Chemical Characterization of Honeysuckle Polyphenols and Their Alleviating Function on Ultraviolet B-Damaged HaCaT Cells by Modulating the Nrf2/NF-κB Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Preparation of Honeysuckle Polyphenols (HPs)
2.3. LC–MS/MS Analysis
2.4. DPPH Radical Scavenging Activity
2.5. Cytoprotection of HPs on UVB-Irradiated Cell Model
2.5.1. Cell Culture and Establishment of UVB-Irradiated Cell Model
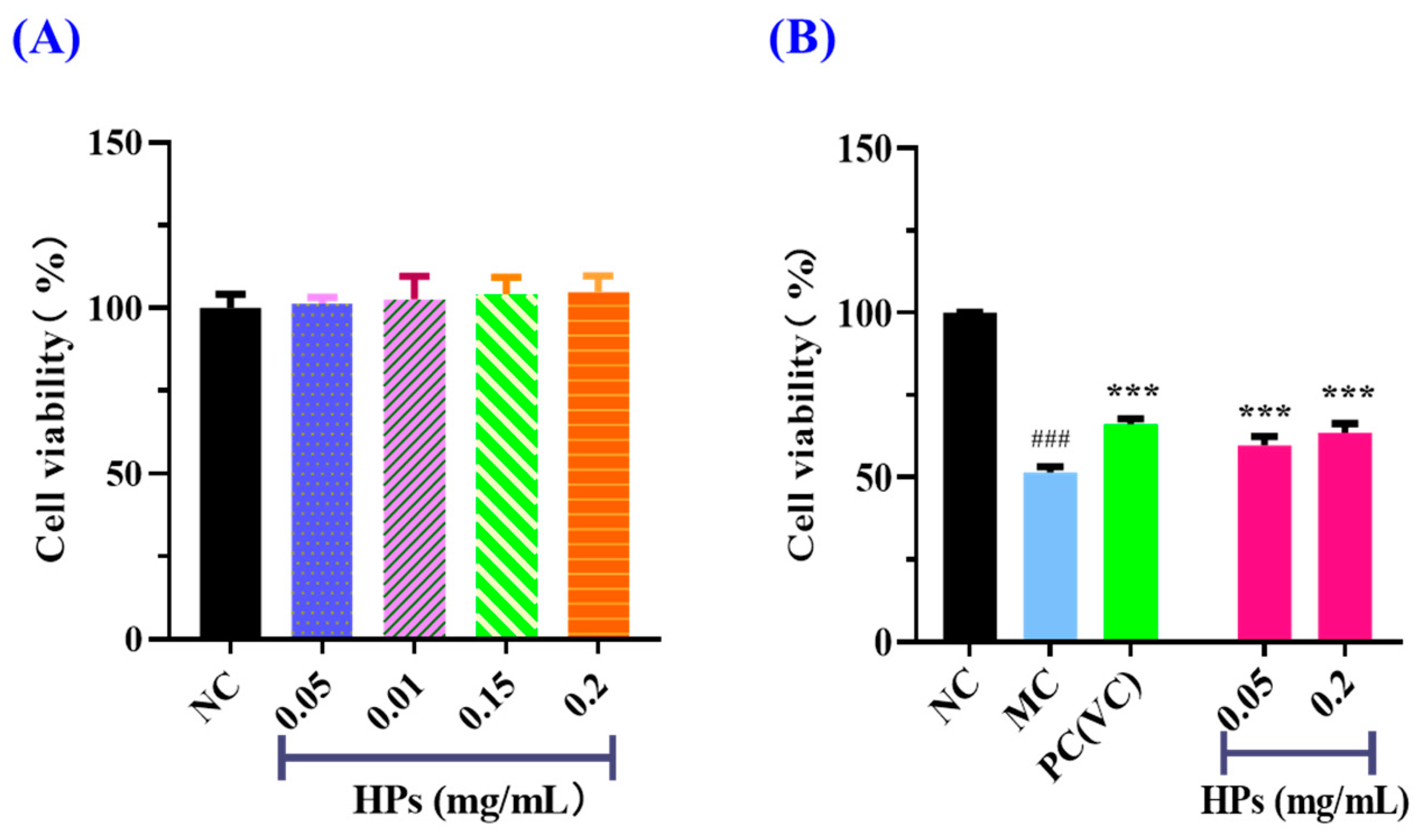
2.5.2. Effect of HPs on the Viability of UVB-Irradiated Cells
2.5.3. Measurement of Intracellular ROS, MDA, and Antioxidant Enzymes
2.5.4. Measurement of Levels of Inflammatory Factors
2.5.5. Measurement of Protein Expression
2.6. Molecular Docking Experiments of Nrf2 with NF-κB (P65)
2.7. Statistical Analysis
3. Results
3.1. Chemical Analysis of HPs by LC–MS/MS
Total Ion Chromatogram of HPs
3.2. Effect of HPs on DPPH Radical Scavenging Activity
3.3. Mitigating Function of HPs on UVB-Irradiated HaCaT Cells through Antioxidant Activity
3.3.1. Establishment of UVB-Irradiated Cell Model
3.3.2. Effects of HPs on the Viability of UVB-Irradiated HaCaT Cells
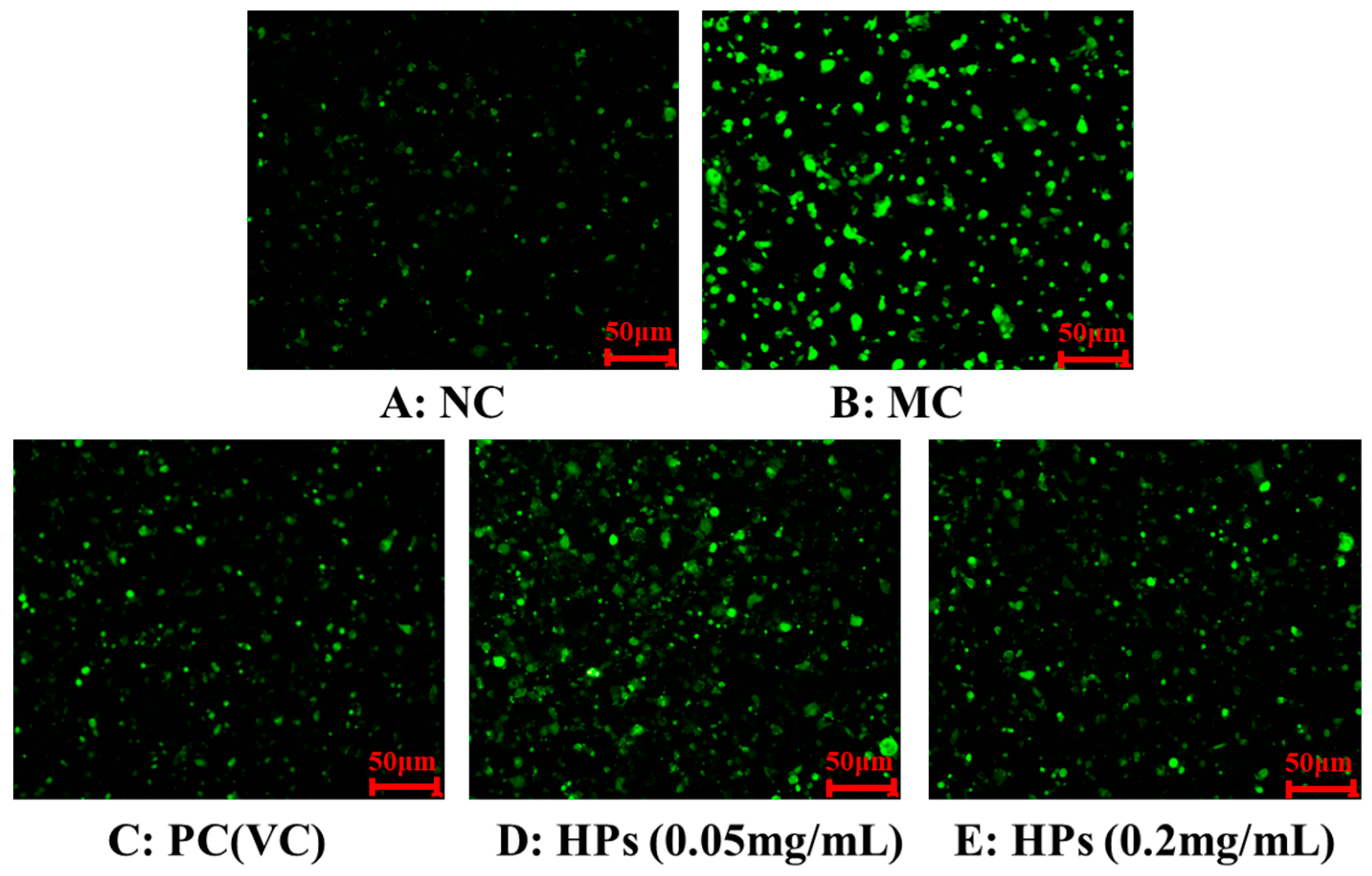
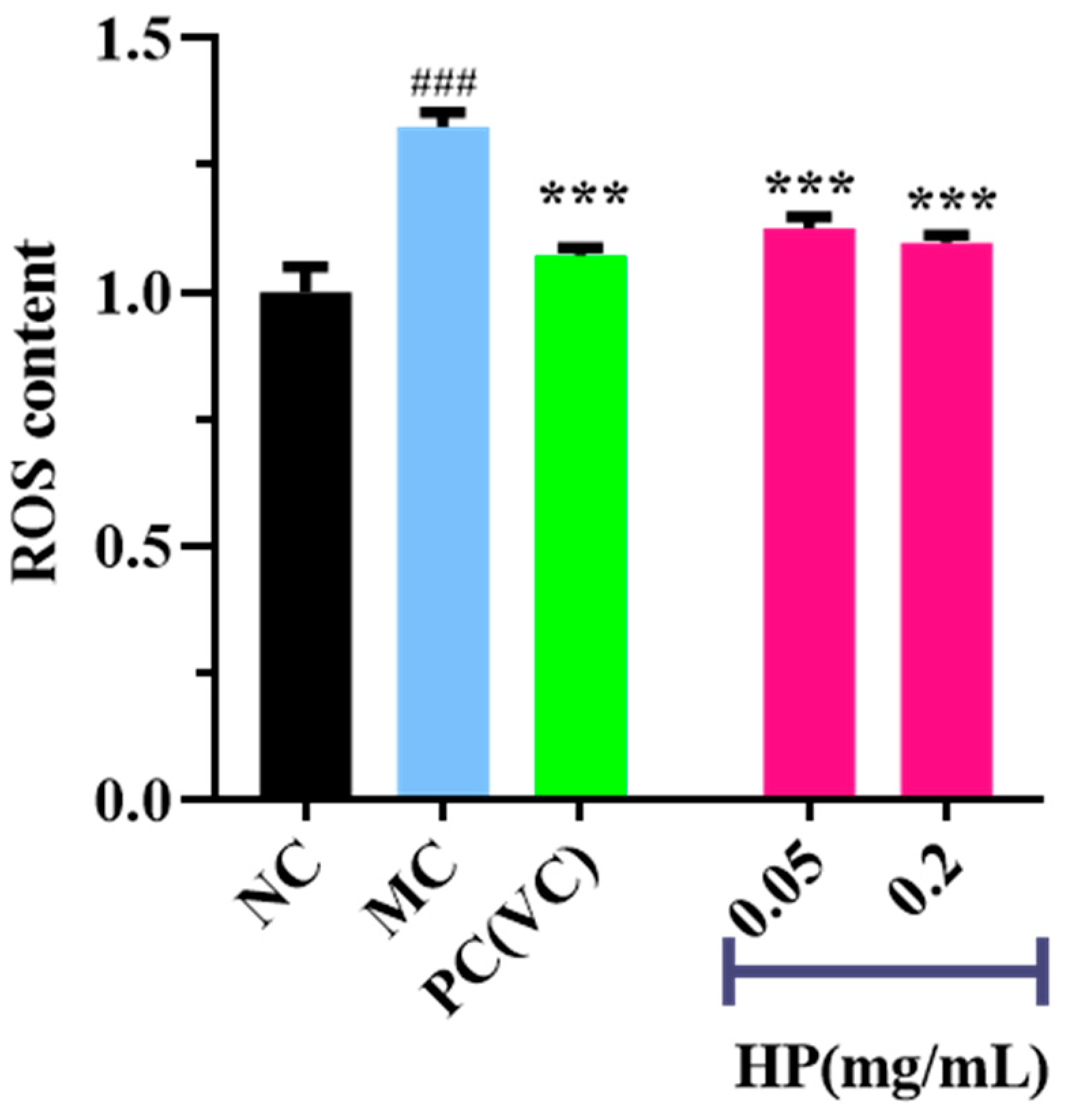
3.3.3. Effects of HPs on ROS Levels in UVB-Irradiated HaCaT Cells
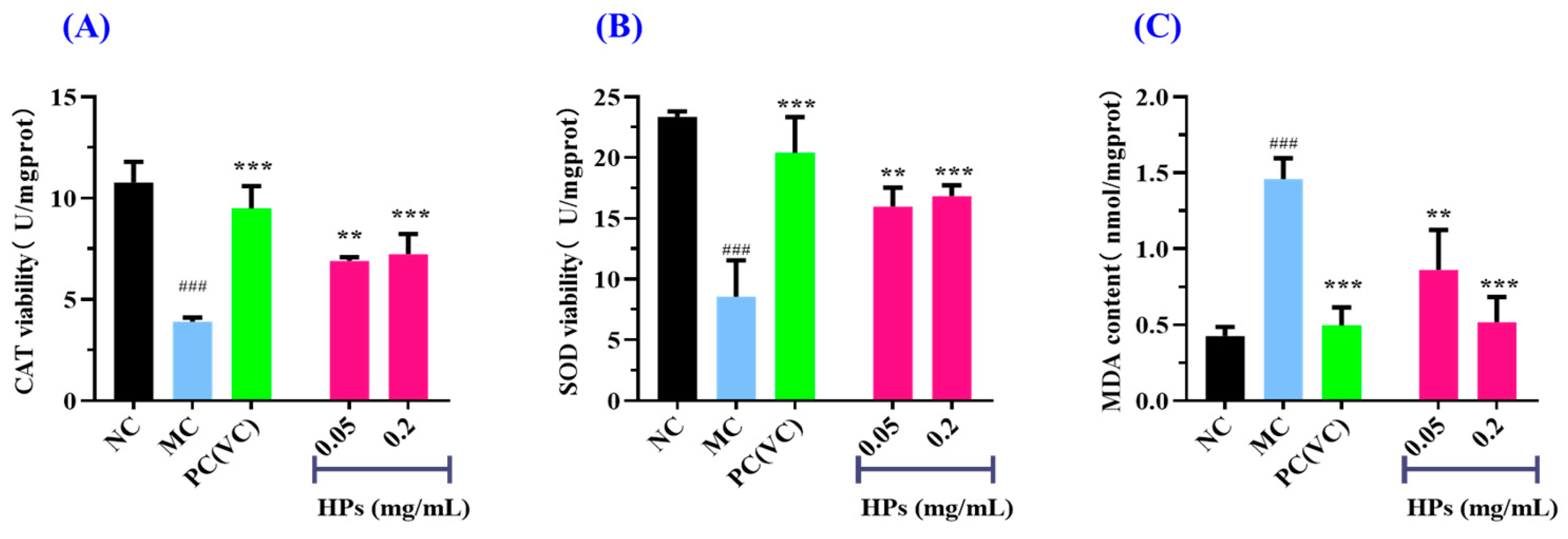
3.3.4. Effects of HPs on Intracellular Oxidase and Oxide Levels in UVB-Irradiated HaCaT Cells
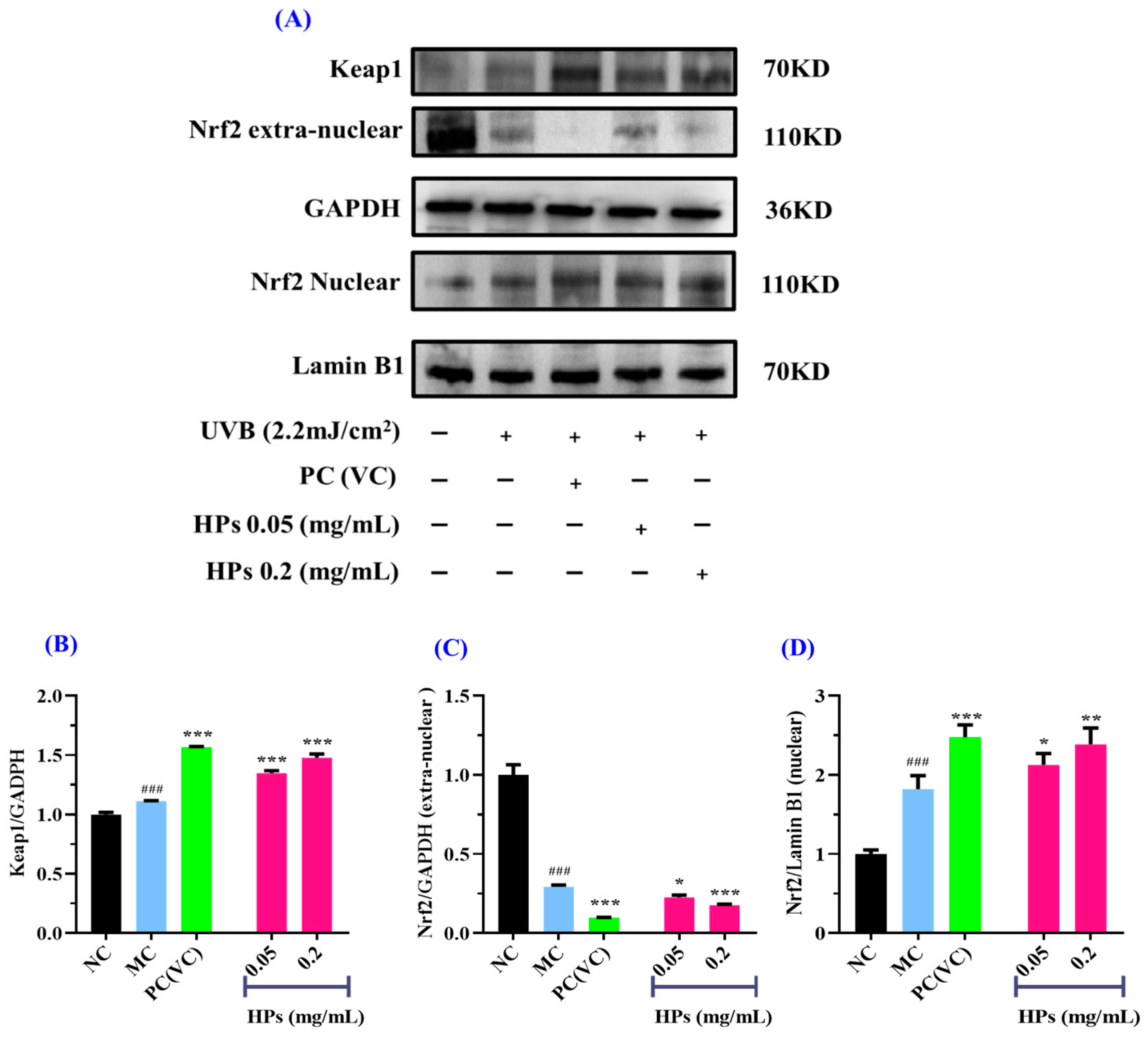
3.3.5. Effect of HPs on the Antioxidant Proteins in UVB-Irradiated HaCaT Cells
3.3.6. Molecular Docking of Chlorogenic Acid, Quercetin, Isorhamnetin, and Luteolin to Nrf2
3.4. Effects of HPs on Intracellular Inflammatory Factors in UVB-Irradiated HaCaT Cells
3.4.1. Effects of HPs on Intracellular TNF-α, IL-6, and IL-1β in UVB-Irradiated HaCaT Cells
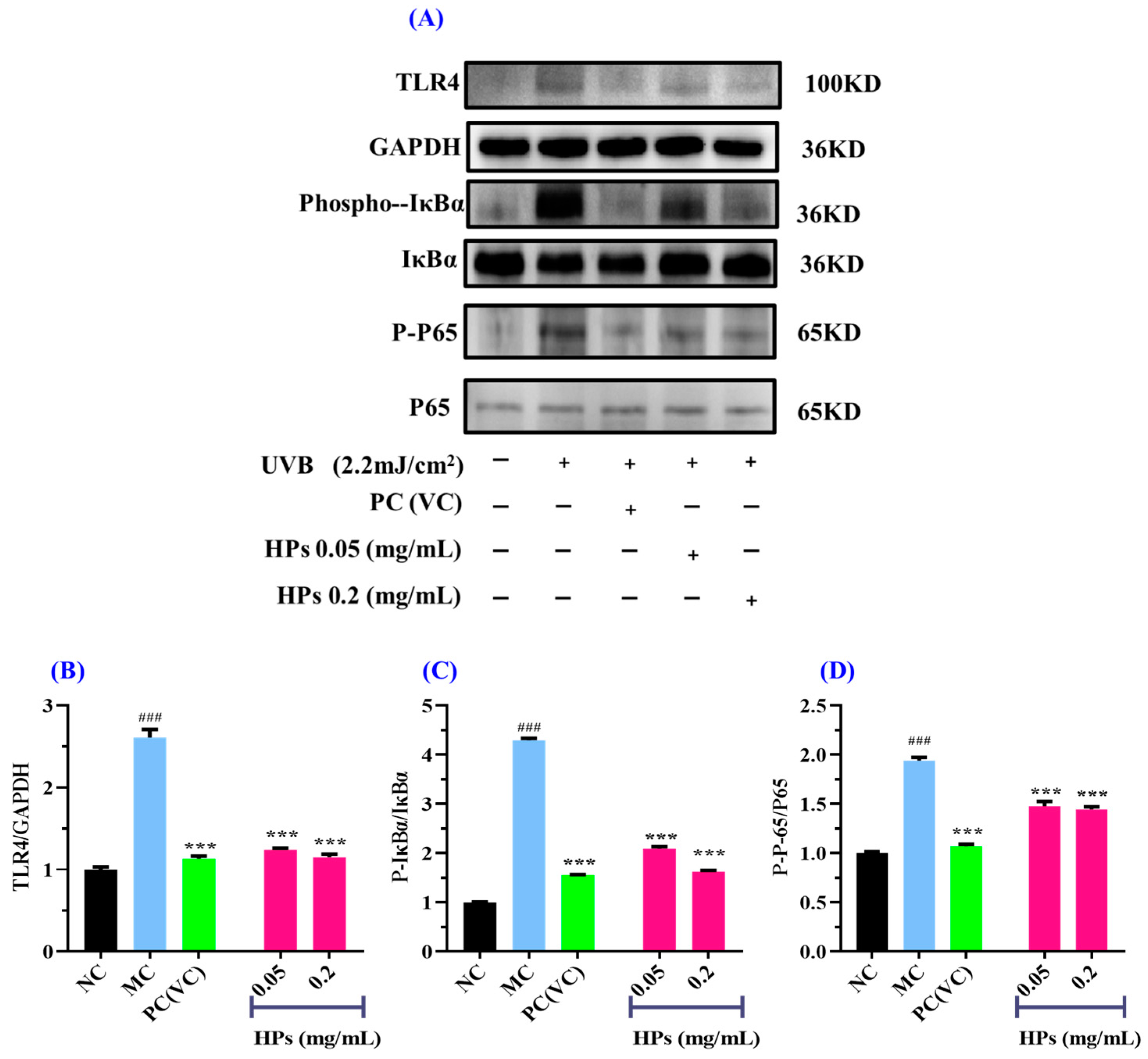
3.4.2. Effect of HPs on Inflammatory Protein Expression in UVB-Irradiated HaCaT Cells
3.4.3. Molecular Docking of Chlorogenic Acid, Quercetin, Isorhamnetin, and Luteolin to NF-κB
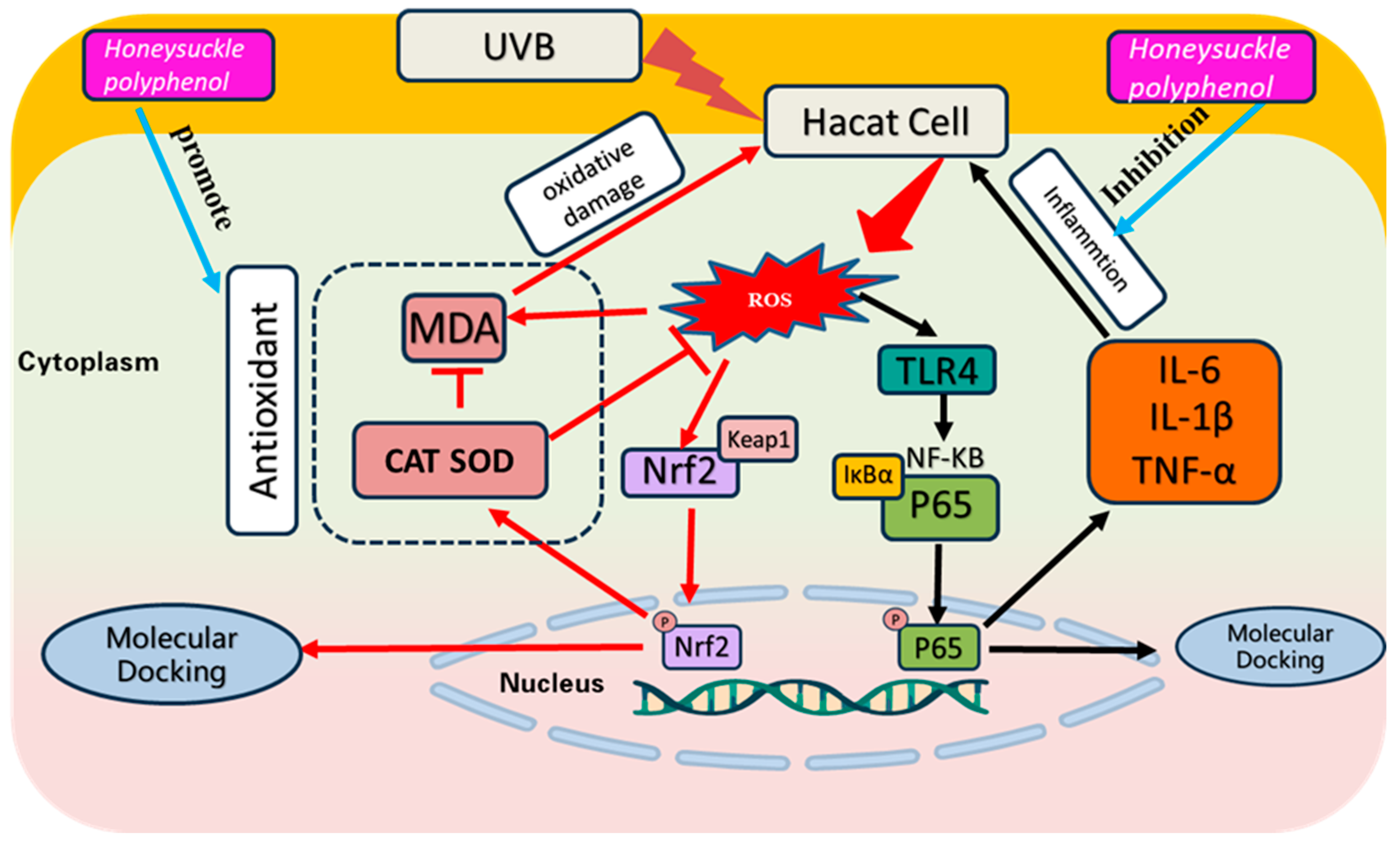
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Afnan, Q.; Kaiser, P.J.; Rafiq, R.A.; Nazir, L.A.; Bhushan, S.; Bhardwaj, S.C.; Sandhir, R.; Tasduq, S.A. Glycyrrhizic acid prevents ultraviolet-B-induced photodamage: A role for mitogen-activated protein kinases, nuclear factor kappa B and mitochondrial apoptotic pathway. Exp. Dermatol. 2016, 25, 440–446. [Google Scholar] [CrossRef]
