A Novel Plant-Based Nutraceutical Combined with Exercise Can Revert Oxidative Status in Plasma and Liver in a Diet-Induced-Obesity Animal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Functional Extracts and Nutraceutical Formulation
2.3. In Vitro Digestion
2.4. Antioxidant Activity
2.5. Mass Spectrophotometry Analysis
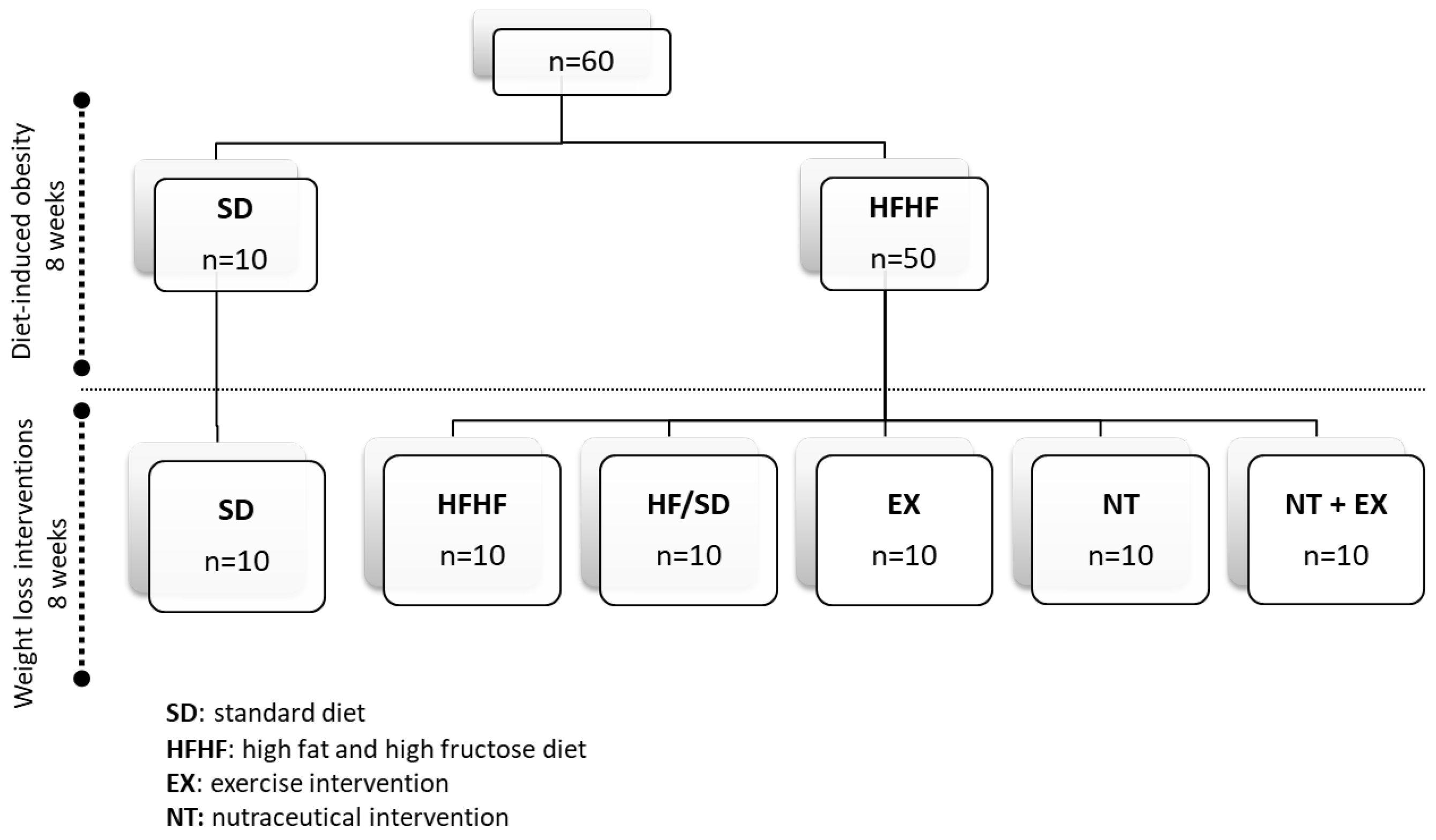
2.6. In Vivo Experimental Design
2.6.1. Animals
2.6.2. Experimental Design
2.6.3. Exercise Protocol
2.6.4. Plasma Analysis
2.6.5. Antioxidant, Detoxifying Enzyme Activity, and Lipid Peroxidation Assays in Liver
2.6.6. Gene Expression Assays
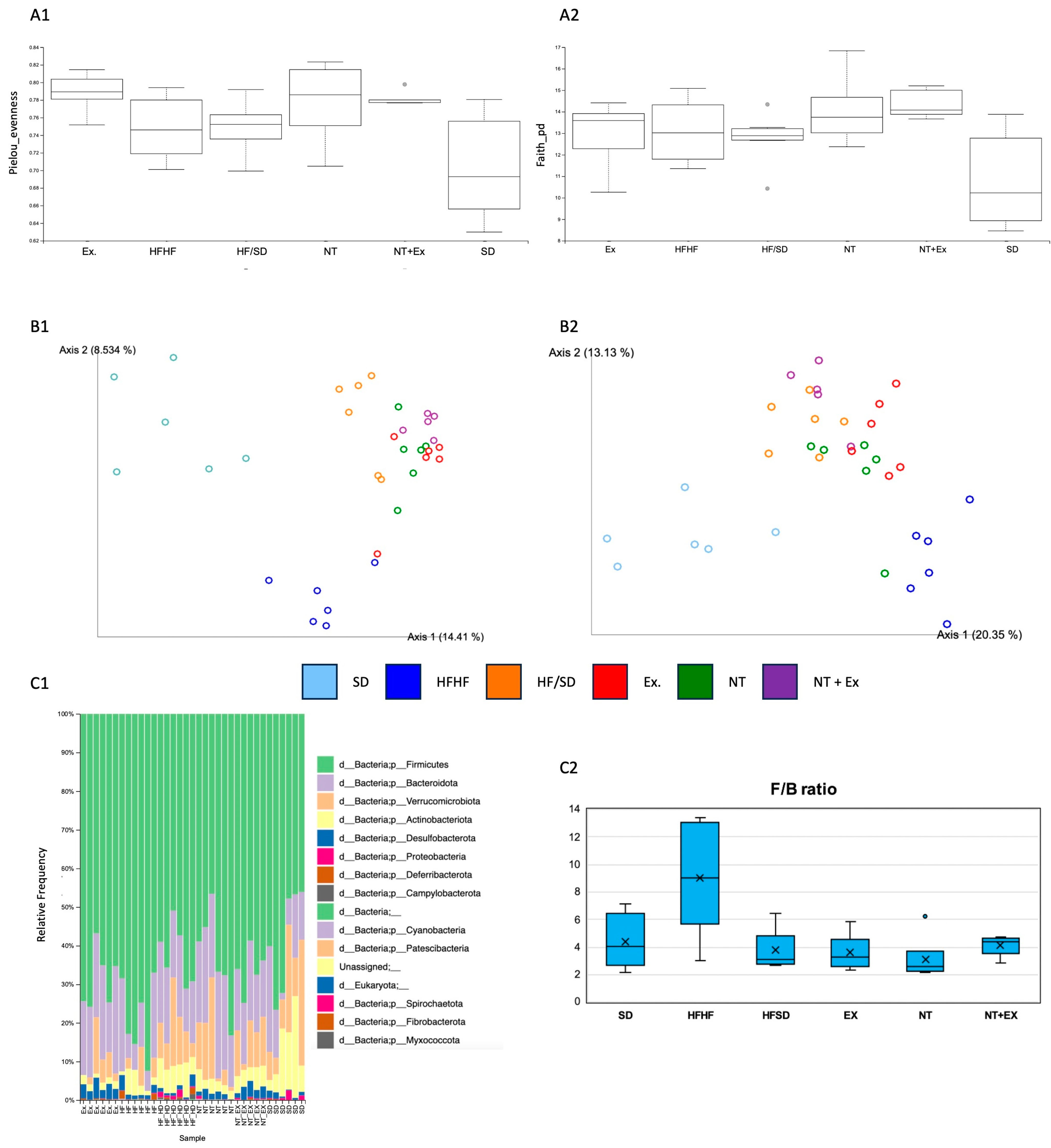
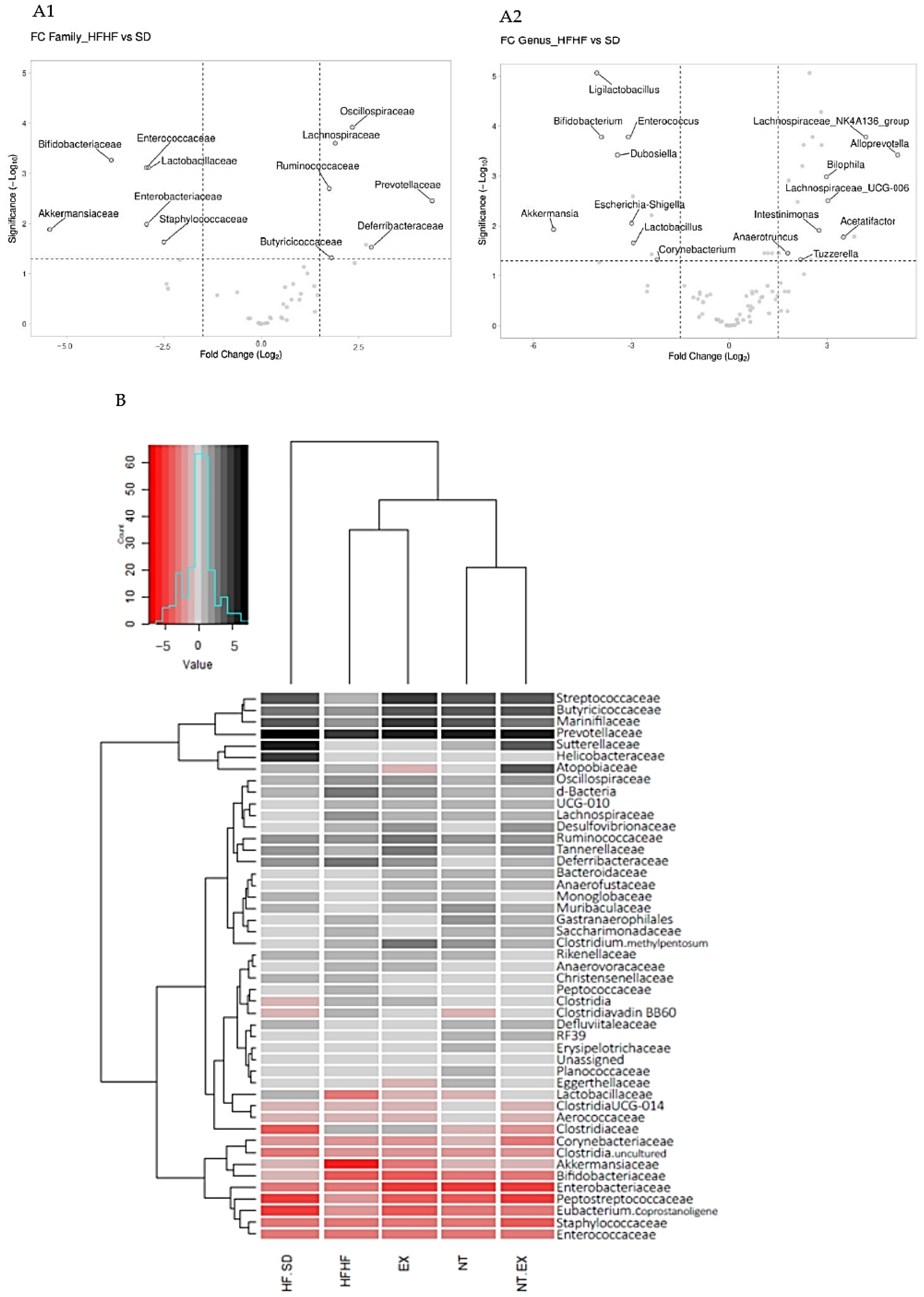
2.6.7. Metagenomic Analysis
Bioinformatic Metagenomic Analysis
2.7. Statistical Analysis
3. Results
3.1. Screening of Antioxidant Capacity in Different Plant Species
3.2. Nutraceutical Composition
| Family | Species | Plant Material | Yield | TPC | ABTS | ICC | LPI |
|---|---|---|---|---|---|---|---|
| (n = 10) | (n = 23) | (mg/g Flour) | (µg GA eq/mg) | (µg GA eq/mg) | (CAU/mg) | (AAU/mg) | |
| Amaryllidaceae | A. ampeloprasum var. porrum | Seeds | 52.8 (0.9) cdef | 4.6 (0.5) a | 2.34 (0.06) abc | 0.67 (0.01) a | 0.46 (0.01) abc |
| Allium cepa | 57.6 (1.1) defg | 6.0 (0.1) ab | 1.36 (0.04) ab | 0.92 (0.00) b | 0.37 (0.01) abc | ||
| Allium cepa | 69.8 (0.4) fgh | 5.8 (0.1) ab | 1.45 (0.01) ab | 0.82 (0.01) ab | 0.22 (0.00) ab | ||
| Apiaceae | Eryngium maritimum | Seeds | 53.4 (0.6) cdef | 26.8 (0.4) e | 2.29 (0.03) abc | 3.74 (0.03) k | 0.22 (0.00) ab |
| Eryngium bourgatii | 39.3 (0.4) abc | 35.2 (0.3) f | 4.93 (0.13) de | 4.64 (0.04) m | 1.14 (0.04) de | ||
| Eryngium campestre | 38.7 (0.8) abc | 28.0 (0.3) e | 3.31 (0.08) bcd | 1.70 (0.04) fg | 2.10 (0.12) gh | ||
| Araceae | Lemna minor | Whole plant | 300.0 (3.8) ñ | 44.7 (0.3) g | 4.31 (0.06) cd | 5.91 (0.06) n | 0.83 (0.03) cd |
| Spirodela polyrhiza | 263.5 (7.2) n | 58.8 (0.8) i | 7.49 (0.35) fg | 3.38 (0.16) j | 1.43 (0.06) ef | ||
| Brassicaceae | Camelina sativa | Seeds | 166.1 (4.9) l | 37.8 (0.9) f | 5.40 (0.17) def | 3.95 (0.08) k | 1.45 (0.09) ef |
