Protective Effects of a Red Orange and Lemon Extract (RLE) on the Hepatotoxicity Induced by Ochratoxin A in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Chemicals and Reagents
2.3. Experimental Design and Sample Collection
2.4. Serum Hepatic Biomarkers Determination
2.5. Determination of Malondialdehyde Levels and SOD, CAT and GPx Activities
2.6. Histological Investigations
2.7. Statistical Analysis
3. Results
3.1. RLE Effects on Hepatic Enzymes and Serum Total Protein Levels in Rats
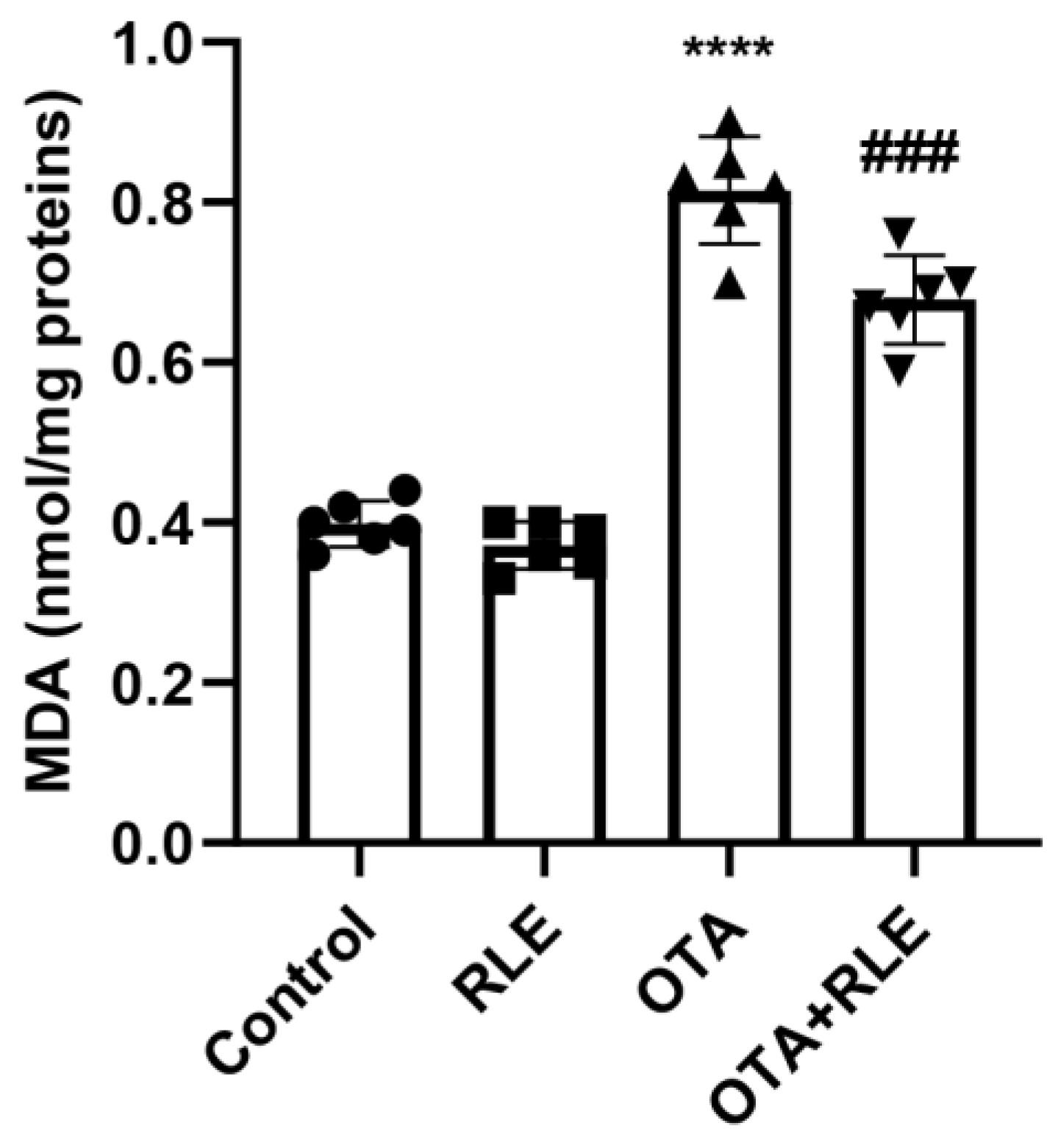
3.2. RLE Co-Treatment Alleviated Lipid Peroxidation in the Liver of OTA-Intoxicated Rats
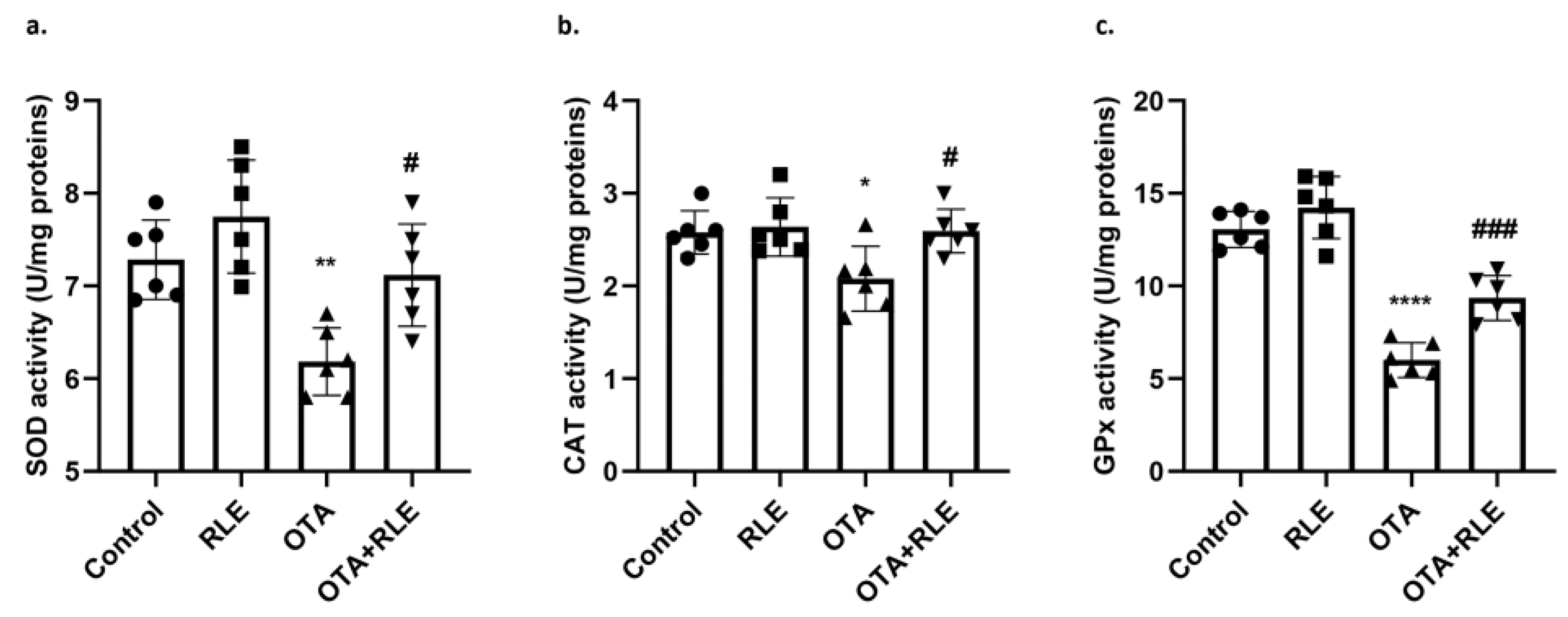
3.3. RLE Mitigates SOD, CAT, and GPx Activities during OTA Intoxication
3.4. Mitigation of Toxic Effects of OTA in Livers’ Histological Sections
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hua, X.; Shi, J.; Jing, N.; Ji, T.; Lv, B.; Liu, L.; Chen, Y. Ochratoxin A: Occurrence and recent advances in detoxification. Toxicon 2022, 210, 11–18. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.-C.; Nebbia, C.S.; et al. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, e06113. [Google Scholar] [CrossRef] [PubMed]
- Khoi, C.-S.; Chen, J.-H.; Lin, T.-Y.; Chiang, C.-K.; Hung, K.-Y. Ochratoxin A-Induced Nephrotoxicity: Up-to-Date Evidence. Int. J. Mol. Sci. 2021, 22, 11237. [Google Scholar] [CrossRef]
- Manderville, R.A.; Wetmore, S.D. Mutagenicity of Ochratoxin A: Role for a Carbon-Linked C8–Deoxyguanosine Adduct? J. Agric. Food Chem. 2017, 65, 7097–7105. [Google Scholar] [CrossRef]
- Ferenczi, S.; Kuti, D.; Cserháti, M.; Krifaton, C.; Szoboszlay, S.; Kukolya, J.; Szőke, Z.; Albert, M.; Kriszt, B.; Kovács, K.J.; et al. Effects of Single and Repeated Oral Doses of Ochratoxin A on the Lipid Peroxidation and Antioxidant Defense Systems in Mouse Kidneys. Toxins 2020, 12, 732. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; You, S.; Song, G. Ochratoxin A exerts neurotoxicity in human astrocytes through mitochondria-dependent apoptosis and intracellular calcium overload. Toxicol. Lett. 2019, 313, 42–49. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Cui, J.; Wu, X.; Li, Y.; Wu, W.; Zhang, X. Ochratoxin A induces reprogramming of glucose metabolism by switching energy metabolism from oxidative phosphorylation to glycolysis in human gastric epithelium GES-1 cells in vitro. Toxicol. Lett. 2020, 333, 232–241. [Google Scholar] [CrossRef]
- Ding, L.; Han, M.; Wang, X.; Guo, Y. Ochratoxin A: Overview of Prevention, Removal, and Detoxification Methods. Toxins 2023, 15, 565. [Google Scholar] [CrossRef]
- Longobardi, C.; Ferrara, G.; Andretta, E.; Montagnaro, S.; Damiano, S.; Ciarcia, R. Ochratoxin A and Kidney Oxidative Stress: The Role of Nutraceuticals in Veterinary Medicine—A Review. Toxins 2022, 14, 398. [Google Scholar] [CrossRef]
- Garcia, C.; Blesso, C.N. Antioxidant properties of anthocyanins and their mechanism of action in atherosclerosis. Free. Radic. Biol. Med. 2021, 172, 152–166. [Google Scholar] [CrossRef]
- Amorini, A.M.; Fazzina, G.; Lazzarino, G.; Tavazzi, B.; Di Pierro, D.; Santucci, R.; Sinibaldi, F.; Galvano, F.; Galvano, G. Activity and mechanism of the antioxidant properties of cyanidin-3-O-beta-glucopyranoside. Free. Radic. Res. 2001, 35, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, P.; Amenta, M.; Ballistreri, G.; Fabroni, S.; Timpanaro, N. Distribution, Antioxidant Capacity, Bioavailability and Biological Properties of Anthocyanin Pigments in Blood Oranges and Other Citrus Species. Molecules 2022, 27, 8675. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Lee, H.J.; Pyo, M.C.; Ryu, D.; Lee, K.-W. Ochratoxin A-Induced Hepatotoxicity through Phase I and Phase II Reactions Regulated by AhR in Liver Cells. Toxins 2019, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Lombari, P.; Salvi, E.; Papale, M.; Giordano, A.; Amenta, M.; Ballistreri, G.; Fabroni, S.; Rapisarda, P.; Capasso, G.; et al. A red orange and lemon by-products extract rich in anthocyanins inhibits the progression of diabetic nephropathy. J. Cell. Physiol. 2019, 234, 23268–23278. [Google Scholar] [CrossRef]
