Updating the Status quo on the Eco-Friendly Approach for Antioxidants Recovered from Plant Matrices Using Cloud Point Extraction
Abstract
:1. Introduction
1.1. Rationale behind the Study
1.2. Cloud Point Extraction (CPE)
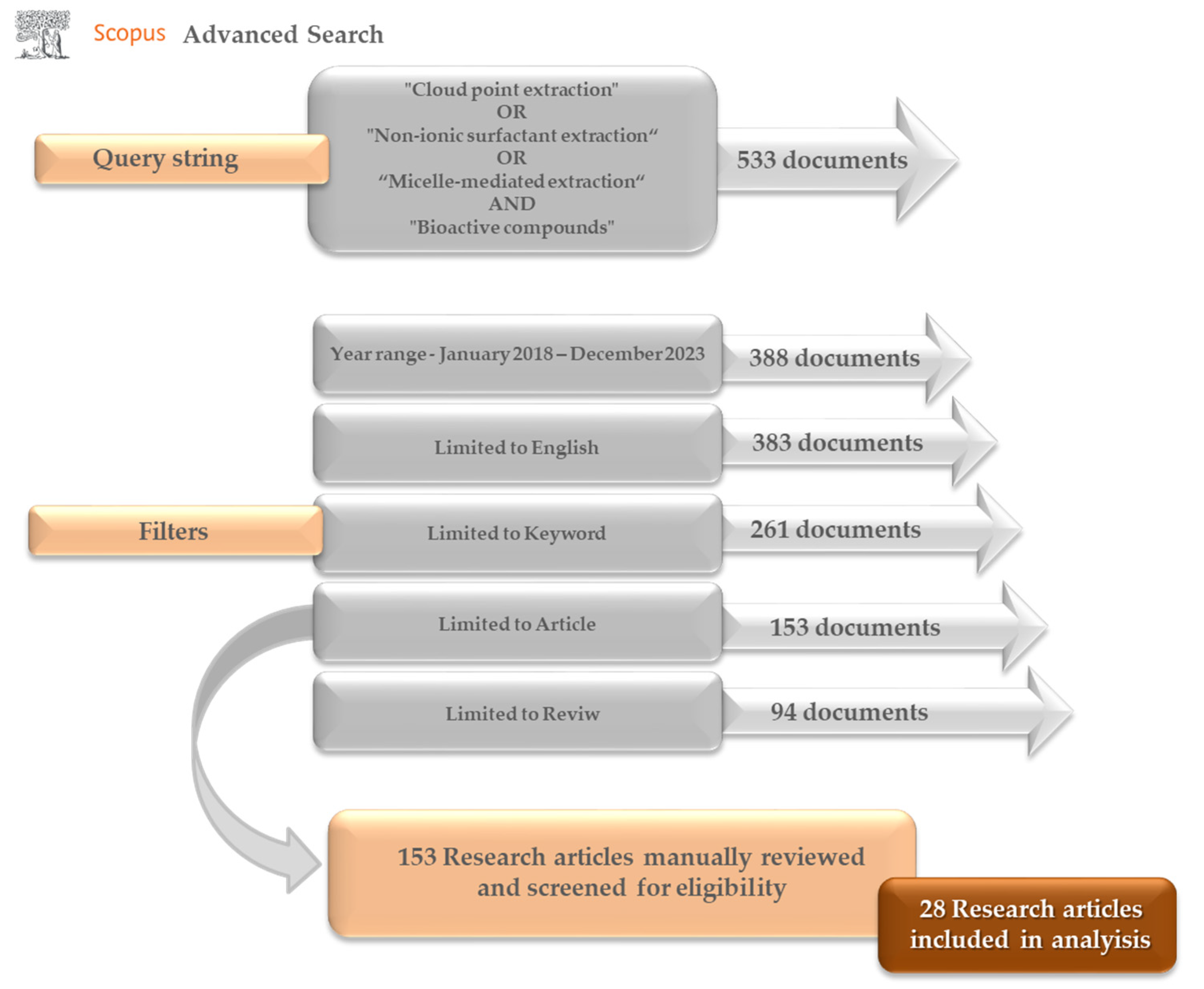
2. Review Methodology—Current Literature Gap or Not?
3. Experimental Protocol of CPE
4. Influencing Parameters
4.1. Surfactants Type and Concentration
4.2. Solution pH Level
4.3. Salting-Out Effect
4.4. Temperature
4.5. Centrifugation
4.6. Recent Examples and Outputs of CPE for Recovering Antioxidants from Different Plant Sources
5. Expanding the Horizons of Cloud Point Extraction: Synergistic Integration with Microwave- and Ultrasonic-Assisted Extraction Techniques
5.1. The Cloud Point Extraction with Microwave-Assisted Extraction (CPE-MAE)
5.2. The Cloud Point Extraction with Ultrasonic-Assisted Extraction (CPE-UAE)
5.3. Recent Examples of CPE-MAE and CPE-UAE for Recovering Bioactive Compounds from Different Plant Sources
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Selvamuthukumaran, M.; Shi, J. Recent Advances in Extraction of Antioxidants from Plant By-Products Processing Industries. Food Qual. Saf. 2017, 1, 61–81. [Google Scholar] [CrossRef]
- Melnyk, A.; Namieśnik, J.; Wolska, L. Theory and Recent Applications of Coacervate-Based Extraction Techniques. TrAC Trends Anal. Chem. 2015, 71, 282–292. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Raiti, J.; Hafidi, A. Cloud Point Extraction of Phenolic Compounds from Pretreated Olive Mill Wastewater. J. Environ. Chem. Eng. 2014, 2, 1480–1486. [Google Scholar] [CrossRef]
- Sun, M.; Wu, Q. Determination of Ultra-Trace Aluminum in Human Albumin by Cloud Point Extraction and Graphite Furnace Atomic Absorption Spectrometry. J. Hazard. Mater. 2010, 176, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wen, S.; Xiang, G. Cloud Point Extraction Combined with Electrothermal Atomic Absorption Spectrometry for the Speciation of Antimony(III) and Antimony(V) in Food Packaging Materials. J. Hazard. Mater. 2010, 175, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, Q. Determination of Trace Bismuth in Human Serum by Cloud Point Extraction Coupled Flow Injection Inductively Coupled Plasma Optical Emission Spectrometry. J. Hazard. Mater. 2011, 192, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhen, W.; Li, Z.; Lian, Y.; Yang, Y. Ultrasonic-Assisted Cloud Point Extraction for Determination of Nickel in Water Samples by Flame Atomic Absorption Spectrometry. Water Sci. Technol. 2012, 66, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, Energy Efficient and Green Cloud Point Extraction: Technology and Applications in Food Processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Mantiniotou, M.; Kalompatsios, D.; Bozinou, E.; Giovanoudis, I.; Lalas, S.I. Exploring the Feasibility of Cloud-Point Extraction for Bioactive Compound Recovery from Food Byproducts: A Review. Biomass 2023, 3, 306–322. [Google Scholar] [CrossRef]
- Mortada, W.I.; Hassanien, M.M.; El-Asmy, A.A. Cloud Point Extraction of Some Precious Metals Using Triton X-114 and a Thioamide Derivative with a Salting-Out Effect. Egypt. J. Basic Appl. Sci. 2014, 1, 184–191. [Google Scholar] [CrossRef]
- Kori, S. Cloud Point Extraction Coupled with Back Extraction: A Green Methodology in Analytical Chemistry. Forensic Sci. Res. 2021, 6, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Gavazov, K.B.; Hagarová, I.; Halko, R.; Andruch, V. Recent Advances in the Application of Nanoparticles in Cloud Point Extraction. J. Mol. Liq. 2019, 281, 93–99. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Ferreira da Mata Cerqueira, U.M.; Ferreira, S.L.C.; Novaes, C.G.; Novais, F.C.; Valasques, G.S.; Novaes da Silva, B. Recent Developments in the Application of Cloud Point Extraction as Procedure for Speciation of Trace Elements. Appl. Spectrosc. Rev. 2022, 57, 338–352. [Google Scholar] [CrossRef]