| B. oleracea var. Gemmifera | 129.5 (3.6) k | 28.6 (0.7) e | 11.60 (0.10) hi | 1.60 (0.02) ef | 0.48 (0.01) abc | ||
| B. oleracea var. Gongyloides | 105.1 (1.0) j | 27.8 (0.3) e | 11.37 (0.11) h | 1.74 (0.01) fg | 0.45 (0.03) abc | ||
| Cucurbitaceae | Cucumis sativus | Seeds | 26.7 (0.4) a | 4.02 (0.08) a | 0.46 (0.01) a | 0.82 (0.02) ab | ND |
| Cucurbita pepo | 50.3 (4.5) cdef | 6.66 (0.14) abc | 1.90 (0.05) ab | 2.49 (0.06) h | 1.53 (0.09) ef | ||
| Fabaceae | Psoralea corylifolia | Seeds | 145.8 (2.1) k | 61.3 (0.5) i | 1.82 (0.03) ab | 2.87 (0.01) i | 0.61 (0.03) bc |
| Cicer arietinum | 107.9 (2.4) j | 2.66 (0.03) a | 0.33 (0.03) a | 1.78 (0.01) fg | 0.13 (0.01) ab | ||
| Pisum sativum | 83.7 (2.5) hi | 6.65 (0.14) abc | 0.37 (0.01) a | 1.24 (0.01) cd | 0.23 (0.01) ab | ||
| Vicia sativa | 87.9 (1.0) i | 23.23 (0.37) e | 7.12 (0.27) efg | 1.81 (0.03) fg | 0.27 (0.03) ab | ||
| Lamiaceae | O. basilicum Ligure Cert | Seeds | 46.9 (0.7) bcde | 62.94 (5.32) i | 14.99 (0.29) j | 4.84 (0.01) m | 3.25 (0.15) h |
| O. basilicum Mammolo | 53.6 (1.6) cdef | 60.6 (3.0) i | 8.96 (0.05) g | 2.30 (0.03) h | 1.62 (0.05) efg | ||
| Sapotaceae | Argania spinosa | Fruit pulp | 338.7 (2.8) o | 100.3 (0.9) j | 51.2 (1.6) l | 2.42 (0.04) h | 0.64 (0.01) bcd |
| Solanaceae | Capsicum annuum | Seeds | 30.1 (1.2) ab | 24.7 (8.7) e | 0.43 (0.05) a | 1.68 (0.03) fg | 0.57 (0.01) abc |
| Capsicum annuum Jalapeño | 65.4 (1.2) fg | 12.3 (4.3) cd | 0.05 (0.01) a | 1.24 (0.03) cd | 0.20 (0.00) ab | ||
| Solanum lycopersicum | 65.2 (2.5) efg | 7.29 (0.32) abc | 0.40 (0.01) a | 0.61 (0.03) a | 1.56 (0.03) ef |
| NT | EtOH AS | EtOH CS | HP SP | HP PC | |
|---|---|---|---|---|---|
| TPC (µg GA eq/mg) | 40.9 (0.1) d | 76.4 (0.2) e | 36.0 (0.8) c | 31.9 (0.7) b | 23.9 (0.3) a |
| ABTS (µg GA eq/mg) | 16.1 (0.4) bc | 26.0 (1.2) d | 9.1 (0.1) a | 16.0 (0.6) b | 18.8 (0.1) c |
| ICC (CAU/mg) | 3.51 (0.04) d | 3.24 (0.01) c | 2.12 (0.01) b | 1.01 (0.01) a | 2.17 (0.01) b |
| IRC (µg GA/mg) | 25.4 (0.4) d | 54.2 (0.1) e | 23.1 (0.1) c | 13.0 (0.1) a | 15.7 (0.1) b |
| LPI (mAAU/mg) | 36.7 (3.9) c | 6.7 (0.2) a | 11.6 (0.6) ab | 15.7 (0.3) b | 11.0 (0.8) ab |
