Beyond Mortality: Exploring the Influence of Plant Phenolics on Modulating Ferroptosis—A Systematic Review
Abstract
:1. Introduction
1.1. Apoptosis
1.2. Ferroptosis
1.2.1. Lipid Metabolism and Ferroptosis
1.2.2. Iron Metabolism and Ferroptosis
1.2.3. Role of Xct-/GPX4 Axis
1.2.4. GCH1/DHFR/BH4 Axis
1.2.5. FSP1/NAD(P)H/CoQ10 Axis
1.2.6. Keap1/Nrf2 Axis
1.2.7. Role of p53 in Ferroptosis
1.3. Plant Secondary Metabolites
2. Methodology
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
3. Results and Discussion
3.1. Studying Influence on Ferroptosis
3.1.1. Studies on Ferroptosis Inhibition
3.1.2. Studies on Ferroptosis Induction
3.2. Polyphenols as Potential Therapeutic Agents in Diverse Diseases via Ferroptosis Modulation
3.2.1. Ferroptosis as Potential Target for Cancer Treatment
3.2.2. Ferroptosis as Potential Target for Diabetes Treatment
3.2.3. Ferroptosis as Potential Target in Treatment of Neurodegenerative Diseases
3.2.4. Ferroptosis as Potential Target in Traumatic Brain Injury Treatment
3.2.5. Ischemia/Reperfusion Injury Treatment by Targeting Ferroptosis
3.2.6. Ferroptosis as Potential Target in Treatment of Liver Diseases
3.2.7. Acute Lung Injury Treatment through Ferroptosis Modulation
| Phenolics | Proposed Mechanism | Model System in Which Effect Was Examined | Reference | |
|---|---|---|---|---|
| In Vitro | In Vivo | |||
| Agrimonolide | Increases ROS and Fe2+ levels and downregulates GPX4 and SLC7A11 | A2780 and SKOV-3 cells | SKOV-3 cells xenograft model in BALB/c mice | [136] |
| Alloimperatorin | Accumulation of Fe, ROS, and MDA, decreased expression of SLC7A11 and GPX4 with increased expression of Keap1 | Breast cancer cells | / | [137] |
| Amentoflavone | Induces ferroptosis through miR-496/ATF2 axis | GSE-1, AGS and HGC-27 cells | AGS cells xenograft model in BALB/c nude mice | [62] |
| Promotes ferroptosis by activation of ROS/AMPK/mTOR pathway | ESC and KLE cell lines | / | [63] | |
| Increased levels of iron, lipid ROS, and MDA, depletion of GSH | U251 and U373 cells | U251 cells xenograft model in BALB/c nude mice | [64] | |
| Apigenin | Causes iron accumulation, lipid peroxidation, GSH depletion, downregulates expression of SLC7A11 and GPX4, upregulates expression of p62, HO-1, and ferritin | Human Ishikawa cells | Ishikawa cells xenograft model in BALB/c nude mice | [52] |
| Nanocomposites Fe2O3/Fe3O4@mSiO2 loaded with apigenin induce ferroptosis by increasing ROS production and downregulation of expression of GPX4, FTH1 and upregulation of expression of COX2 and p53 | A549 and HUVECs | / | [138] | |
| Auriculasin | Increases levels of Fe2+ and MDA, and induces generation of ROS, promotion of expression of Keap1 and AIFM | HCT116 and SW480 cells | / | [69] |
| Baicalin | Promotes Fe2+ accumulation, ROS production, lipid peroxidation, depletes GSH, decreases expression of GPX4 and Xct- through upregulated degradation of Nrf2 | MG63, 143B, hBMSC | MG63 xenograft model in BALB/c nude mice | [54] |
| Promotes ROS production, enhances mRNA expression of TfR1, NOX1 and COX2, suppresses mRNA expression of GPX4, FTH1, and FTL | AGS and SGC-7901 cells | / | [55] | |
| Induces ferroptosis through downregulation of FTH1 | Bladder cancer cells 5637 and KU-19-19 cells | KU-19-19 cells xenograft model in mice | [65] | |
| Bavachin | Increases Fe2+ through increased DMT1 and TfR and decreased FTH and FTL expression, depletes GSH and enhances ROS production and MDA, modulates STAT3/p53/SLC7A11 axis | MG63 and HOS cell lines | / | [139] |
| Bilobetin | Promotes iron accumulation, ROS production and upregulates p53 expression | HCT116, HT29, RKO and LOVO cells | HCT116 xenograft model in BALB/c mice | [140] |
| Chrysin | Induces ferritinophagy-dependent ferroptosis | PANC-1, Capan-2, BxPC-3, AsPC-1 and HPDE6-C7 cells | PANC-1 xenograft model in BALB/c nude mice | [141] |
| Chrysophanol | Accumulation of ROS, downregulation of GPX4 and SLC7A11 | HSC-T6 cell line | / | [128] |
| Curcumin | Induces ferroptosis through GSH/GPX4 and FSP1/CoQ10-NADH pathways | A549 cells | A549 cells xenograft model in NOD/SCID mice | [10] |
| Promotes iron accumulation and lipid peroxidation, upregulates expression of HO-1, and downregulates expression of GPX4 | Nthy-ori-3-1, FTC-133 and FTC-238 cells | / | [58] | |
| Induces ROS production and downregulates expression of GPX4 and FSP1 | SW480 and HCT116 cells | / | [56] | |
| Induces ferroptosis through upregulation of SLC1A5 | MDA-MB-453 and MCF-7 | MCF-7 cells xenograft model in BALB/c nude mice | [61] | |
| Promotes iron accumulation and lipid peroxidation, downregulates GSH, GPX4, and SLC7A11, activates PI3K/Akt/mTOR pathway | HCT-8 cells | / | [59] | |
| Depletion of GSH, accumulation of iron and ROS, lipid peroxidation | A549 and H1299 cells | Lewis lung carcinoma model in C57BL/6 mice | [51] | |
| Induces ferroptosis through ADAMTS18 | Sunitinib-resistant A498 and 786-O cells | / | [60] | |
| Accumulation of intracellular iron, ROS, LOOH, MDA, and upregulation of HO-1 | MCF-7 cells | / | [142] | |
| Danshensu | Promotes ROS production and downregulates expression of GPX4 and SLC7A11 | LX-2 and T6 cells | / | [129] |
| 3,5-dicaffeoylquinic acid | Induces ferroptosis and mitochondrial dysfunction through ROS/AMPK/mTOR pathway | HCT116 and SW480 cells | / | [143] |
| 4,4′-dimethoxychalcone | Promotes iron accumulation, lipid peroxidation, and activates Keap1/Nrf2/HO-1 pathway | A549 cells and 786-O cells | / | [144] |
| Diplacone | Promotes lipid peroxidation ATF3 expression | A549, NK-92 and K562 cells | / | [145] |
| Emodin | Downregulates Notch1/Nrf2/GPX4 pathway | NRK-52E cells | Emodin high-dose induced nephrotoxicity in mice | [146] |
| Erianin | Ferrostatin-1 inhibits cytotoxicity of erianin suggesting activation of ferroptosis | A549 and H1299 cells | A549 cells xenograft model in nude mice | [66] |
| Promotes accumulation of iron and ROS production, induces lipid peroxidation and downregulates expression of GPX4, FTH1, and ferritin | LoVo, HCT116, DLD1, HCT8, SW480, SW620, HT29, Caco2, MC38 cells | HCT116-luc cells subcutaneous and liver metastasis models in BALB/c nude mice | [37] | |
| Promotes iron accumulation and lipid peroxidation and downregulates expression of GPX4, upregulates expression of ALOX12, p53, and METTL3 | CD44+/CD105+ HuRCSCs | Xenograft model in BALB/Cnu/nu mice | [57] | |
| ROS accumulation, GSH depletion, lipid peroxidation, and downregulation of FTH1, GPX4, HO-1, Xct-/SLC7A11, and Nrf2 | RT4 and KU-19-19 cells | KU-19-19 cells xenograft model in BALB/c nude mice | [41] | |
| Induces ferroptosis through activation of Ca2+/CaM signaling pathway | H460 and H1299 cells | H460 cells xenograft model in BALB/c nude mice | [67] | |
| Eriocitrin | Downregulates Nrf2/SLC7A11/GPX4 axis | A549 and H1299 cells | / | [147] |
| Eriodictyol | Downregulates expression of SLC7A11 and GPX4, downregulates Nrf2/HO-1/NQO1 pathway | A2780 and CaoV3 cells | CaoV3 cell xenograft model in BALB/c mice | [148] |
| Esculetin | Promotes iron accumulation and lipid peroxidation through NCOA4 mediated ferritinophagy | HUH7 and HCCLM3 cells | HUH7 cells xenograft model in BALB/c mice | [149] |
| Ferulic acid | Upregulates ACSL4 and HO-1 and downregulates SLC7A11 and GPX4 | TE-4 and EC-1 cells | / | [150] |
| Gallic acid | Downregulates expression of GPX4 and SLC7A11 by inhibiting Wnt/β-catenin pathway | HepG2 cells | / | [151] |
| Potential ferroptosis inducer, and increased ROS and MDA production, reduced activity of GPX. | MDA-MB-231, MCF10A, and A375 cells | / | [152] | |
| Cell death triggered by gallic acid can be prevented by ferroptosis inhibitor DFO | HeLa, SH-SY5Y, and H446 cells | / | [153] | |
| Gambogenic acid | Activates p53/SLC7A11/GPX4 axis | HOS and 143B cells | 143B cells xenograft model in athymic nude mice | [154] |
| Activates AMPKα/SLC7A11/GPX4 pathway | FHC, HCT116, HT29, DLD-1, HCT115 and COLO320DM cells | HCT116 cells xenograft model in BALB/c mice | [40] | |
| Downregulates NEAT1 and induces ferroptosis through SLC7A11/GPX4 axis | A375, B16, B16F10 and A2058 cells | B16F10 xenograft model in C57BL/6 mice | [68] | |
| Induces ferroptosis through p53/SLC7A11/GPX4 axis | A375 and A2058 cells | / | [155] | |
| 6-gingerol | Enhanced production of ROS, depletion of GSH, downregulated expression of Nrf2 and GPX4 | LNCaP, DU145, and PC3 cells | / | [156] |
| Upregulates expression of NCOA4 and TfR1, and downregulates expression of GPX4 and FTH1 | A549 cells | A549 cells xenograft model in BALB/c mice | [157] | |
| Ginkgetin | Promotes iron accumulation, ROS production, and upregulates p53 expression | HCT116, HT29, RKO and LOVO cells | HCT116 xenograft model in BALB/c mice | [140] |
| Enhances cytotoxicity of DDP through induction of ferroptosis, increases levels of free iron, production of ROS, and lipid peroxidation, lowers GSH/GSSG ratio, reduces protein levels of SLC7A11 and GPX4, inhibits Nrf2/HO-1 axis | A549, NCI-H460, SPC-A-1 cells | NSCLC xenograft model in nude mouse | [158] | |
| Honokiol | Promotes lipid peroxidation and upregulates expression of HO-1 | THP-1, U-937, and SMK-1 cells | / | [159] |
| Increases levels of iron and ROS, and downregulates expression of GPX4 | SW48, HT29, LS174T, HCT116, HCT8, RKO, and SW480 cells | RKO cells xenograft model in BALB/c mice | [160] | |
| Icariin | Induces autophagy and ferroptosis through modulation of miR-7/mTOR/SREBP1 pathway | RWPE-1, DU145, and PC-3 cells | / | [161] |
| Icariside II | Upregulates miR-324-3p which downregulates GPX4 | ACHN, A498, 786-O, Caki-1, and 293T cells | Caki-1 cells xenograft model in BALB/c mice | [162] |
| Isoginkgetin | Promotes iron accumulation, ROS production, and upregulates p53 expression | HCT116, HT29, RKO, and LOVO cells | HCT116 xenograft model in BALB/c mice | [140] |
| Isoliquiritigenin | Upregulates expression of HO-1, and downregulates expression of GPX4 | SGC996, NOZ, and L-2F7 cells | NOZ cells xenograft model in BALB/c mice | [163] |
| Downregulates GPX4, and upregulates TfR and DMT1 expression | HSC-T6 cells | CCl4-induced liver-fibrosis model in C57BL/6 mice and 0.06% TA-induced liver fibrosis model in zebrafish | [9] | |
| Isoliquiritin | Promotes iron accumulation and downregulates expression of GPX4 and SLC7A11 | MDA-MB-231 and MCF-7 cells | BALB/c mice | [164] |
| Isoorientin | Inhibits SIRT6/Nrf2/GPX4 signaling pathway | A549 cells | A549 cells xenograft model in BALB/c mice | [165] |
| Isoquercitrin | Downregulates expression of GPX4, HO-1 and SLC7A11 | CNE1 and HNE1 cells | CNE1 cells xenograft model in BALB/c mice | [166] |
| Luteolin | Promotes expression of ACSL4, and downregulates expression of GPX4, SLC7A11, FTH1, and FTL1 | RWPE-1, DU145, PC-3, and LNcaP cells | PC-3 cells xenograft model in BALB/c mice | [167] |
| Acts synergistically with erastin, and upregulates expression of HIC1 which results in downregulated expression of GPX4 | HCT116, SW480, and NCM460 cells | HCT116 cells xenograft model in BALB/c mice | [168] | |
| Lysionotin | Promotes degradation of Nrf2 | HCT116, SW480, HIEC, and NCM460 cells | HCT116 cells xenograft model in athymic nude mice | [169] |
| Nobiletin | Increases levels of iron, MDA, and ROS, decreases levels of GSH and GPX4, enhances the expression of Keap1, and inhibits the expression of Nrf2 and HO-1 | SK-MEL-28 | / | [170] |
| Osthole | Downregulates expression of GPX4 and SLC7A11, and upregulates expression of HO-1 and NCOA4 | HCT116, SW480, and MC38 cells | HCT116 cells xenograft model in C57BL/6J mice | [76] |
| Puerarin | Promotes autophagy-dependent ferroptosis through upregulation of NCOA4 | HT-29 cells | / | [171] |
| Plumbagin | Promotes ROS production and GPX4 degradation | HepG2 and Hep3B cells | / | [172] |
| Quercetin | Downregulates expression of GPX4, and upregulates expression of SLC7A11 and TfR1 | HEC-1-A cells | / | [173] |
| Triggers the release of iron through degradation of ferritin, enhances the production of ROS and lipid peroxidation, increases expression of SLC27A4 and decreases expression of GPX1 | HepG2, Hep3B, MDA-MB-231, HCT116 cell lines | / | [71] | |
| Resveratrol | Promotes ferroptosis through Hsa-miR-335-5p/NFS1/GPX4 pathway | AML-193 and OCI-AML-3 cells | / | [174] |
| Upregulates expression of ACSL4, and downregulates expression of GPX4 and SLC7A11 | Human plasma and lung tissues, PBMCs and H520 cells | / | [175] | |
| Rhamnazin | Downregulates expression of GPX4 | HCC Huh7 and SMMC-7721 cell | / | [176] |
| Robustaflavone A | Accumulation of ROS, downregulation of Nedd4, and upregulation of VDAC2 | MCF-7 cells | / | [177] |
| Rotenone | Promotes iron accumulation, promotes expression of ACSL4, COX2, GPX4 and SLC7A11 | Primary neuron culture | Intracerebral hemorrhage model in ICR mice | [178] |
| Upregulates expression of Keap1, p53, and COX2, and downregulates expression of GPX4, SLC7A11, and FTH1 | NE-4C cells | / | [179] | |
| Inhibits Keap1/Nrf2/SLC7A11/GPX4 axis | BV-2 cells | / | [180] | |
| S-3′-hydroxy-7′, 2′, 4′-trimethoxyisoxane | Inhibits Nrf2/HO-1 signaling pathway | SGC-7901, A549, H460, SW480, BEL-7402, HeLa, HBE, and MCF-7 cells | A549 cells xenograft model in nude mice | [181] |
| Scoparone | Activaties the ROS/JNK/SP1/ACSL4 axis | NHBE, A549, H1299, and PC-9 cells | / | [75] |
| Shikonin | Inhibits Nrf2 signaling pathway | HTR-8/SVneo cells and villous tissue of women suffering from TP and from women with normal pregnancies | / | [182] |
| Downregulates expression of GPX4, and upregulates expression of TfR1, NCOA4, HO-1, and shows synergism with cisplatin | A2780, SKOV3, OVCAR4, A2780/DDP, SKOV3/DDP, and OVCAR4/DDP cells | A2780/DPP cells xenograft model in BALB/c nude mice | [73] | |
| Downregulates expression of GPX4 and promotes expression of ATF3 | SBC-2 and H69 cells | SBC-2 cells xenograft model in nude mice | [74] | |
| Induces GOT1-dependent ferritinophagy | RPMI 8226 and U266 cells | RPMI 8226 cells xenograft model in Nod-SCID mice | [183] | |
| Shikonin-Fe(III) nanoparticles induce iron accumulation, GSH depletion, production of ROS, increase lipid peroxidation and downregulate GPX4 expression | 4T1 and L929 cells | 4T1 xenograft model in BALB/c mice | [184] | |
| Theaflavin-3,3′-Digallate | Induces iron accumulation and lipid peroxidation, downregulates expression of GPX4 and FTH1 | HOS, MG63, and hFOB1.19 cells | HOS cells xenograft model in BALB/c mice | [185] |
| 2,3,5,4′-tetrahydroxystilbene | Upregulates expression of ACSL4 and downregulates expression of GPX4 | DLD-1, HT-29, HCT-116 | HT-29 xenograft model in severe combined immunodeficient (SCID) mice | [53] |
| Tiliroside | Downregulates expression of GPX4 and SLC7A11 and upregulates expression of HO-1 | BT-549, MDA-MB-468, SK-BR-3, MCF-7, and MCF-10A cells | BT-549 cells xenograft model in BALB/c mice | [186] |
| Promotes ubiquitination of Nrf2, and downregulates expression of GPX4 and FTH1 | HepG2, Hep3B SMMC-7721, and L02 cells | HepG2 xenograft model in BALB/c mice | [187] | |
| Typhaneoside | Increases ROS production, and lipid peroxidation and reduces GSH and GPX. Promotes autophagy and autophagic degradation of ferritin | Kas-1, HL60, NB4, K562 cells | HL60 cells xenograft model in BALB/c mice | [38] |
| Wogonin | Inhibits Nrf2/GPX4 pathway | AsPC-1, PANC-1, and HPDE6-C7 cells | PANC-1 cells xenograft model in BALB/c mice | [70] |
| Wogonoside | Modulates SOCS1/p53/SLC7A11 pathway to induce ferroptosis | HSC-t6, AML12, and RAW 264.7 cells | CCl4-induced liver fibrosis model in C57BL/6 mice | [127] |
| Phenolics | Proposed Mechanism | Model System in Which Effect Was Examined | Reference | |
|---|---|---|---|---|
| In Vitro | In Vivo | |||
| Acacetin | Upregulates expression of GPX4 and downregulates expression of ACSL4 | HepG2 cells | High-fat-diet-induced lipid accumulation in liver in C57BL/6 mice | [188] |
| Acetyl zingerone | Activates Nrf2/HO-1 signaling pathway | Primary chondrocytes | Knee osteoarthritis model in C57BL/6J mice | [189] |
| Aloe-emodin | Complexes Fe2+, modulates intracellular iron metabolism, increases expression of Nrf2, GPX4, and SLC7A11 | H9c2 cells | / | [190] |
| Anacardic acid | Downregulates expression of TfR1, and upregulates expression of GPX4 | / | Freeney free-fall impact model-induced traumatic brain injury in ICR mice | [102] |
| Anhydroxysafflor yellow B | Reduces ROS, 4-HNE, and MDA production, reduces Fe2+ levels, increases GSH/GSSG ratio, and upregulates expression of GPX4 and SLC7A11 | PC12 cells | / | [191] |
| Apigenin | Decreases production of ROS and MDA, upregulates activity of SOD, and improves GSH/GSSG ratio, lowers level of Fe2+ | AML12 cells | / | [192] |
| Apigenin-7-O-(-6″-p-coumaroyl)-glucoside | Reduces the level of ROS and Fe2+, inhibits HO-1 and MAO-B | Human umbilical vein endothelial cell line (HUVECs) | Model of intestinal ischemia/reperfusion injury in CL57BL/6J mice | [107] |
| Astringin | Antioxidant activity | bmMSCs | / | [193] |
| Avicularin | Activates Nrf2/HO-1/GPX4 pathway | RAW 246.7 and HepG2 cells | LPS/D-GalN-induced acute liver failure model in C57BL/6 mice | [194] |
| Baicalein | Decreases iron accumulation and lipid peroxidation, upregulates expression of GPX4 and SLC7A11, downregulates expression of ALOX15 | IEC-6 cells | CPT-11-induced gastrointestinal dysfunction in Wistar rats | [195] |
| Decreases levels of ROS and ALOX15 | / | Cardiac arrest model in SD rats | [196] | |
| Inhibits ferroptosis through a decrease in p53 acetylation level by elevating SIRT1 | HK2 cells | Polymyxin B-induced acute kidney injury model in C57BL/6 mice | [197] | |
| Alleviates ferroptosis through AMPK/Nrf2/HO-1 axis | Primary human and mouse chondrocyte | Surgery-induced osteoarthritis model in C57BL/6J mice | [198] | |
| Decreases levels of ROS, MDA, and iron, and restores protein levels of GPX4 | H9c2 cells and primary cardiomyocytes from neonatal SD rats | Langerdorff perfusion system for ischemia/reperfusion model on isolated hearts from SD rats | [119] | |
| Modulates GPX4/ACSL4/ACSL3 axis | HT22 cells | tMCAO-induced cerebral ischemia/reperfusion injury model in C57BL/6 mice | [118] | |
