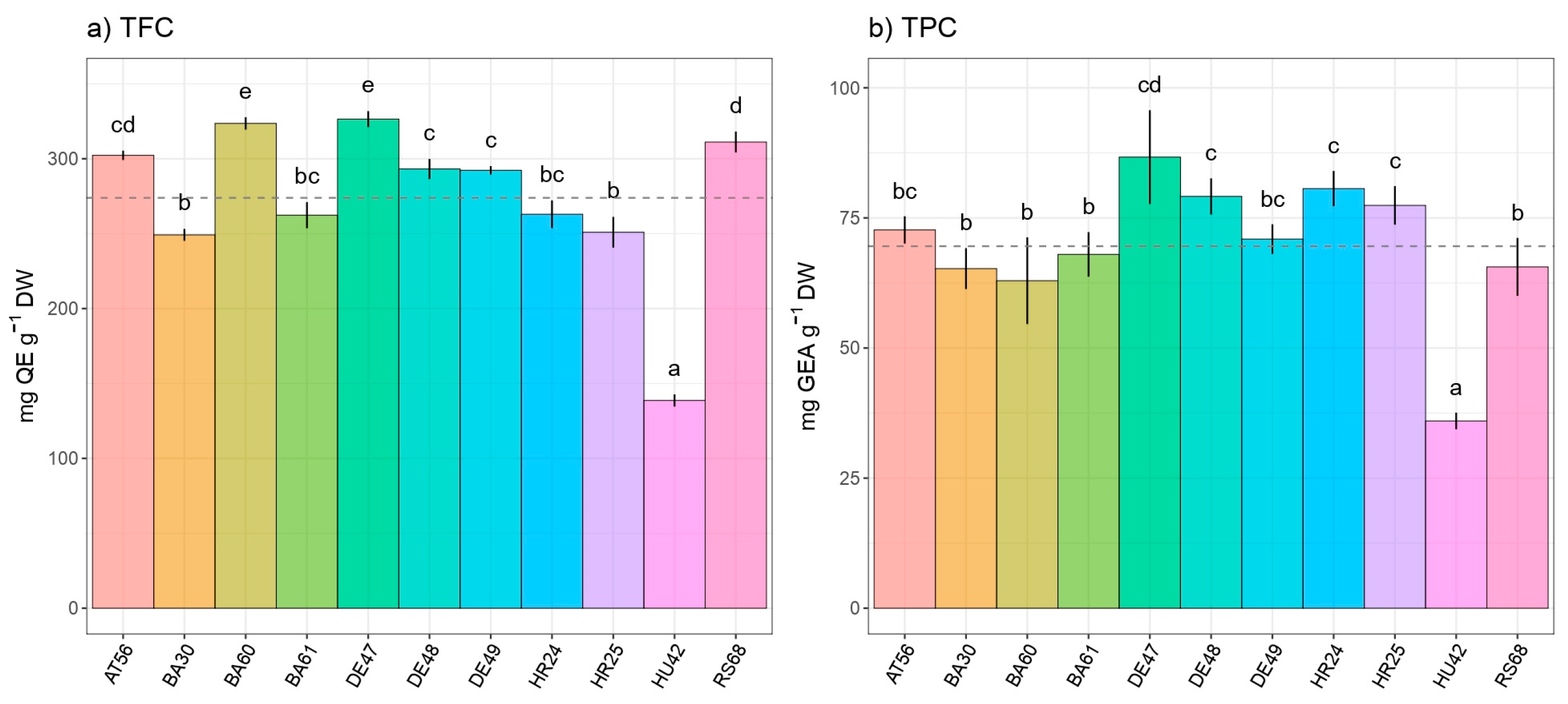
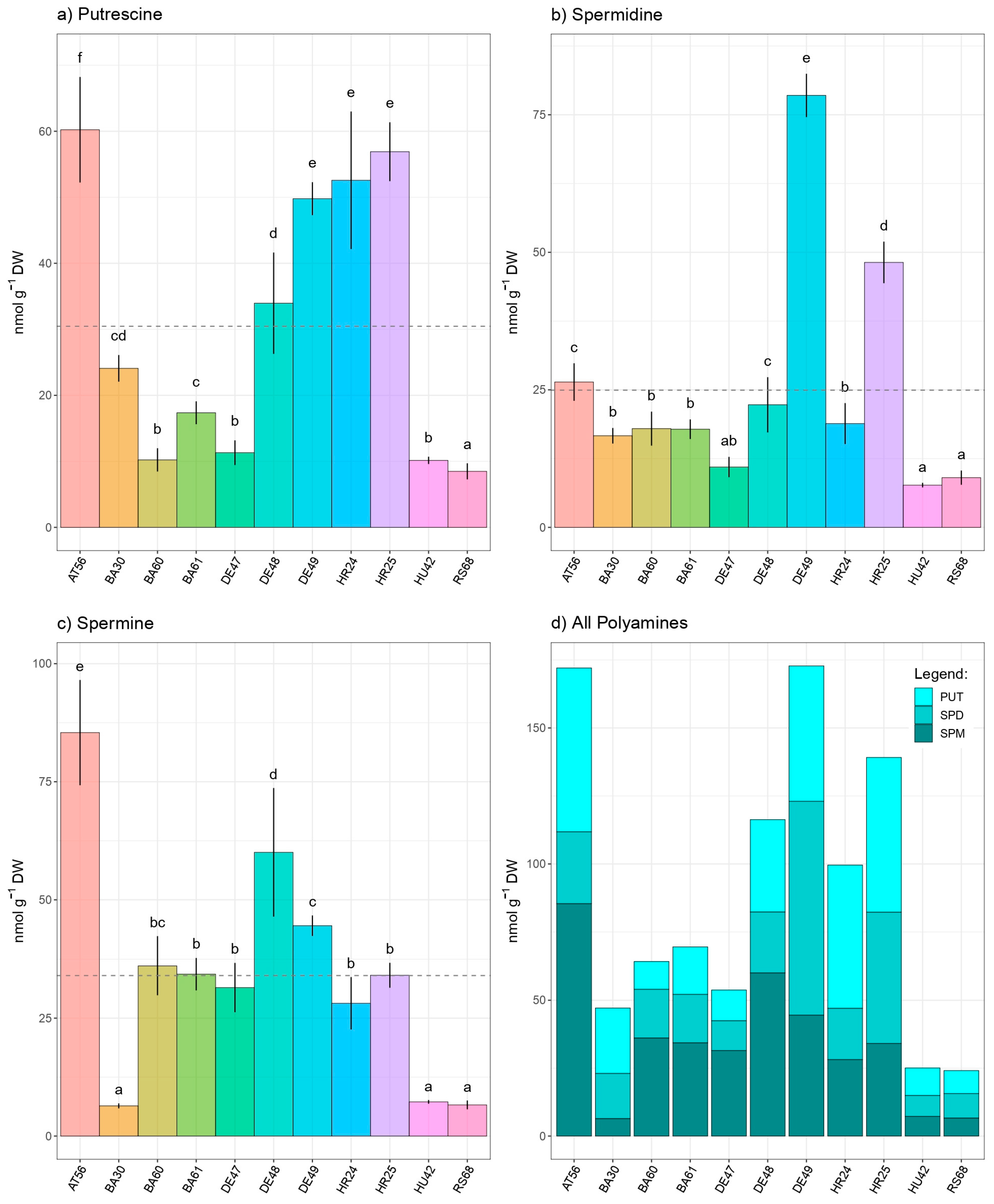
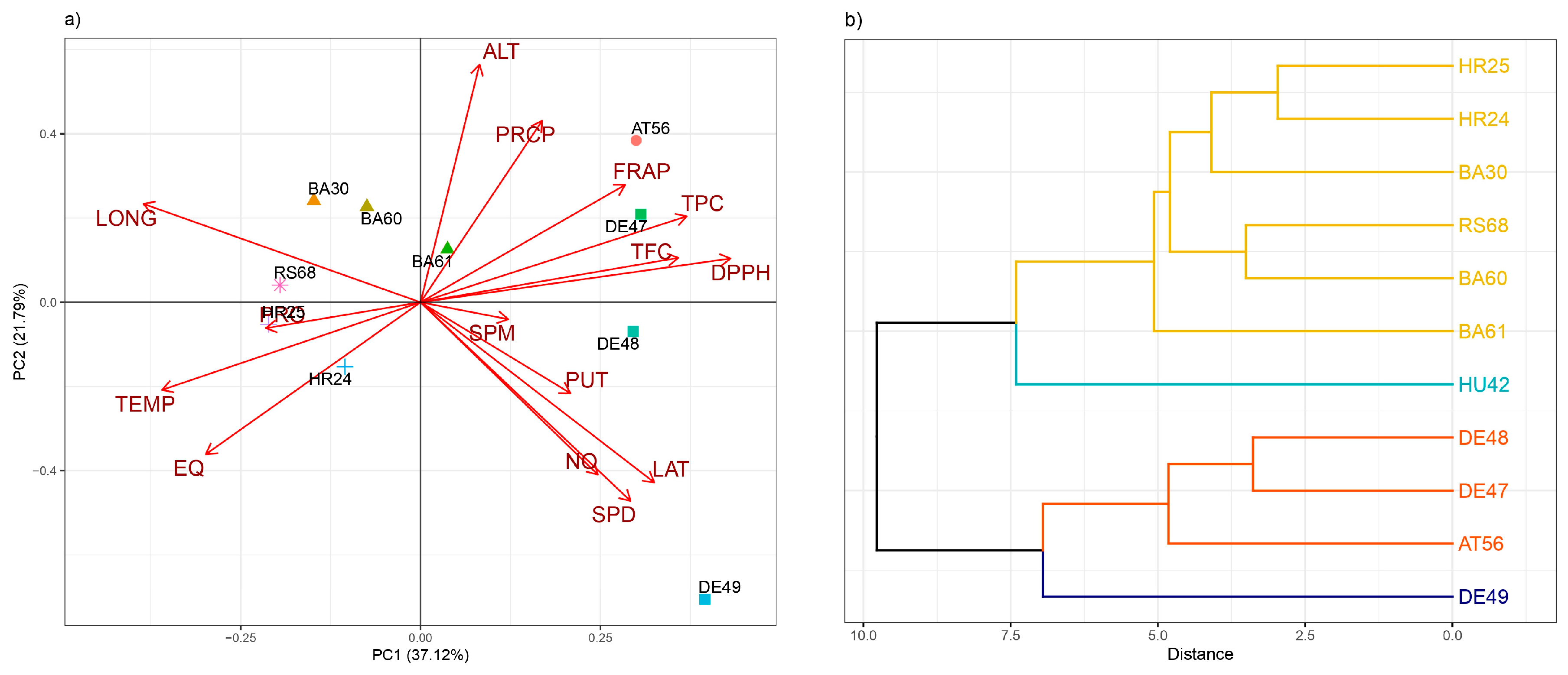
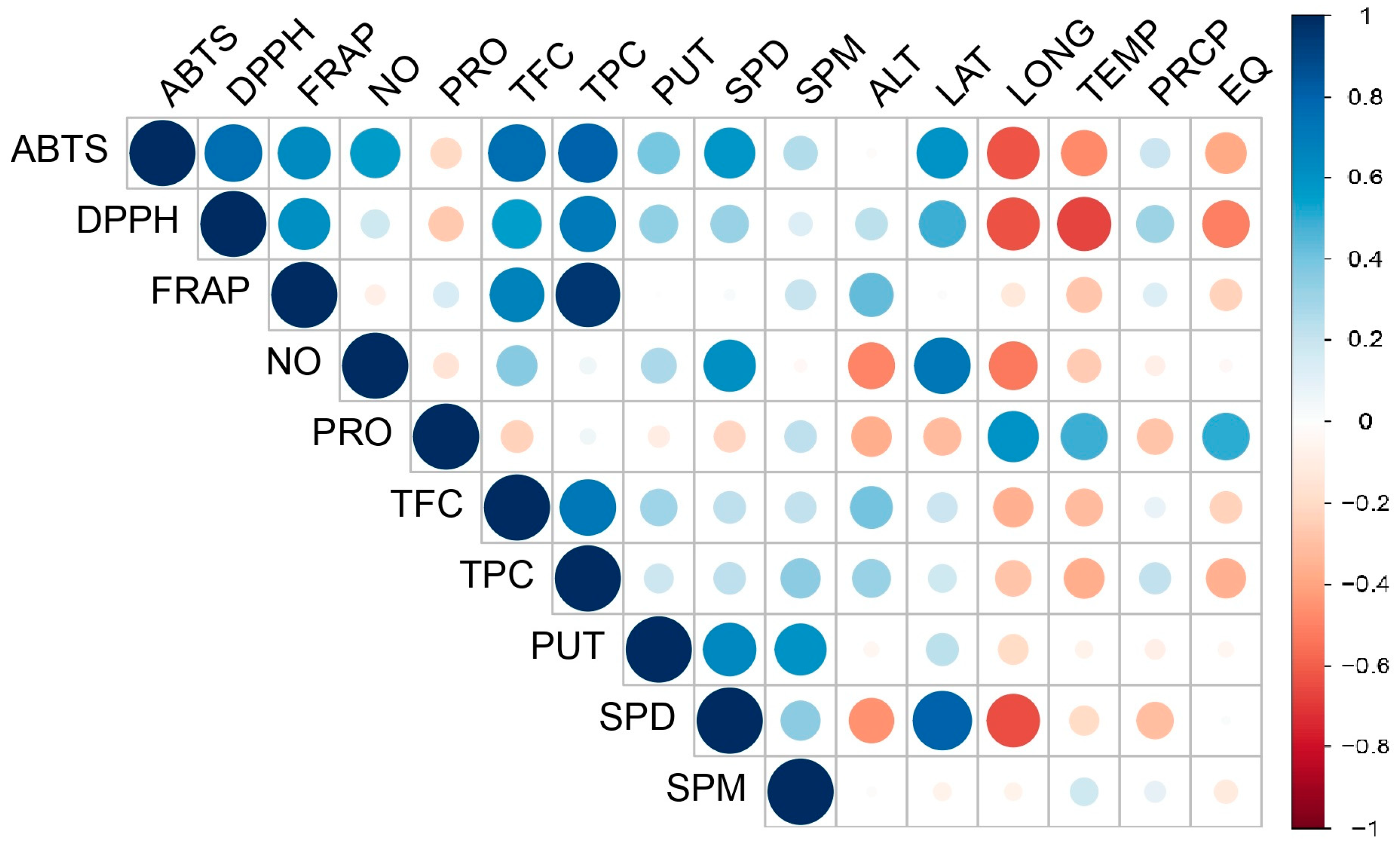
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kutnar, L.; Kermavnar, J.; Pintar, A.M. Climate change and disturbances will shape future temperate forests in the transition zone between Central and SE Europe. Ann. For. Res. 2021, 64, 67–86. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate Change Impacts, Adaptive Capacity, and Vulnerability of European Forest Ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of Tree Species to Heat Waves and Extreme Heat Events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef]
- Mátyás, C.; Berki, I.; Czúcz, B.; Gálos, B.; Móricz, N.; Rasztovits, E. Future of beech in Southeast Europe from the perspective of evolutionary ecology. Acta Silv. Lignaria Hung. 2010, 6, 91–110. [Google Scholar] [CrossRef]
- Dulamsuren, C.; Hauck, M.; Kopp, G.; Ruff, M.; Leuschner, C. European beech responds to climate change with growth decline at lower, and growth increase at higher elevations in the center of its distribution range (SW Germany). Trees 2017, 31, 673–686. [Google Scholar] [CrossRef]
- Tognetti, R.; Lasserre, B.; Di Febbraro, M.; Marchetti, M. Modeling Regional Drought-Stress Indices for Beech Forests in Mediterranean Mountains Based on Tree-Ring Data. Agric. For. Meteorol. 2019, 265, 110–120. [Google Scholar] [CrossRef]
- Gebauer, R.; Plichta, R.; Urban, J.; Volařík, D.; Hajickova, M. The Resistance and Resilience of European Beech Seedlings to Drought Stress during the Period of Leaf Development. Tree Physiol. 2020, 40, 1147–1164. [Google Scholar] [CrossRef]
- Stjepanović, S.; Miletić, B.; Drašković, B.; Tunguz, V. The Impact of Climate Change on the Growth of European Beech at Optimal Altitudes in the Republic of Srpska. Topola 2021, 207, 5–10. [Google Scholar] [CrossRef]
- Pavlović, L.; Stojanović, D.; Mladenović, E.; Lakićević, M.; Orlović, S. Potential Elevation Shift of the European Beech Stands (Fagus sylvatica L.) in Serbia. Front. Plant Sci. 2019, 10, 849. [Google Scholar] [CrossRef]