- Mandal, S.; Lahiri, S. A Review on Extraction, Preconcentration and Speciation of Metal Ions by Sustainable Cloud Point Extraction. Microchem. J. 2022, 175, 107150. [Google Scholar] [CrossRef]
- Azooz, E.A.; Ridha, R.K.; Abdulridha, H.A. The Fundamentals and Recent Applications of Micellar System Extraction for Nanoparticles and Bioactive Molecules: A Review. Nano Biomed. Eng. 2021, 13, 264–278. [Google Scholar] [CrossRef]
- Leite, A.C.; Ferreira, A.M.; Morais, E.S.; Khan, I.; Freire, M.G.; Coutinho, J.A.P. Cloud Point Extraction of Chlorophylls from Spinach Leaves Using Aqueous Solutions of Nonionic Surfactants. ACS Sustain. Chem. Eng. 2018, 6, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Stalikas, C.D. Micelle-Mediated Extraction as a Tool for Separation and Preconcentration in Metal Analysis. TrAC Trends Anal. Chem. 2002, 21, 343–355. [Google Scholar] [CrossRef]
- Paleologos, E.K.; Giokas, D.L.; Karayannis, M.I. Micelle-Mediated Separation and Cloud-Point Extraction. TrAC Trends Anal. Chem. 2005, 24, 426–436. [Google Scholar] [CrossRef]
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Moreno-Cordero, B.; Pérez-Pavón, J.L.; García-Pinto, C.; Fernández Laespada, E. Surfactant Cloud Point Extraction and Preconcentration of Organic Compounds Prior to Chromatography and Capillary Electrophoresis. J. Chromatogr. A 2000, 902, 251–265. [Google Scholar] [CrossRef]
- Mohajeri, E.; Noudeh, G.D. Effect of Temperature on the Critical Micelle Concentration and Micellization Thermodynamic of Nonionic Surfactants: Polyoxyethylene Sorbitan Fatty Acid Esters. J. Chem. 2012, 9, 2268–2274. [Google Scholar] [CrossRef]
- Zhao, G.; Khin, C.C.; Chen, S.B.; Chen, B.-H. Nonionic Surfactant and Temperature Effects on the Viscosity of Hydrophobically Modified Hydroxyethyl Cellulose Solutions. J. Phys. Chem. B 2005, 109, 14198–14204. [Google Scholar] [CrossRef]
- Gortzi, O.; Lalas, S.; Chatzilazarou, A.; Katsoyannos, E.; Papaconstandinou, S.; Dourtoglou, T. Recovery of Natural Antioxidants from Olive Mill Wastewater Using Genapol-X080. JAOCS J. Am. Oil Chem. Soc. 2008, 85, 133–140. [Google Scholar] [CrossRef]
- Bolan, S.; Padhye, L.P.; Mulligan, C.N.; Alonso, E.R.; Saint-Fort, R.; Jasemizad, T.; Wang, C.; Zhang, T.; Rinklebe, J.; Wang, H.; et al. Surfactant-Enhanced Mobilization of Persistent Organic Pollutants: Potential for Soil and Sediment Remediation and Unintended Consequences. J. Hazard. Mater. 2023, 443, 130189. [Google Scholar] [CrossRef]
- Xing, W.; Chen, L. Micelle-Mediated Extraction and Cloud Point Preconcentration of Bergenin from Ardisia japonica. Sep. Purif. Technol. 2013, 110, 57–62. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Extraction and Concentration of Tanshinones in Salvia miltiorrhiza Bunge by Task-Specific Non-Ionic Surfactant Assistance. Food Chem. 2011, 126, 1985–1990. [Google Scholar] [CrossRef]
- Halko, R.; Hagarová, I.; Andruch, V. Innovative Approaches in Cloud-Point Extraction. J. Chromatogr. A 2023, 1701, 464053. [Google Scholar] [CrossRef]
- Di Giacomo, M.; Bertoni, F.A.; Rocha, M.V.; Nerli, B.B.; Rodríguez, F. Cloud Point Extraction Based on Non-Ionic Surfactants: An Ecofriendly Tool for Recovering Papain from Papaya Latex. J. Environ. Chem. Eng. 2022, 10, 108762. [Google Scholar] [CrossRef]
- Śliwa, P.; Śliwa, K. Nanomicellar Extraction of Polyphenols—Methodology and Applications Review. Int. J. Mol. Sci. 2021, 22, 11392. [Google Scholar] [CrossRef]