| Decreases iron and MDA levels, and suppresses expression of HMOX1 and FPN1, and increases expression of GPX4 and SLC7A11 | THP1 cells and human macrophages isolated from endometrium peritoneal fluid | / | [199] | |
| Inhibits NF-κB pathway, and activates Nrf2/HO-1 pathway | HepG2 cells | CCl4-induced acute liver injury in C57BL/6 mice | [121] | |
| Upregulates GPX4, and downregulates TFR1. | HEM-1 cells | / | [200] | |
| Suppression of lipid peroxidation, lover levels of 4-HNE, upregulation of GPX4, and downregulation of LOX12/15 | HT22 cells | FeCl3-induced post-traumatic epilepsy disorder in C57/BL6 mice | [103] | |
| Baicalin | Enhanced expression of GPX4 and SLC7A11, decreased expression of DMT1 | PC12 cells and primary cortical neurons | Type IV collagenase-induced intracerebral hemorrhage model in C57BL/6 mice | [46] |
| Reduces ROS production, and promotes SOD activity and expression of GPX4, lowers iron levels through modulation of iron uptake, storage, and ferritinophagy | H9C2 cells | Myocardial ischemia/reperfusion injury model in male Sprague-Dawley rats | [201] | |
| Decreased levels of Fe2+, MDA and ROS, increased level of GSH and upregulation of GPX4 | Primary rat neurons | Subarachnoid hemorrhage model in Sprague-Dawley rats | [104] | |
| Biochanin A | Downregulates TfR1, upregulates FPN1, and activates Nrf2/system xc-/GPX4 pathway | Primary chondrocytes isolated from C57BL/6 mice | Iron-dextran-induced iron overload and surgery-induced knee osteoarthritis in C57BL/6 mice | [202] |
| Caffeic acid | Downregulates expression of ACSL4 and TfR1 and activates Nrf2 pathway | SK-N-SH cells | pMCAO-induced cerebral ischemia/reperfusion injury | [117] |
| Cannabinol | Inhibits ROS production and lipid peroxidation, increases expression of Nrf2, HO-1, SOD2, GPX4 | HT22, SH-SY5Y, BV2 cells | / | [203] |
| Calycosin | Downregulates expression of ACSL4 and TfR1, and upregulates expression of FTH1 and GPX4 | PC12 cells | tMCAO/R model in SD rats | [204] |
| Upregulates expression of GPX4, and downregulates expression of NCOA4 | HK-2 cells | Db/db mice and db/m mice | [205] | |
| Cardamonin | Increases expression of SLC7A11, GPX4, p53, and decreases expression of iNOS and COX2 | Primary chondrocytes isolated from SD rats | Osteoarthritis model in SD rats | [48] |
| Carthamin yellow | Downregulates expression of ACSL4 and TfR1, and upregulates expression of GPX4 and FTH1 | / | MCAO-induced cerebral ischemia/reperfusion model in SD rats | [206] |
| Chebulagic acid | Inhibits ferroptosis through ROS scavenging and iron chelation | Bone marrow-derived mesenchymal stem cells isolated from SD rats | / | [207] |
| Chebulinic acid | Inhibits ferroptosis through ROS scavenging and iron chelation | Bone marrow-derived mesenchymal stem cells isolated from SD rats | / | [207] |
| Chicoric acid | Activates Nrf2/HO-1 signaling pathway | / | LPS-induced endometriosis in C57BL/6 mice | [208] |
| Possible ferroptosis inhibitor thanks to ability to protect against oxidative damage, reduces ROS and MDA production, increases levels of GSH and SOD, and promotes expression of Nrf2 and HO-1 | / | Acute lung injury model in male BALB/c mice induced by LPS | [133] | |
| Chlorogenic acid | Upregulates expression of GPX4, SLC7A11, and SLC3A2 | Primary cortical neurons isolated from mice | Hypoxic-ischemic brain injury model in neonatal C57BL/6J mice | [209] |
| Decreases Fe 2+ and MDA accumulation and increased GSH, GPX, GST, and CAT | / | Triptolide-induced multi-organ injury in Kunming mice | [210] | |
| Upregulates GPX4, GSH, and NADPH; the proposed mechanism is the activation of the IL6/JAK2/STAT3 pathway which leads to reduced production of hepcidin and enhanced expression of FPN1 in the duodenum | / | Chronic stress-induced duodenal ferroptosis model in Wistar rats | [211] | |
| Chrysin | Upregulates expression of SLC7A11 and GPX4, and downregulates expression of ACSL4, TfR1 and COX2 | / | tMCAO-induced cerebral ischemia/reperfusion model in SD rats | [212] |
| Chrysophanol | Inhibits ferroptosis through upregulated expression of GPX4 | PC12 cells | Alzheimer’s disease model in SD rats | [84] |
| Corilagin | Downregulates expression of ACSL4, upregulates expression of GPX4, and inhibits NCOA4-mediated ferritinophagy | / | Intestinal ischemia/reperfusion injury model in C57BL/6J mice | [213] |
| Curculigoside | Ferroptosis was characterized by iron accumulation, depletion of GSH, ROS, and MDA production with decreased expression of SOD and GPX4, which were attenuated by curculigoside | IEC-6 cells | Model of ulcerative colitis in C57BL/6J mice | [214] |
| Curcumin | Curcumin-OM-MSCs upregulates expression of GPX4, SLC7A11 and FTH1, and downregulates expression ACSL4 | Primary cortical neurons isolated from SD rats | Collagenase IV-induced intracerebral hemorrhage model in SD rats | [50] |
| Downregulates ACSL4 and upregulates GPX4 | / | Ischemia/reperfusion injury model in Wistar rats | [215] | |
| Inhibits ferritinophagy through the NCOA4 pathway and activates the Nrf2 pathway | / | AFB1-induced kidney nephrotoxicity in ducks | [45] | |
| Promotes expression of Nrf2, GPX4, and HO-1 | BRL-3A cells | Hepatocellular degeneration model in TX mice | [216] | |
| Increases expression of SLC7A11 and GPX4 and reduces expression of ACSL4 and TfR1 | / | Ligature wire-induced periodontitis model in C57/BL mice | [217] | |
| Upregulates expression of Nrf2, GPX4, SLC7A11, HO-1, NQO-1, CAT, SOD, FPN1, FTH1, downregulates expression of Keap1, NCOA4, ACSL4, PTG2, TfR1, p53 | / | NH4Cl-inuced ammonia stress in Gibel carp | [218] | |
| Increases expression of SLC7A11, GPX4 and FTH1 through activation of Nrf2 pathway | Primary chondrocytes isolated from BALB/C mice | Erastin-induced knee ferroptosis in BALB/C mice | [219] | |
| Can inhibit multiple cell death mechanisms induced by antimalarial drug mefloquine, including ferroptosis by inhibition of lipid peroxidation | / | Swiss-strain mice infected with Plasmodium berghei | [220] | |
| Enhances nuclear translocation of Nrf2 and enhances expression of GPX4 and HO-1 | H9c2 cells | Streptozotocin-induced diabetes model in New Zealand rabbits | [82] | |
| Mitigated production of ROS, depletion of GSH, increased levels of iron and MDA, downregulation of SLC7A11, GPX, FTH1, and upregulation of TfR induced by cigarette smoke | BEAS-2B cells | Lung injury model induced by cigarette smoke in Sprague-Dawley rats | [221] | |
| Inhibition of generation of ROS and upregulation of Nrf2/HO-1 pathway | HT22 cells | Intracerebral hemorrhage model in C57BL/6 mice | [222] | |
| Upregulation of expression of HO-1 | Proximal murine tubular epithelial cells (MCTs) | Rhabdomyolysis in C57BL/6 mice | [223] | |
| Iron chelation, prevention of GSH depletion, lipid peroxidation, and GPX4 inactivation | MIN6 cells | / | [224] | |
| Potential ferroptosis inhibitor, reduces lipid peroxidation and preserves the activity of CAT, SOD, and GPX | Primary cardiomyocytes from neonatal Sprague-Dawley rats | Doxorubicin-induced cardiotoxicity in Kun-Ming mice | [225] | |
| Cyanidine-3-glucoside | Decreases levels of Fe2+, 4-HNE, MDA, decreases expression of ACSL4, and increases expression of GPX4. AMPK pathway is essential for anti-ferroptosis activity | HK-2 and NRK-52E cells | Renal ischemia/reperfusion injury model in C57BL/6 mice | [226] |
| Downregulation of NCOA4 and TfR, and upregulation of GPX4 and FTH1 | H9c2 cells | Ischemia/reperfusion injury model in Sprague-Dawley rats | [7] | |
| Cynarin | Alleviates iron accumulation and inhibits ROS production and lipid peroxidation, and upregulates expression of GPX4 and Nrf2 | Primary rat nucleus pulpous cells | Caudal intervertebral disc puncture model in SD rats | [227] |
| Daidzein | Activates Nrf2/SLC7A11/GPX4 pathway | Primary hepatocytes | APAP-induced hepatotoxicity in C57BL/6 mice | [228] |
| Dihydromyricetin | Inhibits ferroptosis through LCN2/Xct- axis | / | Collagenase-induced intracerebral hemorrhage model in C57BL/6 mice | [229] |
| Alleviates iron accumulation, inhibits ROS production, decreases expression of ACSL4, increases expression of GPX4, and inhibits p-JNK inflammatory cytokine pathway | / | High-fat-diet- and streptozotocin-induced diabetes in SD rats | [83] | |
| Activates Nrf2 pathway, and upregulates expression of SOD, CAT, GCLC, GCLM, GPX4 | HK-2 cells | Cisplatin-induced acute kidney injury in C57BL/6 mice | [230] | |
| Inhibits lipid peroxidation, upregulates expression of GPX4, and downregulates ACSL4 | HT22 cells | MCAO-induced cerebral ischemia/reperfusion injury model in SD rats | [231] | |
| Possible ferroptosis inhibitor, reduces the production of MDA, increases levels of GSH and SOD, and activates Nrf2/HO-1 pathway | HT-22 cells | / | [232] | |
| Dihydroquercetin | Lowers levels of MDA and ROS, increases RNA and protein levels of SLC7A11 and GPX4, and increases levels of Nrf2 in a dose-dependent manner | HBE cells | Cigarette smoke-induced chronic obstructive pulmonary disease in BALB/c mice | [233] |
| Upregulates expression of GPX4, FTH1 and NCOA4, downregulates LC3, and inhibits ferritinophagy | HBE and MRC-5 cells | Silica-induced lung fibrosis in C57BL/6 mice | [234] | |
| 3,4-dihydroxyphenyletyl alcohol glycoside | Decreases levels of ROS and MDA, increases the level of GSH, restores the activity of CAT and SOD, promotes expression of GPX4, and inhibits expression of HO-1, ERK, NLRP3, Caspase1, and Gasdermine-D | AML12 cells | APAP-induced acute liver failure model in C57BL/6 mice | [235] |
| Diosmetin | Activates SIRT1/Nrf2 pathway | / | S. aureus-induced mastitis in BALB/c mice | [236] |
| Echinatin | Increases expression of GPX4, SLC7A11, SLC3A2, FTH1, FTL, GCLC ang GCMC, activates Nrf2 pathway | Primary human umbilical artery smooth muscle cells | Vascular stiffening, 5/6 nephrectomy, and atherosclerotic mouse models in C57BL/6 mice | [237] |
| Activates Nrf2 pathway | Primary rat hippocampal neurons | Sevoflurane-induced neurotoxicity in SD rats | [90] | |
| Eleutheroside B | Upregulates expression of GPX4, SLC7A11, FTH1, HO-1, and downregulates expression of TfR1 and COX2, activates Nrf2 pathway | / | SD rats exposed to hypobaric hypoxia | [238] |
| Emodin | Activates Nrf2/SLC7A11/GPX4 axis | HK-2 cells | Streptozotocin-induced diabetes in SD rats | [239] |
| Engeletin | Activates Keap1/Nrf2 pathway | Primary BMSCs from SD rats | / | [240] |
| (-)-epigalocatechin-3-gallate | Upregulates expression of GPX4, and downregulates expression of ACSL4 and COX2 | L-02 cells | High-fat-diet-induced hepatic lipotoxicity in C57BL/6 mice | [126] |
| Acts on STAT1/SLC7A11 pathway | A549 and RAW 264.7 cells | Urethane-induced lung cancer in C57BL/6 mice | [241] | |
| Upregulates Nrf2/GPX4 axis | Primary mouse hepatocytes | High-iron-diet-induced iron overload in C57BL/6 mice | [242] | |
| Acts on miR-450b-5p/ACSL4 axis | HL-1 cells | Acute myocardial infarction model in C57BL/6 mice | [243] | |
| Activates Nrf2/HO-1 pathway | NRK-52E, HK-2, mTEC and AML-12 cells | Gentamicin-induced nephrotoxicity in SD rats | [39] | |
| Reduces iron accumulation, oxidative stress, and abnormal lipid metabolism | H9c2 cells and neonatal rat cardiomyocytes | Doxorubicin-induced cardiotoxicity in C57BL/6 mice | [244] | |
| Upregulates expression of GPX4 and FTH1, downregulates expression of ACSL4 and COX2 | Primary cerebellar granule | Spinal cord injury model in rats | [245] | |
| Reduces production of ROS, and increases expression of GPX4 and SLC7A11, these effects are Nrf2 dependent, and Nrf2 inhibitors abolish EGCG protective effect against ferroptosis | HIEC line | Radiation-induced intestinal injury model in C57BL/6J mice | [246] | |
| Iron chelation, prevention of GSH depletion, lipid peroxidation, and GPX4 inactivation | MIN6 cells | / | [224] | |
| Eriodictyol | Inhibits ferroptosis through VDR-mediated activation of the Nrf2/HO-1 pathway | HT-22 hippocampal cells | Alzheimer’s disease model in APPswe/PS1E9 transgenic mice | [89] |
| Possible ferroptosis inhibitor, reduces the production of ROS and MDA, restores activity of SOD and CAT, increases levels of GSH, activates Nrf2/HO-1 pathway | BV-2 cells | LPS triggered oxidative stress in C57BL/6 mice | [88] | |
| Possible inhibitor of ferroptosis, upregulates SOD, CAT, and GPX, and activates Nrf2/HO-1 axis | RGC-5 cells | / | [247] | |
| Farrerol | Upregulates expression of Nrf2, GPX4, SLC7A11 and HO-1 | / | Hypoxic-ischemic encephalopathy in SD rats | [248] |
| Balances iron metabolism and promotes expression of GPX4 and SLC7A11 | Primary tenocytes isolated from rats | Collagenase-induced tendinopathy in SD rats | [249] | |
| Ferulic acid | Activates Nrf2/GPX4 pathway | HT-22 cells | High-fat-diet-induced cognitive impairment in C57BL/6 mice | [84] |
| Activates Nrf2/HO-1 pathway | MLE-12 cells | Acute lung injury model in BALB/c mice | [134] | |
| Activates Nrf2 signaling pathway | MIN6 cells | / | [250] | |
| Upregulates expression of GPX4 and AMPKα2 | / | Myocardial ischemia/reperfusion injury model in SD rats | [251] | |
| Fisetin | Downregulates expression of ACSL4 and COX2, and upregulates expression of GPX4 | TCMK-1 cells | Chronic kidney disease model in mice | [252] |
| Reduces levels of ROS and MDA, and increases the level of GSH, upregulates SIRT1, Nrf2, GPX4, HO-1, and FTH1 | H9c2 cells | Doxorubicin-induced cardiomyopathy in Waster rats | [253] | |
| Formononetin | Enhances expression of SLC7A11 and GPX4, promotes nuclear translocation of Nrf2, and blocks nuclear translocation of Smad3 and ATF3 | Primary mouse renal tubular epithelial cells | Chronic kidney disease model in C57BL/6 mice | [254] |
| Fraxetin | Promotes expression of GPX4 and SLC7A11, and inhibits expression of NCOA4 | MLE-12 cells | Bleomycin-induced pulmonary fibrosis in C57BL/6 mice | [255] |
| Activates AKT/Nrf2/HO-1 pathway | H9c2 cells | Myocardial infarction model in Wistar rats | [256] | |
| Galangin | Upregulates expression of GPX4, FTH1, and SLC7A11 through activation of Nrf2 pathway | Primary rat cardio-myocytes | Myocardial ischemia/reperfusion injury model in C57BL/6 mice | [108] |
| Activates PI3K/AKT/CREB pathway | HT1080 cells | Ischemia/reperfusion injury model in C57BL/6 mice | [109] | |
| Upregulates expression of SLC7A11 and GPX4 | Hippocampal neurons culture | Cerebral ischemia-reperfusion injury model in gerbils | [44] | |
| Gallic acid | Inhibits ferroptosis through modulation of the P2X7-ROS signaling pathway | Primary microglial culture isolated from neonatal SD rats | Chronic comorbid injury and chronic unpredictable mild stimulation model in Sprague-Dawley rats | [257] |
| Gastrodin | Acts on SIRT1/FOXO3A/GPX4 pathway | HK-2 cells | Cisplatin-induced nephrotoxicity in C57BL/6 mice | [258] |
| Activates Nrf2/GPX4 signaling pathway | HT22 cells | BCCAO-induced vascular dementia model in SD rats | [91] | |
| Upregulation of oxidative defense system through Nrf2/HO-1 axis | HT22 cells | / | [92] | |
| Decreases levels of MDA and ROS, raises the level of GSH, and increases GPX activity, upregulates expression of Nrf2, GPX, HO-1, and FPN1 | C6 cell line | / | [47] | |
| Potential ferroptosis inhibitor, upregulates expression of Nrf2 and HO-1 | Liver sinusoidal endothelial cells isolated from C57BL/6 mouses | / | [259] | |
| Geraniin | Iron chelator and ROS scavenger | bmMSC isolated from rat femur and tibia | / | [260] |
| Gingerenone A | Activates Nrf2/GPX4 signaling pathway | HepG2 cells | Dextran sodium sulphate-induced secondary liver injury in C57 mice | [261] |
| 6-Gingerol | Decreases iron and MDA levels, increases the activity of SOD, inhibits expression of FACL4, promotes expression of GPX4, and activates Nrf2/HO-1 axis | H9c2 cells | Streptozotocin and high-fat-diet-induced diabetes mellitus model in C57BL/6 mice | [262] |
| 8-Gingerol | Upregulates expression of GPX4 and downregulates expression of LOX15 | HT22 cells | Spinal cord injury model in SD rats | [263] |
| Glabridin | Upregulates expression of GPX4, SLC7A11, SLC3A2 and downregulates expression of TfR1 | NRK-52E cells | High-fat-diet- and streptozotocin-induced diabetes in SD rats | [81] |
| Gossypol acetic acid | Decreases levels of Fe2+ and ROS, inhibits lipid peroxidation, upregulates GPX4 | H9c2 cells | Cardiac ischemia/reperfusion injury model in Sprague-Dawley rats | [264] |
| Hesperidin | Activates Nrf2 signaling pathway | Primary human nucleus pulposus cells | Disc degeneration model in mice | [265] |
| Honokiol | Upregulates expression of GPX4 and SLC7A11 by activating Nrf2 pathway | RSC96 cells | Streptozotocin-induced diabetes in SD rats | [266] |
| Hydroxysafflor yellow A | Activates HIF-1α/SLC7A11/GPX4 Signaling pathway | H9c2 cells | Myocardial ischemia/reperfusion injury model in C57BL/6 mice | [267] |
| Upregulates expression of SLC7A11 and GPX4, downregulates expression of ACSL4, downregulates expression of miR-429 which additionally upregulates expression of SLC7A11 | HUVECs | High-fat-diet- and streptozotocin-induced diabetes in ApoE−/− C57BL/6 mice | [268] | |
| Reduces ROS, 4-HNE, and MDA production, reduces Fe2+ levels, increases GSH/GSSG ratio, and upregulates expression of GPX4 and SLC7A1 | PC12 cells | / | [191] | |
| Hyperjaponol J | Suppresses RSL-3-induced ferroptosis | HT22 cells | / | [269] |
| Hyperjaponol K | Suppresses RSL-3-induced ferroptosis | HT22 cells | / | [269] |
| Icariin | Activates Nrf2/SLC7A11/GPX4 pathway | / | Methionine choline-deficient-diet-induced nonalcoholic steatohepatitis in C57BL/6J mice | [270] |
| Activates SIRT1/Nrf2/HO-1 signaling pathway | HL-1 cells | Excessive-ethanol-treated C57BL/6 mice | [271] | |
| Upregulates expression of GPX4 and FTH1 | HUVECs | Atherosclerosis model in ApoE−/− C57BL/6 mice | [272] | |
| Alleviates iron accumulation and lipid peroxidation | / | Alzheimer’s disease model in C57BL/6J mice | [93] | |
| Activates Nrf2/HO-1 signaling pathway | Primary endplate chondrocytes | Intervertebral disc degeneration model in C57BL/6 mice | [273] | |
| Decreases level of Fe2+, upregulates Nrf2, GPX4, HO-1, and downregulates ACSL4 | H9c2 cells | / | [274] | |
| Lowers levels of MDA and iron by increasing expression of GPX4, SLC7A11, SLC3A12L, and Nrf2 and decreasing expression of TfR1 and NCOA4 | HUM-CELL-0060 cells | / | [275] | |
