Potential Phytotherapy of DSS-Induced Colitis: Ameliorating Reactive Oxygen Species-Mediated Necroptosis and Gut Dysbiosis with a New Crataegus pinnatifida Bunge Variety—Daehong
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sample and HPLC Analysis of CP and DH
2.2. Animal Study
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Immunohistochemistry (IHC)
2.6. Cytokine Assay
2.7. Cell Permeability Assay
2.8. Levels of ROS Production
2.9. Annexin V-Propidium Iodide Assay
2.10. Immunoblotting
2.11. 16S rRNA Gene Sequencing-Based Microbiome Profiling
2.12. Differential Abundance Analysis for Microbiome Profiles
2.13. Metagenome Analysis
2.14. Statistical Analysis
3. Results
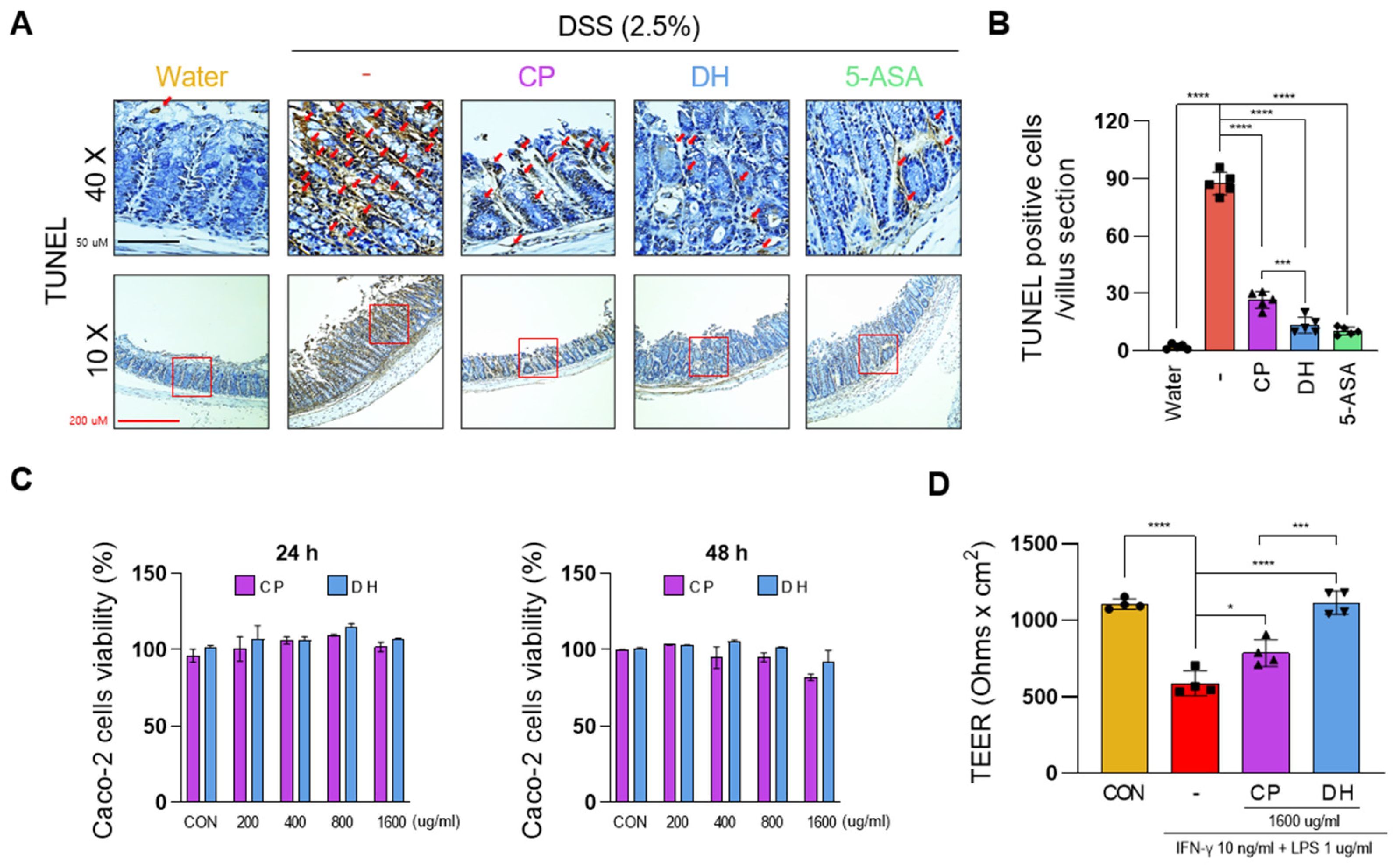
3.1. DH Is More Effective than CP in Alleviating DSS-Induced Colitis
3.2. DH Improves the Paracellular Permeability of Enterocytes Impaired by LPS and INFγ
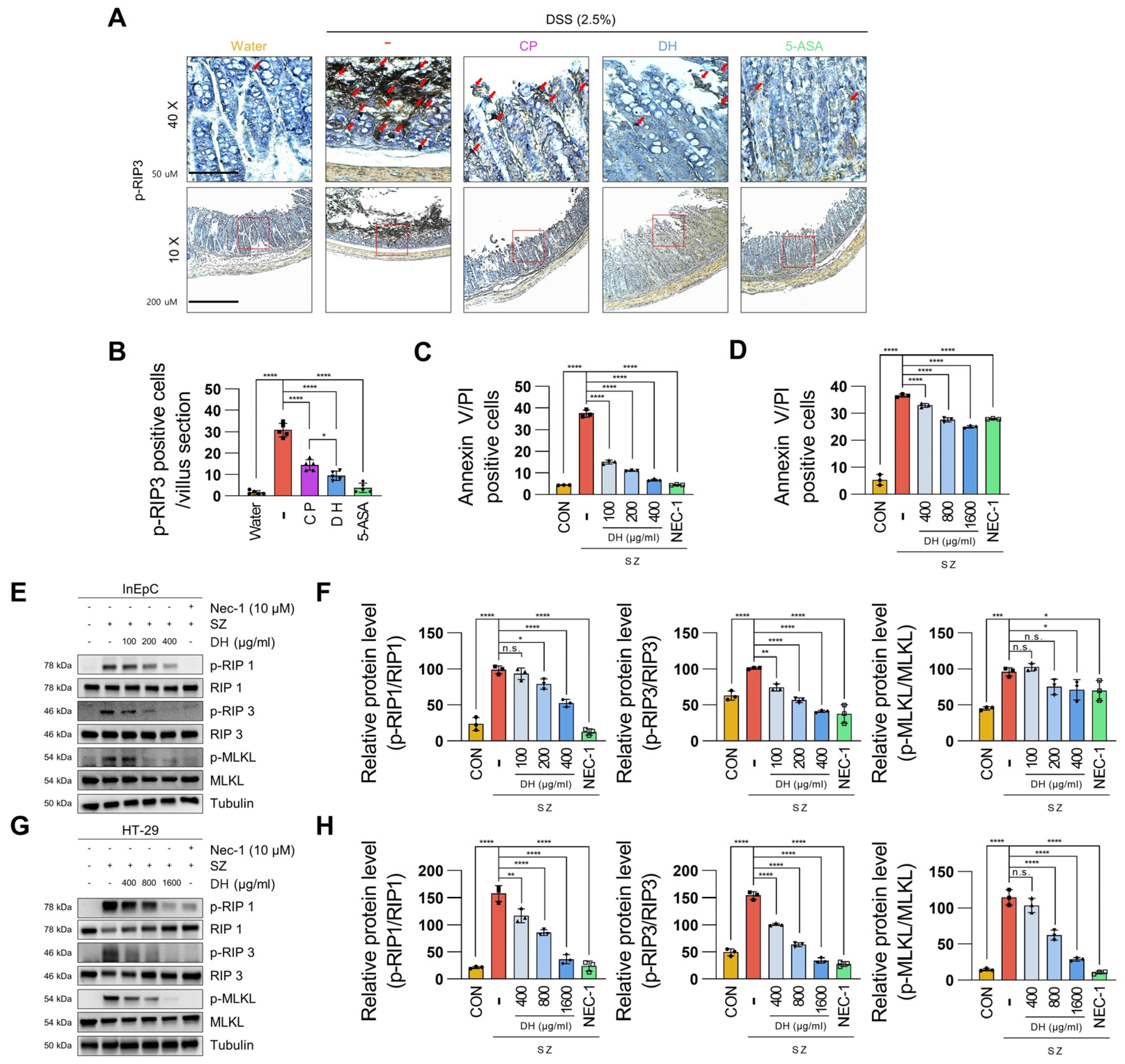
3.3. DH Protects Enterocyte Death by Suppressing Necroptosis of Immune Response in DSS-Induced Colitis
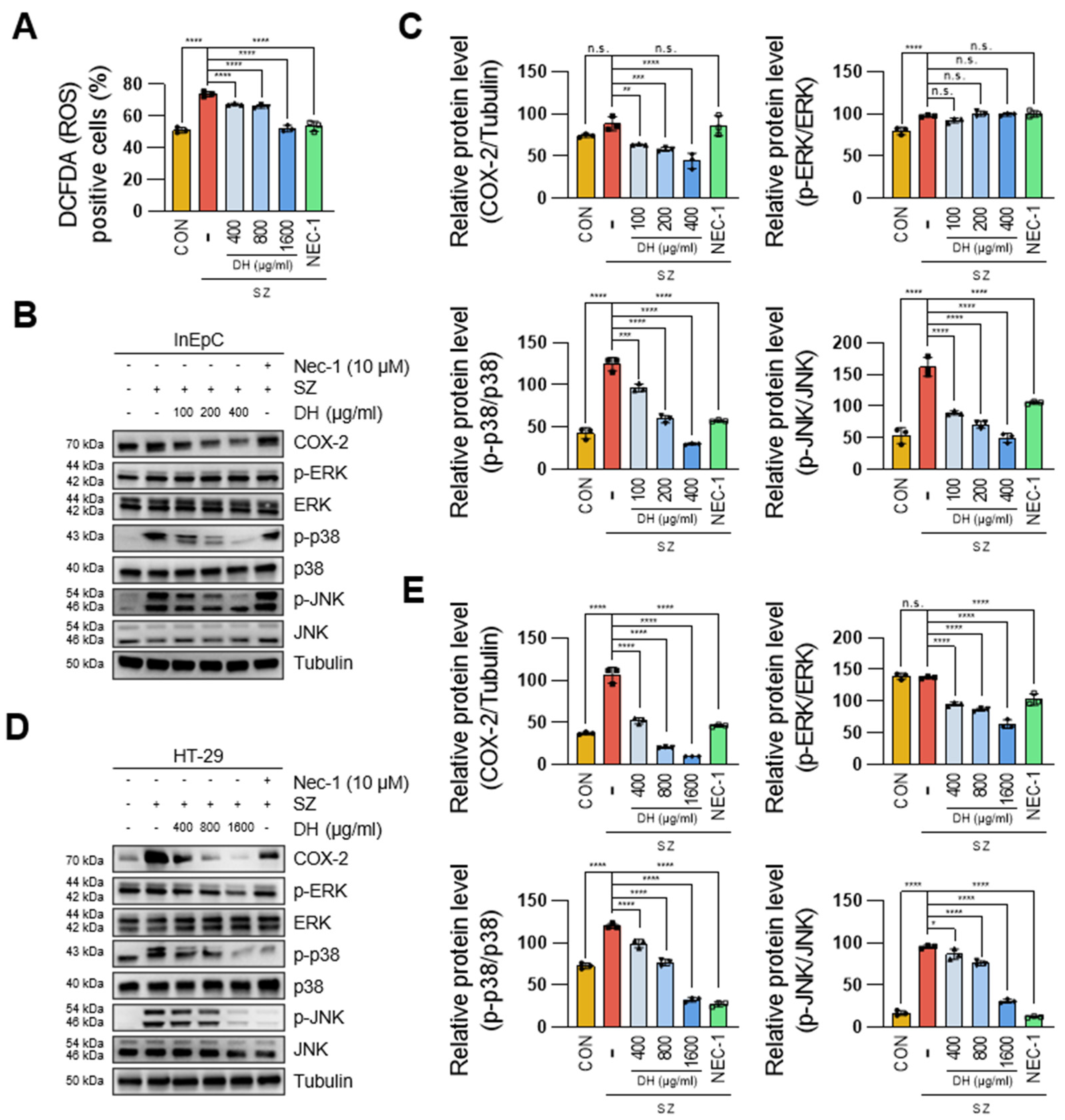
3.4. DH Regulates SZ-Induced ROS Production via the JNK/p38-MAPK/COX2 Pathway
3.5. Epicatechin Is the Bioactive Component of DH Responsible for the Regulation of Necroptosis
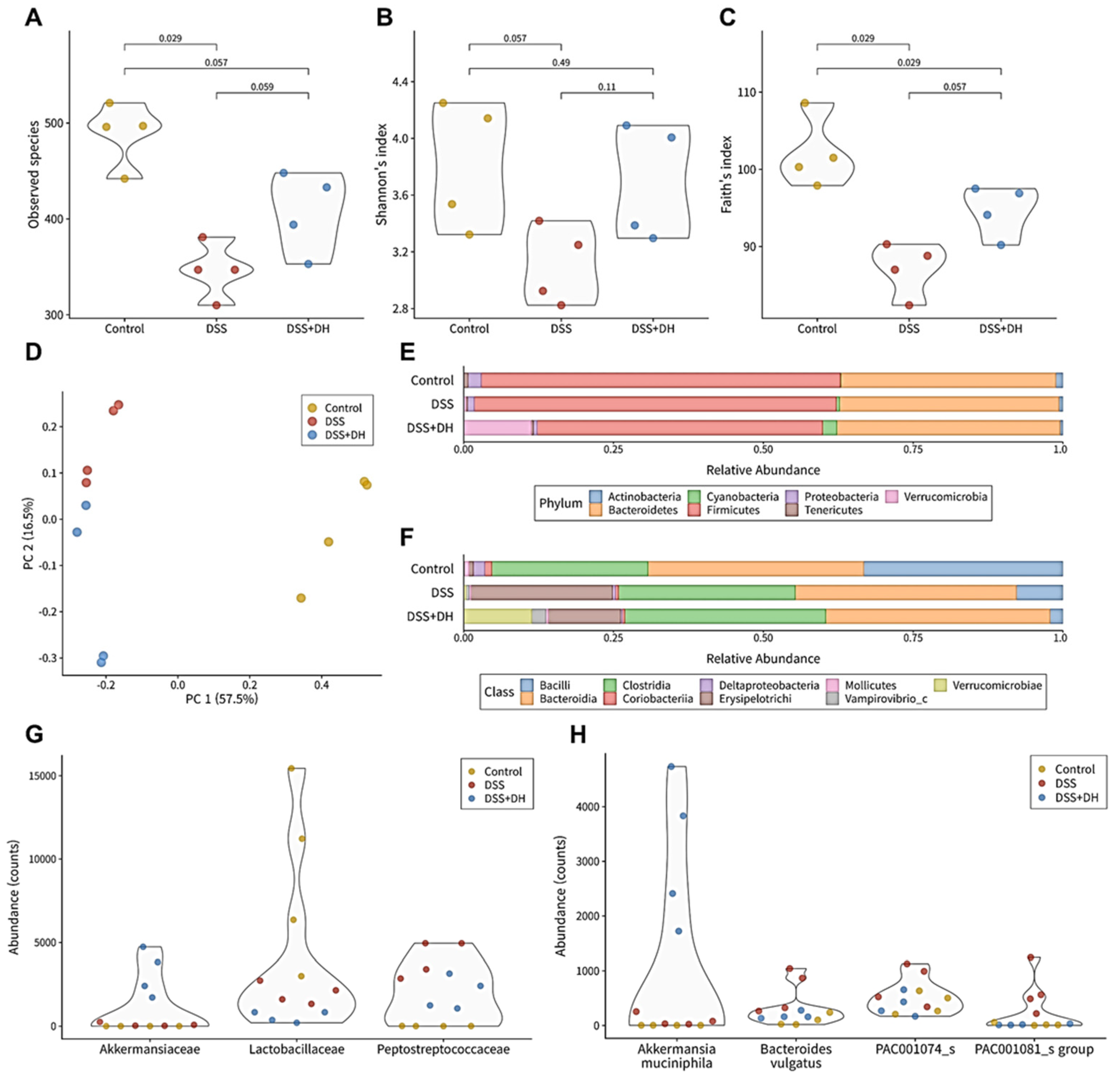