- Sato, N.; Mori, M.; Itabashi, H. Cloud Point Extraction of Cu(II) Using a Mixture of Triton X-100 and Dithizone with a Salting-Out Effect and Its Application to Visual Determination. Talanta 2013, 117, 376–381. [Google Scholar] [CrossRef]
- Sosa Ferrera, Z.; Padrón Sanz, C.; Mahugo Santana, C.; Santana Rodríguez, J.J. The Use of Micellar Systems in the Extraction and Pre-Concentration of Organic Pollutants in Environmental Samples. TrAC Trends Anal. Chem. 2004, 23, 469–479. [Google Scholar] [CrossRef]
- Peng, S.X.; Henson, C.; Strojnowski, M.J.; Golebiowski, A.; Klopfenstein, S.R. Automated High-Throughput Liquid−Liquid Extraction for Initial Purification of Combinatorial Libraries. Anal. Chem. 2000, 72, 261–266. [Google Scholar] [CrossRef]
- Pandit, N.K.; Kanjia, J.; Patel, K.; Pontikes, D.G. Phase Behavior of aqueous Solutions Containing Nonionic Surfactant-Polyethylene Glycol Mixtures. Int. J. Pharm. 1995, 122, 27–33. [Google Scholar] [CrossRef]
- Qin, X.Y.; Meng, J.; Li, X.Y.; Zhou, J.; Sun, X.L.; Wen, A.D. Determination of Venlafaxine in Human Plasma by High-Performance Liquid Chromatography Using Cloud-Point Extraction and Spectrofluorimetric Detection. J. Chromatogr. B 2008, 872, 38–42. [Google Scholar] [CrossRef]
- de Araújo Padilha, C.E.; de Azevedo, J.C.S.; de Sousa, F.C.; de Oliveira, S.D.; de Santana Souza, D.F.; de Oliveira, J.A.; de Macedo, G.R.; dos Santos, E.S. Recovery of Polyphenols from Camu-Camu (Myrciaria dubia H.B.K. McVaugh) Depulping Residue by Cloud Point Extraction. Chin. J. Chem. Eng. 2018, 26, 2471–2476. [Google Scholar] [CrossRef]
- Sazdanić, D.; Atanacković Krstonošić, M.; Ćirin, D.; Cvejić, J.; Alamri, A.; Galanakis, C.M.; Krstonošić, V. Non-Ionic Surfactants-Mediated Green Extraction of Polyphenols from Red Grape Pomace. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100439. [Google Scholar] [CrossRef]
- Zafar, Z.; Bhatti, I.A.; Hanif, M.A.; Zia, M.A. Enhanced Recovery of Phenolics from Acalypha fruticosa by Micelle-Mediated Extraction, Antioxidant, Antimutagenic, Antimicrobial Evaluation, and Chemical Profiling. Biomass Convers. Biorefinery 2022, 244, 1–12. [Google Scholar] [CrossRef]
- Lee, T.Y.; Chow, Y.H.; Chua, B.L. Application of Aqueous Micellar Two-Phase System for Extraction of Bioactive Compounds. In AIP Conference Proceedings, Proceedings of The International Engineering Research Conference—12th Eureca 2019, Selangor Darul Ehsan, Malaysia, 3–4 July 2019; AIP Publishing: Park, MA, USA, 2019; p. 020007. [Google Scholar]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Implementation of Cloud Point Extraction Using Surfactants in the Recovery of Polyphenols from Apricot Cannery Waste. Eng 2023, 4, 1225–1235. [Google Scholar] [CrossRef]
- Alibade, A.; Batra, G.; Bozinou, E.; Salakidou, C.; Lalas, S. Optimization of the Extraction of Antioxidants from Winery Wastes Using Cloud Point Extraction and a Surfactant of Natural Origin (Lecithin). Chem. Pap. 2020, 74, 4517–4524. [Google Scholar] [CrossRef]
- Karadag, A.; Kayacan Cakmakoglu, S.; Metin Yildirim, R.; Karasu, S.; Avci, E.; Ozer, H.; Sagdic, O. Enrichment of Lecithin with Phenolics from Olive Mill Wastewater by Cloud Point Extraction and Its Application in Vegan Salad Dressing. J. Food Process. Preserv. 2022, 46, e16645. [Google Scholar] [CrossRef]
- Kiai, H.; Raiti, J.; El-Abbassi, A.; Hafidi, A. Recovery of Phenolic Compounds from Table Olive Processing Wastewaters Using Cloud Point Extraction Method. J. Environ. Chem. Eng. 2018, 6, 1569–1575. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Voulgaris, A.; Katsoulis, K.; Lalas, S.I.; Roussis, I.G.; Gortzi, O. Development of Enriched Oil with Polyphenols Extracted from Olive Mill Wastewater. Foods 2023, 12, 497. [Google Scholar] [CrossRef] [PubMed]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Isolation of Polyphenols from Two Waste Streams of Clingstone Peach Canneries Utilizing the Cloud Point Extraction Method. Biomass 2023, 3, 291–305. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Mantiniotou, M.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Antioxidant Capacity in Two Different Cultivars of Ripe and Unripe Peaches Utilizing the Cloud-Point Extraction Method. AgriEngineering 2023, 5, 2139–2154. [Google Scholar] [CrossRef]