3.3. Mass Spectrophotometry Identification of Bioactive Compounds
3.4. In Vitro Digestion
3.5. In Vivo Assay
3.5.1. Food Intake and Body Weight
3.5.2. Tissue and Organ Weights


3.5.3. Liver Parameters and Antioxidant Activity
3.5.4. Plasma Insulin and Inflammation Markers
3.5.5. Gene Expression
| SD | HFHF | HF/SD | Ex | NT | NT + Ex | Ex Effect | NT Effect | NT + Ex Interaction | R2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Water (%) | 70.4 (0.4) c | 66.9 (0.8) a | 70.5 (0.3) c | 69.2 (0.2) bc | 68.3 (0.3) ab | 69.9 (0.1) bc | p = 0.295 (1.33%) | p = 0.002 (13.55%) | p < 0.001 (42.63%) | 0.541 |
| Fat (%) | 3.49 (0.14) a | 11.63 (0.45) b | 3.56 (0.10) a | 3.93 (0.25) a | 4.77 (0.31) a | 4.08 (0.10) a | p = 0.60 (1.08%) | p = 0.009 (36.48%) | p = 0.055 (17.00%) | 0.434 |
| CAT | 7.00 (0.14) b | 7.01 (0.26) b | 7.00 (0.15) b | 6.10 (0.11) a | 6.43 (0.1) ab | 6.20 (0.25) ab | p = 0.007 (16.74%) | p = 0.148 (4.53%) | p = 0.090 (6.28%) | 0.213 |
| Mn-SOD | 27.3 (1.8) a | 25.7 (1.3) a | 33.8 (1.1) b | 35.7 (1.3) b | 37.5 (1.2) bc | 42.1 (0.9) c | p = 0.005 (13.59%) | p < 0.001 (30.55%) | p = 0.261 (2.01%) | 0.415 |
| Cu/Zn-SOD | 398.9 (11.6) b | 475.4 (15.0) c | 367.7 (7.8) ab | 369.7 (9.0) ab | 389.4 (6.6) ab | 346.6 (6.6) a | p = 0.013 (14.16%) | p = 0.957 (0.01%) | p = 0.012 (14.24%) | 0.223 |
| t-SOD | 428.0 (22.5) ab | 480.6 (20.7) b | 401.5 (7.9) a | 405.4 (9.5) a | 427.0 (6.7) ab | 388.8 (10.2) a | p = 0.035 (10.40%) | p = 0.524 (0.90%) | p = 0.020 (12.91%) | 0.177 |
| GPx | 4.65 (0.31) a | 5.23 (0.22) ab | 5.58 (0.22) abc | 5.61 (0.24) abc | 6.08 (0.28) bc | 6.35 (0.22) c | p = 0.517 (1.02%) | p = 0.016 (15.11%) | p = 0.607 (0.64%) | 0.096 |
| ’GST | 1684.2 (67.1) a | 2313.7 (116.9) b | 2050.9 (65.5) b | 2105.7 (43.9) b | 1720.9 (81.7) a | 1699.6 (56.9) a | p = 0.861 (0.07%) | p < 0.001 (32.03%) | p = 0.69 (0.34%) | 0.261 |
| SD | HFHF | HF/SD | Ex | NT | NT + Ex | Ex Effect | NT Effect | NT + Ex Interaction | R2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Insulin | 600.7 (53.2) b | 1.096.0 (105.2) c | 353.8 (33.5) a | 315.0 (28.0) a | 244.3 (11.3) a | 204.2 (13.0) a | p = 0.111 (3.44%) | p < 0.001 (26.10%) | p = 0.931 (0.01%) | 0.258 |
