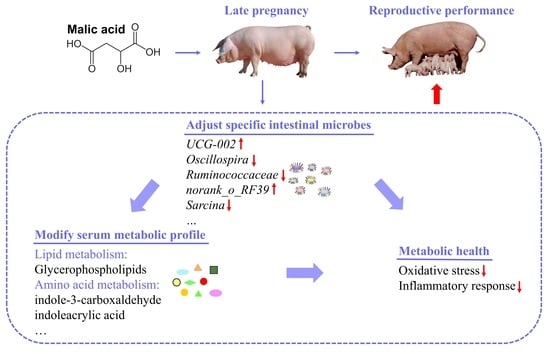
Maternal Malic Acid May Ameliorate Oxidative Stress and Inflammation in Sows through Modulating Gut Microbiota and Host Metabolic Profiles during Late Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Sample Collection
2.3. DNA Extraction, 16S rRNA Sequencing of Gut Microbiota, and Data Analysis
2.4. Untargeted Metabolomics Analysis of Sows’ Serum
2.5. Fecal Short-Chain Fatty Acid (SCFA) Analysis
2.6. Statistical Analysis
3. Results
3.1. Maternal MA Supplementation Modulated the Composition but Not the Richness or Diversity of Gut Microbiota during Late Pregnancy
3.2. Gut Microbiota Alteration May Mechanically Contribute to the MA-Enhanced Antioxidant and Anti-Inflammation Capacity
3.3. MA Reshaped the Functions of Gut Microbiota
3.4. MA Diminished SCFAs in Feces
3.5. MA Mediated the Metabolic Profile in Serum
3.6. Correlation between Serum Metabolites and Fecal Microbiota/Antioxidant or Inflammation Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L.A. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Cao, M.; Li, Y.; Zhuo, Y.; Fang, Z.; Che, L.; Xu, S.; Feng, B.; Lin, Y. Dietary supplementation of Bacillus subtilis PB6 improves sow reproductive performance and reduces piglet birth intervals. Anim. Nutr. 2020, 6, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, C.; Heinonen, M.; Valros, A.; Peltoniemi, O. Environmental and sow-related factors affecting the duration of farrowing. Anim. Reprod. Sci. 2010, 119, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Ruediger, K.; Schulze, M. Post-farrowing stress management in sows by administration of azaperone: Effects on piglets performance. J. Anim. Sci. 2012, 90, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Black, J.; Mullan, B.; Lorschy, M.; Giles, L. Lactation in the sow during heat stress. Livest. Prod. Sci. 1993, 35, 153–170. [Google Scholar] [CrossRef]
- Silanikove, N.; Shapiro, F.; Shinder, D. Acute heat stress brings down milk secretion in dairy cows by up-regulating the activity of the milk-borne negative feedback regulatory system. BMC Physiol. 2009, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Romero-Haro, A.A.; Alonso-Alvarez, C. Oxidative stress experienced during early development influences the offspring phenotype. Am. Nat. 2020, 196, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhou, J.; Xiong, X.; Zou, L.; Kong, X.; Tan, B.; Yin, Y. Differences in Gut Microbial and Serum Biochemical Indices Between Sows with Different Productive Capacities During Perinatal Period. Front. Microbiol. 2019, 10, 3047. [Google Scholar] [CrossRef]
- Hayakawa, T.; Masuda, T.; Kurosawa, D.; Tsukahara, T. Dietary administration of probiotics to sows and/or their neonates improves the reproductive performance, incidence of post-weaning diarrhea and histopathological parameters in the intestine of weaned piglets. Anim. Sci. J. 2016, 87, 1501–1510. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Cheng, C.; Wei, H.; Yu, H.; Xu, C.; Jiang, S.; Peng, J. Metabolic syndrome during perinatal period in sows and the link with gut microbiota and metabolites. Front. Microbiol. 2018, 9, 1989. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ji, Y.; Yin, C.; Deng, M.; Tang, T.; Deng, B.; Ren, W.; Deng, J.; Yin, Y.; Tan, C. Differential analysis of gut microbiota correlated with oxidative stress in sows with high or low litter performance during lactation. Front. Microbiol. 2018, 9, 1665. [Google Scholar] [CrossRef]
