Identification and Functional Validation of Two Novel Antioxidant Peptides in Saffron
Abstract
:1. Introduction
2. Materials and Methods
2.1. Total Protein Extraction of Saffron
2.2. Determination of the Whole Protein of Saffron by SDS-PAGE and Native-PAGE
2.3. Protein Analysis of Saffron by High-Performance Liquid Chromatography (HPLC)
2.4. Identification of Saffron’s Functional Peptides by Liquid Chromatography Mass Spectrometry (LC-MS/MS) and Proteome Sequencing
2.5. In Vitro Antioxidant Acrivity
2.5.1. DPPH Radical Scavenging Activity
2.5.2. 2,2′-Azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) Radical Scavenging Activity Assay
2.5.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.6. H2O2 Induced Oxidative Damage in a HepG2 Cell Model Experiment
2.7. Intracellular Antioxidant Activity Assay
2.8. Determination of Stability of Saffron Antioxidant Peptides
2.9. Statistical Analysis
3. Results
3.1. The Composition and Antioxidant Activity of Total Stigma Protein
3.2. Identification of Peptide Sequences
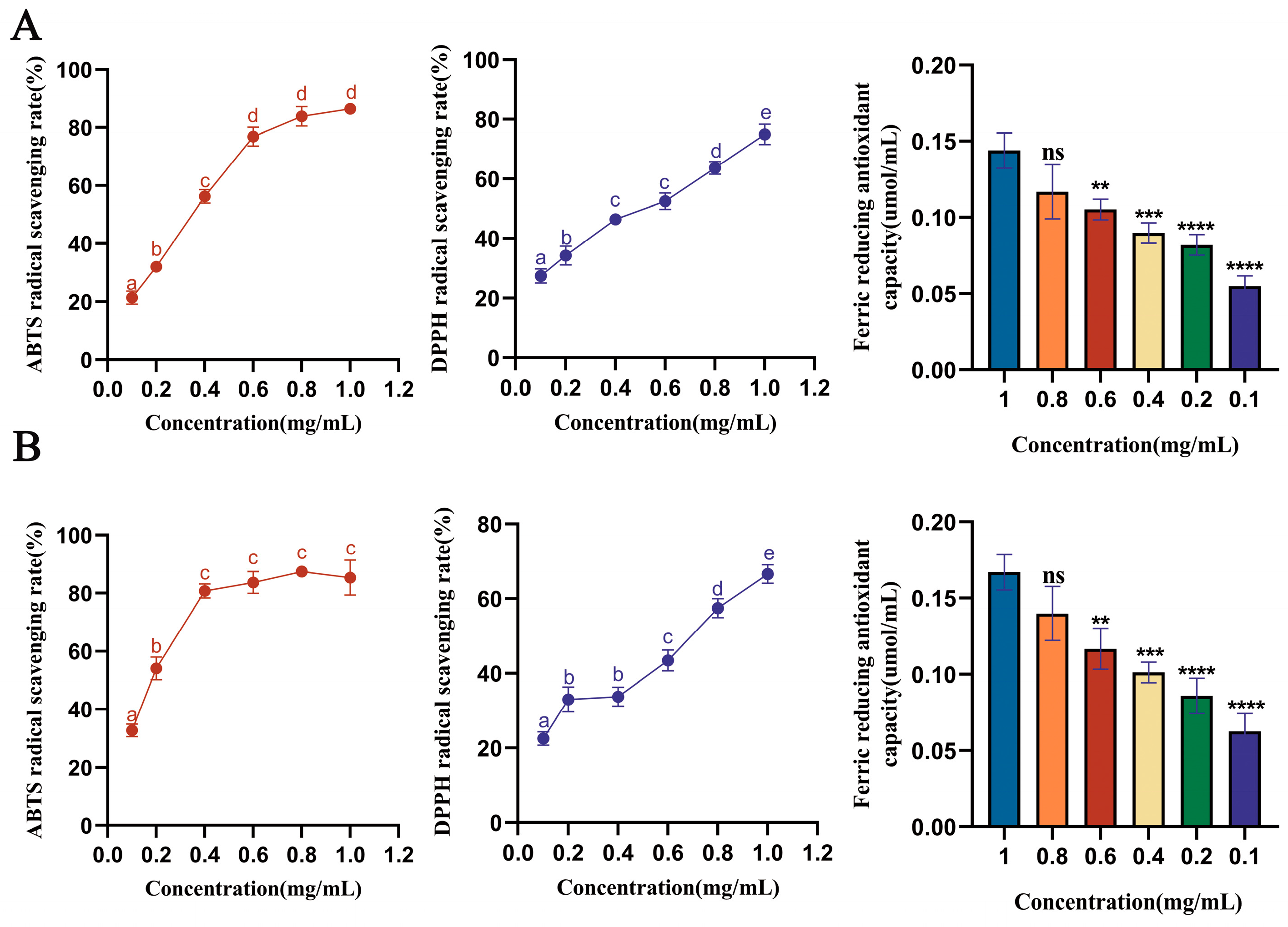
3.3. Analysis of Antioxidant Capacity of Saffron Stigma Active Peptides
3.4. Effect of Saffron Peptides on HepG2 Cells Injured with H2O2
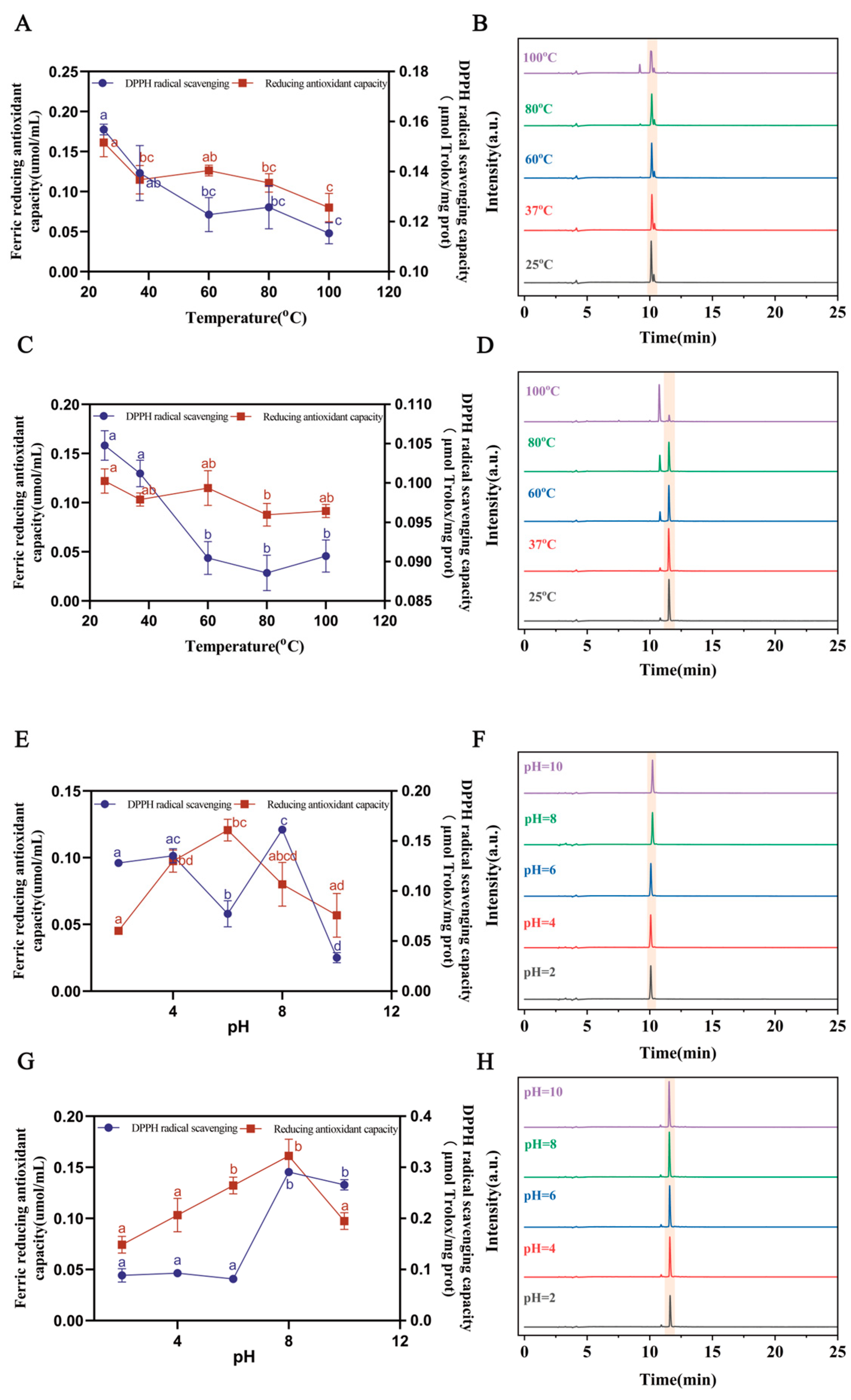