- Qiu, W.J.; Xu, M.Z.; Zhu, X.D.; Ji, Y.H. MicroRNA-27a alleviates IL-1β-induced inflammatory response and articular cartilage degradation via TLR4/NF-κB signaling pathway in articular chondrocytes. Int. Immunopharmacol. 2019, 76, 105839. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Long, Y.; Wang, W.; Zhang, Y.; Du, F.; Zhang, S.; Li, Z.; Deng, J.; Li, J. Photoprotective Effects of Dendrobium nobile Lindl. Polysaccharides against UVB-Induced Oxidative Stress and Apoptosis in HaCaT Cells. Int. J. Mol. Sci. 2023, 24, 6120. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.F.; Xi, Q.H.; Sheng, Y.; Wang, Y.M.; Wang, W.Y.; Chi, C.F.; Wang, B. Antioxidant Peptides from Monkfish Swim Bladders: Ameliorating NAFLD In Vitro by Suppressing Lipid Accumulation and Oxidative Stress via Regulating AMPK/Nrf2 Pathway. Mar. Drugs 2023, 21, 360. [Google Scholar] [CrossRef]
- Zhao, X.J.; Chen, L.; Zhao, Y.; Pan, Y.; Yang, Y.Z.; Sun, Y.; Jiao, R.Q.; Kong, L.D. Polygonum cuspidatum extract attenuates fructose-induced liver lipid accumulation through inhibiting Keap1 and activating Nrf2 antioxidant pathway. Phytomedicine 2019, 63, 152986. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Ci, X. Role of Nrf2 and Its Activators in Respiratory Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 7090534. [Google Scholar] [CrossRef]
- Zheng, S.L.; Wang, Y.Z.; Zhao, Y.Q.; Chi, C.F.; Zhu, W.Y.; Wang, B. High Fischer ratio oligopeptides from hard-shelled mussel: Preparation and hepatoprotective effect against acetaminophen-induced liver injury in mice. Food Biosci. 2023, 53, 102638. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, Z.H.; Li, C.G.; Zhu, Y.J.; Li, Z.; Tang, Y.Z.; Ni, C.L. Apigenin prevents metabolic syndrome in high-fructose diet-fed mice by Keap1-Nrf2 pathway. Biomed. Pharmacother. 2018, 105, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, J.; Juliet Igbokwe, C.; Duan, Y.; Cai, M.; He, Y.; Zhang, H. Oligopeptide of RDPEER from watermelon seeds prevents heat stress-induced liver injury by suppressing oxidative stress and inflammation responses. J. Funct. Foods 2023, 105, 105563. [Google Scholar] [CrossRef]
- Hussein, S.; Kamel, G.A.M. Pioglitazone ameliorates cisplatin-induced testicular toxicity by attenuating oxidative stress and inflammation via TLR4/MyD88/NF-κB signaling pathway. J. Trace Elem. Med. Biol. 2023, 80, 127287. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tang, X.; Zhang, W.; Li, G.; Chen, Y.; Guo, A.; Hu, C. 6-Bromoindirubin-3′-Oxime Suppresses LPS-Induced Inflammation via Inhibition of the TLR4/NF-κB and TLR4/MAPK Signaling Pathways. Inflammation 2019, 42, 2192–2204. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, A.; Muhammadnejad, A.; Dehpour, A.R.; Mehr, S.E.; Akhavan, M.M.; Shirkoohi, R.; Chamanara, M.; Mousavi, S.E.; Rezayat, S.M. Atorvastatin attenuates TNBS-induced rat colitis: The involvement of the TLR4/NF-κB signaling pathway. Inflammopharmacology 2016, 24, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zhao, Y.; Yao, Y.; Wu, N.; Xu, M.; Du, H.; Wu, J.; Tu, Y. Antioxidant Stress and Anti-Inflammatory Activities of Egg White Proteins and Their Derived Peptides: A Review. J. Agric. Food Chem. 2022, 70, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, M.J. Nano-gold displayed anti-inflammatory property via NF-κB pathways by suppressing COX-2 activity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Li, R.J.; Kuang, X.P.; Wang, W.J.; Wan, C.P.; Li, W.X. Comparison of chemical constitution and bioactivity among different parts of Lonicera japonica Thunb. J. Sci. Food Agric. 2020, 100, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, W.; Fu, C.; Song, Y.; Fu, Q. Lonicerae japonicae flos and Lonicerae flos: A systematic review of ethnopharmacology, phytochemistry and pharmacology. Phytochem. Rev. 2020, 19, 1–61. [Google Scholar] [CrossRef]
- Tang, X.; Liu, X.; Zhong, J.; Fang, R. Potential Application of Lonicera japonica Extracts in Animal Production: From the Perspective of Intestinal Health. Front. Microbiol. 2021, 12, 719877. [Google Scholar] [CrossRef]
- Jeong, S.H.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Won, C.K.; Park, K.I.; Kim, E.; Heo, J.D.; Kim, H.W.; Ahn, M.; et al. Potential Antioxidant and Anti-Inflammatory Effects of Lonicera japonica and Citri Reticulatae Pericarpium Polyphenolic Extract (LCPE). Antioxidants 2023, 12, 1582. [Google Scholar] [CrossRef]
- Su, X.; Zhu, Z.H.; Zhang, L.; Wang, Q.; Xu, M.m.; Lu, C.; Zhu, Y.; Zeng, J.; Duan, J.A.; Zhao, M. Anti-inflammatory property and functional substances of Lonicerae Japonicae Caulis. J. Ethnopharmacol. 2021, 267, 113502. [Google Scholar] [CrossRef]
- Hu, Y.D.; Xi, Q.H.; Kong, J.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from the Collagens of Monkfish (Lophius litulon) Swim Bladders: Isolation, Characterization, Molecular Docking Analysis and Activity Evaluation. Mar. Drugs 2023, 21, 516. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comput.-Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef]
- Xu, W.J.; Zhai, J.W.; Cui, Q.; Liu, J.Z.; Luo, M.; Fu, Y.J.; Zu, Y.G. Ultra-turrax based ultrasound-assisted extraction of five organic acids from honeysuckle (Lonicera japonica Thunb.) and optimization of extraction process. Sep. Purif. Technol. 2016, 166, 73–82. [Google Scholar] [CrossRef]