- Wang, H.; Hu, C.; Cheng, C.; Cui, J.; Ji, Y.; Hao, X.; Li, Q.; Ren, W.; Deng, B.; Yin, Y. Unraveling the association of fecal microbiota and oxidative stress with stillbirth rate of sows. Theriogenology 2019, 136, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wei, H.; Ao, J.; Long, G.; Peng, J. Inclusion of konjac flour in the gestation diet changes the gut microbiota, alleviates oxidative stress, and improves insulin sensitivity in sows. Appl. Environ. Microbiol. 2016, 82, 5899–5909. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; de Agüero, M.G.; Ganal-Vonarburg, S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol. 2017, 17, 508–517. [Google Scholar] [CrossRef]
- Li, Q.; Yang, S.; Chen, F.; Guan, W.; Zhang, S. Nutritional strategies to alleviate oxidative stress in sows. Anim. Nutr. 2022, 9, 60–73. [Google Scholar] [CrossRef]
- Reyes-Camacho, D.; Vinyeta, E.; Pérez, J.F.; Aumiller, T.; Criado, L.; Palade, L.M.; Taranu, I.; Folch, J.M.; Calvo, M.A.; Van der Klis, J.D. Phytogenic actives supplemented in hyperprolific sows: Effects on maternal transfer of phytogenic compounds, colostrum and milk features, performance and antioxidant status of sows and their offspring, and piglet intestinal gene expression. J. Anim. Sci. 2020, 98, skz390. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, J.; Chen, Y.; Xu, Q.; Song, P.; Li, Y.; Li, K.; Liu, H. Recent advances in microbial production of L-malic acid. Appl. Microbiol. Biotechnol. 2022, 106, 7973–7992. [Google Scholar] [CrossRef]
- Yousefi, M.; Ghafarifarsani, H.; Raissy, M.; Yilmaz, S.; Vatnikov, Y.A.; Kulikov, E.V. Effects of dietary malic acid supplementation on growth performance, antioxidant and immunological parameters, and intestinal gene expressions in rainbow trout, Oncorhynchus mykiss. Aquaculture 2023, 563, 738864. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, P.; Xia, C.; Cheng, Y.; Guo, X.; Li, Y. Effects of malic acid and citric acid on growth performance, antioxidant capacity, haematology and immune response of Carassius auratus gibelio. Aquacult. Res. 2020, 51, 2766–2776. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, M.; Yan, E.; Wang, Y.; Ma, C.; Zhang, P.; Yin, J. Dietary malic acid supplementation induces skeletal muscle fiber-type transition of weaned piglets and further improves meat quality of finishing pigs. Front. Nutr. 2022, 8, 825495. [Google Scholar] [CrossRef] [PubMed]
- Yan, E.; Wang, Y.; He, L.; Guo, J.; Zhang, X.; Yin, J. Effects of dietary l-malic acid supplementation on meat quality, antioxidant capacity and muscle fiber characteristics of finishing pigs. Foods 2022, 11, 3335. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, G.; Wang, Y.; Yan, E.; He, L.; Guo, J.; Yin, J.; Zhang, X. Maternal consumption of l-malic acid enriched diets improves antioxidant capacity and glucose metabolism in offspring by regulating the gut microbiota. Redox Biol. 2023, 67, 102889. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, X.; Ma, Y.; Cai, S.; Yang, L.; Fan, Y.; Zeng, X.; Qiao, S. Lactobacillus reuteri improves the development and maturation of fecal microbiota in piglets through mother-to-infant microbe and metabolite vertical transmission. Microbiome 2022, 10, 211. [Google Scholar] [CrossRef]
- Hedemann, M.; Flummer, C.; Kristensen, N.; Theil, P. Metabolic profiling of plasma from sows before parturition and during lactation using a liquid chromatography–mass spectrometry-based approach. J. Anim. Sci. 2012, 90, 200–202. [Google Scholar] [CrossRef][Green Version]
- Newbern, D.; Freemark, M. Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 409–416. [Google Scholar] [CrossRef]
- Palm, M.; Axelsson, O.; Wernroth, L.; Larsson, A.; Basu, S. Involvement of inflammation in normal pregnancy. Acta Obstet. Gynecol. Scand. 2013, 92, 601–605. [Google Scholar] [CrossRef]
- Pereira, R.D.; De Long, N.E.; Wang, R.C.; Yazdi, F.T.; Holloway, A.C.; Raha, S. Angiogenesis in the placenta: The role of reactive oxygen species signaling. Biomed. Res. Int. 2015, 2015, 814543. [Google Scholar] [CrossRef]