- Del Castillo, E.M.; Zang, C.S.; Buras, A.; Hacket-Pain, A.; Esper, J.; Serrano-Notivoli, R.; Hartl, C.; Weigel, R.; Klesse, S.; Resco de Dios, V.; et al. Climate-Change-Driven Growth Decline of European Beech Forests. Commun. Biol. 2022, 5, 163. [Google Scholar] [CrossRef]
- Lenormand, T. Gene Flow and the Limits to Natural Selection. Trends Ecol. Evol. 2002, 17, 183–189. [Google Scholar] [CrossRef]
- Schuldt, B.; Knutzen, F.; Delzon, S.; Jansen, S.; Müller-Haubold, H.; Burlett, R.; Clough, Y.; Leuschner, C. How adaptable is the hydraulic system of European beech in the face of climate change-related precipitation reduction? New Phytol. 2016, 210, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Robson, T.M.; Sánchez-Gómez, D.; Cano, F.J.; Aranda, I. Variation in Functional Leaf Traits among Beech Provenances during a Spanish Summer Reflects the Differences in Their Origin. Tree Genet. Genomes 2012, 8, 1111–1121. [Google Scholar] [CrossRef]
- Pšidová, E.; Zivcak, M.; Stojnic, S.; Orlović, S.; Gömöry, D.; Kučerová, J.; Ditmarová, Ľ.; Střelcová, K.; Brestic, M.; Kalaji, H. Altitude of Origin Influences the Responses of PSII Photochemistry on Heat Waves in European Beech (Fagus sylvatica L.). Environ. Exp. Botany 2017, 152, 97–106. [Google Scholar] [CrossRef]
- Štajner, D.; Orlović, S.; Popović, B.M.; Kebert, M.; Stojnić, S.; Klašnja, B. Chemical Parameters of Oxidative Stress Adaptability in Beech. J. Chem. 2012, 2013, e592695. [Google Scholar] [CrossRef]
- Visi-Rajczi, E.; Hofmann, T.; Albert, L.; Mátyás, C. Tracing the Acclimation of European Beech (Fagus sylvatica L.) Populations to Climatic Stress by Analyzing the Antioxidant System. iForest 2021, 14, 95–103. [Google Scholar] [CrossRef]
- Aranda, I.; Sánchez-Gómez, D.; de Miguel Vega, M.; Mancha, J.A.; Guevara, M.A.; Cadahía, E.; Fernández de Simón, M.B. Fagus sylvatica L. Provenances Maintain Different Leaf Metabolic Profiles and Functional Response. Acta Oecol. 2017, 82, 1–9. [Google Scholar] [CrossRef]
- Aranda García, I.; Sánchez-Gómez, D.; Cadahía, E.; Fernández De Simón, M.B. Ecophysiological and Metabolic Response Patterns to Drought under Controlled Condition in Open-Pollinated Maternal Families from a Fagus sylvatica L. Population. Environ. Exp. Bot. 2018, 150, 209–221. [Google Scholar] [CrossRef]