| Icariside II | Activates Nrf2 signaling pathway | primary astrocytes | MCAO-induced cerebral ischemia/reperfusion injury in mice | [276] |
| Isoforsythiaside | Activates Nrf2 signaling pathway | HT22 and BV2 cells | Alzheimer’s disease model in mice | [85] |
| Isoliquiritigenin | Reduces MDA, Fe2+, and NO levels, increases expression of GPX4 and Xct- system, and reduces expression of NCOA4 | HK2 cells | LPS-induced acute kidney injury model in C57BL/6 mice | [277] |
| Isoliquiritin apioside | Inhibits HIF-1α signaling pathway | MLE-2 cells | Intestinal ischemia/reperfusion-induced acute lung injury in C57BL/6 mice | [278] |
| Isoquercetin | Decreases the production of ROS and MDA, increases the activity of SOD and CAT, and inhibits the NOX4/ROS/NF-κB pathway by induction of Nrf2 nuclear translocation | Primary rat hippocampal neuron cell culture | Cerebral ischemia/reperfusion injury model in Sprague-Dawley rats induced by MCAO/R surgery | [279] |
| Isorhamnetin | Possible ferroptosis inhibitor, activates Akt/SIRT1/Nrf2/HO-1 signaling pathway | HT22 cells | / | [280] |
| Upregulates SIRT1, Nrf2, and HO-1, and downregulates NOX2/4 | H9c2 cells | Hypoxia/reoxygenation-induced myocardial injury | [106] | |
| Isorhapontigenin | Inhibits PRDX2/MFN2/ACSL4 signaling pathway | CMECS | Diabetes model in db/db mice | [281] |
| Kaempferol | Activates Nrf2 signaling pathway | L02 cells | Acetaminophen-induced liver injury in BALB/c mice | [125] |
| Activates Nrf2/SLC7A11/GPX4 axis | Primary mouse cortical culture prepared from E16 mouse embryos | / | [120] | |
| Kumatakenin | Prevents iron accumulation through Eno3/IRP1 and inhibits lipid peroxidation | MODE-K cells | DSS-induced acute colitis in C57BL/6 mice | [282] |
| Licochalcone A | Upregulates expression of GPX4, downregulates expression of ACSL4, inhibits Nrf2/HO-1 axis | H9c2 cells | Ischemia/reperfusion model in SD rats | [283] |
| Luteolin | Decreases levels of ROS, MDA, and iron, and restores protein levels of GPX4 | H9c2 cells and primary cardiomyocytes from neonatal SD rats | Langerdorff perfusion system for ischemia/reperfusion model on isolated hearts from SD rats | [119] |
| Loureirin C | Activates Nrf2 signaling pathway | Primary cortical neurons and 5H-SY5Y cells | MCAO/R-induced cerebral ischemia/reperfusion injury in C57BL/6 mice | [116] |
| Methyl ferulic acid | Downregulates expression of ACSL4 and upregulates expression of GPX4 in a NOX4 dependent manner | / | SNI-induced neuropathic pain model in SD rats | [284] |
| Moracin N | Upregulates expression of GPX4, SLC7A11, CAT, SOD2, NFE2L2, HMOX1, GCLC, and GCLM, and downregulates expression of ACSL4, PTGS2, and FTH1 | HT22 cells | / | [285] |
| Naringenin | Activates Nrf2/HO-1 signaling pathway | BEAS-2B cells | AgNPs-induced lung injury in ICR mice | [135] |
| Decreases expression of NOX1, increases expression of GPX4, SLC7A11, FTH, and FPN1 | H9C2 cells | Myocardial ischemia/reperfusion injury model in Sprague-Dawley rats | [115] | |
| Naringin | Targeting of P2Y14 receptors, upregulation of Nrf2/GPX4 pathway | / | Streptozotocin-induced diabetic cardiac autonomic neuropathy model in Sprague-Dawley rats | [77] |
| Nobiletin | Modulates p53/SLC7A11 axis | MLE-12 cells | Heatstroke-induced acute lung injury model in C57BL/6 mice | [286] |
| Downregulates expression of ACSL4 and NCOA4 | H9c2 cells | High-fat-diet- and streptozotocin-induced diabetes model in SD rats | [287] | |
| Activates Nrf2/HO-1 signaling pathway | / | Sepsis-associated acute liver injury in C57BL/6 mice | [122] | |
| Ameliorates oxidative stress and ferroptosis through modulation of GPX4 and FTH1 | / | Renal injury model in C57BL/6J mice | [288] | |
| Phlorizin | Alleviates iron overload and lipid peroxidation, promotes expression of GPX4, and decreases expression of FTH1 and FTL1 | Primary mouse colonic lamina propria cells | Dextran sulphate sodium-induced colitis in C57BL/6J mice | [289] |
| Piceatannol | Antioxidant activity | bmMSCs | / | [193] |
| Pinocembrin | Inhibits ferroptosis through activation of Nrf2 pathway | Primary chondrocytes isolated from C57BL/6 mice | Surgery-induced intervertebral disc degeneration model in C57BL/6 mice | [290] |
| Polydatin | Reduces levels of iron, ROS, and MDA, increases the level of GSH, and GPX4 activity | HK-2 cells | Cisplatin-induced acute kidney injury model in C57BL/6 mice | [291] |
| Increase in GPX4 activity and decreased level of MDA | Neuro2A cells | Traumatic brain injury in male C57BL/6 mice | [100] | |
| Proanthocyanidins | Activates Nrf2/HO-1 signaling pathway | / | MCAO-induced cerebral ischemia/reperfusion injury in ICR mice | [292] |
| Downregulates expression of ACSL4, and upregulates expression of GPX4 and SLC7A11 | / | Virus infection model in mice | [293] | |
| Decreased levels of iron, TBARS, downregulation of ACSL4 and ALOX15B, upregulation of GPX4, Nrf2, and HO-1, and increased level of GSH | / | Spinal cord injury in C57BL/6 mice | [294] | |
| Protocatechualdehyde | Possible inhibitor of ferroptosis thanks to the ability to decrease the production of ROS, 4-HNE, and 8-OHdG, and activate the PKCε/Nrf2/HO-1 signaling pathway | SH-SY5Y cells | Focal cerebral ischemia model in Sprague-Dawley rats induced by MCAO | [295] |
| Protocatechuic acid | Alleviates iron accumulation and lipid peroxidation and promotes expression of GPX4 | Caco-2 cells | Ulcerative colitis model in C57BL/6 mice | [296] |
| Pterostilbene | Activates Nrf2 signaling pathway | / | Diquat-induced intestinal damage in broiler chicks | [297] |
| Promotes expression of Nrf2, GPX4, and HO-1 | COV434 and KGN cells | / | [298] | |
| Puerarin | Inhibits lipid peroxidation and upregulates expression of GPX4 and FTH1 | H9c2 cells | Ischemia/reperfusion injury model in C57BL/6 mice | [49] |
| Downregulates expression of ACSL4, and upregulates expression of GPX4 and FSP1 | HK-2 cells | Ischemia/reperfusion injury model in SD rats | [299] | |
| Upregulates expression of Nrf2, GPX4, SOD, HO-, pAMPK, and PGC1α, and downregulates expression of ACSL4 | / | Subarachnoid hemorrhage model in SD rats | [101] | |
| Upregulates expression of GPX4 and FTH1, and downregulates ACSL4 and TfR1 | / | LPS-induced myocardial injury in SD rats | [300] | |
| Reduces levels of ROS, MDA, and iron, increases level of GSH, increases expression of ACSL7A11, GPX4, FTH1, and decreases expression of NOX1 | A549 cells | / | [132] | |
| Decreases levels of free iron and inhibits lipid peroxidation | H9c2 cells | Heart failure model in Sprague-Dawley rats | [301] | |
| Punicalagin | Potential ferroptosis inhibitor; activates Nrf2/HO-1 signaling pathway | Primary BMSCs | Bone defect model in SD rats | [302] |
| Quercetin | Activates SIRT1/Nrf2/GPX4 pathway | AT2 cells | LPS-induced lung injury in C57BL/6 mice | [130] |
| Activates Nrf2/HO-1 signaling pathway | HK-2 cells | Diabetes model in db/db C57BL/KsJ mice | [78] | |
| Prevents FTH1 degradation by direct interaction with NCOA4 | HepG2 cells | Acrylamide-induced liver injury in C57BL/6J mice | [123] | |
| Activates SIRT1/p53/SLC7A11 pathway | H9c2 cells | Sepsis-induced cardiomyopathy model in SD rats | [303] | |
| Inhibits lipid peroxidation, and upregulates expression of GPX4 and SLC7A11 | RAW 246.7 cells | LPS/ovalbumin-induced neutrophilic asthma in C57BL/6 mice | [304] | |
| Upregulates expression of GPX4 and PGS2, and downregulates expression of Tf and Id2 | Primary oligodendrocyte progenitor cells | Spinal cord injury model in C57BL/6 mice | [305] | |
| Decreases Fe2+ and MDA accumulation, and increases GSH, GPX, GST, and CAT | / | Triptolide-induced multi-organ injury in Kunming mice | [210] | |
| Upregulates expression of GPX4, SLC7A11, FTH1, FPN1, FSP1, and downregulates expression of ACSL4 and TfR1 | / | Deoxynivalenol-induced intestinal damage in BALB/c mice | [306] | |
| Activates Nrf2/HO-1 pathway | HT22 cells | / | [307] | |
| Activates Nrf2 signaling pathway | M17, PC12 and SH-SY5Y cells | MPTP-induced neurotoxicity in C57BL/6 mice | [95] | |
| Activates SIRT1/Nrf2/GPX4/SLC7A11 signaling pathway | HT22 cells | Kainic acid-induced seizures in C57BL/6J mice | [94] | |
| Promotes TFEB-dependent degradation of ferritin | MCF-7 and MDA-MB-231 cells | / | [72] | |
| Activates Nrf2 signaling pathway | Primary BMSCs | / | [308] | |
| Decreases levels of MDA, and increases the level of GSH and GPX4, inhibits ferroptosis through repression of activation transcription factor 3 (ATF3) and repression of HO-1 | NRK-52E and HK-2 cells | Acute kidney injury model in C57BL/6J mice induced by renal ischemia/reperfusion or folic acid | [309] | |
| Lowers levels of ROS and MDA, increases the level of GSH, restores the activity of SOD, and normalizes protein levels of Xct-, GPX4, and VDAC2 | INS-1 cells | Type 2 diabetes mellitus model in C57BL/6J mice induced by high-fat diet and streptozotocin | [310] | |
| Resveratrol | Upregulates expression of Nrf2, GPX4, FTH1, and NQO1, and downregulates expression of TfR1 and p53 | H9c2 cells | 5-FU-induced cardiotoxicity in C57BL/6J mice | [311] |
| Activates SLC7A11/GPX4 axis | MLOY4 cells | Diabetes periodontitis model in C57BL/6 mice | [79] | |
| Upregulates expression of Nrf2, GPX4, SLC7A11, and FTH1 | / | High-intensity-exercise-training-induced intestinal damage in Kunming mice | [312] | |
| Activates SIRT1/p53 signaling pathway | HiPSCs | Heart failure model in C57BL/6J mice | [313] | |
| Activates SIRT3/FoxO3a pathway, promotes expression of GPX4 and FTH1, downregulates expression of ACSL4 | Caco-2 cells | Intestinal ischemia/reperfusion injury model in C57BL/6 mice | [112] | |
| Activates Nrf2/Keap1 signaling pathway | BEAS-2B cells | / | [314] | |
| Activates Nrf2/HO-1 pathway | HT22 cells | / | [307] | |
| Activates SLC7A11/GPX4 pathway | HepG2 cells | / | [315] | |
| Upregulates expression of GPX4 and FTH1, and downregulates expression of TfR1 | H9c2 cells | Myocardial ischemia/reperfusion injury model in SD rats | [111] | |
| Upregulates expression of GPX4 and SLC7A11 | H9c2 cells | Myocardial infarction model in SD rats | [316] | |
| Alleviates iron accumulation, inhibits lipid peroxidation, and raises GSH levels | Primary cardiomyocytes | LPS-induced endotoxemia model in C57BL/6 mice | [317] | |
| Upregulates SIRT1/Nrf2 signaling pathway | H9c2 cells | Sepsis-induced cardiomyopathy model in SD rats | [318] | |
| Decreased levels of iron and ROS, and increased levels of GSH, decreased expression of ACSL4, increased expression of GPX4 and Ferritin | Primary cortical neurons | Focal ischemic brain damage model in SD rats induced by middle cerebral artery occlusion/reperfusion | [110] | |
| Decreases level of MDA, increases level of GSH, downregulates expression of ACSL4 and COX2, and upregulates expression of GPX4 | MIN6 cells | / | [319] | |
| Decreases levels of iron, ROS and MDA, increases GSH, increases expression of GPX4, FPN1, Nrf2 and SLC7A11, and downregulates STAT1 and Keap1 | BV2 cells | / | [180] | |
| Rhein | Activates Nrf2/SLC7A11/GPX4 pathway | HT22 cells | MCAO-induced cerebral ischemia/reperfusion injury in SD rats | [113] |
| Downregulates expression of ACSL4 and TfR1, and upregulates expression of GPX4 and SLC7A11 through modulation of Rac1/NOX1/β-Catenin Axis | MPC5 cells | Diabetic nephropathy in C57BL/6J mice | [80] | |
| Rutin | Decreases Fe 2+ and MDA accumulation and increased GSH, GPX, GST, and CAT | / | Triptolide-induced multi-organ injury in Kunming mice | [210] |
| Activates Nrf2/HO-1 signaling pathway | Hen ovary follicle tissue culture | / | [320] | |
| Salidroside | Promotes expression of GPX4, SOD1 and SOD2, and downregulates expression of ACSL4 by modulating PI3K/AKT signaling pathway | NRK cells | Renal ischemia/reperfusion injury model in SD rats | [321] |
| Inhibits endoplasmic reticulum oxidative stress-related ferroptosis through activation of AMPK/SIRT1 pathway | AML12 cells | APAP-induced acute liver injury in C57BL/6J mice | [124] | |
| Activates Nrf2/GPX4 pathway | / | Alzheimer’s disease model in SAMP8 mice | [96] | |
| Inhibits lipid peroxidation and promotes expression of GPX4 | C2C12 cells, HUVECs and MOVAS cells | Diabetic hindlimb ischemia model in C57BL/6 mice | [322] | |
| Activates Nrf2/SLC7A11/GPX4 pathway | MLE-12 and RAW 264.7 cells | Lung ischemia/reperfusion injury in C57BL/6 mice | [323] | |
| Decreased iron concentration, inhibition of lipid peroxidation and production of 4-HNE, and increased expression of GPX4 | H9c2 cells | Doxorubicin-induced cardiomyopathy in C57BL/6 mice | [324] | |
| Inhibits IL-17A mediated ferroptosis through modulation of Act1/TRAF6/p38 MAPK pathway | / | Hyperoxia-induced acute lung injury in KM mice | [131] | |
| Promotes expression of GPX4, SLC7A11, FPN1 and FTH1, downregulates expression of ACSL4 and TfR1 | / | Aging-related renal fibrosis in SAMP8 mice | [325] | |
| Activates Nrf2/HO-1 signaling pathway | HT22 cells | Alzheimer’s disease model in mice | [97] | |
| Salvianolate | Possible ferroptosis inhibitor, activates Keap1/Nrf2/HO-1 signaling pathway | Primary renal tubular epithelial cells | Renal ischemia/reperfusion injury in C57BL/6J mice | [326] |
| Salvianolic acid A | Modulates HIF-2α/DUOX1/GPX4 pathway | HK-2 cells | NaAsO2-induced kidney injury in C57BL/6J mice | [327] |
| Downregulates expression of ACSL4 and upregulates expression of GPX4 and SLC7A11 and modulates iron metabolism | 661W cells | Iron overload model in Kunming mice | [328] | |
| Salvianolic acid B | Upregulates expression of GPX4 and FTH1, downregulates expression of TfR1 | H9c2 cells | Myocardial ischemia/reperfusion injury model in SD rats | [329] |
| Activates Nrf2 signaling pathway | / | Myocardial infarction model in SD rats | [330] | |
| Potential ferroptosis inhibitor, reduces MDA and H2O2 production, increases the level of GSH, increases the activity of GPX and SOD, and increases expression of Nrf2, HO-1, and NQO-1 | Primary cortical neurons | Subarachnoid hemorrhage model in Sprague-Dawley rats and C57BL/6 mice | [331] | |
| Scutellarein | Promotes expression of GPX4, and prevents overexpression of HO-1 | BEAS-2B cells | LPS/cigarette smoke-induced chronic obstructive pulmonary disease model in C57BL/6 mice | [332] |
| Sennoside A | Alleviates iron accumulation, inhibits lipid peroxidation, and raises GSH levels | BV2 cells | Alzheimer’s disease model in mice | [87] |
| Sesamin | Reduces production of MDA and iron concentration, increases the level of GSH, and activity of SOD, GPX4, increases expression of FPN1 and TfR1, and inhibits expression of FTH1 and FTL | / | PM2.5 induced cardiovascular injury in Sprague-Dawley rats | [333] |
| Silibinin | Downregulates expression of p53 and upregulates expression of SLC7A11 and GPX4 | HT22 cells | Streptozotocin-induced neurotoxicity in SD rats | [99] |
| Upregulates expression of GPX4 and FSP1, downregulates expression COX2, activates PINK1-dependent mitophagy | INS-1 cells | / | [334] | |
| Suberosin | Downregulates expression of ACSL4, LOX, LPCAT3, and upregulates expression of GPX4 | / | Streptozotocin-induced diabetes in SD rats | [335] |
| Syringic acid | Activates Nrf2/HO-1/SLC7A11 pathway | C2C12 cells | Femoral artery ischemia/reperfusion injury in C57BL/6 mice | [336] |
| Tannic acid | Upregulates expression of GPX4, SLC7A11 and ferritin, downregulates expression of ACSL4, TfR1, COX2, LOX, and p53 | / | T2-toxin treated C57BL/6J mice | [337] |
| Tectorigenin | Downregulates expression of ACSL4 and upregulates expression of GPX4 | / | LPS-treated C57BL/6 mice | [338] |
| Upregulates expression of SLC7A11, GPX4, and downregulates expression of NOX4 and activation of Smad3 | Mouse primary renal tubular epithelia cells | Unilateral ureteral obstruction model in C57BL/6 mice | [339] | |
| Tetrahydroxy stilbene glycoside | Activates GSH/GPX4/ROS and Keap1/Nrf2/ARE pathways | / | Alzheimer’s disease model in APP/PS1 mice | [98] |
| Theaflavin-3,3′-Digallate | Upregulates expression of Nrf2, GPX4, FTH1 and HO-1 | Primary culture of human chondrocytes | Osteoarthritis model in SD rats | [340] |
| Thonningianin A | Activates Nrf2/HO-1 signaling pathway | SH-SY5Y cells | 6-hydroxydopamine treated zebrafish | [43] |
| Trilobatin | Activates Nrf2/HO-1/GPX4 pathway | / | Exhaustive exercise-induced fatigue in C57BL/6 mice | [341] |
| Umbelliferone | Downregulation of ACLS4 and upregulation of GPX4, Nrf2 and HO-1 expression | HK-2 cells | Diabetic nephropathy model in C57BLKS/J db/db and C57BLKS/J db/m mice | [342] |
| Vitexin | Downregulates expression of Keap1 and TfR1 and upregulates expression of GPX4, SLC7A11, and HO-1 | Primary cortical neuron cells | MCAO-induced cerebral ischemia/reperfusion injury in SD rats | [114] |
| Activates Keap1/Nrf2/HO-1 pathway | HK2 and NRK-49 F cells | Chronic kidney disease model in C57BL/6J mice | [343] | |
| Upregulates GPX4/SLC7A11 axis | HK-2 cells | High-fat-diet- and streptozotocin-induced diabetic nephropathy in SD rats | [344] | |
| Wedelolactone | Upregulation of GPX4 | Pancreatic acinar cell line AR42J | Tauro-cholate induced acute pancreatitis in Sprague-Dawley rats | [345] |
| Xanthohumol | Decreased lipid peroxidation, ROS neutralization, iron chelation, reduced levels of ACSL4 and Nrf2, and modulation of GPX4 | H9c2 cells | Langendorff hearth perfusion system in rats | [42] |
4. Summary
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ketelut-Carneiro, N.; Fitzgerald, K.A. Apoptosis, Pyroptosis, and Necroptosis-Oh My! The Many Ways a Cell Can Die. J. Mol. Biol. 2022, 434, 167378. [Google Scholar] [CrossRef]
- Debruyne, A.C.; Okkelman, I.A.; Dmitriev, R.I. Balance between the Cell Viability and Death in 3D. Semin. Cell Dev. Biol. 2023, 144, 55–66. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis Turns 10: Emerging Mechanisms, Physiological Functions, and Therapeutic Applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Non-Apoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Lesjak, M.; Simin, N.; Srai, S.K.S. Can Polyphenols Inhibit Ferroptosis? Antioxidants 2022, 11, 150. [Google Scholar] [CrossRef]