3.6. DH Strengthens the Composition of the Fecal Microbiota in DSS-Induced Mice
3.7. DH Supports Beneficial Bacteria and Suppresses Harmful Bacteria in the Fecal Microbiota
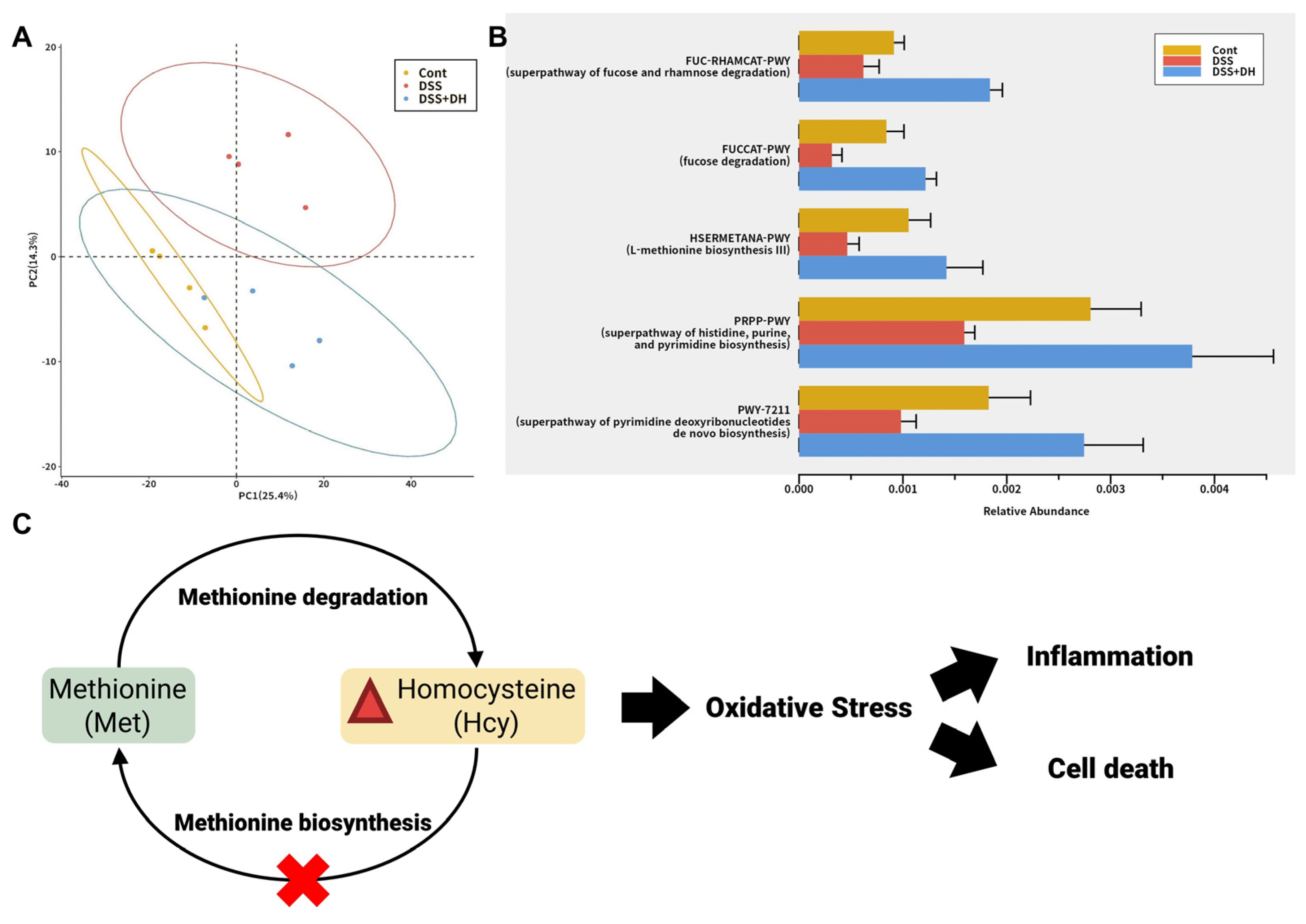
3.8. DH Restores the Functionality of the Gut Microbiota to Improve Gut Health and Reduce Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Kossmann, J. Improving crops for a changing world. Front. Plant Sci. 2021, 12, 728328. [Google Scholar] [CrossRef]
- Li, R.; Luan, F.; Zhao, Y.; Wu, M.; Lu, Y.; Tao, C.; Zhu, L.; Zhang, C.; Wan, L. Crataegus pinnatifida: A botanical, ethnopharmacological, phytochemical, and pharmacological overview. J. Ethnopharmacol. 2023, 301, 115819. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Mehri, S.; Hosseinzadeh, H. The effects of Crataegus pinnatifida (Chinese hawthorn) on metabolic syndrome: A review. Iran. J. Basic Med. Sci. 2019, 22, 460. [Google Scholar] [PubMed]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of disease: Inflammatory bowel diseases. Proc. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.; Chan, F.K. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Xia, B.; Crusius, J.; Meuwissen, S.; Pena, A. Inflammatory bowel disease: Definition, epidemiology, etiologic aspects, and immunogenetic studies. World J. Gastroenterol. 1998, 4, 446. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Ng, S.C. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar] [CrossRef]