- Cadahía, E.; Fernández de Simón, B.; Aranda, I.; Sanz, M.; Sánchez-Gómez, D.; Pinto, E. Non-Targeted Metabolomic Profile of Fagus sylvatica L. Leaves Using Liquid Chromatography with Mass Spectrometry and Gas Chromatography with Mass Spectrometry. Phytochem. Anal. 2015, 26, 171–182. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of Oxidative Stress in Plants: From Classical Chemistry to Cell Biology. Environ. Exp. Botany 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaei, M.; Abhari, F.M. The Role of Plant-Derived Natural Antioxidants in Reduction of Oxidative Stress. Biofactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline Metabolism as Regulatory Hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Kebert, M.; Kostić, S.; Čapelja, E.; Vuksanović, V.; Stojnić, S.; Gavranović Markić, A.; Zlatković, M.; Milović, M.; Galović, V.; Orlović, S. Ectomycorrhizal Fungi Modulate Pedunculate Oak’s Heat Stress Responses through the Alternation of Polyamines, Phenolics, and Osmotica Content. Plants 2022, 11, 3360. [Google Scholar] [CrossRef] [PubMed]
- Kebert, M.; Kostić, S.; Stojnić, S.; Čapelja, E.; Gavranović Markić, A.; Zorić, M.; Kesić, L.; Flors, V. A Fine-Tuning of the Plant Hormones, Polyamines and Osmolytes by Ectomycorrhizal Fungi Enhances Drought Tolerance in Pedunculate Oak. Int. J. Mol. Sci. 2023, 24, 7510. [Google Scholar] [CrossRef]
- Kebert, M.; Vuksanović, V.; Stefels, J.; Bojović, M.; Horák, R.; Kostić, S.; Kovačević, B.; Orlović, S.; Neri, L.; Magli, M.; et al. Species-Level Differences in Osmoprotectants and Antioxidants Contribute to Stress Tolerance of Quercus robur L., and Q. cerris L. Seedlings under Water Deficit and High Temperatures. Plants 2022, 11, 1744. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Cadahía, E.; Aranda, I. Aerial and Underground Organs Display Specific Metabolic Strategies to Cope with Water Stress under Rising Atmospheric CO2 in Fagus sylvatica L. Physiol Plant. 2022, 174, e13711. [Google Scholar] [CrossRef]