- Shan, X.; Lv, Z.-Y.; Yin, M.-J.; Chen, J.; Wang, J.; Wu, Q.-N. The Protective Effect of Cyanidin-3-Glucoside on Myocardial Ischemia-Reperfusion Injury through Ferroptosis. Oxidative Med. Cell. Longev. 2021, 2021, 8880141. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Guan, Q.; Tao, X.; Wang, J.; Li, W. The Role of Ferroptosis in the Development of Acute and Chronic Kidney Diseases. J. Cell Physiol. 2022, 237, 4412–4427. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.; Xie, S.; Lai, Y.; Mo, C.; Zeng, T.; Kuang, S.; Zhou, C.; Zeng, Z.; Chen, Y.; et al. Isoliquiritigenin Alleviates Liver Fibrosis through Caveolin-1-Mediated Hepatic Stellate Cells Ferroptosis in Zebrafish and Mice. Phytomedicine 2022, 101, 154117. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Yan, J.; Hou, A.; Sui, W.; Sun, M. Curcumin Induces Ferroptosis in A549 CD133+ Cells through the GSH-GPX4 and FSP1-CoQ10-NAPH Pathways. Discov. Med. 2023, 35, 251–263. [Google Scholar] [CrossRef]
- Efferth, T.; Oesch, F. Repurposing of Plant Alkaloids for Cancer Therapy: Pharmacology and Toxicology. Semin. Cancer Biol. 2021, 68, 143–163. [Google Scholar] [CrossRef]
- Kerr, J.; Wyllie, A.; Currie, A. Apoptosis: A Basic Biological Phenomenon with Wide-Ranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Riley, J.S.; Bock, F.J. Voices from beyond the Grave: The Impact of Apoptosis on the Microenvironment. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119341. [Google Scholar] [CrossRef]
- Wang, P.-P.; Huang, X.; Yang, M.-W.; Fang, S.-Y.; Hong, F.-F.; Yang, S.-L. Effects of Non-Drug Treatment on Liver Cells Apoptosis during Hepatic Ischemia-Reperfusion Injury. Life Sci. 2021, 275, 119321. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, H.; Gao, J.; Liu, Y.; Li, J.; Wang, J. Molecular Machinery and Interplay of Apoptosis and Autophagy in Coronary Heart Disease. J. Mol. Cell. Cardiol. 2019, 136, 27–41. [Google Scholar] [CrossRef]
- Li, D.; Pi, W.; Sun, Z.; Liu, X.; Jiang, J. Ferroptosis and Its Role in Cardiomyopathy. Biomed. Pharmacother. 2022, 153, 113279. [Google Scholar] [CrossRef]
- Ou, M.; Jiang, Y.; Ji, Y.; Zhou, Q.; Du, Z.; Zhu, H.; Zhou, Z. Role and Mechanism of Ferroptosis in Neurological Diseases. Mol. Metab. 2022, 61, 101502. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Pan, Q.; Zhang, W.; Xiang, J.; Jiang, X. Ferroptosis Is an Autophagic Cell Death Process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Yiannikourides, A.; Latunde-Dada, G.O. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Chen, Z.; Xiong, Z.; Liu, X. Ferroptosis—A New Target of Osteoporosis. Exp. Gerontol. 2022, 165, 111836. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Fan, K.; Chen, Y.; Zhang, G.; Chen, J.; Zhang, Y. Understanding the Mechanistic Regulation of Ferroptosis in Cancer: The Gene Matters. J. Genet. Genom. 2022, 49, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.J.; Frey, A.G.; Palenchar, D.J.; Achar, S.; Bullough, K.Z.; Vashisht, A.; Wohlschlegel, J.A.; Philpott, C.C. A PCBP1-BolA2 Chaperone Complex Delivers Iron for Cytosolic [2Fe-2S] Cluster Assembly. Nat. Chem. Biol. 2019, 15, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Kraft, V.A.N.; Bezjian, C.T.; Pfeiffer, S.; Ringelstetter, L.; Müller, C.; Zandkarimi, F.; Merl-Pham, J.; Bao, X.; Anastasov, N.; Kössl, J.; et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent. Sci. 2020, 6, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Roh, J.-L. Induction of Ferroptosis in Head and Neck Cancer: A Novel Bridgehead for Fighting Cancer Resilience. Cancer Lett. 2022, 546, 215854. [Google Scholar] [CrossRef]
- Anandhan, A.; Dodson, M.; Schmidlin, C.J.; Liu, P.; Zhang, D.D. Breakdown of an Ironclad Defense System: The Critical Role of NRF2 in Mediating Ferroptosis. Cell Chem. Biol. 2020, 27, 436–447. [Google Scholar] [CrossRef]
- Kang, R.; Kroemer, G.; Tang, D. The Tumor Suppressor Protein P53 and the Ferroptosis Network. Free Radic. Biol. Med. 2019, 133, 162–168. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, W. P53 in Ferroptosis Regulation: The New Weapon for the Old Guardian. Cell Death Differ. 2022, 29, 895–910. [Google Scholar] [CrossRef]
- Anjitha, K.S.; Sameena, P.P.; Puthur, J.T. Functional Aspects of Plant Secondary Metabolites in Metal Stress Tolerance and Their Importance in Pharmacology. Plant Stress 2021, 2, 100038. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Fernie, A.R. Diversity: Current and Prospective Secondary Metabolites for Nutrition and Medicine. Curr. Opin. Biotechnol. 2022, 74, 164–170. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Rostom, B.; Karaky, R.; Kassab, I.; Sylla-Iyarreta Veitía, M. Coumarins Derivatives and Inflammation: Review of Their Effects on the Inflammatory Signaling Pathways. Eur. J. Pharmacol. 2022, 922, 174867. [Google Scholar] [CrossRef]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source Plants, Chemistry, Biosynthesis, Pharmacology, Application and Problems Related to Their Clinical Application-A Comprehensive Review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef] [PubMed]
- Robertson, I.; Wai Hau, T.; Sami, F.; Sajid Ali, M.; Badgujar, V.; Murtuja, S.; Saquib Hasnain, M.; Khan, A.; Majeed, S.; Tahir Ansari, M. The Science of Resveratrol, Formulation, Pharmacokinetic Barriers and Its Chemotherapeutic Potential. Int. J. Pharm. 2022, 618, 121605. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on Tannins: Extraction Processes, Applications and Possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Miao, Q.; Deng, W.; Lyu, W.; Sun, Z.; Fan, S.; Qi, M.; Qiu, S.; Zhu, Y.; Lin, J.; Chen, M.; et al. Erianin Inhibits the Growth and Metastasis through Autophagy-Dependent Ferroptosis in KRASG13D Colorectal Cancer. Free Radic. Biol. Med. 2023, 204, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-Y.; Huang, Z.-X.; Chen, G.-Q.; Sheng, F.; Zheng, Y.-S. Typhaneoside Prevents Acute Myeloid Leukemia (AML) through Suppressing Proliferation and Inducing Ferroptosis Associated with Autophagy. Biochem. Biophys. Res. Commun. 2019, 516, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Yang, Y.-R.; Ma, W.-X.; Wang, H.-Y.; Fan, Q.-W.; Wang, Y.-Y.; Li, C.; Wang, J.; Hu, Z.-M.; Wang, X.-F.; et al. Epigallocatechin Gallate Attenuates Gentamicin-Induced Nephrotoxicity by Suppressing Apoptosis and Ferroptosis. Molecules 2022, 27, 8564. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, M.; Zhang, X.; Wang, X.; Su, K.; Xu, Z.; Wang, X.; Yang, Y. Gambogenic Acid Inhibits Proliferation and Ferroptosis by Targeting the miR-1291/FOXA2 and AMPKα/SLC7A11/GPX4 Axis in Colorectal Cancer. Cell Biol. Int. 2023, 47, 1813–1824. [Google Scholar] [CrossRef]
- Xiang, Y.; Chen, X.; Wang, W.; Zhai, L.; Sun, X.; Feng, J.; Duan, T.; Zhang, M.; Pan, T.; Yan, L.; et al. Natural Product Erianin Inhibits Bladder Cancer Cell Growth by Inducing Ferroptosis via NRF2 Inactivation. Front. Pharmacol. 2021, 12, 775506. [Google Scholar] [CrossRef]
- Lin, J.-H.; Yang, K.-T.; Lee, W.-S.; Ting, P.-C.; Luo, Y.-P.; Lin, D.-J.; Wang, Y.-S.; Chang, J.-C. Xanthohumol Protects the Rat Myocardium against Ischemia/Reperfusion Injury-Induced Ferroptosis. Oxidative Med. Cell. Longev. 2022, 2022, 9523491. [Google Scholar] [CrossRef]
- Sun, Y.; He, L.; Wang, W.; Xie, Z.; Zhang, X.; Wang, P.; Wang, L.; Yan, C.; Liu, Z.; Zhao, J.; et al. Activation of Atg7-Dependent Autophagy by a Novel Inhibitor of the Keap1-Nrf2 Protein-Protein Interaction from Penthorum Chinense Pursh. Attenuates 6-Hydroxydopamine-Induced Ferroptosis in Zebrafish and Dopaminergic Neurons. Food Funct. 2022, 13, 7885–7900. [Google Scholar] [CrossRef]
- Guan, X.; Li, Z.; Zhu, S.; Cheng, M.; Ju, Y.; Ren, L.; Yang, G.; Min, D. Galangin Attenuated Cerebral Ischemia-Reperfusion Injury by Inhibition of Ferroptosis through Activating the SLC7A11/GPX4 Axis in Gerbils. Life Sci. 2021, 264, 118660. [Google Scholar] [CrossRef]
- Liu, H.; He, Y.; Gao, X.; Li, T.; Qiao, B.; Tang, L.; Lan, J.; Su, Q.; Ruan, Z.; Tang, Z.; et al. Curcumin Alleviates AFB1-Induced Nephrotoxicity in Ducks: Regulating Mitochondrial Oxidative Stress, Ferritinophagy, and Ferroptosis. Mycotoxin Res. 2023, 39, 437–451. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, Y.; Yang, Y.; Su, S.; Zhou, L.; Lo, P.-C.; Cai, J.; Qiao, Y.; Li, M.; Huang, S.; et al. Baicalin Inhibits Ferroptosis in Intracerebral Hemorrhage. Front. Pharmacol. 2021, 12, 629379. [Google Scholar] [CrossRef]
- Jiang, T.; Chu, J.; Chen, H.; Cheng, H.; Su, J.; Wang, X.; Cao, Y.; Tian, S.; Li, Q. Gastrodin Inhibits H2O2-Induced Ferroptosis through Its Antioxidative Effect in Rat Glioma Cell Line C6. Biol. Pharm. Bull. 2020, 43, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Wang, Y.; Li, L.; Li, X.; Qiu, B.; Hu, Y. Cardamonin Alleviates Chondrocytes Inflammation and Cartilage Degradation of Osteoarthritis by Inhibiting Ferroptosis via P53 Pathway. Food Chem. Toxicol. 2023, 174, 113644. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Peng, S.; Zhou, G.; Chen, S.; Wei, Y.; Xu, J.; Gu, H.; Li, J.; Liu, S.; et al. Puerarin Protects against Myocardial Ischemia/Reperfusion Injury by Inhibiting Ferroptosis. Biol. Pharm. Bull. 2023, 46, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, J.; He, J.; Tan, F.; Lu, M.; Yuan, F.; Zhu, X.; Kong, L. Curcumin Preconditioning Enhances the Neuroprotective Effects of Olfactory Mucosa-Derived Mesenchymal Stem Cells on Experimental Intracerebral Hemorrhage. Heliyon 2023, 9, e17874. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ding, H.; Liang, M.; Chen, X.; Yan, Y.; Wan, N.; Chen, Q.; Zhang, J.; Cao, J. Curcumin Induces Ferroptosis in Non-Small-Cell Lung Cancer via Activating Autophagy. Thorac. Cancer 2021, 12, 1219–1230. [Google Scholar] [CrossRef]
- Liang, Y.; Zhong, Q.; Ma, R.; Ni, Z.; Thakur, K.; Zhang, J.; Wei, Z. Apigenin, a Natural Flavonoid, Promotes Autophagy and Ferroptosis in Human Endometrial Carcinoma Ishikawa Cells in Vitro and in Vivo. Food Sci. Hum. Wellness 2023, 12, 2242–2251. [Google Scholar] [CrossRef]
- Tsai, K.-Y.; Wei, P.-L.; Lee, C.-C.; Makondi, P.T.; Chen, H.-A.; Chang, Y.-Y.; Liu, D.-Z.; Huang, C.-Y.; Chang, Y.-J. 2,3,5,4′-Tetrahydroxystilbene (TG1), a Novel Compound Derived from 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-Glucoside (THSG), Inhibits Colorectal Cancer Progression by Inducing Ferroptosis, Apoptosis, and Autophagy. Biomedicines 2023, 11, 1798. [Google Scholar] [CrossRef]
- Wen, R.; Dong, X.; Zhuang, H.; Pang, F.; Ding, S.; Li, N.; Mai, Y.; Zhou, S.; Wang, J.; Zhang, J. Baicalin Induces Ferroptosis in Osteosarcomas through a Novel Nrf2/xCT/GPX4 Regulatory Axis. Phytomedicine 2023, 116, 154881. [Google Scholar] [CrossRef]
- Yuan, J.; Khan, S.U.; Yan, J.; Lu, J.; Yang, C.; Tong, Q. Baicalin Enhances the Efficacy of 5-Fluorouracil in Gastric Cancer by Promoting ROS-Mediated Ferroptosis. Biomed. Pharmacother. 2023, 164, 114986. [Google Scholar] [CrossRef]
- Miyazaki, K.; Xu, C.; Shimada, M.; Goel, A. Curcumin and Andrographis Exhibit Anti-Tumor Effects in Colorectal Cancer via Activation of Ferroptosis and Dual Suppression of Glutathione Peroxidase-4 and Ferroptosis Suppressor Protein-1. Pharmaceuticals 2023, 16, 383. [Google Scholar] [CrossRef]
- Shen, H.; Geng, Z.; Nie, X.; Liu, T. Erianin Induces Ferroptosis of Renal Cancer Stem Cells via Promoting ALOX12/P53 mRNA N6-Methyladenosine Modification. J. Cancer 2023, 14, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Z.; Xu, J.; Zhang, N.; Chen, J.; Wang, G.; Zhao, Y. Curcumin Induces Ferroptosis in Follicular Thyroid Cancer by Upregulating HO-1 Expression. Oxidative Med. Cell. Longev. 2023, 2023, 6896790. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tan, A.-H.; Li, J. Curcumin Represses Colorectal Cancer Cell Proliferation by Triggering Ferroptosis via PI3K/Akt/mTOR Signaling. Nutr. Cancer 2023, 75, 726–733. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, W.-J.; Peng, Y.-J.; Cheng, S.-D. Curcumin Reverses the Sunitinib Resistance in Clear Cell Renal Cell Carcinoma (ccRCC) through the Induction of Ferroptosis via the ADAMTS18 Gene. Transl. Cancer Res. 2021, 10, 3158–3167. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, Y.; Wang, Y.; Yu, T.; Zhu, C.; Zhang, X.; Guan, J. Curcumin Suppresses Tumorigenesis by Ferroptosis in Breast Cancer. PLoS ONE 2022, 17, e0261370. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Xu, Y.; Gao, E.; Zhang, W.; Zhang, F.; Xiang, Y.; Xu, L.; Dong, F. Amentoflavone Attenuates Cell Proliferation and Induces Ferroptosis in Human Gastric Cancer by miR-496/ATF2 Axis. Chem. Biol. Drug Des. 2023, 102, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhen, P.; Li, D.; Liu, X.; Ding, X.; Liu, H. Amentoflavone Promotes Ferroptosis by Regulating Reactive Oxygen Species (ROS)/5′AMP-Activated Protein Kinase (AMPK)/Mammalian Target of Rapamycin (mTOR) to Inhibit the Malignant Progression of Endometrial Carcinoma Cells. Bioengineered 2022, 13, 13269–13279. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, N.; Wang, H.; Wang, N.; Peng, H.; Wang, J.; Li, Y.; Liu, M.; Li, H.; Zhang, Y.; et al. Amentoflavone Suppresses Cell Proliferation and Induces Cell Death through Triggering Autophagy-Dependent Ferroptosis in Human Glioma. Life Sci. 2020, 247, 117425. [Google Scholar] [CrossRef]
- Kong, N.; Chen, X.; Feng, J.; Duan, T.; Liu, S.; Sun, X.; Chen, P.; Pan, T.; Yan, L.; Jin, T.; et al. Baicalin Induces Ferroptosis in Bladder Cancer Cells by Downregulating FTH1. Acta Pharm. Sin. B 2021, 11, 4045–4054. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Z.; Liu, H. Erianin Suppressed Lung Cancer Stemness and Chemotherapeutic Sensitivity via Triggering Ferroptosis. Environ. Toxicol. 2023, 39, 479–486. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Q.; Feng, J.; Yan, L.; Sun, Y.; Liu, S.; Xiang, Y.; Zhang, M.; Pan, T.; Chen, X.; et al. Erianin, a Novel Dibenzyl Compound in Dendrobium Extract, Inhibits Lung Cancer Cell Growth and Migration via Calcium/Calmodulin-Dependent Ferroptosis. Signal Transduct. Target. Ther. 2020, 5, 51. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, H.; Wu, H.; Liu, C.; Li, S.; Li, B.; Su, J.; Luo, S.; Li, Q. Gambogenic Acid Antagonizes the Expression and Effects of Long Non-Coding RNA NEAT1 and Triggers Autophagy and Ferroptosis in Melanoma. Biomed. Pharmacother. 2022, 154, 113636. [Google Scholar] [CrossRef]
- Wang, C.-X.; Chen, L.-H.; Zhuang, H.-B.; Shi, Z.-S.; Chen, Z.C.; Pan, J.-P.; Hong, Z.-S. Auriculasin Enhances ROS Generation to Regulate Colorectal Cancer Cell Apoptosis, Ferroptosis, Oxeiptosis, Invasion and Colony Formation. Biochem. Biophys. Res. Commun. 2022, 587, 99–106. [Google Scholar] [CrossRef]
- Liu, X.; Peng, X.; Cen, S.; Yang, C.; Ma, Z.; Shi, X. Wogonin Induces Ferroptosis in Pancreatic Cancer Cells by Inhibiting the Nrf2/GPX4 Axis. Front. Pharmacol. 2023, 14, 1129662. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Ma, J.; Li, X.-Y.; Wu, Y.; Shi, H.; Chen, Y.; Lu, G.; Shen, H.-M.; Lu, G.-D.; Zhou, J. Quercetin Induces P53-Independent Cancer Cell Death through Lysosome Activation by the Transcription Factor EB and Reactive Oxygen Species-Dependent Ferroptosis. Br. J. Pharmacol. 2021, 178, 1133–1148. [Google Scholar] [CrossRef]
- An, S.; Hu, M. Quercetin Promotes TFEB Nuclear Translocation and Activates Lysosomal Degradation of Ferritin to Induce Ferroptosis in Breast Cancer Cells. Comput. Intell. Neurosci. 2022, 2022, 5299218. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Zhou, J.; Zhu, Z.; Xu, Q.; Yin, Z.; Wang, Y.; Zheng, Z.; Zhao, H. Shikonin and Cisplatin Synergistically Overcome Cisplatin Resistance of Ovarian Cancer by Inducing Ferroptosis via Upregulation of HMOX1 to Promote Fe2+ Accumulation. Phytomedicine 2023, 112, 154701. [Google Scholar] [CrossRef]
- Qian, X.; Zhu, L.; Xu, M.; Liu, H.; Yu, X.; Shao, Q.; Qin, J. Shikonin Suppresses Small Cell Lung Cancer Growth via Inducing ATF3-Mediated Ferroptosis to Promote ROS Accumulation. Chem. Biol. Interact. 2023, 382, 110588. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wei, Y.; Yang, Q.; Cai, Y.; Zhu, K.; Chen, X. Scoparone Induces Both Apoptosis and Ferroptosis via Multiple Mechanisms in Non-Small-Cell Lung Cancer Cells. Toxicol In Vitro 2023, 91, 105627. [Google Scholar] [CrossRef]
- Zhou, X.; Kang, J.; Zhang, L.; Cheng, Y. Osthole Inhibits Malignant Phenotypes and Induces Ferroptosis in KRAS-Mutant Colorectal Cancer Cells via Suppressing AMPK/Akt Signaling. Cancer Chemother. Pharmacol. 2023, 92, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Pi, L.; Guo, H.; Hu, Z.; Zhou, C.; Hu, Q.; Peng, H.; Xiao, Z.; Zhang, Z.; Wang, M.; et al. Naringin Relieves Diabetic Cardiac Autonomic Neuropathy Mediated by P2Y14 Receptor in Superior Cervical Ganglion. Front. Pharmacol. 2022, 13, 873090. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, Y.; Qiao, Y.; Zheng, Y.; Yu, X.; Liu, F.; Wang, H.; Zheng, B.; Pan, S.; Ren, K.; et al. Quercetin Ameliorates Diabetic Kidney Injury by Inhibiting Ferroptosis via Activating Nrf2/HO-1 Signaling Pathway. Am. J. Chin. Med. 2023, 51, 997–1018. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Pan, S.; Feng, Y.; He, H.; Cheng, S.; Wang, L.; Wang, L.; Pathak, J.L. Resveratrol Alleviates Diabetic Periodontitis-Induced Alveolar Osteocyte Ferroptosis Possibly via Regulation of SLC7A11/GPX4. Nutrients 2023, 15, 2115. [Google Scholar] [CrossRef]
- Xiong, D.; Hu, W.; Han, X.; Cai, Y. Rhein Inhibited Ferroptosis and EMT to Attenuate Diabetic Nephropathy by Regulating the Rac1/NOX1/β-Catenin Axis. Front. Biosci. 2023, 28, 100. [Google Scholar] [CrossRef]
- Tan, H.; Chen, J.; Li, Y.; Li, Y.; Zhong, Y.; Li, G.; Liu, L.; Li, Y. Glabridin, a Bioactive Component of Licorice, Ameliorates Diabetic Nephropathy by Regulating Ferroptosis and the VEGF/Akt/ERK Pathways. Mol. Med. 2022, 28, 58. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Shaohuan, Q.; Pinfang, K.; Chao, S. Curcumin Attenuates Ferroptosis-Induced Myocardial Injury in Diabetic Cardiomyopathy through the Nrf2 Pathway. Cardiovasc. Ther. 2022, 2022, 3159717. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, S.; Li, Q.; Song, Z.; He, J.; Yang, S.; Yan, C.; Ling, H. Dihydromyricetin Alleviates Hippocampal Ferroptosis in Type 2 Diabetic Cognitive Impairment Rats via Inhibiting the JNK-Inflammatory Factor Pathway. Neurosci. Lett. 2023, 812, 137404. [Google Scholar] [CrossRef]