- More, P.R.; Arya, S.S. A Novel, Green Cloud Point Extraction and Separation of Phenols and Flavonoids from Pomegranate Peel: An Optimization Study Using RCCD. J. Environ. Chem. Eng. 2019, 7, 103306. [Google Scholar] [CrossRef]
- Motikar, P.D.; More, P.R.; Arya, S.S. A Novel, Green Environment-Friendly Cloud Point Extraction of Polyphenols from Pomegranate Peels: A Comparative Assessment with Ultrasound and Microwave-Assisted Extraction. Sep. Sci. Technol. 2021, 56, 1014–1025. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Development of a Cloud Point Extraction Technique Based on Lecithin for the Recovery of Carotenoids from Liquid Tomato Wastewater. Waste 2023, 1, 105–114. [Google Scholar] [CrossRef]
- Vieira, F.A.; Ventura, S.P.M. Efficient Extraction of Carotenoids from Sargassum muticum Using Aqueous Solutions of Tween 20. Mar. Drugs 2019, 17, 310. [Google Scholar] [CrossRef]
- Martins, M.; Fernandes, A.P.M.; Torres-Acosta, M.A.; Collén, P.N.; Abreu, M.H.; Ventura, S.P.M. Extraction of Chlorophyll from Wild and Farmed Ulva Spp. Using Aqueous Solutions of Ionic Liquids. Sep. Purif. Technol. 2021, 254, 117589. [Google Scholar] [CrossRef]
- Fontana, A.R.; Camargo, A.B.; Altamirano, J.C. Coacervative Microextraction Ultrasound-Assisted Back-Extraction Technique for Determination of Organophosphates Pesticides in Honey Samples by Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2010, 1217, 6334–6341. [Google Scholar] [CrossRef]
- Wu, L.; Hu, M.; Li, Z.; Song, Y.; Yu, C.; Zhang, Y.; Zhang, H.; Yu, A.; Ma, Q.; Wang, Z. Determination of Triazine Herbicides in Fresh Vegetables by Dynamic Microwave-Assisted Extraction Coupled with Homogeneous Ionic Liquid Microextraction High Performance Liquid Chromatography. Anal. Bioanal. Chem. 2015, 407, 1753–1762. [Google Scholar] [CrossRef]
- Du, X.; Yuan, J.; Cao, H.; Ye, L.; Ma, A.; Du, J.; Pan, J. Ultrasound-Assisted Micellar Cleanup Coupled with Large-Volume-Injection Enrichment for the Analysis of Polar Drugs in Blood and Zebrafish Samples. Ultrason. Sonochem. 2022, 85, 105998. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Luo, L.; Wei, W.; Wang, Q.; Zhang, J. Quantitative Detection of Zinc Oxide Nanoparticle in Environmental Water by Cloud Point Extraction Combined ICP-MS. Adsorpt. Sci. Technol. 2021, 2021, e9958422. [Google Scholar] [CrossRef]
- Simitchiev, K.; Stefanova, V.; Kmetov, V.; Andreev, G.; Kovachev, N.; Canals, A. Microwave-Assisted Cloud Point Extraction of Rh, Pd and Pt with 2-Mercaptobenzothiazole as Preconcentration Procedure Prior to ICP-MS Analysis of Pharmaceutical Products. J. Anal. At. Spectrom. 2008, 23, 717–726. [Google Scholar] [CrossRef]
- Jia, G.; Lv, C.; Zhu, W.; Qiu, J.; Wang, X.; Zhou, Z. Applicability of Cloud Point Extraction Coupled with Microwave-Assisted Back-Extraction to the Determination of Organophosphorous Pesticides in Human Urine by Gas Chromatography with Flame Photometry Detection. J. Hazard. Mater. 2008, 159, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Sikalos, T.I.; Paleologos, E.K. Cloud Point Extraction Coupled with Microwave or Ultrasonic Assisted Back Extraction as a Preconcentration Step Prior to Gas Chromatography. Anal. Chem. 2005, 77, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- Zygoura, P.D.; Paleologos, E.K.; Riganakos, K.A.; Kontominas, M.G. Determination of Diethylhexyladipate and Acetyltributylcitrate in Aqueous Extracts after Cloud Point Extraction Coupled with Microwave Assisted Back Extraction and Gas Chromatographic Separation. J. Chromatogr. A 2005, 1093, 29–35. [Google Scholar] [CrossRef]