- Damiano, S.; Iovane, V.; Squillacioti, C.; Mirabella, N.; Prisco, F.; Ariano, A.; Amenta, M.; Giordano, A.; Florio, S.; Ciarcia, R. Red orange and lemon extract prevents the renal toxicity induced by ochratoxin A in rats. J. Cell. Physiol. 2020, 235, 5386–5393. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Oriente, F.; Cabaro, S.; Liotti, A.; Longo, M.; Parrillo, L.; Pagano, T.B.; Raciti, G.A.; Penkov, D.; Paciello, O.; Miele, C.; et al. PREP1 deficiency downregulates hepatic lipogenesis and attenuates steatohepatitis in mice. Diabetologia 2013, 56, 2713–2722. [Google Scholar] [CrossRef]
- Veteläinen, R.L.; Bennink, R.J.; de Bruin, K.; van Vliet, A.; van Gulik, T.M. Hepatobiliary function assessed by 99mTc-mebrofenin cholescintigraphy in the evaluation of severity of steatosis in a rat model. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1107–1114. [Google Scholar] [CrossRef]
- Sorrenti, V.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Bognanno, M.; Galvano, F. Toxicity of Ochratoxin A and its modulation by antioxidants: A review. Toxins 2013, 5, 1742–1766. [Google Scholar] [CrossRef]
- Maggiolino, A.; Bragaglio, A.; Salzano, A.; Rufrano, D.; Claps, S.; Sepe, L.; Damiano, S.; Ciarcia, R.; Dinardo, F.; Hopkins, D.; et al. Dietary supplementation of suckling lambs with anthocyanins: Effects on growth, carcass, oxidative and meat quality traits. Anim. Feed Sci. Technol. 2021, 276, 114925. [Google Scholar] [CrossRef]
- Fonseca, L.D.; Eler, J.P.; Pereira, M.A.; Rosa, A.F.; Alexandre, P.A.; Moncau, C.T.; Salvato, F.; Rosa-Fernandes, L.; Palmisano, G.; Ferraz, J.B.S.; et al. Liver proteomics unravel the metabolic pathways related to Feed Efficiency in beef cattle. Sci. Rep. 2019, 9, 5364. [Google Scholar] [CrossRef] [PubMed]