- Liu, H.; Hou, C.; Li, N.; Zhang, X.; Zhang, G.; Yang, F.; Zeng, X.; Liu, Z.; Qiao, S. Microbial and metabolic alterations in gut microbiota of sows during pregnancy and lactation. FASEB J. 2019, 33, 4490–4501. [Google Scholar] [CrossRef]
- Zhou, P.; Zhao, Y.; Zhang, P.; Li, Y.; Gui, T.; Wang, J.; Jin, C.; Che, L.; Li, J.; Lin, Y. Microbial mechanistic insight into the role of inulin in improving maternal health in a pregnant sow model. Front. Microbiol. 2017, 8, 2242. [Google Scholar] [CrossRef]
- Kim, M.; Morrison, M.; Yu, Z. Status of the phylogenetic diversity census of ruminal microbiomes. FEMS Microbiol. Ecol. 2011, 76, 49–63. [Google Scholar] [CrossRef]
- Becker, A.A.; Hesta, M.; Hollants, J.; Janssens, G.P.; Huys, G. Phylogenetic analysis of faecal microbiota from captive cheetahs reveals underrepresentation of Bacteroidetes and Bifidobacteriaceae. BMC Microbiol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Komaroff, A.L. The microbiome and risk for obesity and diabetes. JAMA 2017, 317, 355–356. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Wang, W.; Huang, C.; Hu, Y.; Johnston, L.; Wang, F. Dietary supplementation with fine-grinding wheat bran improves lipid metabolism and inflammatory response via modulating the gut microbiota structure in pregnant sow. Front. Microbiol. 2022, 13, 835950. [Google Scholar] [CrossRef]
- He, J.; Guo, H.; Zheng, W.; Xue, Y.; Zhao, R.; Yao, W. Heat stress affects fecal microbial and metabolic alterations of primiparous sows during late gestation. J. Anim. Sci. Biotechnol. 2019, 10, 84. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Zhang, L.; Yang, Y.; Lin, Y.; Zhuo, Y.; Fang, Z.; Che, L.; Feng, B.; Xu, S. Maternal dietary fiber composition during gestation induces changes in offspring antioxidative capacity, inflammatory response, and gut microbiota in a sow model. Int. J. Mol. Sci. 2019, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, Y.; Gao, K.; Wen, X.; Hu, S.; Wang, L.; Jiang, Z.; Xiao, H. Maternal resveratrol regulates the growth performance, antioxidant capacity, and intestinal health of suckling piglets through intestinal microorganisms at high summer temperatures. Front. Nutr. 2022, 9, 971496. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, T.; Arfken, A.; Summers, K. Enteroendocrine peptides, growth, and the microbiome during the porcine weaning transition. Anim. Microbiome 2022, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, L.; Li, Z.; Yin, J.; Tan, B.; Chen, J.; Jiang, Q.; Ma, X. Evolution of the Gut Microbiota and Its Fermentation Characteristics of Ningxiang Pigs at the Young Stage. Animals 2021, 11, 638. [Google Scholar] [CrossRef]
- Ali, Q.; Ma, S.; Farooq, U.; Niu, J.; Li, F.; Li, D.; Wang, Z.; Sun, H.; Cui, Y.; Shi, Y. Pasture intake protects against commercial diet-induced lipopolysaccharide production facilitated by gut microbiota through activating intestinal alkaline phosphatase enzyme in meat geese. Front. Immunol. 2022, 13, 1041070. [Google Scholar] [CrossRef]
- Ayyanna, R.; Ankaiah, D.; Arul, V. Anti-inflammatory and antioxidant properties of probiotic bacterium Lactobacillus mucosae AN1 and Lactobacillus fermentum SNR1 in Wistar albino rats. Front. Microbiol. 2018, 9, 3063. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M. Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. mBio 2017, 8, e00470-17. [Google Scholar] [CrossRef] [PubMed]
- Owens, L.A.; Colitti, B.; Hirji, I.; Pizarro, A.; Jaffe, J.E.; Moittié, S.; Bishop-Lilly, K.A.; Estrella, L.A.; Voegtly, L.J.; Kuhn, J.H. A Sarcina bacterium linked to lethal disease in sanctuary chimpanzees in Sierra Leone. Nat. Commun. 2021, 12, 763. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S. Spirochete flagella and motility. Biomolecules 2020, 10, 550. [Google Scholar] [CrossRef]