- Mei, Z.; Hong, Y.; Yang, H.; Cai, S.; Hu, Y.; Chen, Q.; Yuan, Z.; Liu, X. Ferulic Acid Alleviates High Fat Diet-Induced Cognitive Impairment by Inhibiting Oxidative Stress and Apoptosis. Eur. J. Pharmacol. 2023, 946, 175642. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, H.; Liu, H.; Chen, S.; Guo, H.; Ma, S.; Han, W.; Li, Y.; Wang, D. Isoforsythiaside Confers Neuroprotection against Alzheimer’s Disease by Attenuating Ferroptosis and Neuroinflammation in Vivo and in Vitro. Food Sci. Hum. Wellness 2023, 12, 1730–1742. [Google Scholar] [CrossRef]
- Luo, J.; Lu, Q.; Sun, B.; Shao, N.; Huang, W.; Hu, G.; Cai, B.; Si, W. Chrysophanol Improves Memory Impairment and Cell Injury by Reducing the Level of Ferroptosis in Aβ25–35 Treated Rat and PC12 Cells. 3 Biotech 2023, 13, 348. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Huang, B.; Huang, R. Sennoside A Restrains TRAF6 Level to Modulate Ferroptosis, Inflammation and Cognitive Impairment in Aging Mice with Alzheimer’s Disease. Int. Immunopharmacol. 2023, 120, 110290. [Google Scholar] [CrossRef]
- He, P.; Yan, S.; Wen, X.; Zhang, S.; Liu, Z.; Liu, X.; Xiao, C. Eriodictyol Alleviates Lipopolysaccharide-Triggered Oxidative Stress and Synaptic Dysfunctions in BV-2 Microglial Cells and Mouse Brain. J. Cell. Biochem. 2019, 120, 14756–14770. [Google Scholar] [CrossRef]
- Li, L.; Li, W.-J.; Zheng, X.-R.; Liu, Q.-L.; Du, Q.; Lai, Y.-J.; Liu, S.-Q. Eriodictyol Ameliorates Cognitive Dysfunction in APP/PS1 Mice by Inhibiting Ferroptosis via Vitamin D Receptor-Mediated Nrf2 Activation. Mol. Med. 2022, 28, 11. [Google Scholar] [CrossRef]
- Xu, Z.; You, Y.; Tang, Q.; Zeng, H.; Zhao, T.; Wang, J.; Li, F. Echinatin Mitigates Sevoflurane-Induced Hippocampal Neurotoxicity and Cognitive Deficits through Mitigation of Iron Overload and Oxidative Stress. Pharm. Biol. 2022, 60, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, E.; Yang, H.; Chen, Y.; Tao, L.; Xu, Y.; Chen, T.; Shen, X. Gastrodin Ameliorates Cognitive Dysfunction in Vascular Dementia Rats by Suppressing Ferroptosis via the Regulation of the Nrf2/Keap1-GPx4 Signaling Pathway. Molecules 2022, 27, 6311. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Cheng, H.; Su, J.; Wang, X.; Wang, Q.; Chu, J.; Li, Q. Gastrodin Protects against Glutamate-Induced Ferroptosis in HT-22 Cells through Nrf2/HO-1 Signaling Pathway. Toxicol In Vitro 2020, 62, 104715. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, Y.; Qin, Z.; Pei, H.; Zhai, L.; Guan, Q.; Wu, S.; Shen, H. Icariin Improves Cognitive Impairment by Inhibiting Ferroptosis of Nerve Cells. Aging 2023, 15, 11546–11553. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Zhao, W.; Lowe, S.; Bentley, R.; Hu, G.; Mei, H.; Jiang, X.; Sun, C.; Wu, Y.; Liu, Y. Quercetin Alleviates Kainic Acid-Induced Seizure by Inhibiting the Nrf2-Mediated Ferroptosis Pathway. Free Radic. Biol. Med. 2022, 191, 212–226, Corrigendum in Free Radic. Biol. Med. 2022, 193, 80. [Google Scholar] [CrossRef]
- Lin, Z.-H.; Liu, Y.; Xue, N.-J.; Zheng, R.; Yan, Y.-Q.; Wang, Z.-X.; Li, Y.-L.; Ying, C.-Z.; Song, Z.; Tian, J.; et al. Quercetin Protects against MPP+/MPTP-Induced Dopaminergic Neuron Death in Parkinson’s Disease by Inhibiting Ferroptosis. Oxidative Med. Cell. Longev. 2022, 2022, 7769355. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, L.; Zeng, Y.; Wang, Y.; Pei, T.; Xie, Z.; Xiong, Q.; Wei, H.; Li, W.; Li, J.; et al. Salidroside Alleviates Cognitive Impairment by Inhibiting Ferroptosis via Activation of the Nrf2/GPX4 Axis in SAMP8 Mice. Phytomedicine 2023, 114, 154762. [Google Scholar] [CrossRef]
- Yang, S.; Xie, Z.; Pei, T.; Zeng, Y.; Xiong, Q.; Wei, H.; Wang, Y.; Cheng, W. Salidroside Attenuates Neuronal Ferroptosis by Activating the Nrf2/HO1 Signaling Pathway in Aβ1-42-Induced Alzheimer’s Disease Mice and Glutamate-Injured HT22 Cells. Chin. Med. 2022, 17, 82. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Wu, Q.; Wang, S.; Yang, S.; Li, X.; Chen, N.; Li, L.; Zhang, L. Tetrahydroxy Stilbene Glycoside Ameliorates Alzheimer’s Disease in APP/PS1 Mice via Glutathione Peroxidase Related Ferroptosis. Int. Immunopharmacol. 2021, 99, 108002. [Google Scholar] [CrossRef]
- Liu, P.; Chen, W.; Kang, Y.; Wang, C.; Wang, X.; Liu, W.; Hayashi, T.; Qiu, Z.; Mizuno, K.; Hattori, S.; et al. Silibinin Ameliorates STING-Mediated Neuroinflammation via Downregulation of Ferroptotic Damage in a Sporadic Alzheimer’s Disease Model. Arch. Biochem. Biophys. 2023, 744, 109691. [Google Scholar] [CrossRef]
- Huang, L.; He, S.; Cai, Q.; Li, F.; Wang, S.; Tao, K.; Xi, Y.; Qin, H.; Gao, G.; Feng, D. Polydatin Alleviates Traumatic Brain Injury: Role of Inhibiting Ferroptosis. Biochem. Biophys. Res. Commun. 2021, 556, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, H.; Hu, Y.; Zhou, C.; Wu, J.; Wu, Y.; Wang, H.; Lenahan, C.; Huang, L.; Nie, S.; et al. Puerarin Attenuates Oxidative Stress and Ferroptosis via AMPK/PGC1α/Nrf2 Pathway after Subarachnoid Hemorrhage in Rats. Antioxidants 2022, 11, 1259. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Guo, J.; Ma, Y.; Li, J.; Ji, H.; Chen, Z.; Zheng, J. Anacardic Acid Improves Neurological Deficits in Traumatic Brain Injury by Anti-Ferroptosis and Anti-Inflammation. Exp. Neurol. 2023, 370, 114568. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.-Q.; Jia, J.-N.; Sun, Q.-Y.; Zhou, H.-H.; Jin, W.-L.; Mao, X.-Y. Baicalein Exerts Neuroprotective Effects in FeCl3-Induced Posttraumatic Epileptic Seizures via Suppressing Ferroptosis. Front. Pharmacol. 2019, 10, 638. [Google Scholar] [CrossRef]
- Zheng, B.; Zhou, X.; Pang, L.; Che, Y.; Qi, X. Baicalin Suppresses Autophagy-Dependent Ferroptosis in Early Brain Injury after Subarachnoid Hemorrhage. Bioengineered 2021, 12, 7794–7804. [Google Scholar] [CrossRef]
- Salvadori, M.; Rosso, G.; Bertoni, E. Update on Ischemia-Reperfusion Injury in Kidney Transplantation: Pathogenesis and Treatment. World J. Transplant. 2015, 5, 52–67. [Google Scholar] [CrossRef]
- Zhao, T.-T.; Yang, T.-L.; Gong, L.; Wu, P. Isorhamnetin Protects against Hypoxia/Reoxygenation-Induced Injure by Attenuating Apoptosis and Oxidative Stress in H9c2 Cardiomyocytes. Gene 2018, 666, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-D.; Ye, W.; Tian, W.; Meng, J.-R.; Zhang, M.; Sun, Y.; Zhang, H.-N.; Wang, S.-J.; Wu, K.-H.; Liu, C.-X.; et al. Old Targets, New Strategy: Apigenin-7-O-β-d-(-6″-p-Coumaroyl)-Glucopyranoside Prevents Endothelial Ferroptosis and Alleviates Intestinal Ischemia-Reperfusion Injury through HO-1 and MAO-B Inhibition. Free Radic. Biol. Med. 2022, 184, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Liu, H.; Yang, C.; Mo, H.; Wang, X.; Song, X.; Jiang, L.; Deng, P.; Chen, R.; Wu, P.; et al. Galangin Attenuates Myocardial Ischemic Reperfusion-Induced Ferroptosis by Targeting Nrf2/Gpx4 Signaling Pathway. Drug Des. Dev. Ther. 2023, 17, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xue, R.; Geng, Y.; Zhang, S. Galangin Inhibited Ferroptosis through Activation of the PI3K/AKT Pathway in Vitro and in Vivo. FASEB J. 2022, 36, e22569. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Huang, J.; Chen, Y.; Li, X.; Wen, J.; Tian, M.; Ren, J.; Zhou, L.; Yang, Q. Resveratrol Pretreatment Protects Neurons from Oxygen-Glucose Deprivation/Reoxygenation and Ischemic Injury through Inhibiting Ferroptosis. Biosci. Biotechnol. Biochem. 2022, 86, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tan, Y.; Ouyang, S.; He, J.; Liu, L. Resveratrol Protects against Myocardial Ischemia-Reperfusion Injury via Attenuating Ferroptosis. Gene 2022, 808, 145968. [Google Scholar] [CrossRef]
- Wang, X.; Shen, T.; Lian, J.; Deng, K.; Qu, C.; Li, E.; Li, G.; Ren, Y.; Wang, Z.; Jiang, Z.; et al. Resveratrol Reduces ROS-Induced Ferroptosis by Activating SIRT3 and Compensating the GSH/GPX4 Pathway. Mol. Med. 2023, 29, 137. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, T.; Zhang, W.-Y.; Huang, S.-R.; Hu, Y.; Sun, J. Rhein Attenuates Cerebral Ischemia-Reperfusion Injury via Inhibition of Ferroptosis through NRF2/SLC7A11/GPX4 Pathway. Exp. Neurol. 2023, 369, 114541. [Google Scholar] [CrossRef]
- Guo, L.; Shi, L. Vitexin Improves Cerebral Ischemia-reperfusion Injury by Attenuating Oxidative Injury and Ferroptosis via Keap1/Nrf2/HO-1signaling. Neurochem. Res. 2023, 48, 980–995. [Google Scholar] [CrossRef]
- Xu, S.; Wu, B.; Zhong, B.; Lin, L.; Ding, Y.; Jin, X.; Huang, Z.; Lin, M.; Wu, H.; Xu, D. Naringenin Alleviates Myocardial Ischemia/Reperfusion Injury by Regulating the Nuclear Factor-Erythroid Factor 2-Related Factor 2 (Nrf2)/System Xc-/Glutathione Peroxidase 4 (GPX4) Axis to Inhibit Ferroptosis. Bioengineered 2021, 12, 10924–10934. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mi, Y.; Wang, Y.; Meng, Q.; Xu, L.; Liu, Y.; Zhou, D.; Wang, Y.; Liang, D.; Li, W.; et al. Loureirin C Inhibits Ferroptosis after Cerebral Ischemia Reperfusion through Regulation of the Nrf2 Pathway in Mice. Phytomedicine 2023, 113, 154729. [Google Scholar] [CrossRef]
- Li, X.-N.; Shang, N.-Y.; Kang, Y.-Y.; Sheng, N.; Lan, J.-Q.; Tang, J.-S.; Wu, L.; Zhang, J.-L.; Peng, Y. Caffeic Acid Alleviates Cerebral Ischemic Injury in Rats by Resisting Ferroptosis via Nrf2 Signaling Pathway. Acta Pharmacol. Sin. 2023, 45, 248–267. [Google Scholar] [CrossRef]
- Li, M.; Meng, Z.; Yu, S.; Li, J.; Wang, Y.; Yang, W.; Wu, H. Baicalein Ameliorates Cerebral Ischemia-Reperfusion Injury by Inhibiting Ferroptosis via Regulating GPX4/ACSL4/ACSL3 Axis. Chem. Biol. Interact. 2022, 366, 110137. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-C.; Lin, J.-H.; Lee, W.-S.; Liu, C.-H.; Lin, T.-Y.; Yang, K.-T. Baicalein and Luteolin Inhibit Ischemia/Reperfusion-Induced Ferroptosis in Rat Cardiomyocytes. Int. J. Cardiol. 2023, 375, 74–86. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhai, Y.; Chen, J.; Xu, X.; Wang, H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules 2021, 11, 923. [Google Scholar] [CrossRef]
- Dai, C.; Li, H.; Wang, Y.; Tang, S.; Velkov, T.; Shen, J. Inhibition of Oxidative Stress and ALOX12 and NF-κB Pathways Contribute to the Protective Effect of Baicalein on Carbon Tetrachloride-Induced Acute Liver Injury. Antioxidants 2021, 10, 976. [Google Scholar] [CrossRef]
- Huang, W.; Chen, H.; He, Q.; Xie, W.; Peng, Z.; Ma, Q.; Huang, Q.; Chen, Z.; Liu, Y. Nobiletin Protects against Ferroptosis to Alleviate Sepsis-Associated Acute Liver Injury by Modulating the Gut Microbiota. Food Funct. 2023, 14, 7692–7704. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, K.; Wang, J.; He, K.; Zhou, X.; Nie, S. Quercetin Alleviates Acrylamide-Induced Liver Injury by Inhibiting Autophagy-Dependent Ferroptosis. J. Agric. Food Chem. 2023, 71, 7427–7439. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, L.; Zhang, X.; Ying, K.; Zhou, R.; Cai, W.; Wu, X.; Jiang, H.; Xu, Q.; Miao, D.; et al. Salidroside Ameliorates Acetaminophen-Induced Acute Liver Injury through the Inhibition of Endoplasmic Reticulum Stress-Mediated Ferroptosis by Activating the AMPK/SIRT1 Pathway. Ecotoxicol. Environ. Saf. 2023, 262, 115331. [Google Scholar] [CrossRef]
- Li, H.; Weng, Q.; Gong, S.; Zhang, W.; Wang, J.; Huang, Y.; Li, Y.; Guo, J.; Lan, T. Kaempferol Prevents Acetaminophen-Induced Liver Injury by Suppressing Hepatocyte Ferroptosis via Nrf2 Pathway Activation. Food Funct. 2023, 14, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-B.; Chu, X.-L.; Jin, Y.-X.; Jiang, J.-J.; Zhao, X.; Yu, M. Epigallocatechin Gallate Alleviates High-Fat Diet-Induced Hepatic Lipotoxicity by Targeting Mitochondrial ROS-Mediated Ferroptosis. Front. Pharmacol. 2023, 14, 1148814. [Google Scholar] [CrossRef]
- Liu, G.; Wei, C.; Yuan, S.; Zhang, Z.; Li, J.; Zhang, L.; Wang, G.; Fang, L. Wogonoside Attenuates Liver Fibrosis by Triggering Hepatic Stellate Cell Ferroptosis through SOCS1/P53/SLC7A11 Pathway. Phytother. Res. 2022, 36, 4230–4243. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-Y.; Chiu, V.; Hsieh, P.-C.; Huang, C.-Y.; Huang, S.J.; Tzeng, I.-S.; Tsai, F.-M.; Chen, M.-L.; Liu, C.-T.; Chen, Y.-R. Chrysophanol Attenuates Hepatitis B Virus X Protein-Induced Hepatic Stellate Cell Fibrosis by Regulating Endoplasmic Reticulum Stress and Ferroptosis. J. Pharmacol. Sci. 2020, 144, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Su, Z.; Xu, J.-H.; Ko, C.-Y. Danshensu Attenuated Lipopolysaccharide-Induced LX-2 and T6 Cells Activation through Regulation of Ferroptosis. Food Sci. Nutr. 2023, 11, 344–349. [Google Scholar] [CrossRef]
- Deng, S.; Li, J.; Li, L.; Lin, S.; Yang, Y.; Liu, T.; Zhang, T.; Xie, G.; Wu, D.; Xu, Y. Quercetin Alleviates Lipopolysaccharide-induced Acute Lung Injury by Inhibiting Ferroptosis via the Sirt1/Nrf2/Gpx4 Pathway. Int. J. Mol. Med. 2023, 52, 118. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zuo, Z.; Di, X.; Huang, Y.; Gong, G.; Xu, B.; Wang, L.; Zhang, X.; Liang, Z.; Hou, Y.; et al. Salidroside Attenuates HALI via IL-17A-Mediated Ferroptosis of Alveolar Epithelial Cells by Regulating Act1-TRAF6-P38 MAPK Pathway. Cell Commun. Signal. 2022, 20, 183. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, H.; Chen, Z. Puerarin Inhibits Ferroptosis and Inflammation of Lung Injury Caused by Sepsis in LPS Induced Lung Epithelial Cells. Front. Pediatr. 2021, 9, 706327. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Ci, X.; Cheng, H.; Yu, Q.; Li, D. Chicoric Acid Alleviates Lipopolysaccharide-Induced Acute Lung Injury in Mice through Anti-Inflammatory and Anti-Oxidant Activities. Int. Immunopharmacol. 2019, 66, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, J.; Yao, S.; Zheng, J.; Gong, X.; Xiao, B. Ferulic Acid Alleviates Alveolar Epithelial Barrier Dysfunction in Sepsis-Induced Acute Lung Injury by Activating the Nrf2/HO-1 Pathway and Inhibiting Ferroptosis. Pharm. Biol. 2022, 60, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, M.; Wu, H.; Fan, W.; Zhang, J.; Su, W.; Wang, Y.; Li, P. Naringenin Attenuates Inflammation, Apoptosis, and Ferroptosis in Silver Nanoparticle-Induced Lung Injury through a Mechanism Associated with Nrf2/HO-1 Axis: In Vitro and in Vivo Studies. Life Sci. 2022, 311, 121127. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Wang, H.; Ding, P.; Wang, C. Agrimonolide Inhibits Cancer Progression and Induces Ferroptosis and Apoptosis by Targeting SCD1 in Ovarian Cancer Cells. Phytomedicine 2022, 101, 154102. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, R.-F.; Li, J.; Yu, K.; Bi, K.-X. Alloimperatorin Activates Apoptosis, Ferroptosis, and Oxeiptosis to Inhibit the Growth and Invasion of Breast Cancer Cells in Vitro. Biochem. Cell Biol. 2022, 100, 213–222. [Google Scholar] [CrossRef]
- Liu, R.; Rong, G.; Liu, Y.; Huang, W.; He, D.; Lu, R. Delivery of Apigenin-Loaded Magnetic Fe2O3/Fe3O4@mSiO2 Nanocomposites to A549 Cells and Their Antitumor Mechanism. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111719. [Google Scholar] [CrossRef]
- Luo, Y.; Gao, X.; Zou, L.; Lei, M.; Feng, J.; Hu, Z. Bavachin Induces Ferroptosis through the STAT3/P53/SLC7A11 Axis in Osteosarcoma Cells. Oxidative Med. Cell. Longev. 2021, 2021, 1783485. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Y.; Yao, F.; Li, H.; Yang, Y.; Li, X.; Bai, Z.; Hu, Y.; Wang, P.; Xu, X. Ginkgo Biflavones Cause P53 Wild-Type Dependent Cell Death in a Transcription-Independent Manner of P53. J. Nat. Prod. 2023, 86, 346–356. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, C.; Zhong, W.; Wang, Q.; Zhang, D.; Zhang, J.; Xie, S.; Xu, M. Chrysin Induces Autophagy-Dependent Ferroptosis to Increase Chemosensitivity to Gemcitabine by Targeting CBR1 in Pancreatic Cancer Cells. Biochem. Pharmacol. 2021, 193, 114813. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, J.; Zhou, Y.; Gao, Q.; Wang, R.; Fu, Y.; Zheng, L.; Yu, H. Transcriptome Investigation and In Vitro Verification of Curcumin-Induced HO-1 as a Feature of Ferroptosis in Breast Cancer Cells. Oxidative Med. Cell. Longev. 2020, 2020, 3469840. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, X.; Zhao, L.; Ye, K.; Wang, S.; Lin, C. 3,5-diCQA Suppresses Colorectal Cancer Cell Growth through ROS/AMPK/mTOR Mediated Mitochondrial Dysfunction and Ferroptosis. Cell Cycle 2023, 22, 1951–1968. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, T.; Zhao, Y.; Meng, X.; Ding, W.; Wang, Q.; Liu, C.; Deng, H. Flavonoid 4,4′-Dimethoxychalcone Induced Ferroptosis in Cancer Cells by Synergistically Activating Keap1/Nrf2/HMOX1 Pathway and Inhibiting FECH. Free Radic. Biol. Med. 2022, 188, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.S.; Ryu, H.W.; Song, Y.N.; Kang, M.-J.; Huh, Y.H.; Park, J.-Y.; Oh, S.M.; Lee, S.-Y.; Park, Y.J.; Kim, D.-Y.; et al. Diplacone Isolated from Paulownia Tomentosa Mature Fruit Induces Ferroptosis-Mediated Cell Death through Mitochondrial Ca2+ Influx and Mitochondrial Permeability Transition. Int. J. Mol. Sci. 2023, 24, 7057. [Google Scholar] [CrossRef]
- Xing, M.; Ma, X.; Wang, X.; Wang, H.; Xie, M.; Zhang, Z.; Zhou, J. Emodin Disrupts the Notch1/Nrf2/GPX4 Antioxidant System and Promotes Renal Cell Ferroptosis. J. Appl. Toxicol. 2023, 43, 1702–1718. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Lai, K.; Deng, Y.; Lu, Z.; Song, C.; Wang, W.; Xu, C.; Li, N.; Geng, Q. Eriocitrin Inhibits Epithelial-Mesenchymal Transformation (EMT) in Lung Adenocarcinoma Cells via Triggering Ferroptosis. Aging 2023, 15, 10089–10104. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Tie, H.; Tian, W.; Zhao, Y.; Qin, L.; Guo, S.; Li, Q.; Bao, C. Eriodictyol Regulated Ferroptosis, Mitochondrial Dysfunction, and Cell Viability via Nrf2/HO-1/NQO1 Signaling Pathway in Ovarian Cancer Cells. J. Biochem. Mol. Toxicol. 2023, 37, e23368. [Google Scholar] [CrossRef]
- Xiu, Z.; Li, Y.; Fang, J.; Han, J.; Li, S.; Li, Y.; Yang, X.; Song, G.; Li, Y.; Jin, N.; et al. Inhibitory Effects of Esculetin on Liver Cancer Through Triggering NCOA4 Pathway-Mediation Ferritinophagy in Vivo and in Vitro. J. Hepatocell. Carcinoma 2023, 10, 611–629. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Tang, J.; Wang, R. Ferulic Acid Mitigates Growth and Invasion of Esophageal Squamous Cell Carcinoma through Inducing Ferroptotic Cell Death. Dis. Markers 2022, 2022, 4607966. [Google Scholar] [CrossRef]