- Zhao, F.; Du, L.; Wang, J.; Liu, H.; Zhao, H.; Lyu, L.; Wang, W.; Wu, W.; Li, W. Polyphenols from Prunus mume: Extraction, purification, and anticancer activity. Food Funct. 2023, 14, 4380–4391. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Huang, S.; Zhang, L.; Ge, Z.; Sun, L.; Zong, W. Purification of Polyphenols from Distiller’s Grains by Macroporous Resin and Analysis of the Polyphenolic Components. Molecules 2019, 24, 1284. [Google Scholar] [CrossRef]
- Mohsen, S.M.; Ammar, A.S.M. Total phenolic contents and antioxidant activity of corn tassel extracts. Food Chem. 2009, 112, 595–598. [Google Scholar] [CrossRef]
- Cai, W.W.; Hu, X.M.; Wang, Y.M.; Chi, C.F.; Wang, B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, pH, and Simulated Gastrointestinal Digestion Treatments. Mar. Drugs 2022, 20, 626. [Google Scholar] [CrossRef]
- Li, G.; Chu, M.; Tong, Y.; Liang, Y.; Wang, S.; Ma, C.; Wang, Z.; Zhou, W. Protective Effects of Hippophae rhamnoides L. Phenylpropanoids on Doxorubicin-Induced Cardiotoxicity in Zebrafish. Molecules 2022, 27, 8858. [Google Scholar] [CrossRef]
- Cui, B.; Wang, Y.; Jin, J.; Yang, Z.; Guo, R.; Li, X.; Yang, L.; Li, Z. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxidative Med. Cell. Longev. 2022, 2022, 6037303. [Google Scholar] [CrossRef]
- Alekhya Sita, G.J.; Gowthami, M.; Srikanth, G.; Krishna, M.M.; Rama Sireesha, K.; Sajjarao, M.; Nagarjuna, K.; Nagarjuna, M.; Chinnaboina, G.K.; Mishra, A.; et al. Protective role of luteolin against bisphenol A-induced renal toxicity through suppressing oxidative stress, inflammation, and upregulating Nrf2/ARE/HO-1 pathway. IUBMB Life 2019, 71, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Lohakul, J.; Soontrapa, K.; Sampattavanich, S.; Akarasereenont, P.; Panich, U. Activation of Nrf2 Reduces UVA-Mediated MMP-1 Upregulation via MAPK/AP-1 Signaling Cascades: The Photoprotective Effects of Sulforaphane and Hispidulin. J. Pharmacol. Exp. Ther. 2017, 360, 388–398. [Google Scholar] [CrossRef]
- Tonolo, F.; Moretto, L.; Grinzato, A.; Fiorese, F.; Folda, A.; Scalcon, V.; Ferro, S.; Arrigoni, G.; Bellamio, M.; Feller, E.; et al. Fermented Soy-Derived Bioactive Peptides Selected by a Molecular Docking Approach Show Antioxidant Properties Involving the Keap1/Nrf2 Pathway. Antioxidants 2020, 9, 1306. [Google Scholar] [CrossRef]
- Wang, S.; Yang, L.; Hou, A.; Liu, S.; Yang, L.; Kuang, H.; Jiang, H. Screening hepatoprotective effective components of Lonicerae japonica Flos based on the spectrum-effect relationship and its mechanism exploring. Food Sci. Hum. Wellness 2023, 12, 283–294. [Google Scholar] [CrossRef]
- Chen, G.L.; Fan, M.X.; Wu, J.L.; Li, N.; Guo, M.Q. Antioxidant and anti-inflammatory properties of flavonoids from lotus plumule. Food Chem. 2019, 277, 706–712. [Google Scholar] [CrossRef]