| TNFα | 2.86 (0.14) b | 3.82 (0.24) c | 3.36 (0.23) bc | 1.21 (0.01) a | 1.44 (0.09) a | 1.69 (0.07) a | p < 0.001 (26.02%) | p < 0.001 (12.89%) | p < 0.001 (36.47%) | 0.741 |
| IL-6 | 20.5 (0.4) b | 20.3 (0.8) b | 17.5 (0.8) b | 12.0 (1.2) a | 10.4 (0.5) a | 11.2 (1.0) a | p < 0.001 (6.95%) | p = 0.013 (16.34%) | p = 0.002 (10.85%) | 0.334 |
| Resistin | 14,098.8 (673.2) d | 19,344.9 (967.2) e | 12,070.7 (698.3) cd | 10,574.1 (498.2) bc | 7021.8 (337.6) a | 8539.8 (500.1) ab | p = 0.909 (0.01%) | p < 0.001 (38.83%) | p = 0.008 (7.23%) | 0.435 |
| SD | HFHF | HF/SD | EX | NT | NT + Ex | |
|---|---|---|---|---|---|---|
| Liver gene expression | ||||||
| Cat | 1.00 (0.13) a | 1.11 (0.10) a | 1.73 (0.06) b | 1.84 (0.13) b | 2.09 (0.25) bc | 2.54 (0.19) c |
| Gpx | 1.00 (0.08) a | 1.04 (0.18) a | 1.45 (0.07) a | 1.22 (0.12) a | 1.44 (0.38) a | 1.25 (0.19) a |
| Sod1 | 1.00 (0.05) bc | 1.19 (0.03) c | 0.81 (0.05) ab | 1.06 (0.13) bc | 0.62 (0.05) a | 1.06 (0.07) bc |
| Sod2 | 1.00 (0.06) a | 1.11 (0.07) a | 1.40 (0.06) b | 1.53 (0.06) b | 1.07 (0.05) a | 1.71 (0.10) c |
| Nqo1 | 1.00 (0.09) ab | 0.44 (0.06) a | 1.69 (0.21) bc | 0.61 (0.04) a | 0.85 (0.22) a | 1.85 (0.25) c |
| Gsta2 | 1.00 (0.27) ab | 1.07 (0.27) ab | 1.39 (0.08) abc | 0.76 (0.02) a | 1.68 (0.05) bc | 1.87 (0.23) c |
| Tnfα | 1.00 (0.08) ab | 1.76 (0.23) c | 1.01 (0.11) ab | 0.61 (0.05) a | 0.86 (0.07) a | 1.46 (0.05) bc |
| Plantaris muscle gene expression | ||||||
| Cat | 1.00 (0.08) b | 1.13 (0.08) b | 0.57 (0.01) a | 0.61 (0.04) a | 1.53 (0.09) c | 1.55 (0.07) c |
| Gpx | 1.00 (0.07) a | 1.70 (0.21) a | 4.04 (0.49) b | 3.02 (0.21) b | 1.41 (0.15) a | 1.03 (0.08) a |
| Sod1 | 1.00 (0.05) cd | 0.98 (0.10) d | 0.64 (0.03) ab | 0.52 (0.04) a | 0.76 (0.02) bc | 1.16 (0.04) d |
| Sod2 | 1.00 (0.06) bc | 0.92 (0.06) b | 0.60 (0.01) a | 0.85 (0.07) b | 1.13 (0.04) c | 1.44 (0.04) d |
3.5.6. Metagenomic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesidad y Sobrepeso. Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 24 January 2022).