- Stallmach, A.; Hagel, S.; Bruns, T. Adverse effects of biologics used for treating IBD. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 167–182. [Google Scholar] [CrossRef]
- Beaugerie, L.; Rahier, J.-F.; Kirchgesner, J. Predicting, preventing, and managing treatment-related complications in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1324–1335.e1322. [Google Scholar] [CrossRef]
- Parian, A.; Limketkai, B.N. Dietary supplement therapies for inflammatory bowel disease: Crohn’s disease and ulcerative colitis. Curr. Pharm. Des. 2016, 22, 180–188. [Google Scholar] [CrossRef]
- Rossi, R.E.; Whyand, T.; Murray, C.D.; Hamilton, M.I.; Conte, D.; Caplin, M.E. The role of dietary supplements in inflammatory bowel disease: A systematic review. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1357–1364. [Google Scholar] [CrossRef]
- Ma, L.; Lang, Y.; Xin, X.; Zhao, W.; Zhou, Q.; Wang, J.; Dong, S. Polysaccharides extracted from hawthorn (Crataegus pinnatifida) exhibiting protective effects against DSS/AOM-induced colorectal cancer in vivo. J. Funct. Foods 2023, 107, 105618. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.D.; Reid, J.N.; Macklaim, J.M.; McMurrough, T.A.; Edgell, D.R.; Gloor, G.B. Unifying the analysis of high-throughput sequencing datasets: Characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome 2014, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Peddada, S.D. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef]
- Nearing, J.T.; Douglas, G.M.; Hayes, M.G.; MacDonald, J.; Desai, D.K.; Allward, N.; Jones, C.M.; Wright, R.J.; Dhanani, A.S.; Comeau, A.M. Microbiome differential abundance methods produce different results across 38 datasets. Nat. Commun. 2022, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The MetaCyc database of metabolic pathways and enzymes—A 2019 update. Nucleic Acids Res. 2020, 48, D445–D453. [Google Scholar] [CrossRef] [PubMed]
- Clapper, M.L.; Gary, M.A.; Coudry, R.A.; Litwin, S.; Chang, W.-C.L.; Devarajan, K.; Lubet, R.A.; Cooper, H.S. 5-aminosalicylic acid inhibits colitis-associated colorectal dysplasias in the mouse model of azoxymethane/dextran sulfate sodium-induced colitis. Inflamm. Bowel Dis. 2008, 14, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Vereecke, L.; Beyaert, R.; van Loo, G. Enterocyte death and intestinal barrier maintenance in homeostasis and disease. Trends Mol. Med. 2011, 17, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Vandenabeele, P.; Krysko, D.V. Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity 2013, 38, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Vandenabeele, P. Necroptosis and its role in inflammation. Nature 2015, 517, 311–320. [Google Scholar] [CrossRef]