- Guo, X.; Yu, X.; Zheng, B.; Zhang, L.; Zhang, F.; Zhang, Y.; Li, J.; Pu, G.; Zhang, L.; Wu, H. Network Pharmacology-Based Identification of Potential Targets of Lonicerae japonicae Flos Acting on Anti-Inflammatory Effects. BioMed Res. Int. 2021, 2021, 5507003. [Google Scholar] [CrossRef]
- Guo, A.L.; Chen, L.M.; Wang, Y.M.; Liu, X.Q.; Zhang, Q.W.; Gao, H.M.; Wang, Z.M.; Xiao, W.; Wang, Z.Z. Influence of Sulfur Fumigation on the Chemical Constituents and Antioxidant Activity of Buds of Lonicera japonica. Molecules 2014, 19, 16640–16655. [Google Scholar] [CrossRef]
- Kong, J.; Hu, X.M.; Cai, W.W.; Wang, Y.M.; Chi, C.F.; Wang, B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs (II): Protective Function on UVB-Irradiated HaCaT Cells through Antioxidant and Anti-Apoptotic Mechanisms. Mar. Drugs 2023, 21, 105. [Google Scholar] [CrossRef]
- Li, L.; Fu, J.; Sun, J.; Liu, D.; Chen, C.; Wang, H.; Hou, Y.; Xu, Y.; Pi, J. Is Nrf2-ARE a potential target in NAFLD mitigation? Curr. Opin. Toxicol. 2019, 13, 35–44. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Chen, Q.M. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic. Biol. Med. 2022, 179, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Su, Z.y.; Kong, A.-N.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef]
- Wu, S.; He, X.; Wu, X.; Qin, S.; He, J.; Zhang, S.; Hou, D.X. Inhibitory effects of blue honeysuckle (Lonicera caerulea L) on adjuvant-induced arthritis in rats: Crosstalk of anti-inflammatory and antioxidant effects. J. Funct. Foods 2015, 17, 514–523. [Google Scholar] [CrossRef]
- Kopacz, A.; Kloska, D.; Forman, H.J.; Jozkowicz, A.; Grochot-Przeczek, A. Beyond repression of Nrf2: An update on Keap1. Free Radic. Biol. Med. 2020, 157, 63–74. [Google Scholar] [CrossRef]
- Lo, S.C.; Li, X.; Henzl, M.T.; Beamer, L.J.; Hannink, M. Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. EMBO J. 2006, 25, 3605–3617. [Google Scholar] [CrossRef]
- Park, J.Y.; Chung, T.W.; Jeong, Y.J.; Kwak, C.H.; Ha, S.H.; Kwon, K.M.; Abekura, F.; Cho, S.H.; Lee, Y.C.; Ha, K.T.; et al. Ascofuranone inhibits lipopolysaccharide–induced inflammatory response via NF-kappaB and AP-1, p-ERK, TNF-α, IL-6 and IL-1β in RAW 264.7 macrophages. PLoS ONE 2017, 12, e0171322. [Google Scholar] [CrossRef]
- Thoma, A.; Lightfoot, A.P. NF-κB and Inflammatory Cytokine Signalling: Role in Skeletal Muscle Atrophy. In Muscle Atrophy; Xiao, J., Ed.; Springer: Singapore, 2018; Volume 1088, pp. 267–279. [Google Scholar]
- Asadzadeh Manjili, F.; Yousefi-Ahmadipour, A.; Kazemi Arababadi, M. The roles played by TLR4 in the pathogenesis of multiple sclerosis; A systematic review article. Immunol. Lett. 2020, 220, 63–70. [Google Scholar] [CrossRef]