- Hruby, A.; Manson, J.E.; Qi, L.; Malik, V.S.; Rimm, E.B.; Sun, Q.; Willett, W.C.; Hu, F.B. Determinants and Consequences of Obesity. Am. J. Public Health 2016, 106, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Hung, H.-Y. Recent Advances in Natural Anti-Obesity Compounds and Derivatives Based on In Vivo Evidence: A Mini-Review. Eur. J. Med. Chem. 2022, 237, 114405. [Google Scholar] [CrossRef] [PubMed]
- Gjermeni, E.; Kirstein, A.S.; Kolbig, F.; Kirchhof, M.; Bundalian, L.; Katzmann, J.L.; Laufs, U.; Blüher, M.; Garten, A.; Le Duc, D. Obesity–An Update on the Basic Pathophysiology and Review of Recent Therapeutic Advances. Biomolecules 2021, 11, 1426. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhao, H.; Song, H. Shared Signaling Pathways and Targeted Therapy by Natural Bioactive Compounds for Obesity and Type 2 Diabetes. Crit. Rev. Food Sci. Nutr. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Li, N.; Guenancia, C.; Rigal, E.; Hachet, O.; Chollet, P.; Desmoulins, L.; Leloup, C.; Rochette, L.; Vergely, C. Short-Term Moderate Diet Restriction in Adulthood Can Reverse Oxidative, Cardiovascular and Metabolic Alterations Induced by Postnatal Overfeeding in Mice. Sci. Rep. 2016, 6, 30817. [Google Scholar] [CrossRef]
- Yzydorczyk, C.; Li, N.; Rigal, E.; Chehade, H.; Mosig, D.; Armengaud, J.B.; Rolle, T.; Krishnasamy, A.; Orozco, E.; Siddeek, B.; et al. Calorie Restriction in Adulthood Reduces Hepatic Disorders Induced by Transient Postnatal Overfeeding in Mice. Nutrients 2019, 11, 2796. [Google Scholar] [CrossRef]
- D’Anneo, A.; Lauricella, M. Natural and Synthetic Compounds for Management, Prevention and Treatment of Obesity. Int. J. Mol. Sci. 2022, 23, 2890. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Sánchez-Castillo, D.R.; Cariño-Cervantes, Y.Y.; Torre-Villalvazo, I.; Tovar-Palacio, C.; Vásquez-Reyes, S.; Furuzawa-Carballeda, J.; Acevedo-Carabantes, J.A.; Camacho-Corona, M.D.R.; Guzmán-Mar, J.L.; et al. Vachellia farnesiana Pods or a Polyphenolic Extract Derived from Them Exert Immunomodulatory, Metabolic, Renoprotective, and Prebiotic Effects in Mice Fed a High-Fat Diet. Int. J. Mol. Sci. 2023, 24, 7984. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, B.; Wang, N.; Zuo, Z.; Wei, H.; Zhao, F. A Novel Peptide Protects against Diet-Induced Obesity by Suppressing Appetite and Modulating the Gut Microbiota. Gut 2023, 72, 686–698. [Google Scholar] [CrossRef]
- Saxton, S.N.; Withers, S.B.; Heagerty, A.M. Emerging Roles of Sympathetic Nerves and Inflammation in Perivascular Adipose Tissue. Cardiovasc. Drugs Ther. 2019, 33, 245–259. [Google Scholar] [CrossRef]
- Lopez Trinidad, L.M.; Martinez, R.; Kapravelou, G.; Galisteo, M.; Aranda, P.; Porres, J.M.; Lopez-Jurado, M. Caloric Restriction, Physical Exercise, and CB1 Receptor Blockade as an Efficient Combined Strategy for Bodyweight Control and Cardiometabolic Status Improvement in Male Rats. Sci. Rep. 2021, 11, 4286. [Google Scholar] [CrossRef] [PubMed]
- Kapravelou, G.; Martínez, R.; Andrade, A.M.; López Chaves, C.; López-Jurado, M.; Aranda, P.; Arrebola, F.; Cañizares, F.J.; Galisteo, M.; Porres, J.M. Improvement of the Antioxidant and Hypolipidaemic Effects of Cowpea Flours (Vigna unguiculata) by Fermentation: Results of In Vitro and In Vivo Experiments. J. Sci. Food Agric. 2015, 95, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, F.; Daroch, M.; Tang, J. Positive Effects of Duckweed Polycultures on Starch and Protein Accumulation. Biosci. Rep. 2016, 36, e00380. [Google Scholar] [CrossRef]
- Sumathi, S.; Srimathi, P. Seed Development and Maturation Studies in Chinese Medicinal Plant Babchi (Psoralea corylifolia). Seed Sci. Technol. 2015, 43, 80–89. [Google Scholar] [CrossRef]
- Kapravelou, G.; Martínez, R.; Andrade, A.M.; Sánchez, C.; Chaves, C.L.; López-Jurado, M.; Aranda, P.; Cantarero, S.; Arrebola, F.; Fernández-Segura, E.; et al. Health Promoting Effects of Lupin (Lupinus albus var. multolupa) Protein Hydrolyzate and Insoluble Fiber in a Diet-Induced Animal Experimental Model of Hypercholesterolemia. Food Res. Int. 2013, 54, 1471–1481. [Google Scholar] [CrossRef]
- Porres, J.M.; Aranda, P.; López-Jurado, M.; Urbano, G. Nutritional Evaluation of Protein, Phosphorus, Calcium and Magnesium Bioavailability from Lupin (Lupinus albus var. multolupa)-Based Diets in Growing Rats: Effect of α-Galactoside Oligosaccharide Extraction and Phytase Supplementation. Br. J. Nutr. 2006, 95, 1102–1111. [Google Scholar] [CrossRef]
- Martínez, R.; García-Beltrán, A.; Kapravelou, G.; Mesas, C.; Cabeza, L.; Perazzoli, G.; Guarnizo, P.; Rodríguez-López, A.; Andrés Vallejo, R.; Galisteo, M.; et al. In Vivo Nutritional Assessment of the Microalga Nannochloropsis Gaditana and Evaluation of the Antioxidant and Antiproliferative Capacity of Its Functional Extracts. Mar. Drugs 2022, 20, 318. [Google Scholar] [CrossRef]