- Yang, S.; Fang, X.; Duan, L.; Yang, S.; Lei, Z.; Wen, X. Comparison of Ultrasound-Assisted Cloud Point Extraction and Ultrasound-Assisted Dispersive Liquid Liquid Microextraction for Copper Coupled with Spectrophotometric Determination. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 148, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Temel, N.K.; Gürkan, R. Extraction, Preconcentration, and Quantification of Low Levels of Free Formaldehyde from Some Beverage Matrices by Combination of Ultrasound-Assisted-Cloud Point Extraction with Spectrophotometry. Food Anal. Methods 2017, 10, 4024–4037. [Google Scholar] [CrossRef]
- Altunay, N.; Gürkan, R.; Orhan, U. Indirect Determination of the Flavor Enhancer Maltol in Foods and Beverages through Flame Atomic Absorption Spectrometry after Ultrasound Assisted-Cloud Point Extraction. Food Chem. 2017, 235, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Biata, N.R.; Mashile, G.P.; Ramontja, J.; Mketo, N.; Nomngongo, P.N. Application of Ultrasound-Assisted Cloud Point Extraction for Preconcentration of Antimony, Tin and Thallium in Food and Water Samples Prior to ICP-OES Determination. J. Food Compos. Anal. 2019, 76, 14–21. [Google Scholar] [CrossRef]
- Mai, X.; Liu, Y.; Tang, X.; Wang, L.; Lin, Y.; Zeng, H.; Luo, L.; Fan, H.; Li, P. Sequential Extraction and Enrichment of Flavonoids from Euonymus alatus by Ultrasonic-Assisted Polyethylene Glycol-Based Extraction Coupled to Temperature-Induced Cloud Point Extraction. Ultrason. Sonochem. 2020, 66, 105073. [Google Scholar] [CrossRef] [PubMed]
- Supharoek, S.; Weerasuk, B.; Siriangkhawut, W.; Grudpan, K.; Ponhong, K. Ultrasound-Assisted One-Pot Cloud Point Extraction for Iron Determination Using Natural Chelating Ligands from Dipterocarpus intricatus Dyer Fruit. Molecules 2022, 27, 5697. [Google Scholar] [CrossRef] [PubMed]
- Gürkan, R.; Zengin, H.B. Application of Ultrasound Assisted-Cloud Point Extraction Coupled with Spectrophotometry for the Selective Extraction/Pre-Concentration of Low Levels of Inorganic Hg (as Hg22+/Hg2+) from Liquid Matrices. Int. J. Environ. Anal. Chem. 2023, 1–21. [Google Scholar] [CrossRef]
- Campillo, N.; Marín, J.; Viñas, P.; Garrido, I.; Fenoll, J.; Hernández-Córdoba, M. Microwave Assisted Cloud Point Extraction for the Determination of Vitamin K Homologues in Vegetables by Liquid Chromatography with Tandem Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 6658–6664. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Meng, Y.; Wang, Z.-L.; Cao, L.; Liu, C.; Gao, M.-Z.; Zhao, C.-J.; Fu, Y.-J. Sustainable and Efficient Surfactant-Based Microwave-Assisted Extraction of Target Polyphenols and Furanocoumarins from Fig (Ficus carica L.) leaves. J. Mol. Liq. 2020, 318, 114196. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Bulutlu, C.; Gürkan, R. Application of Simple, Fast and Eco-Friendly Ultrasound-Assisted-Cloud Point Extraction for Pre-Concentration of Zinc, Nickel and Cobalt from Foods and Vegetables Prior to Their Flame Atomic Absorption Spectrometric Determinations. Int. J. Environ. Anal. Chem. 2018, 98, 655–675. [Google Scholar] [CrossRef]
- Guo, N.; Jiang, Y.-W.; Kou, P.; Liu, Z.-M.; Efferth, T.; Li, Y.-Y.; Fu, Y.-J. Application of Integrative Cloud Point Extraction and Concentration for the Analysis of Polyphenols and Alkaloids in Mulberry Leaves. J. Pharm. Biomed. Anal. 2019, 167, 132–139. [Google Scholar] [CrossRef]