- Coutinho-Wolino, K.S.; Almeida, P.P.; Mafra, D.; Stockler-Pinto, M.B. Bioactive compounds modulating Toll-like 4 receptor (TLR4)-mediated inflammation: Pathways involved and future perspectives. Nutr. Res. 2022, 107, 96–116. [Google Scholar] [CrossRef]
- Culhuac, E.B.; Maggiolino, A.; Elghandour, M.M.M.Y.; De Palo, P.; Salem, A.Z.M. Antioxidant and Anti-Inflammatory Properties of Phytochemicals Found in the Yucca Genus. Antioxidants 2023, 12, 574. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S.J.C. Shared principles in NF-kappa B signaling. Cell 2008, 132, 344–622. [Google Scholar] [CrossRef]
- Yunhe, F.; Bo, L.; Xiaosheng, F.; Fengyang, L.; Dejie, L.; Zhicheng, L.; Depeng, L.; Yongguo, C.; Xichen, Z.; Naisheng, Z.; et al. The effect of magnolol on the toll-like receptor 4/nuclear factor kappa B signaling pathway in lipopolysaccharide-induced acute lung injury in mice. Eur. J. Pharmacol. 2012, 689, 255–261. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Ma, M.; Zhang, M.; Li, L.; Chen, W.; Li, S. NaAsO2 regulates TLR4/MyD88/NF-κB signaling pathway through DNMT1/SOCS1 to cause apoptosis and inflammation in hepatic BRL-3A cells. Biol. Trace Elem. Res. 2023, 202, 258–267. [Google Scholar] [CrossRef]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef]
- Rola, R.C.; Marins, L.F.; Nery, L.E.M.; da Rosa, C.E.; Sandrini, J.Z. Responses to ROS inducer agents in zebrafish cell line: Differences between copper and UV-B radiation. Fish Physiol. Biochem. 2014, 40, 1817–1825. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.M.; Qiu, Y.T.; Chi, C.F.; Luo, H.Y.; Wang, B. Gelatin From Cartilage of Siberian Sturgeon (Acipenser baerii): Preparation, Characterization, and Protective Function on Ultraviolet-A-Injured Human Skin Fibroblasts. Front. Mar. Sci 2022, 9, 925407. [Google Scholar] [CrossRef]
- Zhou, F.; Huang, X.; Pan, Y.; Cao, D.; Liu, C.; Liu, Y.; Chen, A. Resveratrol protects HaCaT cells from ultraviolet B-induced photoaging via upregulation of HSP27 and modulation of mitochondrial caspase-dependent apoptotic pathway. Biochem. Biophys. Res. Commun. 2018, 499, 662–668. [Google Scholar] [CrossRef]
- Souto, E.B.; Zielinska, A.; Souto, S.B.; Durazzo, A.; Lucarini, M.; Santini, A.; Silva, A.M.; Atanasov, A.G.; Marques, C.; Andrade, L.N.; et al. (+)-Limonene 1,2-Epoxide-Loaded SLNs: Evaluation of Drug Release, Antioxidant Activity, and Cytotoxicity in an HaCaT Cell Line. Int. J. Mol. Sci. 2020, 21, 1449. [Google Scholar] [CrossRef]