- Martínez, R.; Mesas, C.; Guzmán, A.; Galisteo, M.; López-Jurado, M.; Prados, J.; Melguizo, C.; Bermúdez, F.; Porres, J.M. Bioavailability and Biotransformation of Linolenic Acid from Basil Seed Oil as a Novel Source of Omega-3 Fatty Acids Tested on a Rat Experimental Model. Food Funct. 2022, 13, 7614–7628. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and Its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Dinis, T.C.; Maderia, V.M.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Duh, P.-D.; Tu, Y.-Y.; Yen, G.-C. Antioxidant Activity of Water Extract of Harng Jyur (Chrysanthemum morifolium Ramat). LWT Food Sci. Technol. 1999, 32, 269–277. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- European Parliament. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes Text with EEA Relevance; European Union: Brussels, Belgium, 2010; Volume L276, pp. 33–79. [Google Scholar]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Yang, G.; Meng, B.; Yi, Z.; Yang, G.; Chen, M.; Hou, P.; Wang, H.; Xu, X. Moderate-Intensity Physical Exercise Affects the Exercise Performance and Gut Microbiota of Mice. Front. Cell. Infect. Microbiol. 2021, 11, 712381. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Kim, M.; Ogwu, V. A Modified Catalase Assay Suitable for a Plate Reader and for the Analysis of Brain Cell Cultures. J. Neurosci. Methods 1996, 67, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A.; Sunde, R.A.; Schwartz, G.L.; Hoekstra, W.G. Glutathione Peroxidase Activity in Rat Lens and Other Tissues in Relation to Dietary Selenium Intake. Exp. Eye Res. 1974, 18, 563–569. [Google Scholar] [CrossRef]
- Ukeda, H.; Maeda, S.; Ishii, T.; Sawamura, M. Spectrophotometric Assay for Superoxide Dismutase Based on Tetrazolium Salt 3′-{1-[(Phenylamino)-Carbonyl]-3,4-Tetrazolium}-Bis(4-Methoxy-6-Nitro)Benzenesulfonic Acid Hydrate Reduction by Xanthine–Xanthine Oxidase. Anal. Biochem. 1997, 251, 206–209. [Google Scholar] [CrossRef]
- Terrón-Camero, L.C.; Gordillo-González, F.; Salas-Espejo, E.; Andrés-León, E. Comparison of Metagenomics and Metatranscriptomics Tools: A Guide to Making the Right Choice. Genes 2022, 13, 2280. [Google Scholar] [CrossRef]
- Andrews, S. Babraham Bioinformatics—FastQC. A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 5 September 2023).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Paulson, J.N.; Stine, O.C.; Bravo, H.C.; Pop, M. Differential Abundance Analysis for Microbial Marker-Gene Surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef]
- Martínez, R.; Guzmán, A.; Kapravelou, G.; Melguizo, C.; Bermúdez, F.; Prados, J.; López-Jurado, M.; Porres, J.M. Argan Pulp as a Novel Functional Ingredient with Beneficial Effects on Multiple Metabolism Biomarkers. J. Funct. Foods 2023, 110, 105864. [Google Scholar] [CrossRef]
- Chen, L.; Chen, S.; Sun, P.; Liu, X.; Zhan, Z.; Wang, J. Psoralea corylifolia L.: A Comprehensive Review of Its Botany, Traditional Uses, Phytochemistry, Pharmacology, Toxicology, Quality Control and Pharmacokinetics. Chin. Med. 2023, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Choi, S.-I.; Hong, E.; Kim, G.-H. Psoralea corylifolia L. Extract Ameliorates Nonalcoholic Fatty Liver Disease in Free-Fatty-Acid-Incubated HEPG2 Cells and in High-Fat Diet-Fed Mice. J. Food Sci. 2020, 85, 2216–2226. [Google Scholar] [CrossRef] [PubMed]
- Pagliuso, D.; Palacios Jara, C.E.; Grandis, A.; Lam, E.; Pena Ferreira, M.J.; Buckeridge, M.S. Flavonoids from Duckweeds: Potential Applications in the Human Diet. RSC Adv. 2020, 10, 44981–44988. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.J.; Costa de Camargo, A.; Shahidi, F. Phenolic Profiles and Antioxidant Activity of Defatted Camelina and Sophia Seeds. Food Chem. 2018, 240, 917–925. [Google Scholar] [CrossRef]
- Tsykalo, T.O.; Trzhetsynskyi, S.D. The Study of Hypoglycemic and Hypolipidemic Activity of Camelina sativa (L.) Crantz Extracts in Rats under Conditions of High-Fructose Diet. Ceska Slov. Farm. 2020, 69, 137–142. [Google Scholar]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Shen, L.; Yang, Y.; Zhang, J.; Feng, L.; Zhou, Q. Diacylated Anthocyanins from Purple Sweet Potato (Ipomoeabatatas L.) Attenuate Hyperglycemia and Hyperuricemia in Mice Induced by a High-Fructose/High-Fat Diet. J. Zhejiang Univ. Sci. B 2023, 24, 587–601. [Google Scholar] [CrossRef] [PubMed]