- Temel, N.K.; Gürkan, R. Preconcentration and Determination of Trace Vanadium(V) in Beverages by Combination of Ultrasound Assisted-Cloud Point Extraction with Spectrophotometry. Acta Chim. Slov. 2018, 65, 138–149. [Google Scholar] [CrossRef]
- Xu, X.; Huang, L.; Wu, Y.; Yang, L.; Huang, L. Synergic Cloud-Point Extraction Using [C4mim][PF6] and Triton X-114 as Extractant Combined with HPLC for the Determination of Rutin and Narcissoside in Anoectochilus roxburghii (Wall.) Lindl. and Its Compound Oral Liquid. J. Chromatogr. B 2021, 1168, 122589. [Google Scholar] [CrossRef] [PubMed]
- Optimization of Ultrasound-Assisted Cloud Point Extraction of Polyphenols from Pomegranate Peels—ProQuest. Available online: https://www.proquest.com/openview/a6721097817c00d85365c4cfeb536855/1?pq-origsite=gscholar&cbl=136084 (accessed on 26 January 2024).
- Ji, Y.; Wu, L.; Lv, R.; Wang, H.; Song, S.; Cao, M. Facile Cloud Point Extraction for the Separation and Determination of Phenolic Acids from Dandelion. ACS Omega 2021, 6, 13508–13515. [Google Scholar] [CrossRef] [PubMed]



| Category | Surfactant Examples | Properties |
|---|---|---|
| Non-ionic | Polyoxyethylenes (Genapol X-080, Triton X-100, Triton X-114, Tween 80) | Uncharged hydrophilic head |
| Anionic | Sodium dodecyl sulfate, ammonium lauryl sulfate, sodium laureth sulfate | The hydrophilic group contains an anionic moiety, such as carboxylate, sulfonate, or sulfate |
| Cationic | Cetyl trimethylammonium bromide, methylbenzethonium, benzalkoniu | The hydrophilic head contains positive groups, such as quaternary ammonium |
| Zwiter anionic | 4-(Dodecyldimethyl ammonium) butyrate, erucyl amidopropyl betaine | Cationic, anionic, or neutral, depending on the solution’s pH |
| Plant Material | Target Group of Antioxidants | Surfactant Type | Surfactant Concentration | Temperature (°C) | pH | Time (min) | Solid–Liquid Ratio | Salt | Salt Concentration (% w/v) | Centrifugation Speed (rpm) | Centrifugation Time (min) | CPE Step | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Camu camu residue | Polyphenols | Triton X-114 | 7% w/v | 30 | 3.2 | 180 | - | NaCl | 6 | - | - | 1 | [36] |
| Red grape pomace | Brij S20 and Poloxamer 407 | 3% w/v | 25 | 4 | 45 | 1:10 w/v | - | - | 3500 | 20 | [37] | ||
| Acalypha fruticosa powder | Tween 20 | 8 mM | 70–80 | - | 30 | 1:100 w/v | KCl | 2 | 4000 | 10 | [38] | ||
| Carica papaya leaves | Pluronic L-61 | 10% w/w | 40 | - | 10 | 0.1% (w/w) | - | - | 10,000 | [39] | |||
| Apricot cannery wastewater | Peg 8000 | 2% w/v | 65 | 3.5 | 20 | - | NaCl | 3 | 3500 | 5 | 2 | [40] | |
| Wine sludge waste | Lecithin | 5% w/v | 40 | 3 | 30 | - | NaCl | 5 | 3500 | 15 | 3 | [41] | |
| Olive mill wastewater | 12.5% w/v | 3.5 | 10 | 4500 | 5 | [42] | |||||||
| 3% w/w | 30 | [46] | |||||||||||
| Olive process wastewater | Tween 80 | 10% w/v | 70 | 2 | 30 | - | - | - | - | - | 1 | [43] | |
| Peach waste streams | 5% w/v | 65 | 3.5 | 20 | NaCl | 3 | 3500 | 5 | 2 | [45] | |||
| Unripe and ripe peaches | 45 | 2.5 | 6 | [46] | |||||||||
| Pomegranate peel | Polyphenols, flavonoids | Triton X-114 | 8.22% w/v | 36.80 | 4 | 30 | 0.5 g/50 mL | NaCl | 4 | 8000 | 10 | 1 | [47] |
| 8% w/v | 55 | 4.5 | 1:30 w/v | 14 | 12,000 | [48] | |||||||
| Tomato wastewater | Carotenoids | Lecithin | 1 or 2% w/v | 45 | 3.5 | 20 | - | NaCl | 35.6 | 4500 | 5 | 3 or 2 | [49] |
| Brown microalgae | Tween 20 | 0.046 mol/L | 25 | - | 140 | 0.02 mg/mL | - | - | 5000 | 40 | 1 | [50] | |
| Green microalgae | Chlorophylls | C26H56ClP | 250 mM | 25 | - | 30 | 0.01 g/mL | - | - | 5000 | 30 | 1 | [51] |
| Spinach leaves | C11-C13 9EO’s | 12.4 mM | 41 | - | 30 | 0.07 w/w | - | - | - | - | 1 | [18] |
| Operation Parameters | Parameter Arrays | Short Explanation |
|---|---|---|