- Liu, T.; Xia, Q.; Lv, Y.; Wang, Z.; Zhu, S.; Qin, W.; Yang, Y.; Liu, T.; Wang, X.; Zhao, Z.; et al. ErZhiFormula prevents UV-induced skin photoaging by Nrf2/HO-1/NQO1 signaling: An in vitro and in vivo studies. J. Ethnopharmacol. 2023, 309, 115935. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, M.; Wang, X.; Yan, Y.; Chen, Y.; Wu, W.; Zhang, L.; Zhang, L. Antioxidative Effect of Quetiapine on Acute Ultraviolet-B-Induced Skin and HaCaT Cell Damage. Int. J. Mol. Sci. 2018, 19, 953. [Google Scholar] [CrossRef]
- Miao, H.; Zhang, Y.; Huang, Z.; Lu, B.; Ji, L. Lonicera japonica Attenuates Carbon Tetrachloride-Induced Liver Fibrosis in Mice: Molecular Mechanisms of Action. Am. J. Chin. Med. 2019, 47, 351–367. [Google Scholar] [CrossRef]
- Han, S.J.; Min, H.J.; Yoon, S.C.; Ko, E.A.; Park, S.J.; Yoon, J.H.; Shin, J.S.; Seo, K.Y. HMGB1 in the pathogenesis of ultraviolet-induced ocular surface inflammation. Cell Death Dis. 2015, 6, e1863. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, H.; Yin, J.; Shi, Y.; Tan, J.; Zheng, L.; Wang, C.; Li, X.; Xue, M.; Liu, J.; et al. TLR4 participates in sympathetic hyperactivity Post-MI in the PVN by regulating NF-κB pathway and ROS production. Redox Biol. 2019, 24, 101186. [Google Scholar] [CrossRef]
- Agnihotri, P.; Deka, H.; Chakraborty, D.; Monu, n.; Saquib, M.; Kumar, U.; Biswas, S. Anti-inflammatory potential of selective small compounds by targeting TNF-α & NF-κB signaling: A comprehensive molecular docking and simulation study. J. Biomol. Struct. Dyn. 2023, 41, 13815–13828. [Google Scholar] [PubMed]
- Suo, S.K.; Zheng, S.L.; Chi, C.F.; Luo, H.Y.; Wang, B. Novel angiotensin-converting enzyme inhibitory peptides from tuna byproducts—Milts: Preparation, characterization, molecular docking study, and antioxidant function on H2O2-damaged human umbilical vein endothelial cells. Front. Nutr. 2022, 9, 957778. [Google Scholar] [CrossRef] [PubMed]







| Metabolite | Mode | m/z | Retention Time (min) | Formula | Fragmentation Score | CAS ID |
|---|---|---|---|---|---|---|
| 3-O-Feruloylquinic acid | POS | 369.12 | 3.31 | C17H20O9 | 93.8 | 62929-69-5 |
| Chlorogenic acid | NEG | 353.09 | 3.76 | C16H18O9 | 70.8 | 327-97-9 |
| Quercetin | POS | 303.05 | 4.04 | C15H10O7 | 88.9 | 117-39-5 |
| Isoquercitrin | POS | 465.10 | 4.04 | C21H20O12 | 97.2 | 482-35-9 |
| Isorhamnetin | POS | 317.07 | 4.45 | C16H12O7 | 88.5 | 480-19-3 |
| Kaempferol-3-O-rutinoside | POS | 595.17 | 4.47 | C27H30O15 | 95.5 | 17297-56-2 |
| Kaempferol-3-O-glucoside | POS | 449.11 | 4.48 | C21H20O11 | 92.7 | 480-10-4 |
| Hyperoside | POS | 465.10 | 4.59 | C21H20O12 | 97.7 | 482-36-0 |
| Kaempferol | POS | 287.06 | 4.61 | C15H10O6 | 90.6 | 520-18-3 |
| Quercetin-3-glucoside | POS | 463.09 | 4.65 | C21H20O12 | 94.9 | 482-35-9 |