- Vettorazzi, A.; de Trocóniz, I.F.; González-Peñas, E.; Arbillaga, L.; Corcuera, L.-A.; Gil, A.G.; de Cerain, A.L. Kidney and liver distribution of ochratoxin A in male and female F344 rats. Food Chem. Toxicol. 2011, 49, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; González, D.; Hobloss, Z.; Brackhagen, L.; Myllys, M.; Friebel, A.; Seddek, A.-L.; Marchan, R.; Cramer, B.; Humpf, H.-U.; et al. Inhibition of cytochrome P450 enhances the nephro- and hepatotoxicity of ochratoxin A. Arch. Toxicol. 2022, 96, 3349–3361. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.M.; Abbassa, A.H. The opposing effect of ascorbic acid (vitamin C) on ochratoxin toxicity in Nile tilapia (Oreochromis niloticus). Acta Polenica 2009, 2, 18–22. [Google Scholar]
- Wang, W.; Zhai, S.; Xia, Y.; Wang, H.; Ruan, D.; Zhou, T.; Zhu, Y.; Zhang, H.; Zhang, M.; Ye, H.; et al. Ochratoxin A induces liver inflammation: Involvement of intestinal microbiota. Microbiome 2019, 7, 151. [Google Scholar] [CrossRef]
- Novi, S.; Vestuto, V.; Campiglia, P.; Tecce, N.; Bertamino, A.; Tecce, M.F. Anti-Angiogenic Effects of Natural Compounds in Diet-Associated Hepatic Inflammation. Nutrients 2023, 15, 2748. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wu, F.; Tian, J.; Guo, X.; An, R. Protective effects of compound ammonium glycyrrhizin, L-arginine, silymarin and glucurolactone against liver damage induced by ochratoxin A in primary chicken hepatocytes. Mol. Med. Rep. 2018, 18, 2551–2560. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Ambade, A.; Mandrekar, P. Oxidative stress and inflammation: Essential partners in alcoholic liver disease. Int. J. Hepatol. 2012, 2012, 853175. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Mobashar, M.; Hummel, J.; Blank, R.; Südekum, K.-H. Ochratoxin A in ruminants—A review on its degradation by gut microbes and effects on animals. Toxins 2010, 2, 809–839. [Google Scholar] [CrossRef] [PubMed]