- Kebert, M.; Rapparini, F.; Neri, L.; Bertazza, G.; Orlović, S.; Biondi, S. Copper-Induced Responses in Poplar Clones Are Associated with Genotype- and Organ-Specific Changes in Peroxidase Activity and Proline, Polyamine, ABA, and IAA Levels. J. Plant Growth Regul. 2017, 36, 131–147. [Google Scholar] [CrossRef]
- Kebert, M.; Kostić, S.; Vuksanović, V.; Gavranović Markić, A.; Kiprovski, B.; Zorić, M.; Orlović, S. Metal- and Organ-Specific Response to Heavy Metal-Induced Stress Mediated by Antioxidant Enzymes’ Activities, Polyamines, and Plant Hormones Levels in Populus Deltoides. Plants 2022, 11, 3246. [Google Scholar] [CrossRef]
- Xia, L.; Ma, X.-D.; Cheng, Y.; Junxiang, L.; Zou, J.; Zhai, F.; Zhenyuan, S.; Lei, H. Transcriptomic and Metabolomic Insights into the Adaptive Response of Salix viminalis to Phenanthrene. Chemosphere 2020, 262, 127573. [Google Scholar] [CrossRef]
- Bachrach, U. Naturally Occurring Polyamines: Interaction with Macromolecules. Curr. Protein Pept. Sci. 2005, 6, 559–566. [Google Scholar] [CrossRef]
- Pottosin, I.; Shabala, S. Polyamines Control of Cation Transport across Plant Membranes: Implications for Ion Homeostasis and Abiotic Stress Signaling. Front. Plant Sci. 2014, 5, 154. [Google Scholar] [CrossRef] [PubMed]
- Amooaghaie, R.; Moghym, S. Effect of Polyamines on Thermotolerance and Membrane Stability of Soybean Seedling. Afr. J. Biotechnol. 2011, 10, 9677–9682. [Google Scholar] [CrossRef]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small Amines with Large Effects on Plant Abiotic Stress Tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef] [PubMed]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and Abiotic Stress in Plants: A Complex Relationship. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef] [PubMed]
- Tun, N.N.; Santa-Catarina, C.; Begum, T.; Silveira, V.; Handro, W.; Floh, E.I.S.; Scherer, G.F.E. Polyamines Induce Rapid Biosynthesis of Nitric Oxide (NO) in Arabidopsis Thaliana Seedlings. Plant Cell Physiol. 2006, 47, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Gerlin, L.; Baroukh, C.; Genin, S. Polyamines: Double Agents in Disease and Plant Immunity. Trends Plant. Sci. 2021, 26, 1061–1071. [Google Scholar] [CrossRef]