| Astragalin | POS | 449.11 | 5.18 | C21H20O11 | 91.5 | 480-10-4 |
| Ferulic Acid | NEG | 193.05 | 5.49 | C10H10O4 | 98.5 | 537-98-4 |
| Luteolin | NEG | 285.04 | 5.56 | C15H10O6 | 75.8 | 491-70-3 |
| Genistein | POS | 271.06 | 5.58 | C15H10O5 | 90.7 | 529-59-9 |
| Biochanin A | NEG | 283.06 | 5.68 | C16H12O5 | 94.3 | 491-80-5 |
| Binding Ligand | Amino Acid Residue That Interacts | Docking Score |
|---|---|---|
| Chlorogenic Acid | Hydrogen bonding: Gly603 | −8.6 kcal/mol |
| Quercetin | Hydrogen bonding: Gly462 Electrostatic interaction: Arg415 and Ala556 | −9.5 kcal/mol |
| Isorhamnetin | Hydrogen bonding: Gly462 Electrostatic interaction: Arg415, and Ala556 | −8.9 kcal/mol |
| Luteolin | Electrostatic interaction: Arg415, and Ala556 | −9.4 kcal/mol |
| Binding Ligand | Amino Acid Residue That Interacts | Docking Score |
|---|---|---|
| Chlorogenic Acid | Hydrogen Bonding: Arg237, Arg232, Glu233, and Arg239 Electrostatic interaction: Leu263 and Cys149 | −7.7 kcal/mol |
| Quercetin | Hydrogen bonding: Asn240, Glu179, Arg239, Ser176, and PRO147 Electrostatic interaction: Leu263 and His183 Hydrophobic force: Cys149 | −7.7 kcal/mol |
| Isorhamnetin | Hydrogen bonding: Cys149, Glu184l Arg239, Tyr227, and PRO147 Electrostatic interaction: Leu263, His183, and Phe163 | −7.7 kcal/mol |
| Luteolin | hydrogen bonding: Cys149, Glu179, Arg239, Ser176, and Asn240 Hydrophobic force: Leu263 and His183 | −7.7 kcal/mol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.-L.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Chemical Characterization of Honeysuckle Polyphenols and Their Alleviating Function on Ultraviolet B-Damaged HaCaT Cells by Modulating the Nrf2/NF-κB Signaling Pathways. Antioxidants 2024, 13, 294. https://doi.org/10.3390/antiox13030294
Zheng S-L, Wang Y-M, Chi C-F, Wang B. Chemical Characterization of Honeysuckle Polyphenols and Their Alleviating Function on Ultraviolet B-Damaged HaCaT Cells by Modulating the Nrf2/NF-κB Signaling Pathways. Antioxidants. 2024; 13(3):294. https://doi.org/10.3390/antiox13030294
Chicago/Turabian StyleZheng, Shuo-Lei, Yu-Mei Wang, Chang-Feng Chi, and Bin Wang. 2024. "Chemical Characterization of Honeysuckle Polyphenols and Their Alleviating Function on Ultraviolet B-Damaged HaCaT Cells by Modulating the Nrf2/NF-κB Signaling Pathways" Antioxidants 13, no. 3: 294. https://doi.org/10.3390/antiox13030294
APA StyleZheng, S.-L., Wang, Y.-M., Chi, C.-F., & Wang, B. (2024). Chemical Characterization of Honeysuckle Polyphenols and Their Alleviating Function on Ultraviolet B-Damaged HaCaT Cells by Modulating the Nrf2/NF-κB Signaling Pathways. Antioxidants, 13(3), 294. https://doi.org/10.3390/antiox13030294