| Cloud Point Temperature | Process | The temperature at which phase separation occurs is a critical parameter. Optimization ensures that the CPT is conducive to the efficient extraction of the target analytes. |
| Temperature | The temperature during microwave irradiation should be controlled to avoid degradation of analytes and to optimize the phase separation process. | |
| Stirring or Agitation | Stirring or agitation of the sample during extraction can enhance mass transfer and improve efficiency. | |
| Microwave Power and Irradiation Time | Microwave power and irradiation time directly influence the heating and extraction efficiency. Optimization prevents sample degradation and achieves maximal extraction yields. | |
| Instrumentation Parameters | Specific parameters of the microwave instrument, such as frequency and mode of irradiation, need to be optimized for compatibility with the CPE. | |
| pH of the Extraction Medium | The pH of the extraction medium affects the solubility of analytes and the stability of micelles. Optimal pH conditions should be established for efficient extraction. | |
| Sample Pretreatment | Sample | Preparing the sample through appropriate pretreatment methods, such as grinding or homogenization, can impact the accessibility of analytes during extraction. |
| Sample Matrix Characteristics | The nature of the sample matrix, including its complexity, viscosity, and potential interference, must be considered for the effective CPE-MAE | |
| Sample Size | The amount of sample used can impact the extraction efficiency. Optimization involves determining the optimal sample size for the given system. | |
| Surfactant Type and Concentration | Surfactant | The choice of surfactant significantly affects the CPE. Selection based on its critical micelle concentration and compatibility with microwave irradiation is crucial. |
| Co-Surfactant/Additives | The addition of co-surfactants or other additives may enhance the solubilization of certain analytes or improve phase separation, contributing to overall extraction efficiency. | |
| Surfactant-to-Sample Ratio | The ratio of surfactant to the sample is critical for achieving phase separation and maximizing the concentration of analytes in the surfactant-rich phase. | |
| Nature of Analytes | Analytes | The physicochemical properties of the target analytes, such as solubility and volatility, influence their extraction behavior. Understanding these properties is crucial for optimizing extraction conditions. |
| Extraction Solvent and Volume | The choice of extraction solvent, its compatibility with surfactants, and the volume used influence the extraction efficiency. Optimization ensures an appropriate solvent for the target analytes. | |
| Microwave Vessel Material | Safety | The choice of vessel material for microwave irradiation can influence the heating efficiency and should be considered during optimization. |
| Safety Precautions | Ensuring proper safety measures during microwave irradiation is crucial to prevent accidents and ensure the integrity of the extraction process. |
| Ultrasound-Related Parameters | Short Explanation |
|---|---|
| Ultrasound Frequency | Higher frequencies are associated with smaller cavitation bubbles but may have limited penetration. Lower frequencies penetrate deeper but may result in larger bubbles. Optimization involves selecting a frequency that balances efficient cavitation and penetration based on the nature of the sample matrix and desired analyte extraction. |
| Ultrasound Intensity | It influences cavitation effects and heating during extraction. Optimization involves determining the level that promotes effective cavitation without causing excessive sample heating or degradation. |