- Kebert, M.; Kostić, S.; Rašeta, M.; Stojanović, D.V.; Stojnić, S.; Orlović, S. Species-Specific Level Variation in Polyamines in Coniferous and Deciduous Woody Plant Species in Urban Areas. Horticulture 2023, 9, 1157. [Google Scholar] [CrossRef]
- Stojnić, S.; Viscosi, V.; Stefanović Marković, M.; Ivanković, M.; Orlović, S.; Tognetti, R.; Cocozza, C.; Vasić, V.; Anna, L. Spatial Patterns of Leaf Shape Variation in European Beech (Fagus sylvatica L.) Provenances. Trees 2022, 36, 497–511. [Google Scholar] [CrossRef]
- Zepner, L.; Karrasch, P.; Wiemann, F.; Bernard, L. ClimateCharts.Net—An Interactive Climate Analysis Web Platform. Int. J. Digit. Earth 2021, 14, 338–356. [Google Scholar] [CrossRef]
- Ellenberg, H.H. Vegetation Ecology of Central Europe; Cambridge University Press: Cambridge, UK, 2009; ISBN 978-0-521-11512-4. [Google Scholar]
- Fang, J.; Lechowicz, M.J. Climatic Limits for the Present Distribution of Beech (Fagus L.) Species in the World. J. Biogeogr. 2006, 33, 1804–1819. [Google Scholar] [CrossRef]
- Pernar, N.; Vukelić, J.; Bakšić, D.; Baričević, D.; Perković, I.; Miko, S.; Vrbek, B. Soil Properties in Beech-Fir Forests on Mt. Medvednica (NW Croatia). Period. Biol. 2009, 111, 427–434. [Google Scholar]
- Hengl, T.; de Jesus, J.M.; MacMillan, R.A.; Batjes, N.H.; Heuvelink, G.B.M.; Ribeiro, E.; Samuel-Rosa, A.; Kempen, B.; Leenaars, J.G.B.; Walsh, M.G.; et al. SoilGrids1km—Global Soil Information Based on Automated Mapping. PLoS ONE 2014, 9, e105992. [Google Scholar] [CrossRef] [PubMed]
- Wuehlisch, G. Technical Guidelines for Genetic Conservation and Use for European Beech (Fagus sylvatica); Biodiversity International: Rome, Italy, 2008; pp. 1–6. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Scaramagli, S.; Biondi, S.; Capitani, F.; Gerola, P.; Altamura, M.M.; Torrigiani, P. Polyamine Conjugate Levels and Ethylene Biosynthesis: Inverse Relationship with Vegetative Bud Formation in Tobacco Thin Layers. Physiol. Plant. 1999, 105, 366–375. [Google Scholar] [CrossRef]