- Xie, J.; Wang, H.; Xie, W.; Liu, Y.; Chen, Y. Gallic Acid Promotes Ferroptosis in Hepatocellular Carcinoma via Inactivating Wnt/β-Catenin Signaling Pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2023, in press. [Google Scholar] [CrossRef]
- Khorsandi, K.; Kianmehr, Z.; Hosseinmardi, Z.; Hosseinzadeh, R. Anti-Cancer Effect of Gallic Acid in Presence of Low Level Laser Irradiation: ROS Production and Induction of Apoptosis and Ferroptosis. Cancer Cell Int. 2020, 20, 18. [Google Scholar] [CrossRef]
- Tang, H.M.; Cheung, P.C.K. Gallic Acid Triggers Iron-Dependent Cell Death with Apoptotic, Ferroptotic, and Necroptotic Features. Toxins 2019, 11, 492. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Li, J.; Yang, X.; Huang, J.; Ji, C.; Li, X.; Li, L.; Zhou, J.; Hu, Y. Gambogenic Acid Induces Cell Death in Human Osteosarcoma through Altering Iron Metabolism, Disturbing the Redox Balance, and Activating the P53 Signaling Pathway. Chem. Biol. Interact. 2023, 382, 110602. [Google Scholar] [CrossRef]
- Wang, M.; Li, S.; Wang, Y.; Cheng, H.; Su, J.; Li, Q. Gambogenic Acid Induces Ferroptosis in Melanoma Cells Undergoing Epithelial-to-Mesenchymal Transition. Toxicol. Appl. Pharmacol. 2020, 401, 115110. [Google Scholar] [CrossRef]
- Liu, C.-M.; An, L.; Wu, Z.; Ouyang, A.-J.; Su, M.; Shao, Z.; Lin, Y.; Liu, X.; Jiang, Y. 6-Gingerol Suppresses Cell Viability, Migration and Invasion via Inhibiting EMT, and Inducing Autophagy and Ferroptosis in LPS-Stimulated and LPS-Unstimulated Prostate Cancer Cells. Oncol. Lett. 2022, 23, 187. [Google Scholar] [CrossRef]
- Tsai, Y.; Xia, C.; Sun, Z. The Inhibitory Effect of 6-Gingerol on Ubiquitin-Specific Peptidase 14 Enhances Autophagy-Dependent Ferroptosis and Anti-Tumor in Vivo and in Vitro. Front. Pharmacol. 2020, 11, 598555. [Google Scholar] [CrossRef]
- Lou, J.-S.; Zhao, L.-P.; Huang, Z.-H.; Chen, X.-Y.; Xu, J.-T.; Tai, W.C.-S.; Tsim, K.W.K.; Chen, Y.-T.; Xie, T. Ginkgetin Derived from Ginkgo Biloba Leaves Enhances the Therapeutic Effect of Cisplatin via Ferroptosis-Mediated Disruption of the Nrf2/HO-1 Axis in EGFR Wild-Type Non-Small-Cell Lung Cancer. Phytomedicine 2021, 80, 153370. [Google Scholar] [CrossRef]
- Lai, X.; Sun, Y.; Zhang, X.; Wang, D.; Wang, J.; Wang, H.; Zhao, Y.; Liu, X.; Xu, X.; Song, H.; et al. Honokiol Induces Ferroptosis by Upregulating HMOX1 in Acute Myeloid Leukemia Cells. Front. Pharmacol. 2022, 13, 897791. [Google Scholar] [CrossRef]
- Guo, C.; Liu, P.; Deng, G.; Han, Y.; Chen, Y.; Cai, C.; Shen, H.; Deng, G.; Zeng, S. Honokiol Induces Ferroptosis in Colon Cancer Cells by Regulating GPX4 Activity. Am. J. Cancer Res. 2021, 11, 3039–3054. [Google Scholar]
- Xu, W.; Ding, J.; Li, B.; Sun, T.; You, X.; He, Q.; Sheng, W. Effects of Icariin and Curcumol on Autophagy, Ferroptosis, and Lipid Metabolism Based on miR-7/m-TOR/SREBP1 Pathway on Prostate Cancer. Biofactors 2023, 49, 438–456. [Google Scholar] [CrossRef]
- Yu, R.; Zhou, Y.; Shi, S.; Wang, X.; Huang, S.; Ren, Y. Icariside II Induces Ferroptosis in Renal Cell Carcinoma Cells by Regulating the miR-324-3p/GPX4 Axis. Phytomedicine 2022, 102, 154182. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Wang, X.; Zhu, Q.; Liu, L.; Qiu, S.; Zou, L.; Liu, K.; Li, G.; Miao, H.; et al. Isoliquiritigenin Induces HMOX1 and GPX4-Mediated Ferroptosis in Gallbladder Cancer Cells. Chin. Med. J. 2023, 136, 2210–2220. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Zhang, J.; Luo, C. Isoliquiritin Modulates Ferroptosis via NF-κB Signaling Inhibition and Alleviates Doxorubicin Resistance in Breast Cancer. Immunopharmacol. Immunotoxicol. 2023, 45, 443–454. [Google Scholar] [CrossRef]
- Feng, S.; Li, Y.; Huang, H.; Huang, H.; Duan, Y.; Yuan, Z.; Zhu, W.; Mei, Z.; Luo, L.; Yan, P. Isoorientin Reverses Lung Cancer Drug Resistance by Promoting Ferroptosis via the SIRT6/Nrf2/GPX4 Signaling Pathway. Eur. J. Pharmacol. 2023, 954, 175853. [Google Scholar] [CrossRef]
- Luo, X.; Gong, Y.; Jiang, Q.; Wang, Q.; Li, S.; Liu, L. Isoquercitrin Promotes Ferroptosis and Oxidative Stress in Nasopharyngeal Carcinoma via the AMPK/NF-κB Pathway. J. Biochem. Mol. Toxicol. 2023, 38, e23542. [Google Scholar] [CrossRef]
- Fu, W.; Xu, L.; Chen, Y.; Zhang, Z.; Chen, S.; Li, Q.; You, X. Luteolin Induces Ferroptosis in Prostate Cancer Cells by Promoting TFEB Nuclear Translocation and Increasing Ferritinophagy. Prostate 2023, 84, 223–236. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, L.; Chen, H.; Zheng, Y.; Tan, X.; Zhang, G.; Jiang, R.; Yu, H.; Lin, S.; Wei, Y.; et al. Luteolin Exhibits Synergistic Therapeutic Efficacy with Erastin to Induce Ferroptosis in Colon Cancer Cells through the HIC1-Mediated Inhibition of GPX4 Expression. Free Radic. Biol. Med. 2023, 208, 530–544. [Google Scholar] [CrossRef]
- Gao, Z.; Jiang, J.; Hou, L.; Ji, F. Lysionotin Induces Ferroptosis to Suppress Development of Colorectal Cancer via Promoting Nrf2 Degradation. Oxidative Med. Cell. Longev. 2022, 2022, 1366957. [Google Scholar] [CrossRef]
- Feng, S.; Zhou, Y.; Huang, H.; Lin, Y.; Zeng, Y.; Han, S.; Huang, K.; Liu, Q.; Zhu, W.; Yuan, Z.; et al. Nobiletin Induces Ferroptosis in Human Skin Melanoma Cells Through the GSK3β-Mediated Keap1/Nrf2/HO-1 Signalling Pathway. Front. Genet. 2022, 13, 865073. [Google Scholar] [CrossRef]
- Lian, G.; Huang, X.-X.; Zeng, Y. Puerarin Induces Ferroptosis in Colorectal Cancer Cells via Triggering NCOA4 Upregulation. Nutr. Cancer 2023, 75, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Yan, D.; Jiang, B.; Xue, Q.; Chen, X.; Huang, Q.; Qi, L.; Tang, D.; Chen, X.; Liu, J. Plumbagin Is a Novel GPX4 Protein Degrader That Induces Apoptosis in Hepatocellular Carcinoma Cells. Free Radic. Biol. Med. 2023, 203, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, Q.; Ma, M.; Guo, H. Quercetin Inhibits the Progression of Endometrial HEC-1-A Cells by Regulating Ferroptosis-a Preliminary Study. Eur. J. Med. Res. 2022, 27, 292. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, W.; Sheng, Y.; Sun, J.; Wen, D. Resveratrol Drives Ferroptosis of Acute Myeloid Leukemia Cells through Hsa-miR-335-5p/NFS1/GPX4 Pathway in a ROS-Dependent Manner. Cell. Mol. Biol. 2023, 69, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Minchao, K.; Jizhao, W.; Rui, Z.; Guangjian, Z.; Jin, Z.; Meihe, L. Resveratrol Improves the Cytotoxic Effect of CD8 +T Cells in the Tumor Microenvironment by Regulating HMMR/Ferroptosis in Lung Squamous Cell Carcinoma. J. Pharm. Biomed. Anal. 2023, 229, 115346. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Liu, Y.; Zheng, S. Rhamnazin Inhibits Hepatocellular Carcinoma Cell Aggressiveness in Vitro via Glutathione Peroxidase 4-Dependent Ferroptosis. Tohoku J. Exp. Med. 2022, 258, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhou, X.; Li, J.; Yao, X.-C.; Liu, W.-L.; Kang, F.-H.; Zou, Z.-X.; Xu, K.-P.; Xu, P.-S.; Tan, G.-S. Identification of a New Natural Biflavonoids against Breast Cancer Cells Induced Ferroptosis via the Mitochondrial Pathway. Bioorganic Chem. 2021, 109, 104744. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Z.; Tang, H.; Chen, B.; Cai, Y.; Wei, Y.; Zhao, W.; Wu, Z.B.; Shang, H. Mitochondrial Inhibitor Rotenone Triggers and Enhances Neuronal Ferroptosis Following Intracerebral Hemorrhage. ACS Chem. Neurosci. 2023, 14, 1071–1079. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Feng, Y.; Nie, X.; Liu, Q.; Du, X.; Wu, Y.; Liu, T.; Zhu, X. Rotenone, an Environmental Toxin, Causes Abnormal Methylation of the Mouse Brain Organoid’s Genome and Ferroptosis. Int. J. Med. Sci. 2022, 19, 1184–1197. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Shen, Y.; Xiao, H. Resveratrol Attenuates Rotenone-Induced Inflammation and Oxidative Stress via STAT1 and Nrf2/Keap1/SLC7A11 Pathway in a Microglia Cell Line. Pathol. Res. Pract. 2021, 225, 153576. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, S.; Zhang, X.; Zhao, H. S-3′-Hydroxy-7′, 2′, 4′-Trimethoxyisoxane, a Novel Ferroptosis Inducer, Promotes NSCLC Cell Death through Inhibiting Nrf2/HO-1 Signaling Pathway. Front. Pharmacol. 2022, 13, 973611. [Google Scholar] [CrossRef]
- Lai, Y.; Zeng, F.; Chen, Z.; Feng, M.; Huang, Y.; Qiu, P.; Zeng, L.; Ke, Y.; Deng, G.; Gao, J. Shikonin Could Be Used to Treat Tubal Pregnancy via Enhancing Ferroptosis Sensitivity. Drug Des. Dev. Ther. 2022, 16, 2083–2099. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fu, H.; Fang, L.; Chai, H.; Gao, T.; Chen, Z.; Qian, S. Shikonin Induces Ferroptosis in Multiple Myeloma via GOT1-Mediated Ferritinophagy. Front. Oncol. 2022, 12, 1025067. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Shi, W.; Liu, S.; Liu, H.; Liu, Y.; Ge, P.; Zhang, H. Fe(III)-Shikonin Supramolecular Nanomedicine for Combined Therapy of Tumor via Ferroptosis and Necroptosis. Adv. Healthc. Mater. 2022, 11, e2101926. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Lin, X.; Yang, C.; Chen, Z.; Wang, L.; Li, Q.; Ma, J.; Zhan, F.; Wang, Y.; Yan, J.; et al. Theaflavin-3,3′-Digallate Plays a ROS-Mediated Dual Role in Ferroptosis and Apoptosis via the MAPK Pathway in Human Osteosarcoma Cell Lines and Xenografts. Oxidative Med. Cell. Longev. 2022, 2022, 8966368. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhao, J.-F.; Wang, Y.-M.; Wu, X.; Ye, L. Tiliroside Induces Ferroptosis to Repress the Development of Triple-Negative Breast Cancer Cells. Tissue Cell 2023, 83, 102116. [Google Scholar] [CrossRef]
- Yang, C.; Lu, T.; Liu, M.; Yuan, X.; Li, D.; Zhang, J.; Zhou, L.; Xu, M. Tiliroside Targets TBK1 to Induce Ferroptosis and Sensitize Hepatocellular Carcinoma to Sorafenib. Phytomedicine 2023, 111, 154668. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, H.; Miao, J.; Sheng, Q.; Xu, J.; Gao, Z.; Zhang, X.; Song, Y.; Chen, K. The Natural Flavone Acacetin Protects against High-Fat Diet-Induced Lipid Accumulation in the Liver via the Endoplasmic Reticulum Stress/Ferroptosis Pathway. Biochem. Biophys. Res. Commun. 2023, 640, 183–191. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Miao, C.; Yin, G.; Zhang, Z.; Sun, R.; Ni, S. Acetyl Zingerone Ameliorates Osteoarthritis by Inhibiting Chondrocyte Programmed Cell Death. Mol. Med. Rep. 2023, 28, 202. [Google Scholar] [CrossRef]
- He, Y.; Xi, J.; Fang, J.; Zhang, B.; Cai, W. Aloe-Emodin Alleviates Doxorubicin-Induced Cardiotoxicity via Inhibition of Ferroptosis. Free Radic. Biol. Med. 2023, 206, 13–21. [Google Scholar] [CrossRef]
- Chen, G.; Li, C.; Zhang, L.; Yang, J.; Meng, H.; Wan, H.; He, Y. Hydroxysafflor Yellow A and Anhydrosafflor Yellow B Alleviate Ferroptosis and Parthanatos in PC12 Cells Injured by OGD/R. Free Radic. Biol. Med. 2022, 179, 1–10. [Google Scholar] [CrossRef]
- Han, D.; Yao, Y.; Chen, L.; Miao, Z.; Xu, S. Apigenin Ameliorates Di(2-Ethylhexyl) Phthalate-Induced Ferroptosis: The Activation of Glutathione Peroxidase 4 and Suppression of Iron Intake. Food Chem. Toxicol. 2022, 164, 113089. [Google Scholar] [CrossRef]
- Chen, B.; Li, X.; Ouyang, X.; Liu, J.; Liu, Y.; Chen, D. Comparison of Ferroptosis-Inhibitory Mechanisms between Ferrostatin-1 and Dietary Stilbenes (Piceatannol and Astringin). Molecules 2021, 26, 1092. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Zhu, W.; Fu, J.; Liang, A.; Zheng, T.; Wen, Z.; Wu, X.; Peng, Y.; Yuan, S.; Wu, X. Avicularin Alleviates Acute Liver Failure by Regulation of the TLR4/MyD88/NF-κB and Nrf2/HO-1/GPX4 Pathways to Reduce Inflammation and Ferroptosis. J. Cell. Mol. Med. 2023, 27, 3326–3338. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Zou, Y.; Zhou, W.; Wang, Y. Baicalein, a Component of Banxia Xiexin Decoction, Alleviates CPT-11-Induced Gastrointestinal Dysfunction by Inhibiting ALOX15-Mediated Ferroptosis. Chem. Biol. Drug Des. 2023, 102, 1568–1577. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, F.; Wang, P.; Ran, Y.; Liu, C.; Lu, J.; Zhang, M.; Yao, L. Baicalein Relieves Brain Injury via Inhibiting Ferroptosis and Endoplasmic Reticulum Stress in a Rat Model of Cardiac Arrest. Shock 2023, 59, 434–441. [Google Scholar] [CrossRef]
- Yu, M.; Li, H.; Wang, B.; Wu, Z.; Wu, S.; Jiang, G.; Wang, H.; Huang, Y. Baicalein Ameliorates Polymyxin B-Induced Acute Renal Injury by Inhibiting Ferroptosis via Regulation of SIRT1/P53 Acetylation. Chem. Biol. Interact. 2023, 382, 110607. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shen, K.; Yu, H.; Fan, W. Baicalein Limits Osteoarthritis Development by Inhibiting Chondrocyte Ferroptosis. Free Radic. Biol. Med. 2023, 196, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.-H.; Li, S.-Q.; Ke, J.-Y.; Wang, Y.; Zhao, M.-Z.; Li, J.; Li, M.-Q.; Zhu, Z.-L. Baicalein Relieves Ferroptosis-Mediated Phagocytosis Inhibition of Macrophages in Ovarian Endometriosis. Curr. Issues Mol. Biol. 2022, 44, 6189–6204. [Google Scholar] [CrossRef]
- Yang, M.; Li, X.; Li, H.; Zhang, X.; Liu, X.; Song, Y. Baicalein Inhibits RLS3-Induced Ferroptosis in Melanocytes. Biochem. Biophys. Res. Commun. 2021, 561, 65–72. [Google Scholar] [CrossRef]
- Fan, Z.; Cai, L.; Wang, S.; Wang, J.; Chen, B. Baicalin Prevents Myocardial Ischemia/Reperfusion Injury Through Inhibiting ACSL4 Mediated Ferroptosis. Front. Pharmacol. 2021, 12, 628988. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yang, J.; Pan, Z.; Zhang, G.; Chen, B.; Li, S.; Xiao, J.; Tan, F.; Wang, Z.; Chen, P.; et al. Biochanin A Protects against Iron Overload Associated Knee Osteoarthritis via Regulating Iron Levels and NRF2/System Xc-/GPX4 Axis. Biomed. Pharmacothery 2023, 157, 113915. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Soriano-Castell, D.; Kepchia, D.; Duggan, B.M.; Currais, A.; Schubert, D.; Maher, P. Cannabinol Inhibits Oxytosis/Ferroptosis by Directly Targeting Mitochondria Independently of Cannabinoid Receptors. Free Radic. Biol. Med. 2022, 180, 33–51, Corrigendum in Free Radic. Biol. Med. 2022, 184, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, Z.; Yan, M.; Zhang, Q.; Jiang, T.; Xue, J. Calycosin Decreases Cerebral Ischemia/Reperfusion Injury by Suppressing ACSL4-Dependent Ferroptosis. Arch. Biochem. Biophys. 2023, 734, 109488. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Shen, P.; Wang, C.; Gao, J.; Ye, C.; Wu, F. Calycosin Plays a Protective Role in Diabetic Kidney Disease through the Regulation of Ferroptosis. Pharm. Biol. 2022, 60, 990–996. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, L.; Tang, P.; Chen, D.; Li, Y.; Li, J.; Bao, C. Carthamin Yellow Improves Cerebral Ischemia-reperfusion Injury by Attenuating Inflammation and Ferroptosis in Rats. Int. J. Mol. Med. 2021, 47, 52. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Zhang, W.; Hua, Y.; Chen, B.; Wu, Q.; Chen, D.; Liu, S.; Li, X. Ferroptosis-Inhibitory Difference between Chebulagic Acid and Chebulinic Acid Indicates Beneficial Role of HHDP. Molecules 2021, 26, 4300. [Google Scholar] [CrossRef]
- Wang, K.; Gao, S.; Wang, J.; Yu, F.; Ye, C. Protective Effects of Chicoric Acid on LPS-Induced Endometritis in Mice via Inhibiting Ferroptosis by Nrf2/HO-1 Signal Axis. Int. Immunopharmacol. 2022, 113, 109435. [Google Scholar] [CrossRef]
- Li, L.-Y.; Wang, Q.; Deng, L.; Lin, Z.; Lin, J.-J.; Wang, X.-Y.; Shen, T.-Y.; Zheng, Y.-H.; Lin, W.; Li, P.-J.; et al. Chlorogenic Acid Alleviates Hypoxic-Ischemic Brain Injury in Neonatal Mice. Neural Regen. Res. 2023, 18, 568–576. [Google Scholar] [CrossRef]
- Wu, X.; Wang, J.; Li, B.; Gong, M.; Cao, C.; Song, L.; Qin, L.; Wang, Y.; Zhang, Y.; Li, Y. Chlorogenic Acid, Rutin, and Quercetin from Lysimachia Christinae Alleviate Triptolide-Induced Multi-Organ Injury in Vivo by Modulating Immunity and AKT/mTOR Signal Pathway to Inhibit Ferroptosis and Apoptosis. Toxicol. Appl. Pharmacol. 2023, 467, 116479. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Yang, T.; Wang, H.; Zhao, S.; Sun, N.; Chen, Y.; Zhang, H.; Fan, H. Chlorogenic Acid Alleviates Chronic Stress-Induced Duodenal Ferroptosis via the Inhibition of the IL-6/JAK2/STAT3 Signaling Pathway in Rats. J. Agric. Food Chem. 2022, 70, 4353–4361. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Jiao, J.; Yan, M.; Wang, J.; Li, Q.; Shabuerjiang, L.; Lu, Y.; Song, Q.; Bi, L.; Huang, G.; et al. Chrysin Protects against Cerebral Ischemia-Reperfusion Injury in Hippocampus via Restraining Oxidative Stress and Transition Elements. Biomed. Pharmacother. 2023, 161, 114534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Liu, G.; Han, Q.; Diao, Y.; Liu, J. Corilagin Attenuates Intestinal Ischemia/Reperfusion Injury in Mice by Inhibiting Ferritinophagy-Mediated Ferroptosis through Disrupting NCOA4-Ferritin Interaction. Life Sci. 2023, 334, 122176. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, W.; Wang, J.; Bai, X. Curculigoside Inhibits Ferroptosis in Ulcerative Colitis through the Induction of GPX4. Life Sci. 2020, 259, 118356. [Google Scholar] [CrossRef]
- Kar, F.; Yıldız, F.; Hacioglu, C.; Kar, E.; Donmez, D.B.; Senturk, H.; Kanbak, G. LoxBlock-1 or Curcumin Attenuates Liver, Pancreas and Cardiac Ferroptosis, Oxidative Stress and Injury in Ischemia/Reperfusion-Damaged Rats by Facilitating ACSL/GPx4 Signaling. Tissue Cell 2023, 82, 102114. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Yan, H.; Wu, H.; Cao, S.; Zhao, W.; Dong, T.; Zhou, A. Protective Effect of Curcumin on Hepatolenticular Degeneration through Copper Excretion and Inhibition of Ferroptosis. Phytomedicine 2023, 113, 154539. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, H.; Huang, W.; Liu, Z.; Chen, Z.; Zhao, X.; Ding, T.; Qin, W.; Shen, Y. Curcumin Attenuates Periodontal Injury via Inhibiting Ferroptosis of Ligature-Induced Periodontitis in Mice. Int. J. Mol. Sci. 2023, 24, 9835. [Google Scholar] [CrossRef]
- Wu, L.; Dong, B.; Chen, Q.; Wang, Y.; Han, D.; Zhu, X.; Liu, H.; Zhang, Z.; Yang, Y.; Xie, S.; et al. Effects of Curcumin on Oxidative Stress and Ferroptosis in Acute Ammonia Stress-Induced Liver Injury in Gibel Carp (Carassius Gibelio). Int. J. Mol. Sci. 2023, 24, 6441. [Google Scholar] [CrossRef]