- García-Beltrán, A.; Martínez, R.; Porres, J.M.; Arrebola, F.; Artero, I.R.; Galisteo, M.; Aranda, P.; Kapravelou, G.; López-Jurado, M. Novel insights and mechanisms of diet-induced obesity: Mid-term versus long-term effects on hepatic transcriptome and antioxidant capacity in Sprague-Dawley rats. Life Sci. 2023, 324, 121746. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.J.; Lang, M.J.; Johnson, J.M.; Watanabe, S.; McLaughlin, K.L.; Maschek, J.A.; Verkerke, A.R.P.; Siripoksup, P.; Chaix, A.; Cox, J.E.; et al. Weight Loss Increases Skeletal Muscle Mitochondrial Energy Efficiency in Obese Mice. Life Metab. 2023, 2, load014. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, L.; Li, Q.; Song, C.; Han, N.; Yan, T.; Zhang, L.; Ren, D.; Zhao, Y.; Yang, X. Caloric Restriction, Friend or Foe: Effects on Metabolic Status in Association with the Intestinal Microbiome and Metabolome. J. Agric. Food Chem. 2022, 70, 14061–14072. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; López-Trinidad, L.M.; Kapravelou, G.; Arrebola, F.; Galisteo, M.; Aranda, P.; Porres, J.M.; López-Jurado, M. A Combined Healthy Strategy for Successful Weight Loss, Weight Maintenance and Improvement of Hepatic Lipid Metabolism. J. Nutr. Biochem. 2020, 85, 108456. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Muñoz, M.; De la Fuente-Fernández, M.; Román-Carmena, M.; Amor, S.; Iglesias-de la Cruz, M.C.; García-Laínez, G.; Llopis, S.; Martorell, P.; Verdú, D.; Serna, E.; et al. Supplementation with a New Standardized Extract of Green and Black Tea Exerts Antiadipogenic Effects and Prevents Insulin Resistance in Mice with Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 8521. [Google Scholar] [CrossRef]
- D’Antongiovanni, V.; Fornai, M.; Benvenuti, L.; Di Salvo, C.; Pellegrini, C.; Cappelli, F.; Masi, S.; Antonioli, L. Dietary Supplement, Containing the Dry Extract of Curcumin, Emblica and Cassia, Counteracts Intestinal Inflammation and Enteric Dysmotility Associated with Obesity. Metabolites 2023, 13, 410. [Google Scholar] [CrossRef]
- Geng, L.; Liao, B.; Jin, L.; Huang, Z.; Triggle, C.R.; Ding, H.; Zhang, J.; Huang, Y.; Lin, Z.; Xu, A. Exercise Alleviates Obesity-Induced Metabolic Dysfunction via Enhancing FGF21 Sensitivity in Adipose Tissues. Cell Rep. 2019, 26, 2738–2752.e4. [Google Scholar] [CrossRef]
- Lasker, S.; Rahman, M.M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.B.; Subhan, N.; Ahsan, G.U.; et al. High-Fat Diet-Induced Metabolic Syndrome and Oxidative Stress in Obese Rats Are Ameliorated by Yogurt Supplementation. Sci. Rep. 2019, 9, 20026. [Google Scholar] [CrossRef] [PubMed]
- Diniz, T.A.; de Lima Junior, E.A.; Teixeira, A.A.; Biondo, L.A.; da Rocha, L.A.F.; Valadão, I.C.; Silveira, L.S.; Cabral-Santos, C.; de Souza, C.O.; Rosa Neto, J.C. Aerobic Training Improves NAFLD Markers and Insulin Resistance through AMPK-PPAR-α Signaling in Obese Mice. Life Sci. 2021, 266, 118868. [Google Scholar] [CrossRef]
- Wen, F.; Dai, P.; Song, Z.; Jin, C.; Ji, X.; Hou, J.; Liu, N. Alleviating Effect of Mulberry Leaf 1-Deoxynojirimycin on Resistin-Induced Hepatic Steatosis and Insulin Resistance in Mice. J. Physiol. Pharmacol. 2022, 73, 745–754. [Google Scholar] [CrossRef]
- Cao, H.; Li, C.; Wang, L.; Ziadlou, R.; Grad, S.; Zhang, Y.; Cheng, Y.; Lai, Y.; Yao, X.; Alini, M.; et al. Effect and Mechanism of Psoralidin on Promoting Osteogenesis and Inhibiting Adipogenesis. Phytomedicine 2019, 61, 152860. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Pei, R.; Raghuvanshi, R.; Liu, Z.; Bolling, B.W. Yogurt Supplementation Attenuates Insulin Resistance in Obese Mice by Reducing Metabolic Endotoxemia and Inflammation. J. Nutr. 2023, 153, 703–712. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Tao, S.-Y.; Wu, Y.-X.; An, T.; Lv, B.-H.; Liu, J.-X.; Liu, Y.-T.; Jiang, G.-J. Quinoa Reduces High-Fat Diet-Induced Obesity in Mice via Potential Microbiota-Gut-Brain-Liver Interaction Mechanisms. Microbiol. Spectr. 2022, 10, e00329-22. [Google Scholar] [CrossRef]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, D.; Wu, J.; Liu, J.; Zhou, Y.; Tan, Y.; Feng, W.; Peng, C. Interactions between Gut Microbiota and Polyphenols: A Mechanistic and Metabolomic Review. Phytomedicine 2023, 119, 154979. [Google Scholar] [CrossRef]
- Kendig, M.D.; Leigh, S.; Hasebe, K.; Kaakoush, N.O.; Westbrook, R.F.; Morris, M.J. Obesogenic Diet Cycling Produces Graded Effects on Cognition and Microbiota Composition in Rats. Mol. Nutr. Food Res. 2023, 67, 2200809. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, Y.; Li, D.; Zhang, S.; Wu, Y.; Zhang, Q.; Bai, W. New Insights into the Mechanisms of High-Fat Diet Mediated Gut Microbiota in Chronic Diseases. iMeta 2023, 2, e69. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Martínez-Cuesta, M.C.; Del Campo, R.; Garriga-García, M.; Peláez, C.; Requena, T. Taxonomic Characterization and Short-Chain Fatty Acids Production of the Obese Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 598093. [Google Scholar] [CrossRef]