- Karsauliya, K.; Yahavi, C.; Pandey, A.; Bhateria, M.; Sonker, A.K.; Pandey, H.; Sharma, M.; Singh, S.P. Co-occurrence of mycotoxins: A review on bioanalytical methods for simultaneous analysis in human biological samples, mixture toxicity and risk assessment strategies. Toxicon 2022, 218, 25–39. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Mahmood, M.; Khan, M.Z.U.; Talha, H.M.A.; Sajid, M.; Rafique, K.; Naveed, S.; Faas, J.; Artavia, J.I.; Sulyok, M.; et al. Co-occurrence of mycotoxins and other fungal metabolites in total mixed rations of cows from dairy farms in Punjab, Pakistan. Mycotoxin Res. 2023, 39, 421–436. [Google Scholar] [CrossRef] [PubMed]

| Group | ALT (U/L) | AST (U/L) | ALP (U/L) | Total Protein (g/dL) |
|---|---|---|---|---|
| Control | 43.29 ± 5.2 | 106.72 ± 1.41 | 24.45 ± 6.0 | 79.51 ± 3.40 |
| RLE | 38.07 ± 4.1 | 99.40 ± 5.8 | 20.48 ± 5.0 | 83.46 ± 1.42 |
| OTA | 105.24 ± 61 **** | 185.88 ± 4.3 **** | 91.28 ± 46 **** | 48.15 ± 2.47 **** |
| OTA + RLE | 81.23 ± 59 #### | 54.69 ± 5.9 #### | 61.26 ± 4.9 #### | 82.09 ± 2.33 #### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longobardi, C.; Damiano, S.; Vaccaro, E.; Ballistreri, G.; Restucci, B.; Paciello, O.; Florio, S.; Ciarcia, R. Protective Effects of a Red Orange and Lemon Extract (RLE) on the Hepatotoxicity Induced by Ochratoxin A in Rats. Antioxidants 2024, 13, 289. https://doi.org/10.3390/antiox13030289
Longobardi C, Damiano S, Vaccaro E, Ballistreri G, Restucci B, Paciello O, Florio S, Ciarcia R. Protective Effects of a Red Orange and Lemon Extract (RLE) on the Hepatotoxicity Induced by Ochratoxin A in Rats. Antioxidants. 2024; 13(3):289. https://doi.org/10.3390/antiox13030289
Chicago/Turabian StyleLongobardi, Consiglia, Sara Damiano, Emanuela Vaccaro, Gabriele Ballistreri, Brunella Restucci, Orlando Paciello, Salvatore Florio, and Roberto Ciarcia. 2024. "Protective Effects of a Red Orange and Lemon Extract (RLE) on the Hepatotoxicity Induced by Ochratoxin A in Rats" Antioxidants 13, no. 3: 289. https://doi.org/10.3390/antiox13030289