- Zhou, Y.; Jia, Z.; Wang, J.; Huang, S.; Yang, S.; Xiao, S.; Xia, D.; Zhou, Y. Curcumin Reverses Erastin-Induced Chondrocyte Ferroptosis by Upregulating Nrf2. Heliyon 2023, 9, e20163. [Google Scholar] [CrossRef]
- Olanlokun, J.O.; Abiodun, W.O.; Ebenezer, O.; Koorbanally, N.A.; Olorunsogo, O.O. Curcumin Modulates Multiple Cell Death, Matrix Metalloproteinase Activation and Cardiac Protein Release in Susceptible and Resistant Plasmodium Berghei-Infected Mice. Biomed. Pharmacother. 2022, 146, 112454. [Google Scholar] [CrossRef]
- Tang, X.; Li, Z.; Yu, Z.; Li, J.; Zhang, J.; Wan, N.; Zhang, J.; Cao, J. Effect of Curcumin on Lung Epithelial Injury and Ferroptosis Induced by Cigarette Smoke. Hum. Exp. Toxicol. 2021, 40, S753–S762. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Han, M.; Li, R.; Zhou, L.; Zhang, Y.; Duan, L.; Su, S.; Li, M.; Wang, Q.; Chen, T.; et al. Curcumin Nanoparticles Inhibiting Ferroptosis for the Enhanced Treatment of Intracerebral Hemorrhage. Int. J. Nanomed. 2021, 16, 8049–8065. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Hue, M.; García-Caballero, C.; Palomino-Antolín, A.; Rubio-Navarro, A.; Vázquez-Carballo, C.; Herencia, C.; Martín-Sanchez, D.; Farré-Alins, V.; Egea, J.; Cannata, P.; et al. Curcumin Reduces Renal Damage Associated with Rhabdomyolysis by Decreasing Ferroptosis-Mediated Cell Death. FASEB J. 2019, 33, 8961–8975. [Google Scholar] [CrossRef]
- Kose, T.; Vera-Aviles, M.; Sharp, P.A.; Latunde-Dada, G.O. Curcumin and (−)- Epigallocatechin-3-Gallate Protect Murine MIN6 Pancreatic Beta-Cells against Iron Toxicity and Erastin-Induced Ferroptosis. Pharmaceuticals 2019, 12, 26. [Google Scholar] [CrossRef]
- He, H.; Luo, Y.; Qiao, Y.; Zhang, Z.; Yin, D.; Yao, J.; You, J.; He, M. Curcumin Attenuates Doxorubicin-Induced Cardiotoxicity via Suppressing Oxidative Stress and Preventing Mitochondrial Dysfunction Mediated by 14-3-3γ. Food Funct. 2018, 9, 4404–4418. [Google Scholar] [CrossRef]
- Du, Y.-W.; Li, X.-K.; Wang, T.-T.; Zhou, L.; Li, H.-R.; Feng, L.; Ma, H.; Liu, H.-B. Cyanidin-3-Glucoside Inhibits Ferroptosis in Renal Tubular Cells after Ischemia/Reperfusion Injury via the AMPK Pathway. Mol. Med. 2023, 29, 42. [Google Scholar] [CrossRef]
- Zhang, P.; Rong, K.; Guo, J.; Cui, L.; Kong, K.; Zhao, C.; Yang, H.; Xu, H.; Qin, A.; Ma, P.; et al. Cynarin Alleviates Intervertebral Disc Degeneration via Protecting Nucleus Pulposus Cells from Ferroptosis. Biomed. Pharmacother. 2023, 165, 115252. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wu, R.; Wang, F.; Li, S.; Li, L.; Li, Y.; Qin, P.; Wei, M.; Yang, J.; Wu, J.; et al. Liberation of Daidzein by Gut Microbial β-Galactosidase Suppresses Acetaminophen-Induced Hepatotoxicity in Mice. Cell Host Microbe 2023, 31, 766–780.e7. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Chen, S.; Yang, J.; Jing, J.; Li, J.; Wu, X.; Wang, J.; Wang, J.; Zhang, G.; et al. Dihydromyricetin Attenuates Intracerebral Hemorrhage by Reversing the Effect of LCN2 via the System Xc- Pathway. Phytomedicine 2023, 115, 154756. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, M.; Wang, W.; Zhou, S.; Yu, M.; Qiu, X.; Jiang, S.; Wang, X.; Tang, C.; Li, S.; et al. Dihydromyricetin Attenuates Cisplatin-Induced Acute Kidney Injury by Reducing Oxidative Stress, Inflammation and Ferroptosis. Toxicol. Appl. Pharmacol. 2023, 473, 116595. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, T.; Li, P.; Wang, D.; Liu, T.; Xu, S. Dihydromyricetin Attenuates Cerebral Ischemia Reperfusion Injury by Inhibiting SPHK1/mTOR Signaling and Targeting Ferroptosis. Drug Des. Dev. Ther. 2022, 16, 3071–3085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Zhang, H.; Zeng, T. Dihydromyricetin Inhibits Oxidative Stress and Apoptosis in Oxygen and Glucose Deprivation/Reoxygenation-induced HT22 Cells by Activating the Nrf2/HO-1 Pathway. Mol. Med. Rep. 2021, 23, 397. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, Y.; Luo, L.; Zong, D.; Li, H.; Zeng, Z.; Cui, Y.; Meng, W.; Chen, Y. Dihydroquercetin Suppresses Cigarette Smoke Induced Ferroptosis in the Pathogenesis of Chronic Obstructive Pulmonary Disease by Activating Nrf2-Mediated Pathway. Phytomedicine 2022, 96, 153894. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Sun, Y.; Zhou, N.; Wu, W.; Zheng, W.; Wang, Y. Dihydroquercetin Attenuates Silica-Induced Pulmonary Fibrosis by Inhibiting Ferroptosis Signaling Pathway. Front. Pharmacol. 2022, 13, 845600. [Google Scholar] [CrossRef]
- Liu, T.; Yang, L.; Gao, H.; Zhuo, Y.; Tu, Z.; Wang, Y.; Xun, J.; Zhang, Q.; Zhang, L.; Wang, X. 3,4-Dihydroxyphenylethyl Alcohol Glycoside Reduces Acetaminophen-Induced Acute Liver Failure in Mice by Inhibiting Hepatocyte Ferroptosis and Pyroptosis. PeerJ 2022, 10, e13082. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jin, L.; Yang, B. Diosmetin Alleviates S. aureus-Induced Mastitis by Inhibiting SIRT1/GPX4 Mediated Ferroptosis. Life Sci. 2023, 331, 122060. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, S.; Wang, J.; Liu, J.; Liu, Y.; Zhou, S.; Li, X.; Han, L.; Pang, W.; Yao, W.; et al. Echinatin Maintains Glutathione Homeostasis in Vascular Smooth Muscle Cells to Protect against Matrix Remodeling and Arterial Stiffening. Matrix Biol. 2023, 119, 1–18. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Z.; Pei, C.; Zhao, S.; Jia, N.; Huang, D.; Wang, X.; Wu, Y.; Shi, S.; He, Y.; et al. Eleutheroside B Ameliorated High Altitude Pulmonary Edema by Attenuating Ferroptosis and Necroptosis through Nrf2-Antioxidant Response Signaling. Biomed. Pharmacother. 2022, 156, 113982. [Google Scholar] [CrossRef]
- Ji, J.; Tao, P.; Wang, Q.; Cui, M.; Cao, M.; Xu, Y. Emodin Attenuates Diabetic Kidney Disease by Inhibiting Ferroptosis via Upregulating Nrf2 Expression. Aging 2023, 15, 7673–7688. [Google Scholar] [CrossRef]
- Huang, L.; Bian, M.; Lu, S.; Wang, J.; Yu, J.; Jiang, L.; Zhang, J. Engeletin Alleviates Erastin-Induced Oxidative Stress and Protects against Ferroptosis via Nrf2/Keap1 Pathway in Bone Marrow Mesenchymal Stem Cells. Tissue Cell 2023, 82, 102040. [Google Scholar] [CrossRef]
- Li, F.; Hao, S.; Gao, J.; Jiang, P. EGCG Alleviates Obesity-Exacerbated Lung Cancer Progression by STAT1/SLC7A11 Pathway and Gut Microbiota. J. Nutr. Biochem. 2023, 120, 109416. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, A.; Tan, L.; Tang, D.; Chen, W.; Lai, X.; Gu, K.; Chen, J.; Chen, D.; Tang, Q. Epigallocatechin-3-Gallate Alleviates Liver Oxidative Damage Caused by Iron Overload in Mice through Inhibiting Ferroptosis. Nutrients 2023, 15, 1993. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, N.; Gan, X.; Chen, L.; Wang, R.; Liang, R.; Jian, J. EGCG Attenuated Acute Myocardial Infarction by Inhibiting Ferroptosis via miR-450b-5p/ACSL4 Axis. Phytomedicine 2023, 119, 154999. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, L.; Qiao, Y.; Yang, B.; Yin, D.; He, M. Epigallocatechin-3-Gallate Pretreatment Alleviates Doxorubicin-Induced Ferroptosis and Cardiotoxicity by Upregulating AMPKα2 and Activating Adaptive Autophagy. Redox Biol. 2021, 48, 102185. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Chen, L.; Duan, Y.; Kuang, X.; Peng, Z.; Li, C.; Li, Y.; Xiao, Y.; Jin, H.; et al. EGCG Modulates PKD1 and Ferroptosis to Promote Recovery in ST Rats. Transl. Neurosci. 2020, 11, 173–181. [Google Scholar] [CrossRef]
- Xie, L.-W.; Cai, S.; Zhao, T.-S.; Li, M.; Tian, Y. Green Tea Derivative (-)-Epigallocatechin-3-Gallate (EGCG) Confers Protection against Ionizing Radiation-Induced Intestinal Epithelial Cell Death Both in Vitro and in Vivo. Free Radic. Biol. Med. 2020, 161, 175–186. [Google Scholar] [CrossRef]
- Lv, P.; Yu, J.; Xu, X.; Lu, T.; Xu, F. Eriodictyol Inhibits High Glucose-Induced Oxidative Stress and Inflammation in Retinal Ganglial Cells. J. Cell. Biochem. 2019, 120, 5644–5651. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Sun, P.; Zhu, W.; Chen, Y.; Chen, M.; Yang, X.; Du, X.; Zhao, Y. Farrerol Alleviates Hypoxic-Ischemic Encephalopathy by Inhibiting Ferroptosis in Neonatal Rats via the Nrf2 Pathway. Physiol. Res. 2023, 72, 511–520. [Google Scholar] [CrossRef]
- Wu, Y.; Qian, J.; Li, K.; Li, W.; Yin, W.; Jiang, H. Farrerol Alleviates Collagenase-Induced Tendinopathy by Inhibiting Ferroptosis in Rats. J. Cell. Mol. Med. 2022, 26, 3483–3494. [Google Scholar] [CrossRef]
- Kose, T.; Sharp, P.A.; Latunde-Dada, G.O. Upregulation of Nrf2 Signalling and the Inhibition of Erastin-Induced Ferroptosis by Ferulic Acid in MIN6 Cells. Int. J. Mol. Sci. 2022, 23, 15886. [Google Scholar] [CrossRef]
- Liu, X.; Qi, K.; Gong, Y.; Long, X.; Zhu, S.; Lu, F.; Lin, K.; Xu, J. Ferulic Acid Alleviates Myocardial Ischemia Reperfusion Injury Via Upregulating AMPKα2 Expression-Mediated Ferroptosis Depression. J. Cardiovasc. Pharmacol. 2021, 79, 489–500. [Google Scholar] [CrossRef]
- Wang, B.; Yang, L.-N.; Yang, L.-T.; Liang, Y.; Guo, F.; Fu, P.; Ma, L. Fisetin Ameliorates Fibrotic Kidney Disease in Mice via Inhibiting ACSL4-Mediated Tubular Ferroptosis. Acta Pharmacol. Sin. 2023, 45, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, X.; Pi, W.; Zhang, Y.; Yu, L.; Xu, C.; Sun, Z.; Jiang, J. Fisetin Attenuates Doxorubicin-Induced Cardiomyopathy In Vivo and In Vitro by Inhibiting Ferroptosis Through SIRT1/Nrf2 Signaling Pathway Activation. Front. Pharmacol. 2021, 12, 808480. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Ni, Y.; Gong, Y.; Kang, X.; Guo, H.; Liu, X.; Li, J.; Wang, L. Formononetin Ameliorates Ferroptosis-Associated Fibrosis in Renal Tubular Epithelial Cells and in Mice with Chronic Kidney Disease by Suppressing the Smad3/ATF3/SLC7A11 Signaling. Life Sci. 2023, 315, 121331. [Google Scholar] [CrossRef]
- Zhai, X.; Zhu, J.; Li, J.; Wang, Z.; Zhang, G.; Nie, Y. Fraxetin Alleviates BLM-Induced Idiopathic Pulmonary Fibrosis by Inhibiting NCOA4-Mediated Epithelial Cell Ferroptosis. Inflamm. Res. 2023, 72, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, H.; Wang, H.; Pang, J.; Zhou, Y. Fraxetin Attenuates Ferroptosis in Myocardial Infarction via AKT/Nrf2/HO-1 Signaling. Am. J. Transl. Res. 2021, 13, 10315–10327. [Google Scholar] [PubMed]
- Yang, R.; Shi, L.; Si, H.; Hu, Z.; Zou, L.; Li, L.; Xu, X.; Schmalzing, G.; Nie, H.; Li, G.; et al. Gallic Acid Improves Comorbid Chronic Pain and Depression Behaviors by Inhibiting P2X7 Receptor-Mediated Ferroptosis in the Spinal Cord of Rats. ACS Chem. Neurosci. 2023, 14, 667–676. [Google Scholar] [CrossRef]
- Qiu, C.-W.; Chen, B.; Zhu, H.-F.; Liang, Y.-L.; Mao, L.-S. Gastrodin Alleviates Cisplatin Nephrotoxicity by Inhibiting Ferroptosis via the SIRT1/FOXO3A/GPX4 Signaling Pathway. J. Ethnopharmacol. 2023, 319, 117282. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, B.; Huang, H.; Qu, S.; Yang, S.; Zeng, Z. Gastrodin Induced HO-1 and Nrf2 up-Regulation to Alleviate H2O2-Induced Oxidative Stress in Mouse Liver Sinusoidal Endothelial Cells through P38 MAPK Phosphorylation. Braz. J. Med. Biol. Res. 2018, 51, e7439, Erratum in Braz. J. Med. Biol. Res. 2019, 52, e7439erratum. [Google Scholar] [CrossRef]
- Chen, B.; Li, X.; Liu, J.; Li, Y.; Dai, W.; Chen, Y.; Chen, D. Ferroptosis-Inhibitory Effect and Possible Mechanisms of Ellagitannin Geraniin. ChemistryOpen 2021, 10, 737–739. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, S.; Chen, Z.; Liu, Y.; Pei, C.; Huang, H.; Hou, S.; Ning, W.; Liang, J. Gingerenone A Alleviates Ferroptosis in Secondary Liver Injury in Colitis Mice via Activating Nrf2-Gpx4 Signaling Pathway. J. Agric. Food Chem. 2022, 70, 12525–12534. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, J.; Wu, G.; Hu, Z.; Ying, P.; Bao, Z.; Ding, Z.; Tan, X. 6-Gingerol Alleviates Ferroptosis and Inflammation of Diabetic Cardiomyopathy via the Nrf2/HO-1 Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 3027514. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, M.; Zheng, S.; Huang, R.; Wen, G.; Zhou, P.; Wang, W.; Zhou, S.; Jiang, X.; Liu, S.; et al. Mesoporous Polydopamine Delivering 8-Gingerol for the Target and Synergistic Treatment to the Spinal Cord Injury. J. Nanobiotechnology 2023, 21, 192. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Yang, K.-T.; Ting, P.-C.; Luo, Y.-P.; Lin, D.-J.; Wang, Y.-S.; Chang, J.-C. Gossypol Acetic Acid Attenuates Cardiac Ischemia/Reperfusion Injury in Rats via an Antiferroptotic Mechanism. Biomolecules 2021, 11, 1667. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, R.; Yan, C.; Sun, K.; Gao, L.; Zheng, B.; Shi, J. Hesperidin Mitigates Oxidative Stress-Induced Ferroptosis in Nucleus Pulposus Cells via Nrf2/NF-κB Axis to Protect Intervertebral Disc from Degeneration. Cell Cycle 2023, 22, 1196–1214. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Jiang, W.; Ye, C.; Hu, T.; Yu, Q.; Meng, M.; Sun, L.; Liang, J.; Chen, Y. Honokiol Attenuates High Glucose-Induced Peripheral Neuropathy via Inhibiting Ferroptosis and Activating AMPK/SIRT1/PGC-1α Pathway in Schwann Cells. Phytother. Res. 2023, 37, 5787–5802. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Peng, Y.; Li, J.; Wang, L.; Zhu, X.; Wang, N.; Yang, D.; Zhou, X.; Chang, D. Hydroxysafflor Yellow A Alleviates Acute Myocardial Ischemia/Reperfusion Injury in Mice by Inhibiting Ferroptosis via the Activation of the HIF-1α/SLC7A11/GPX4 Signaling Pathway. Nutrients 2023, 15, 3411. [Google Scholar] [CrossRef]
- Rong, J.; Li, C.; Zhang, Q.; Zheng, G.; Fan, W.; Pan, Z.; Shi, S. Hydroxysafflor Yellow A Inhibits Endothelial Cell Ferroptosis in Diabetic Atherosclerosis Mice by Regulating miR-429/SLC7A11. Pharm. Biol. 2023, 61, 404–415. [Google Scholar] [CrossRef]
- Peng, X.; Tan, Q.; Zhou, H.; Xu, J.; Gu, Q. Discovery of Phloroglucinols from Hypericum Japonicum as Ferroptosis Inhibitors. Fitoterapia 2021, 153, 104984. [Google Scholar] [CrossRef]
- Choi, J.; Choi, H.; Chung, J. Icariin Supplementation Suppresses the Markers of Ferroptosis and Attenuates the Progression of Nonalcoholic Steatohepatitis in Mice Fed a Methionine Choline-Deficient Diet. Int. J. Mol. Sci. 2023, 24, 12510. [Google Scholar] [CrossRef]
- Yu, L.-M.; Dong, X.; Huang, T.; Zhao, J.-K.; Zhou, Z.-J.; Huang, Y.-T.; Xu, Y.-L.; Zhao, Q.-S.; Wang, Z.-S.; Jiang, H.; et al. Inhibition of Ferroptosis by Icariin Treatment Attenuates Excessive Ethanol Consumption-Induced Atrial Remodeling and Susceptibility to Atrial Fibrillation, Role of SIRT1. Apoptosis 2023, 28, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.; Mao, C.; Zhang, C.; Ma, W.; Tang, J.; Xiang, D.; Qi, X. Icariin Alleviates Ferroptosis-Related Atherosclerosis by Promoting Autophagy in Xo-LDL-Induced Vascular Endothelial Cell Injury and Atherosclerotic Mice. Phytother. Res. 2023, 37, 3951–3963. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Sun, L.; Yang, G.; Wang, W.; Liu, X.; Du, T.; Chen, F.; Jing, X.; Cui, X. Icariin Protects Vertebral Endplate Chondrocytes against Apoptosis and Degeneration via Activating Nrf-2/HO-1 Pathway. Front. Pharmacol. 2022, 13, 937502. [Google Scholar] [CrossRef]
- Liu, X.-J.; Lv, Y.-F.; Cui, W.-Z.; Li, Y.; Liu, Y.; Xue, Y.-T.; Dong, F. Icariin Inhibits Hypoxia/Reoxygenation-Induced Ferroptosis of Cardiomyocytes via Regulation of the Nrf2/HO-1 Signaling Pathway. FEBS Open Bio 2021, 11, 2966–2976. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhang, R. Icariin Enhances Cell Survival in Lipopolysaccharide-Induced Synoviocytes by Suppressing Ferroptosis via the Xc-/GPX4 Axis. Exp. Ther. Med. 2021, 21, 72. [Google Scholar] [CrossRef]
- Gao, J.; Ma, C.; Xia, D.; Chen, N.; Zhang, J.; Xu, F.; Li, F.; He, Y.; Gong, Q. Icariside II Preconditioning Evokes Robust Neuroprotection against Ischaemic Stroke, by Targeting Nrf2 and the OXPHOS/NF-κB/Ferroptosis Pathway. Br. J. Pharmacol. 2023, 180, 308–329. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, H.; Xiao, Q.; Li, L.; Zhong, X.; Zhang, J.; Wang, F.; Li, G.; Wang, L.; Li, Y. Isoliquiritigenin Attenuates Septic Acute Kidney Injury by Regulating Ferritinophagy-Mediated Ferroptosis. Ren. Fail. 2021, 43, 1551–1560. [Google Scholar] [CrossRef]
- Zhongyin, Z.; Wei, W.; Juan, X.; Guohua, F. Isoliquiritin Apioside Relieves Intestinal Ischemia/Reperfusion-Induced Acute Lung Injury by Blocking Hif-1α-Mediated Ferroptosis. Int. Immunopharmacol. 2022, 108, 108852. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, H.; Zhang, J.; Yan, M. Isoquercetin Attenuates Oxidative Stress and Neuronal Apoptosis after Ischemia/Reperfusion Injury via Nrf2-Mediated Inhibition of the NOX4/ROS/NF-κB Pathway. Chem. Biol. Interact. 2018, 284, 32–40. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, L.; Wang, Y.; Ding, J.; Wang, R. Isorhamnetin Alleviates High Glucose-Aggravated Inflammatory Response and Apoptosis in Oxygen-Glucose Deprivation and Reoxygenation-Induced HT22 Hippocampal Neurons Through Akt/SIRT1/Nrf2/HO-1 Signaling Pathway. Inflammation 2021, 44, 1993–2005. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Yin, M.; Li, Y.; Chen, C.; Zhang, J.; Sun, K.; Kong, X.; Chen, Z.; Qian, J. Isorhapontigenin Attenuates Cardiac Microvascular Injury in Diabetes via the Inhibition of Mitochondria-Associated Ferroptosis Through PRDX2-MFN2-ACSL4 Pathways. Diabetes 2023, 72, 389–404. [Google Scholar] [CrossRef]
- Arenbaoligao; Guo, X.; Xiong, J.; Zhang, S.; Yang, Y.; Chen, D.; Xie, Y. Kumatakenin Inhibited Iron-Ferroptosis in Epithelial Cells from Colitis Mice by Regulating the Eno3-IRP1-Axis. Front. Pharmacol. 2023, 14, 1127931. [Google Scholar] [CrossRef]
- Lin, J.-H.; Yang, K.-T.; Ting, P.-C.; Lee, W.-S.; Lin, D.-J.; Chang, J.-C. Licochalcone a Improves Cardiac Functions after Ischemia-Reperfusion via Reduction of Ferroptosis in Rats. Eur. J. Pharmacol. 2023, 957, 176031. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, R.; Qi, W.; Jia, L.; Ma, K.; Si, J.; Yin, J.; Zhao, Y.; Dai, Z.; Yin, J. Methyl Ferulic Acid Alleviates Neuropathic Pain by Inhibiting Nox4-Induced Ferroptosis in Dorsal Root Ganglia Neurons in Rats. Mol. Neurobiol. 2023, 60, 3175–3189. [Google Scholar] [CrossRef]