- Biondi, S.; Antognoni, F.; Marincich, L.; Lianza, M.; Tejos, R.; Ruiz, K.B. The Polyamine “Multiverse” and Stress Mitigation in Crops: A Case Study with Seed Priming in Quinoa. Sci. Hortic. 2022, 304, 111292. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to Total Antioxidant Activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Spectrophotometric Determination of Antioxidant Activity. Redox Rep. 1996, 2, 161–171. [Google Scholar] [CrossRef]
- Hensley, K.; Fedynyshyn, J.; Ferrell, S.; Floyd, R.A.; Gordon, B.; Grammas, P.; Hamdheydari, L.; Mhatre, M.; Mou, S.; Pye, Q.N.; et al. Message and Protein-Level Elevation of Tumor Necrosis Factor Alpha (TNF Alpha) and TNF Alpha-Modulating Cytokines in Spinal Cords of the G93A-SOD1 Mouse Model for Amyotrophic Lateral Sclerosis. Neurobiol. Dis. 2003, 14, 74–80. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colometric Methods. J. Food Drug Anal. 2020, 10, 3. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2023. Available online: https://rpkgs.datanovia.com/rstatix/ (accessed on 7 December 2023).
- Wickham, H. Ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Galili, T. Dendextend: An R Package for Visualizing, Adjusting and Comparing Trees of Hierarchical Clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef] [PubMed]
- Chmura, D.; Rozkowski, R. Variability of Beech Provenances in Spring and Autumn Phenology. Silvae Genet. 2002, 51, 123–127. [Google Scholar]
- Stojnić, S.; Orlović, S.; Miljković, D.; von Wuehlisch, G. Intra- and Interprovenance Variations in Leaf Morphometric Traits in European beech (Fagus sylvatica L.). Arch. Biol. Sci. 2016, 68, 781–788. [Google Scholar] [CrossRef]
- Konnert, M.; Ruetz, W. Genetic Variation of Beech (Fagus sylvatica L.) Provenances in an International Beech Provenance Trial. For. Genet. 2001, 8, 173–184. [Google Scholar]
- Harter, D.E.V.; Nagy, L.; Backhaus, S.; Beierkuhnlein, C.; Fussi, B.; Huber, G.; Jentsch, A.; Konnert, M.; Thiel, D.; Kreyling, J. A Comparison of Genetic Diversity and Phenotypic Plasticity among European Beech (Fagus sylvatica L.) Populations from Bulgaria and Germany under Drought and Temperature Manipulation. Int. J. Plant Sci. 2015, 176, 232–244. [Google Scholar] [CrossRef]