- Mølbak, L.; Klitgaard, K.; Jensen, T.K.; Fossi, M.; Boye, M. Identification of a novel, invasive, not-yet-cultivated Treponema sp. in the large intestine of pigs by PCR amplification of the 16S rRNA gene. J. Clin. Microbiol. 2006, 44, 4537–4540. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wan, F.; Wu, Y.; Liu, L.; Liu, Y.; Zhong, R.; Chen, L.; Zhang, H. Caffeic acid supplementation ameliorates intestinal injury by modulating intestinal microbiota in LPS-challenged piglets. Food Funct. 2023, 14, 7705–7717. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X.; Zhang, L.; Wu, J.; Zhao, S.; Jiao, T. Effect of Oregano Oil and Cobalt Lactate on Sheep In Vitro Digestibility, Fermentation Characteristics and Rumen Microbial Community. Animals 2022, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; He, W.; Zhang, H.; Wang, J.; Qi, G.; Guo, N.; Zhang, X.; Wu, S. Bio-Fermented Malic Acid Facilitates the Production of High-Quality Chicken via Enhancing Muscle Antioxidant Capacity of Broilers. Antioxidants 2022, 11, 2309. [Google Scholar] [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, W.L., Jr. Short chain fatty acids and their receptors: New metabolic targets. Transl. Res. 2013, 161, 131–140. [Google Scholar] [CrossRef]
- Konikoff, T.; Gophna, U. Oscillospira: A central, enigmatic component of the human gut microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef]
- Keren, N.; Konikoff, F.M.; Paitan, Y.; Gabay, G.; Reshef, L.; Naftali, T.; Gophna, U. Interactions between the intestinal microbiota and bile acids in gallstones patients. Environ. Microbiol. Rep. 2015, 7, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Du, E.; Chen, F.; Tao, W.; Zhao, N.; Huang, S.; Guo, W.; Huang, J.; Wei, J. Maternal Magnolol Supplementation during Pregnancy and Lactation Promotes Antioxidant Capacity, Improves Gut Health, and Alters Gut Microbiota and Metabolites of Weanling Piglets. Metabolites 2023, 13, 797. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Monnerie, S.; Comte, B.; Ziegler, D.; Morais, J.A.; Pujos-Guillot, E.; Gaudreau, P. Metabolomic and lipidomic signatures of metabolic syndrome and its physiological components in adults: A systematic review. Sci. Rep. 2020, 10, 669. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Feng, L.; Jiang, Q.; Wang, W.; Tang, X.; Yin, Y. Intestinal tryptophan metabolism in disease prevention and swine production. Anim. Nutr. 2023, 15, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, M.; Luo, C.; Kolde, R.; d’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A. Indoleacrylic acid produced by commensal peptostreptococcus species suppresses inflammation. Cell Host Microbe 2017, 22, 25–37.e26. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Xiang, H.; Guo, M.; Li, S.; Liu, M.; Yao, J. The Tryptophan Metabolite Indole-3-Carboxaldehyde Alleviates Mice with DSS-Induced Ulcerative Colitis by Balancing Amino Acid Metabolism, Inhibiting Intestinal Inflammation, and Improving Intestinal Barrier Function. Molecules 2023, 28, 3704. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, M.; Su, J.; Li, Y.; Long, J.; Chu, J.; Wan, X.; Cao, Y.; Li, Q. Lipid Metabolism and Cancer. Life 2022, 12, 784. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zhang, T.Y.; Luo, L.; Shi, Y.Q.; Bai, F.J.; Jiang, D.N. Impact of Dietary L-Malic Acid Supplementation on Growth, Feed Utilization, Ash Deposition, and Hepatic Lipid Metabolism of Juvenile Genetically Improved Farmed Tilapia, Oreochromis niloticus. J. World Aquacult. Soc. 2017, 48, 563–573. [Google Scholar] [CrossRef]
- Watanabe, S.; Togashi, S.-i.; Takahashi, N.; Fukui, T. L-tryptophan as an antioxidant in human placenta extract. J. Nutr. Sci. Vitaminol. 2002, 48, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, H.; Bai, M.; Gao, J.; Wu, X.; Yin, Y. Redox properties of tryptophan metabolism and the concept of tryptophan use in pregnancy. Int. J. Mol. Sci. 2017, 18, 1595. [Google Scholar] [CrossRef]