- Wen, L.; Zhou, T.; Jiang, Y.; Gong, L.; Yang, B. Identification of Prenylated Phenolics in Mulberry Leaf and Their Neuroprotective Activity. Phytomedicine 2021, 90, 153641. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xie, W.; Peng, Z.; Liu, Y.; Li, H.; Huang, W. Nobiletin Ameliorates Heatstroke-Induced Acute Lung Injury by Inhibiting Ferroptosis via P53/SLC7A11 Pathway. Shock 2023, 61, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Tian, L.; Zhang, Y.; Qiu, Z.; Lei, S.; Xia, Z.-Y. Nobiletin Alleviates Myocardial Ischemia-Reperfusion Injury via Ferroptosis in Rats with Type-2 Diabetes Mellitus. Biomed. Pharmacother. 2023, 163, 114795. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-H.; Yang, S.-F.; Cheng, C.-C.; Hsu, K.-C.; Chen, Y.-S.; Chen, Y.-Y.; Wang, C.-W.; Guan, S.-S.; Wu, C.-T. Nobiletin Alleviates Ferroptosis-Associated Renal Injury, Inflammation, and Fibrosis in a Unilateral Ureteral Obstruction Mouse Model. Biomedicines 2022, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, D.; Huang, Y.; Chen, L.; Li, Y.; Yang, Z.; Fu, S.; Hu, G. Phlorizin Mitigates Dextran Sulfate Sodium-Induced Colitis in Mice by Modulating Gut Microbiota and Inhibiting Ferroptosis. J. Agric. Food Chem. 2023, 71, 16043–16056. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Yang, H.; Jing, X.; Wang, W.; Liu, X.; Zhang, B.; Liu, X.; Shao, Y.; Cui, X. Activation of the Nrf-2 Pathway by Pinocembrin Safeguards Vertebral Endplate Chondrocytes against Apoptosis and Degeneration Caused by Oxidative Stress. Life Sci. 2023, 333, 122162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, P.; Wang, T.-T.; Du, Y.-W.; Chen, Y.; Li, Z.; He, M.-L.; Feng, L.; Li, H.-R.; Han, X.; et al. Polydatin Attenuates Cisplatin-Induced Acute Kidney Injury by Inhibiting Ferroptosis. Oxidative Med. Cell. Longev. 2022, 2022, 9947191. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, J.; Yao, Z.-M.; Sun, X.-R.; Tong, X.-H.; Hu, M.; Zhang, Y.; Dong, S.-Y. Procyanidins Alleviated Cerebral Ischemia/Reperfusion Injury by Inhibiting Ferroptosis via the Nrf2/HO-1 Signaling Pathway. Molecules 2023, 28, 3582. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-W.; Du, Y.; Ma, S.-S.; Shi, Y.-C.; Xu, H.-C.; Deng, L.; Chen, X.-Y. Proanthocyanidins Attenuates Ferroptosis against Influenza-Induced Acute Lung Injury in Mice by Reducing IFN-γ. Life Sci. 2023, 314, 121279. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yin, C.; Zhang, Z.; Tang, H.; Shen, W.; Zha, X.; Gao, M.; Sun, J.; Xu, X.; Chen, Q. Proanthocyanidin Promotes Functional Recovery of Spinal Cord Injury via Inhibiting Ferroptosis. J. Chem. Neuroanat. 2020, 107, 101807. [Google Scholar] [CrossRef]
- Guo, C.; Wang, S.; Duan, J.; Jia, N.; Zhu, Y.; Ding, Y.; Guan, Y.; Wei, G.; Yin, Y.; Xi, M.; et al. Protocatechualdehyde Protects Against Cerebral Ischemia-Reperfusion-Induced Oxidative Injury Via Protein Kinase Cε/Nrf2/HO-1 Pathway. Mol. Neurobiol. 2017, 54, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, X.; Zhou, F.; Xiao, S.; Zhong, L.; Hu, S.; Zhou, Z.; Li, L.; Tan, Y. Protocatechuic Acid Alleviates Dextran-Sulfate-Sodium-Induced Ulcerative Colitis in Mice via the Regulation of Intestinal Flora and Ferroptosis. Molecules 2023, 28, 3775. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Li, Y.; Wang, T. Pterostilbene Confers Protection against Diquat-Induced Intestinal Damage with Potential Regulation of Redox Status and Ferroptosis in Broiler Chickens. Oxidative Med. Cell. Longev. 2023, 2023, 8258354. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, Q.L.; Li, Z.H.; Ji, R.; Wang, J.Y.; Cao, M.L.; Mu, X.F.; Zhang, Y.; Guo, D.Y.; Yang, J. Pterostilbene Ameliorates Oxidative Damage and Ferroptosis in Human Ovarian Granulosa Cells by Regulating the Nrf2/HO-1 Pathway. Arch. Biochemy Biophys. 2023, 738, 109561. [Google Scholar] [CrossRef]
- Jian, J.; Wang, D.; Xiong, Y.; Wang, J.; Zheng, Q.; Jiang, Z.; Zhong, J.; Yang, S.; Wang, L. Puerarin Alleviated Oxidative Stress and Ferroptosis during Renal Fibrosis Induced by Ischemia/Reperfusion Injury via TLR4/Nox4 Pathway in Rats. Acta Cir. Bras. 2023, 38, e382523. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, J.; Chen, Y.; Liu, Y.; Tang, X.; Xia, P.; Yu, P.; Yu, S. Puerarin Protects against Sepsis-Induced Myocardial Injury through AMPK-Mediated Ferroptosis Signaling. Aging 2022, 14, 3617–3632. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, C.; Li, H.; Chen, X.; Ding, Y.; Xu, S. Puerarin Protects against Heart Failure Induced by Pressure Overload through Mitigation of Ferroptosis. Biochem. Biophys. Res. Commun. 2018, 497, 233–240. [Google Scholar] [CrossRef]
- Huang, L.; Lu, S.; Bian, M.; Wang, J.; Yu, J.; Ge, J.; Zhang, J.; Xu, Q. Punicalagin Attenuates TNF-α-Induced Oxidative Damage and Promotes Osteogenic Differentiation of Bone Mesenchymal Stem Cells by Activating the Nrf2/HO-1 Pathway. Exp. Cell Res. 2023, 430, 113717. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhao, X.; Chen, Q.; Wang, X.; Wu, Y.; Zhao, H. Quercetin Ameliorates Ferroptosis of Rat Cardiomyocytes via Activation of the SIRT1/P53/SLC7A11 Signaling Pathway to Alleviate Sepsis-induced Cardiomyopathy. Int. J. Mol. Med. 2023, 52, 116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, R.; Peng, W.; Zhao, X.; Bai, W.; Hu, C. Quercetin Alleviates Ferroptosis Accompanied by Reducing M1 Macrophage Polarization during Neutrophilic Airway Inflammation. Eur. J. Pharmacol. 2023, 938, 175407. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Wang, M.; Chen, H.; Li, Y.; Wei, W.; Liu, X.; Wu, Y.; Luo, S.; Liu, X.; et al. Quercetin Prevents the Ferroptosis of OPCs by Inhibiting the Id2/Transferrin Pathway. Chem. Biol. Interact. 2023, 381, 110556. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Jiang, M.; Hong, X.; Fu, Y.; Chen, Y.; Wu, H.; Sun, Y.; Wang, X.; Zhou, E.; Wang, J.; et al. Quercetin Alleviates Deoxynivalenol-Induced Intestinal Damage by Suppressing Inflammation and Ferroptosis in Mice. J. Agric. Food Chem. 2023, 71, 10761–10772. [Google Scholar] [CrossRef]
- Kato, K.; Takahashi, M.; Oh-hashi, K.; Ando, K.; Hirata, Y. Quercetin and Resveratrol Inhibit Ferroptosis Independently of Nrf2–ARE Activation in Mouse Hippocampal HT22 Cells. Food Chem. Toxicol. 2023, 172, 113586. [Google Scholar] [CrossRef]
- Lan, D.; Qi, S.; Yao, C.; Li, X.; Liu, H.; Wang, D.; Wang, Y. Quercetin Protects Rat BMSCs from Oxidative Stress via Ferroptosis. J. Mol. Endocrinol. 2022, 69, 401–413. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin Alleviates Acute Kidney Injury by Inhibiting Ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef]
- Li, D.; Jiang, C.; Mei, G.; Zhao, Y.; Chen, L.; Liu, J.; Tang, Y.; Gao, C.; Yao, P. Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients 2020, 12, 2954. [Google Scholar] [CrossRef]
- Li, D.; Song, C.; Zhang, J.; Zhao, X. Resveratrol Alleviated 5-FU-Induced Cardiotoxicity by Attenuating GPX4 Dependent Ferroptosis. J. Nutr. Biochem. 2023, 112, 109241. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, X.; Ding, B.; Zi, M.; Ma, Y. Resveratrol Attenuated High Intensity Exercise Training-Induced Inflammation and Ferroptosis via Nrf2/FTH1/GPX4 Pathway in Intestine of Mice. Turk. J. Med. Sci. 2023, 53, 446–454. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, S.; Tang, B.; Kang, P.; Zhang, H.; Shi, C. Resveratrol Inhibits Ferroptosis and Decelerates Heart Failure Progression via Sirt1/P53 Pathway Activation. J. Cell. Mol. Med. 2023, 27, 3075–3089. [Google Scholar] [CrossRef]
- Huang, W.; Yu, L.; Cai, W.; Ma, C. Resveratrol Protects BEAS-2B Cells against Erastin-Induced Ferroptosis through the Nrf2/Keap1 Pathway. Planta Med. 2023, 89, 408–415. [Google Scholar] [CrossRef]
- Wang, P.; Yao, Q.; Zhu, D.; Yang, X.; Chen, Q.; Lu, Q.; Liu, A. Resveratrol Protects against Deoxynivalenol-Induced Ferroptosis in HepG2 Cells. Toxicology 2023, 494, 153589. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, M.; Qin, C.; Wang, Z.; Chen, J.; Wang, R.; Hu, J.; Zou, Q.; Niu, X. Resveratrol Attenuate Myocardial Injury by Inhibiting Ferroptosis Via Inducing KAT5/GPX4 in Myocardial Infarction. Front. Pharmacol. 2022, 13, 906073. [Google Scholar] [CrossRef]
- Wang, X.; Simayi, A.; Fu, J.; Zhao, X.; Xu, G. Resveratrol Mediates the miR-149/HMGB1 Axis and Regulates the Ferroptosis Pathway to Protect Myocardium in Endotoxemia Mice. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E21–E32. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Cao, G.; Lin, L.; Zhang, Y.; Luo, X.; Ma, X.; Aiyisake, A.; Cheng, Q. Resveratrol Attenuates Sepsis-Induced Cardiomyopathy in Rats through Anti-Ferroptosis via the Sirt1/Nrf2 Pathway. J. Investig. Surg. 2023, 36, 2157521. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, L.; Chen, H.; Wei, S.; Yao, K.; Sun, X.; Yang, G.; Jiang, L.; Zhang, C.; Wang, N.; et al. Resveratrol Protected Acrolein-Induced Ferroptosis and Insulin Secretion Dysfunction via ER-Stress- Related PERK Pathway in MIN6 Cells. Toxicology 2022, 465, 153048. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, S.; Zhao, A.; Mi, Y.; Zhang, C. Protective Effect of Rutin on Ferroptosis-Induced Oxidative Stress in Aging Laying Hens through Nrf2/HO-1 Signaling. Cell Biol. Int. 2023, 47, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wang, Y.; Liu, Y.; Li, C. Salidroside Inhibits Renal Ischemia/Reperfusion Injury-induced Ferroptosis by the PI3K/AKT Signaling Pathway. Exp. Ther. Med. 2023, 26, 507. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Luo, L.; Kasim, V.; Wu, S. Salidroside Facilitates Therapeutic Angiogenesis in Diabetic Hindlimb Ischemia by Inhibiting Ferroptosis. Biomed. Pharmacother. 2023, 159, 114245. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Luo, J.; Zhang, J.; Sang, A.; Cheng, Z.; Li, X. Salidroside Postconditioning Attenuates Ferroptosis-Mediated Lung Ischemia-Reperfusion Injury by Activating the Nrf2/SLC7A11 Signaling Axis. Int. Immunopharmacol. 2023, 115, 109731, Corrigendum in Int. Immunopharmacol. 2023, 117, 110002. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, J.; Le, Y.; Pan, J.; Liu, Y.; Liu, Z.; Wang, C.; Dou, X.; Lu, D. Salidroside Inhibits Doxorubicin-Induced Cardiomyopathy by Modulating a Ferroptosis-Dependent Pathway. Phytomedicine 2022, 99, 153964. [Google Scholar] [CrossRef]
- Yang, S.; Pei, T.; Wang, L.; Zeng, Y.; Li, W.; Yan, S.; Xiao, W.; Cheng, W. Salidroside Alleviates Renal Fibrosis in SAMP8 Mice by Inhibiting Ferroptosis. Molecules 2022, 27, 8039. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Cui, S.; Ma, H.; Zhu, P.; Li, N.; Zhang, X.; Zhang, L.; Xuan, L.; Li, J. Salvianolate Ameliorates Renal Tubular Injury through the Keap1/Nrf2/ARE Pathway in Mouse Kidney Ischemia-Reperfusion Injury. J. Ethnopharmacol. 2022, 293, 115331. [Google Scholar] [CrossRef]
- Yang, D.; Xia, X.; Xi, S. Salvianolic Acid A Attenuates Arsenic-Induced Ferroptosis and Kidney Injury via HIF-2α/DUOX1/GPX4 and Iron Homeostasis. Sci. Total Environ. 2023, 907, 168073. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Q.; Jian, W.; Han, X.; Zhang, Y.; Zeng, Y.; Liu, R.; Wang, Q.; Song, Q. Protective Benefits of Salvianic Acid A against Retinal Iron Overload by Inhibition of Ferroptosis. Biomed. Pharmacother. 2023, 165, 115140. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Mao, C.; Zhang, C.; Zhang, M.; Gong, J.; Wang, X. Salvianolic Acid B Inhibits Ferroptosis and Apoptosis during Myocardial Ischemia/Reperfusion Injury via Decreasing the Ubiquitin-Proteasome Degradation of GPX4 and the ROS-JNK/MAPK Pathways. Molecules 2023, 28, 4117. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, X.; Wang, S.; Zhang, Y.; Wang, Y.; Ding, Y.; Shen, J.; Zhao, J.; Qin, H.; Xu, Y.; et al. Protective Effects of Salvianolic Acid B on Rat Ferroptosis in Myocardial Infarction through Upregulating the Nrf2 Signaling Pathway. Int. Immunopharmacol. 2022, 112, 109257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Q.; Lu, Y.; Wan, J.; Dai, H.; Zhou, X.; Lv, S.; Chen, X.; Zhang, X.; Hang, C.; et al. Cerebroprotection by Salvianolic Acid B after Experimental Subarachnoid Hemorrhage Occurs via Nrf2- and SIRT1-Dependent Pathways. Free Radic. Biol. Med. 2018, 124, 504–516. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Wang, L.; Liu, Y.; Chen, H.; Hu, Q.; Xie, C.; Meng, X.; Shen, X. Scutellarein Alleviates Chronic Obstructive Pulmonary Disease through Inhibition of Ferroptosis by Chelating Iron and Interacting with Arachidonate 15-Lipoxygenase. Phytother. Res. 2023, 37, 4587–4606. [Google Scholar] [CrossRef]
- Ren, J.; Yin, B.; Li, X.; Zhu, S.; Deng, J.; Sun, Y.; Zhang, Z.; Guo, Z.; Pei, H.; Zhang, F.; et al. Sesamin Attenuates PM2.5-Induced Cardiovascular Injury by Inhibiting Ferroptosis in Rats. Food Funct. 2021, 12, 12671–12682. [Google Scholar] [CrossRef]
- Du, Q.; Wu, X.; Ma, K.; Liu, W.; Liu, P.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. Silibinin Alleviates Ferroptosis of Rat Islet β Cell INS-1 Induced by the Treatment with Palmitic Acid and High Glucose through Enhancing PINK1/Parkin-Mediated Mitophagy. Arch. Biochem. Biophys. 2023, 743, 109644. [Google Scholar] [CrossRef]
- Iqbal, S.; Jabeen, F.; Kahwa, I.; Omara, T. Suberosin Alleviates Thiazolidinedione-Induced Cardiomyopathy in Diabetic Rats by Inhibiting Ferroptosis via Modulation of ACSL4-LPCAT3 and PI3K-AKT Signaling Pathways. Cardiovasc. Toxicol. 2023, 23, 295–304. [Google Scholar] [CrossRef]
- Wang, F.; Qi, Y.; Gao, Y.; Wang, Z.; Shen, X.; Wu, H. Syringic Acid Suppresses Ferroptosis of Skeletal Muscle Cells to Alleviate Lower Limb Ischemia/Reperfusion Injury in Mice via the HMGB1 Pathway. Chem. Biol. Drug Des. 2023, 102, 1387–1398. [Google Scholar] [CrossRef]
- Wang, J.; Qu, J.; Liu, S.; Xu, Q.; Li, X.; Zhu, Y.; Liu, X.; Yi, J.; Yuan, Z.; Huang, P.; et al. Tannic Acid Ameliorates Systemic Glucose and Lipid Metabolic Impairment Induced by Low-Dose T-2 Toxin Exposure. J. Agric. Food Chem. 2023, 71, 12574–12586. [Google Scholar] [CrossRef]
- Fang, X.; Fu, W.; Zou, B.; Zhang, F. Tectorigenin Relieved Sepsis-Induced Myocardial Ferroptosis by Inhibiting the Expression of Smad3. Toxicol. Res. 2023, 12, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, J.; Zhu, B.; Fan, J.; Hu, Q.; Wang, L. Tectorigenin Protects against Unilateral Ureteral Obstruction by Inhibiting Smad3-Mediated Ferroptosis and Fibrosis. Phytother. Res. 2022, 36, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ni, S.; Xu, N.; Yin, G.; Yu, Y.; Zhou, B.; Zhao, G.; Wang, L.; Zhu, R.; Jiang, S.; et al. Theaflavin-3,3′-Digallate Inhibits Erastin-Induced Chondrocytes Ferroptosis via the Nrf2/GPX4 Signaling Pathway in Osteoarthritis. Oxidative Med. Cell. Longev. 2022, 2022, 3531995. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wei, Y.; Zhang, Y.; Xu, F.; Ma, C.; Gong, Q.; Gao, J.; Xu, Y. Trilobatin, a Naturally Occurring Food Additive, Ameliorates Exhaustive Exercise-Induced Fatigue in Mice: Involvement of Nrf2/ARE/Ferroptosis Signaling Pathway. Front. Pharmacol. 2022, 13, 913367. [Google Scholar] [CrossRef]
- Jin, T.; Chen, C. Umbelliferone Delays the Progression of Diabetic Nephropathy by Inhibiting Ferroptosis through Activation of the Nrf-2/HO-1 Pathway. Food Chem. Toxicol. 2022, 163, 112892. [Google Scholar] [CrossRef]
- Song, J.; Wang, H.; Sheng, J.; Zhang, W.; Lei, J.; Gan, W.; Cai, F.; Yang, Y. Vitexin Attenuates Chronic Kidney Disease by Inhibiting Renal Tubular Epithelial Cell Ferroptosis via NRF2 Activation. Mol. Med. 2023, 29, 147. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Wang, H.; Chen, Y. Vitexin Ameliorated Diabetic Nephropathy via Suppressing GPX4-Mediated Ferroptosis. Eur. J. Pharmacol. 2023, 951, 175787. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Sui, J.; Dong, X.; Jing, B.; Gao, Z. Wedelolactone Alleviates Acute Pancreatitis and Associated Lung Injury via GPX4 Mediated Suppression of Pyroptosis and Ferroptosis. Free Radic. Biol. Med. 2021, 173, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Moral, S.; Verardo, V.; Robert, P.; Segura-Carretero, A.; Martínez-Férez, A. 13—Nanoencapsulation Strategies Applied to Maximize Target Delivery of Intact Polyphenols. In Encapsulations; Grumezescu, A.M., Ed.; Nanotechnology in the Agri-Food Industry; Academic Press: Cambridge, MA, USA, 2016; pp. 559–595. ISBN 978-0-12-804307-3. [Google Scholar]
- Lesjak, M.; Srai, K.S.S. Role of Dietary Flavonoids in Iron Homeostasis. Pharmaceuticals 2019, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Schorpp, K.; Samaga, D.; Unger, K.; Hadian, K.; Stockwell, B.R. Machine Learning Classifies Ferroptosis and Apoptosis Cell Death Modalities with TfR1 Immunostaining. ACS Chem. Biol. 2022, 17, 654–660. [Google Scholar] [CrossRef]
- Schorpp, K.; Bessadok, A.; Biibosunov, A.; Rothenaigner, I.; Strasser, S.; Peng, T.; Hadian, K. CellDeathPred: A Deep Learning Framework for Ferroptosis and Apoptosis Prediction Based on Cell Painting. Cell Death Discov. 2023, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Chen, X.; Huang, F. Machine Learning Revealed Ferroptosis Features and Ferroptosis-Related Gene-Based Immune Microenvironment in Lung Adenocarcinoma. Chem. Biol. Interact. 2023, 378, 110471. [Google Scholar] [CrossRef] [PubMed]
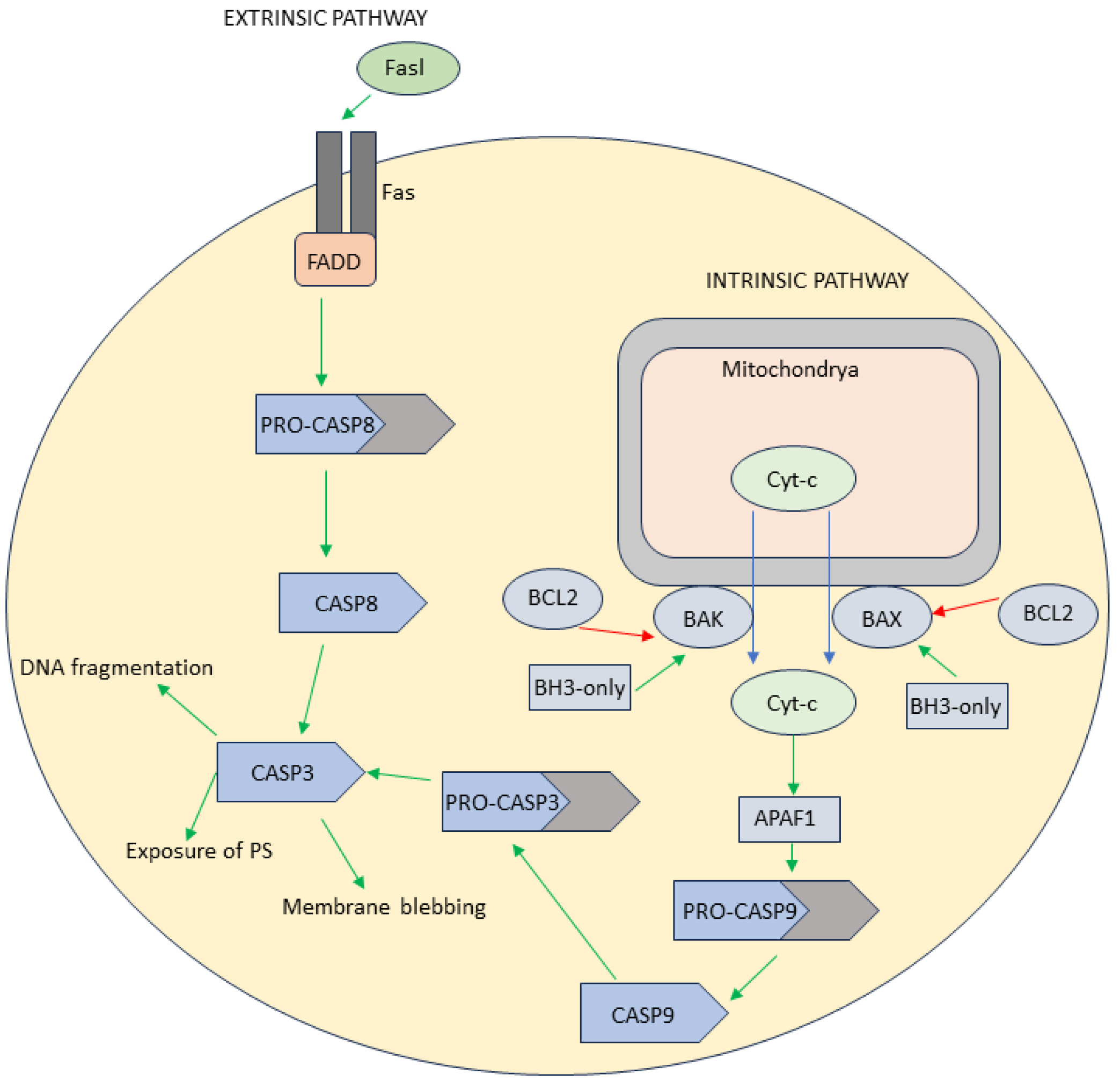


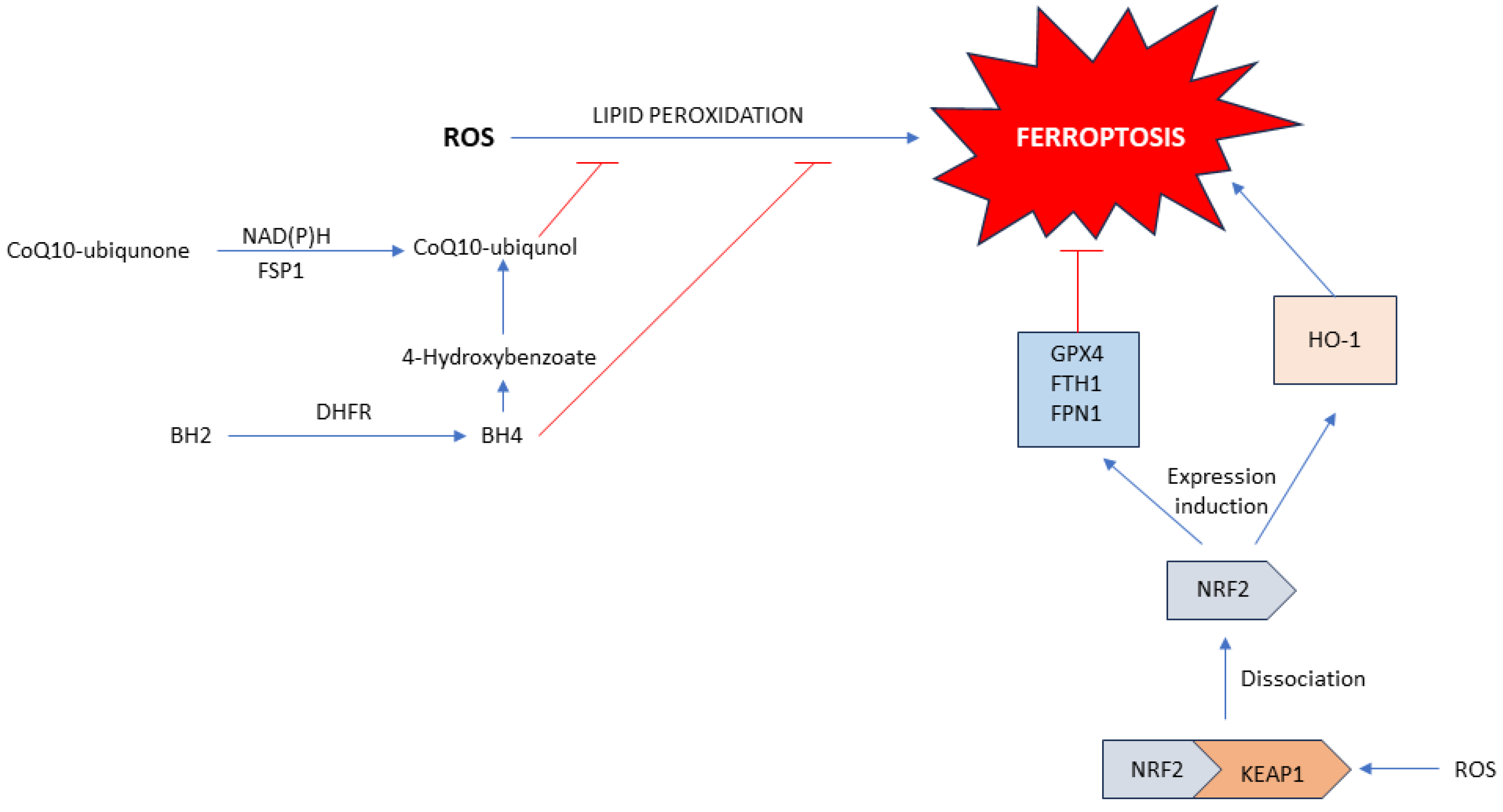
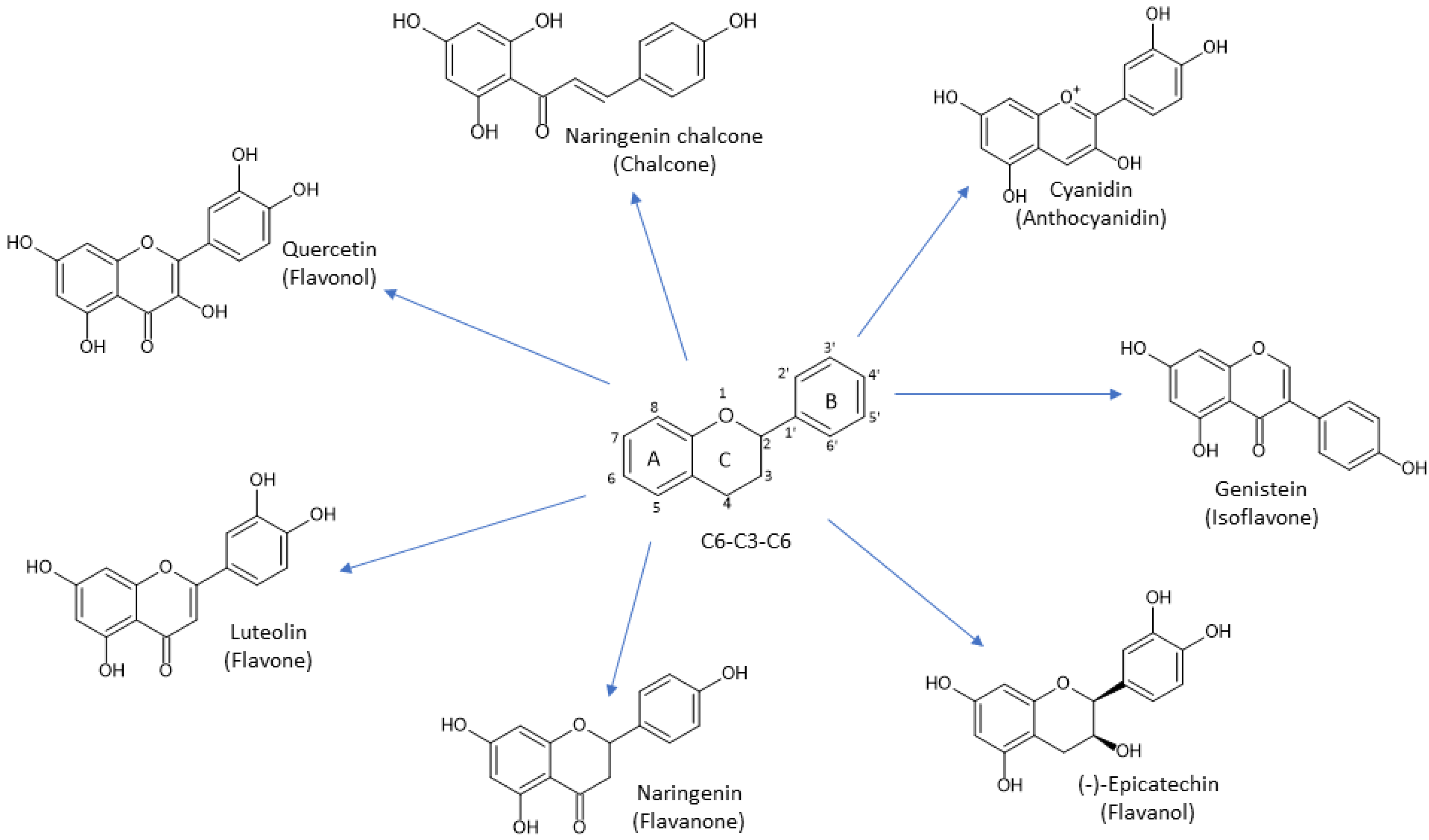
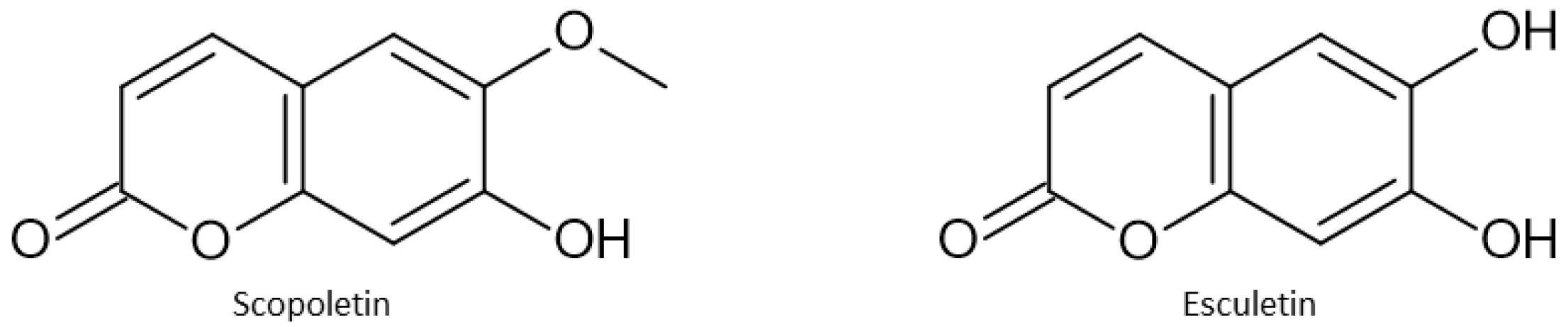


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Živanović, N.; Lesjak, M.; Simin, N.; Srai, S.K.S. Beyond Mortality: Exploring the Influence of Plant Phenolics on Modulating Ferroptosis—A Systematic Review. Antioxidants 2024, 13, 334. https://doi.org/10.3390/antiox13030334
Živanović N, Lesjak M, Simin N, Srai SKS. Beyond Mortality: Exploring the Influence of Plant Phenolics on Modulating Ferroptosis—A Systematic Review. Antioxidants. 2024; 13(3):334. https://doi.org/10.3390/antiox13030334
Chicago/Turabian StyleŽivanović, Nemanja, Marija Lesjak, Nataša Simin, and Surjit K. S. Srai. 2024. "Beyond Mortality: Exploring the Influence of Plant Phenolics on Modulating Ferroptosis—A Systematic Review" Antioxidants 13, no. 3: 334. https://doi.org/10.3390/antiox13030334
APA StyleŽivanović, N., Lesjak, M., Simin, N., & Srai, S. K. S. (2024). Beyond Mortality: Exploring the Influence of Plant Phenolics on Modulating Ferroptosis—A Systematic Review. Antioxidants, 13(3), 334. https://doi.org/10.3390/antiox13030334