- Seifert, S.; Vornam, B.; Finkeldey, R. A set of 17 single nucleotide polymorphism (SNP) markers for European beech (Fagus sylvatica L.). Conserv. Genet. Resour. 2012, 4, 1045–1047. [Google Scholar] [CrossRef][Green Version]
- Xu, L. The Effect of Polyamineon Flower Bud Differentiation and Bud Germination of Chrysanthemum. Shandong Agric. Univ. 2015, 2, 31–36. [Google Scholar]
- Mustafavi, S.H.; Badi, H.N.; Sękara, A.; Mehrafarin, A.; Janda, T.; Ghorbanpour, M.; Rafiee, H. Polyamines and Their Possible Mechanisms Involved in Plant Physiological Processes and Elicitation of Secondary Metabolites. Acta Physiol. Plant. 2018, 40, 102. [Google Scholar] [CrossRef]
- Shao, J.; Huang, K.; Batool, M.; Idrees, F.; Afzal, R.; Haroon, M.; Noushahi, H.A.; Wu, W.; Hu, Q.; Lu, X.; et al. Versatile Roles of Polyamines in Improving Abiotic Stress Tolerance of Plants. Front. Plant Sci. 2022, 13, 1003155. [Google Scholar] [CrossRef]
- Yamamoto, A.; Shim, I.-S.; Fujihara, S. Inhibition of Putrescine Biosynthesis Enhanced Salt Stress Sensitivity and Decreased Spermidine Content in Rice Seedlings. Biol. Plant. 2017, 61, 385–388. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tahara, M.; Yamada, Y.; Mitsudomi, Y.; Koga, K. Characterization of the Polyamine Biosynthetic Pathways and Salt Stress Response in Brachypodium Distachyon. J. Plant Growth Regul. 2017, 37, 625–634. [Google Scholar] [CrossRef]
- Cai, G.; Sobieszczuk-Nowicka, E.; Aloisi, I.; Fattorini, L.; Serafini-Fracassini, D.; Del Duca, S. Polyamines Are Common Players in Different Facets of Plant Programmed Cell Death. Amino Acids. 2015, 47, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Do, P.T.; Degenkolbe, T.; Erban, A.; Heyer, A.G.; Kopka, J.; Köhl, K.I.; Hincha, D.K.; Zuther, E. Dissecting Rice Polyamine Metabolism under Controlled Long-Term Drought Stress. PLoS ONE 2013, 8, e60325. [Google Scholar] [CrossRef] [PubMed]
- Serafini-Fracassini, D.; Del Duca, S. Transglutaminases: Widespread Cross-Linking Enzymes in Plants. Ann. Bot. 2008, 102, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Szalai, G.; Gondor, O.K.; Janda, T. Unfinished Story of Polyamines: Role of Conjugation, Transport and Light-Related Regulation in the Polyamine Metabolism in Plants. Plant Sci. 2021, 308, 110923. [Google Scholar] [CrossRef]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines Are Important in Abiotic Stress Signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef]