- Lee, K.-I.; Kim, H.J.; Kim, H.; Kim, M.-S.; Kim, J.I.; Park, K.-S. Magnolia officinalis Bark Extract Prevents Enterocyte Death in a Colitis Mouse Model by Inhibiting ROS-Mediated Necroptosis. Antioxidants 2022, 11, 2435. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of gut microbiota in inflammatory bowel disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Insights into the role of Erysipelotrichaceae in the human host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef]
- Priya, S.; Burns, M.B.; Ward, T.; Mars, R.A.; Adamowicz, B.; Lock, E.F.; Kashyap, P.C.; Knights, D.; Blekhman, R. Identification of shared and disease-specific host gene–microbiome associations across human diseases using multi-omic integration. Nat. Microbiol. 2022, 7, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Duan, C.; Wu, J.; Wang, Z.; Tan, C.; Hou, L.; Qian, W.; Han, C.; Hou, X. Fucose promotes intestinal stem cell-mediated intestinal epithelial development through promoting Akkermansia-related propanoate metabolism. Gut Microbes 2023, 15, 2233149. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Mills, R.H.; Dulai, P.S.; Vázquez-Baeza, Y.; Sauceda, C.; Daniel, N.; Gerner, R.R.; Batachari, L.E.; Malfavon, M.; Zhu, Q.; Weldon, K. Multi-omics analyses of the ulcerative colitis gut microbiome link Bacteroides vulgatus proteases with disease severity. Nat. Microbiol. 2022, 7, 262–276. [Google Scholar] [CrossRef]
- Han, S.-K.; Shin, Y.-J.; Lee, D.-Y.; Kim, K.M.; Yang, S.-J.; Kim, D.S.; Choi, J.-W.; Lee, S.; Kim, D.-H. Lactobacillus rhamnosus HDB1258 modulates gut microbiota-mediated immune response in mice with or without lipopolysaccharide-induced systemic inflammation. BMC Microbiol. 2021, 21, 146. [Google Scholar] [CrossRef]
- Sitkin, S.; Vakhitov, T.; Kononova, S.; Skalinskaya, M.; Pokrotnieks, J. Gut microbiota-mediated pleiotropic effects of fucose can improve inflammatory bowel disease by modulating bile acid metabolism and enhancing propionate production. Inflamm. Bowel Dis. 2021, 27, e10–e11. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Koklesova, L.; Mazurakova, A.; Samec, M.; Biringer, K.; Samuel, S.M.; Büsselberg, D.; Kubatka, P.; Golubnitschaja, O. Homocysteine metabolism as the target for predictive medical approach, disease prevention, prognosis, and treatments tailored to the person. EPMA J. 2021, 12, 477–505. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, J.; Bing, Y.; Yan, W.; Zhu, Y.; Xia, B.; Chen, M. Diet-induced hyperhomocysteinaemia increases intestinal inflammation in an animal model of colitis. J. Crohn’s Colitis 2015, 9, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, A.; Guéant, J.L.; Peyrin-Biroulet, L. Meta-analysis: Hyperhomocysteinaemia in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2011, 34, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, M.; Negroni, A.; Stronati, L.; Vitali, R.; Prete, E.; Bertin, J.; Gough, P.J.; Aloi, M.; Cucchiara, S. Necroptosis is active in children with inflammatory bowel disease and contributes to heighten intestinal inflammation. Off. J. Am. Coll. Gastroenterol. ACG 2014, 109, 279–287. [Google Scholar] [CrossRef]
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 2019, 17, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Franzosa, E.A.; Lloyd-Price, J.; McIver, L.J.; Schwager, R.; Poon, T.W.; Ananthakrishnan, A.N.; Andrews, E.; Barron, G.; Lake, K. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat. Microbiol. 2018, 3, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Geerlings, S.Y.; Aalvink, S.; de Vos, W.M.; Belzer, C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 637–642. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-I.; Jo, Y.; Yuk, H.J.; Kim, S.-Y.; Kim, H.; Kim, H.J.; Hwang, S.-K.; Park, K.