- Nikolaus, S.; Schulte, B.; Al-Massad, N.; Thieme, F.; Schulte, D.M.; Bethge, J.; Rehman, A.; Tran, F.; Aden, K.; Häsler, R. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology 2017, 153, 1504–1516.e1502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tan, C.; Xin, Z.; Huang, S.; Ma, J.; Zhang, M.; Shu, G.; Luo, H.; Deng, B.; Jiang, Q. Uplc-orbitrap-ms/ms combined with biochemical analysis to determine the growth and development of mothers and fetuses in different gestation periods on Tibetan sow model. Front. Nutr. 2022, 9, 836938. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Deng, F.; Yuan, M.; Chen, M.; Zeng, L.; Ouyang, Y.; Chen, X.; Zhao, B.; Yang, Z.; Tian, Z. Metabolomics reveals the defense mechanism of histidine supplementation on high-salt exposure-induced hepatic oxidative stress. Life Sci. 2023, 314, 121355. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.B.; de Andrade, R.B.; Gemelli, T.; Oliveira, L.S.; Campos, A.G.; Dutra-Filho, C.S.; Wannmacher, C.M.D. Effect of histidine administration to female rats during pregnancy and lactation on enzymes activity of phosphoryltransfer network in cerebral cortex and hippocampus of the offspring. Metab. Brain Dis. 2012, 27, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, Y.; Ji, H.; Li, L.; Liu, H.; Wang, S.; Zhang, D.; Yin, J.; Wang, J.; Zhang, X. Chenodeoxycholic Acid Improves Embryo Implantation and Metabolic Health through Modulating Gut Microbiota–Host Metabolites Interaction during Early Pregnancy. Antioxidants 2023, 13, 8. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, B.; Cai, S.; Zeng, X.; Ye, Q.; Mao, X.; Zhang, S.; Zeng, X.; Ye, C.; Qiao, S. Metabolic disorder of amino acids, fatty acids and purines reflects the decreases in oocyte quality and potential in sows. J. Proteom. 2019, 200, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wu, Y.; Huang, L.; Lin, R.; Li, Z.; Wang, X.; Wu, P.; Huang, L. Mechanism of the Effect of Compound Anoectochilus roxburghii (Wall.) Lindl. Oral Liquid in Treating Alcoholic Rat Liver Injury by Metabolomics. Drug Des. Devel. Ther. 2023, 17, 3409–3428. [Google Scholar] [CrossRef] [PubMed]
- Heidari, R.; Mohammadi, H.; Ghanbarinejad, V.; Ahmadi, A.; Ommati, M.M.; Niknahad, H.; Jamshidzadeh, A.; Azarpira, N.; Abdoli, N. Proline supplementation mitigates the early stage of liver injury in bile duct ligated rats. J. Basic. Clin. Physiol. Pharmacol. 2018, 30, 91–101. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Zhao, Y.; Li, S.; Chang, Z.; Liu, H.; Zhang, D.; Wang, S.; Zhang, X.; Wang, J. Maternal Malic Acid May Ameliorate Oxidative Stress and Inflammation in Sows through Modulating Gut Microbiota and Host Metabolic Profiles during Late Pregnancy. Antioxidants 2024, 13, 253. https://doi.org/10.3390/antiox13020253
Chen M, Zhao Y, Li S, Chang Z, Liu H, Zhang D, Wang S, Zhang X, Wang J. Maternal Malic Acid May Ameliorate Oxidative Stress and Inflammation in Sows through Modulating Gut Microbiota and Host Metabolic Profiles during Late Pregnancy. Antioxidants. 2024; 13(2):253. https://doi.org/10.3390/antiox13020253
Chicago/Turabian StyleChen, Meixia, Ying Zhao, Shuang Li, Zhuo Chang, Hui Liu, Dongyan Zhang, Sixin Wang, Xin Zhang, and Jing Wang. 2024. "Maternal Malic Acid May Ameliorate Oxidative Stress and Inflammation in Sows through Modulating Gut Microbiota and Host Metabolic Profiles during Late Pregnancy" Antioxidants 13, no. 2: 253. https://doi.org/10.3390/antiox13020253
APA StyleChen, M., Zhao, Y., Li, S., Chang, Z., Liu, H., Zhang, D., Wang, S., Zhang, X., & Wang, J. (2024). Maternal Malic Acid May Ameliorate Oxidative Stress and Inflammation in Sows through Modulating Gut Microbiota and Host Metabolic Profiles during Late Pregnancy. Antioxidants, 13(2), 253. https://doi.org/10.3390/antiox13020253