- Navakoudis, E.; Kotzabasis, K. Polyamines: A Bioenergetic Smart Switch for Plant Protection and Development. J. Plant Physiol. 2022, 270, 153618. [Google Scholar] [CrossRef]
- Majumdar, R.; Barchi, B.; Turlapati, S.A.; Gagne, M.; Minocha, R.; Long, S.; Minocha, S.C. Glutamate, Ornithine, Arginine, Proline, and Polyamine Metabolic Interactions: The Pathway is Regulated at the Post-Transcriptional Level. Front. Plant Sci. 2016, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry Behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kebert, M.; Stojnić, S.; Rašeta, M.; Kostić, S.; Vuksanović, V.; Ivanković, M.; Lanšćak, M.; Markić, A.G. Variations in Proline Content, Polyamine Profiles, and Antioxidant Capacities among Different Provenances of European Beech (Fagus sylvatica L.). Antioxidants 2024, 13, 227. https://doi.org/10.3390/antiox13020227
Kebert M, Stojnić S, Rašeta M, Kostić S, Vuksanović V, Ivanković M, Lanšćak M, Markić AG. Variations in Proline Content, Polyamine Profiles, and Antioxidant Capacities among Different Provenances of European Beech (Fagus sylvatica L.). Antioxidants. 2024; 13(2):227. https://doi.org/10.3390/antiox13020227
Chicago/Turabian StyleKebert, Marko, Srđan Stojnić, Milena Rašeta, Saša Kostić, Vanja Vuksanović, Mladen Ivanković, Miran Lanšćak, and Anđelina Gavranović Markić. 2024. "Variations in Proline Content, Polyamine Profiles, and Antioxidant Capacities among Different Provenances of European Beech (Fagus sylvatica L.)" Antioxidants 13, no. 2: 227. https://doi.org/10.3390/antiox13020227
APA StyleKebert, M., Stojnić, S., Rašeta, M., Kostić, S., Vuksanović, V., Ivanković, M., Lanšćak, M., & Markić, A. G. (2024). Variations in Proline Content, Polyamine Profiles, and Antioxidant Capacities among Different Provenances of European Beech (Fagus sylvatica L.). Antioxidants, 13(2), 227. https://doi.org/10.3390/antiox13020227