-S. Potential Phytotherapy of DSS-Induced Colitis: Ameliorating Reactive Oxygen Species-Mediated Necroptosis and Gut Dysbiosis with a New Crataegus pinnatifida Bunge Variety—Daehong. Antioxidants 2024, 13, 340. https://doi.org/10.3390/antiox13030340
Lee K-I, Jo Y, Yuk HJ, Kim S-Y, Kim H, Kim HJ, Hwang S-K, Park K-S. Potential Phytotherapy of DSS-Induced Colitis: Ameliorating Reactive Oxygen Species-Mediated Necroptosis and Gut Dysbiosis with a New Crataegus pinnatifida Bunge Variety—Daehong. Antioxidants. 2024; 13(3):340. https://doi.org/10.3390/antiox13030340
Chicago/Turabian StyleLee, Kang-In, Yousang Jo, Heung Joo Yuk, Sun-Young Kim, Hyungjun Kim, Hye Jin Kim, Soo-Keol Hwang, and Ki-Sun Park. 2024. "Potential Phytotherapy of DSS-Induced Colitis: Ameliorating Reactive Oxygen Species-Mediated Necroptosis and Gut Dysbiosis with a New Crataegus pinnatifida Bunge Variety—Daehong" Antioxidants 13, no. 3: 340. https://doi.org/10.3390/antiox13030340
APA StyleLee, K.-I., Jo, Y., Yuk, H. J., Kim, S.-Y., Kim, H., Kim, H. J., Hwang, S.-K., & Park, K.-S. (2024). Potential Phytotherapy of DSS-Induced Colitis: Ameliorating Reactive Oxygen Species-Mediated Necroptosis and Gut Dysbiosis with a New Crataegus pinnatifida Bunge Variety—Daehong. Antioxidants, 13(3), 340. https://doi.org/10.3390/antiox13030340