- Krajmalnik-Brown, R.; Ilhan, Z.-E.; Kang, D.-W.; DiBaise, J.K. Effects of Gut Microbes on Nutrient Absorption and Energy Regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef]
- Caricilli, A.M.; Saad, M.J.A. The Role of Gut Microbiota on Insulin Resistance. Nutrients 2013, 5, 829–851. [Google Scholar] [CrossRef]
- Ruan, S.; Gao, X.; Li, B.; Tian, J. The Synergic Effects and Mechanism of KGM–DMY Complex in the Prevention of Obesity and Enhancement of Fatigue Resistance in Mice. Food Funct. 2023, 14, 2607–2620. [Google Scholar] [CrossRef]
- Yu, Q.; Yu, F.; Li, Q.; Zhang, J.; Peng, Y.; Wang, X.; Li, T.; Yin, N.; Sun, G.; Ouyang, H.; et al. Anthocyanin-Rich Butterfly Pea Flower Extract Ameliorating Low-Grade Inflammation in a High-Fat-Diet and Lipopolysaccharide-Induced Mouse Model. J. Agric. Food Chem. 2023, 71, 11941–11956. [Google Scholar] [CrossRef]
- Wang, X.; Duan, C.; Li, Y.; Lu, H.; Guo, K.; Ge, X.; Chen, T.; Shang, Y.; Liu, H.; Zhang, D. Sodium Butyrate Reduces Overnutrition-Induced Microglial Activation and Hypothalamic Inflammation. Int. Immunopharmacol. 2022, 111, 109083. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Shapiro, J.A.; Church, T.R.; Miller, G.; Trinh-Shevrin, C.; Yuen, E.; Friedlander, C.; Hayes, R.B.; Ahn, J. A Taxonomic Signature of Obesity in a Large Study of American Adults. Sci. Rep. 2018, 8, 9749. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jiang, X.; Cao, M.; Ge, J.; Bao, Q.; Tang, L.; Chen, Y.; Li, L. Altered Fecal Microbiota Correlates with Liver Biochemistry in Nonobese Patients with Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2016, 6, 32002. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.S.C.; Seguro, C.S.; Naves, M.M.V. Gut Microbiota and Physical Exercise in Obesity and Diabetes—A Systematic Review. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 863–877. [Google Scholar] [CrossRef] [PubMed]
| Stage | Time | % VO2 Max |
|---|---|---|
| Warm-up | 4′ | 20 |
| Exercise protocol | ||
| 1X | 3′ | 30 → 65 |
| 1′ | 65 | |
| 4X | 1′ 30″ | 30 → 65 |
| 30″ | 65 | |
| 1′ | rest |
| DIALYZED | RETAINED | |||
|---|---|---|---|---|
| Blank | NT | Blank | NT | |
| Dializability (%) | - | 86.6 (1.9) | - | - |
| TPC (µg GA eq/mg) | 7.21 (0.23) | 39.8 (0.7) * | 8.24 (0.4) | 43.7 (2.1) * |
| ABTS (µg GA eq/mg) | 8.96 (0.23) | 34.5 (0.5) * | 7.05 (0.27) | 21.7 (0.4) * |
| ICC (CAU/mg) | 0.59 (0.03) | 2.95 (0.14) * | 0.41 (0.02) | 3.87 (0.13) * |
| IRC (µg GA eq/mg) | 0.80 (0.11) | 10.1 (0.5) * | 1.53 (0.16) | 11.1 (0.4) * |
| SD | HFHF | HF/SD | Ex | NT | NT + Ex | |
|---|---|---|---|---|---|---|
| Liver | 5.13 (0.06) bc | 5.27 (0.15) c | 4.70 (0.07) ab | 4.58 (0.11) a | 4.62 (0.10) a | 4.40 (0.12) a |
| Kidneys | 0.68 (0.01) ab | 0.72 (0.01) bc | 0.75 (0.01) c | 0.75 (0.01) c | 0.71 (0.01) bc | 0.67 (0.01) a |
| Heart | 0.60 (0.01) a | 0.66 (0.01) b | 0.63 (0.01) ab | 0.63 (0.01) ab | 0.64 (0.01) ab | 0.61 (0.01) a |
| Spleen | 0.30 (0.02) a | 0.40 (0.01) b | 0.33 (0.02) a | 0.30 (0.02) a | 0.32 (0.01) a | 0.30 (0.01) a |
| Caecum | 0.24 (0.01) bc | 0.19 (0.01) a | 0.24 (0.00) bc | 0.22 (0.01) ab | 0.26 (0.01) c | 0.27 (0.01) c |
| Colon | 0.38 (0.01) a | 0.50 (0.02) bc | 0.46 (0.01) b | 0.54 (0.01) bc | 0.48 (0.02) b | 0.56 (0.01) c |
| Epididymal fat | 2.44 (0.19) a | 8.39 (0.40) b | 2.69 (0.18) a | 2.30 (0.10) a | 2.72 (0.16) a | 2.39 (0.23) a |
| Abdominal fat | 0.62 (0.04) a | 2.99 (0.29) b | 0.68 (0.03) a | 0.53 (0.05) a | 0.83 (0.08) a | 0.58 (0.07) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Carrasco, A.; Kapravelou, G.; López-Jurado, M.; Bermúdez, F.; Andrés-León, E.; Terrón-Camero, L.C.; Prados, J.; Melguizo, C.; Porres, J.M.; Martínez, R. A Novel Plant-Based Nutraceutical Combined with Exercise Can Revert Oxidative Status in Plasma and Liver in a Diet-Induced-Obesity Animal Model. Antioxidants 2024, 13, 274. https://doi.org/10.3390/antiox13030274
Guzmán-Carrasco A, Kapravelou G, López-Jurado M, Bermúdez F, Andrés-León E, Terrón-Camero LC, Prados J, Melguizo C, Porres JM, Martínez R. A Novel Plant-Based Nutraceutical Combined with Exercise Can Revert Oxidative Status in Plasma and Liver in a Diet-Induced-Obesity Animal Model. Antioxidants. 2024; 13(3):274. https://doi.org/10.3390/antiox13030274
Chicago/Turabian StyleGuzmán-Carrasco, Ana, Garyfallia Kapravelou, María López-Jurado, Francisco Bermúdez, Eduardo Andrés-León, Laura C. Terrón-Camero, José Prados, Consolación Melguizo, Jesus M. Porres, and Rosario Martínez. 2024. "A Novel Plant-Based Nutraceutical Combined with Exercise Can Revert Oxidative Status in Plasma and Liver in a Diet-Induced-Obesity Animal Model" Antioxidants 13, no. 3: 274. https://doi.org/10.3390/antiox13030274