| Duration of Ultrasound Exposure | The duration directly influences the efficiency of analyte release from the matrix. Optimization of irradiation time involves finding the balance between sufficient extraction and minimizing sample degradation. Shorter irradiation times may not fully exploit cavitation effects, while excessively long times may lead to undesired effects. |
| Cavitation rate | Cavitation is the formation, growth, and collapse of bubbles in a liquid medium. It creates localized microenvironments with high temperatures and pressures, facilitating the release of analytes from the sample matrix. Longer irradiation times may enhance cavitation effects, but careful control is necessary to avoid excessive heating, which can degrade sensitive analytes. |
| Plant Material | Target Bioactives | Surfactant Type | Surfactant Concentration | Temperature (°C) | pH | Time (min) | Solid–Liquid Ratio | Salt | Salt Concentration (% w/v) | Centrifugation Speed (rpm) | Centrifugation Time (min) | CPE Step | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPE-MAE | |||||||||||||
| Vegetables * | Vitamin K | Triton X-45 | 15% w/v | 38 | 7 | 10–20 | nd | NaCl | 0.04 | 3500 | 3 | 1 | [67] |
| fig (Ficus carica L.) leaves | Polyphenols and furanocoumarins | PEG8000 | 2.5% w/v | 40 | nd | 10.27 | 19.95 mL/g | / | / | 12,000 | 10 | 1 | [68] |
| Pomegranate peels | Polyphenols | Triton X-114 | 8% w/v | 55 | 4.5 | 30 | 1:70 | NaCl | 14 | 12,000 | 10 | 2–3 | [48] |
| CPE-UAE | |||||||||||||
| foods and vegetables ** | zinc, nickel and cobalt | Igepal CO-630 | 0.2% w/v | 50 | 5 | 10 | nd | nd | nd | 4000 | 5 | 1 | [69] |
| Mulberry leaves | polyphenols and alkaloids | Triton X-114 | 3% w/w | nd | nd | nd | 1:35 | NaCl | 0.05 M | 3800 | 5 | 1 | [70] |
| Euonymus alatus | flavonoids | PEG-400 | 16% w/w | 55 | 3.5 | 15 | 1:60 | (NH4)2SO4 | 6.7 | 4000 | 5 | 2 | [64] |
| edible vegetal oils and vinegar | Vanadium types (V) and (IV) | Triton X-114 | 0.001–0.01074% w/v | 40 | 4 | 5 | nd | NaNO3 | 0.15 mol/L | 4000 | 10 | 1 | [71] |
| Anoectochilus roxburghii (Wall.) Lindl. | rutin and narcissoside | 20% [C4 mim] [PF6] and Triton X-114 | [C4 mim] [PF6]:Triton X-114 = 2:23 | 45 | 3 | 10 | 1:60 | NaCl | 0.25 g/mL | 4000 | 10 | 1 | [72] |
| Green vegetables *** | iron | Triton X-114 | 0.3% w/w | 45 | 5.5 | nd | nd | / | / | 5000 | 10 | nd | [65] |
| Clingstone Peach Canneries waste | polyphenols | Tween 80 | 10% w/w | 65 | 3.5 | 20 | NaCl | 3 | 4500 | 20 | 2 | [45] | |
| Pomegranate peel | Triton X-110 | 10% w/w | 70 | 4 | 40 | 1:40 | NaCl | 14 | 6000 | 20 | 1 | [73] | |
| Triton X-114 | 8% w/v | 55 | 4.5 | 30 | 1:70 | 14 | 12,000 | 10 | 2–3 | [48] | |||
| Dandelion | 5% w/v | 60 | 3.5 | nd | 10 | 6000 | 5 | 1 | [74] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Travičić, V.; Cvanić, T.; Šovljanski, O.; Erceg, T.; Perović, M.; Stupar, A.; Ćetković, G. Updating the Status quo on the Eco-Friendly Approach for Antioxidants Recovered from Plant Matrices Using Cloud Point Extraction. Antioxidants 2024, 13, 280. https://doi.org/10.3390/antiox13030280
Travičić V, Cvanić T, Šovljanski O, Erceg T, Perović M, Stupar A, Ćetković G. Updating the Status quo on the Eco-Friendly Approach for Antioxidants Recovered from Plant Matrices Using Cloud Point Extraction. Antioxidants. 2024; 13(3):280. https://doi.org/10.3390/antiox13030280
Chicago/Turabian StyleTravičić, Vanja, Teodora Cvanić, Olja Šovljanski, Tamara Erceg, Milica Perović, Alena Stupar, and Gordana Ćetković. 2024. "Updating the Status quo on the Eco-Friendly Approach for Antioxidants Recovered from Plant Matrices Using Cloud Point Extraction" Antioxidants 13, no. 3: 280. https://doi.org/10.3390/antiox13030280