3.5. The Stability of Saffron Antioxidant Peptides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, B.; Li, S.; Yang, J.; Lin, D.; Feng, Y.; Lu, J.; Shao, Q. Phytochemistry, pharmacology, and potential clinical applications of saffron: A review. J. Ethnopharmacol. 2021, 281, 114555. [Google Scholar] [CrossRef]
- Aissa, R.; Ibourki, M.; Ait Bouzid, H.; Bijla, L.; Oubannin, S.; Sakar, E.H.; Jadouali, S.; Hermansyah, A.; Goh, K.W.; Ming, L.C.; et al. Phytochemistry, quality control and medicinal uses of Saffron (Crocus sativus L.): An updated review. J. Med. Life 2023, 16, 822–836. [Google Scholar]
- Rahaiee, S.; Moini, S.; Hashemi, M.; Shojaosadati, S.A. Evaluation of antioxidant activities of bioactive compounds and various extracts obtained from saffron (Crocus sativus L.): A review. J. Food Sci. Technol. 2015, 52, 1881–1888. [Google Scholar] [CrossRef]
- Arzi, L.; Hoshyar, R. Saffron anti-metastatic properties, ancient spice novel application. Crit. Rev. Food Sci. Nutr. 2022, 62, 3939–3950. [Google Scholar] [CrossRef] [PubMed]
- Abirami, R.G.; Kowsalya, S. Quantification and correlation study on derived phenols and antioxidant activity of seaweeds from Gulf of Mannar. J. Herbs Spices Med. Plants 2017, 23, 9–17. [Google Scholar] [CrossRef]
- Esmaealzadeh, D.; Moodi Ghalibaf, A.; Shariati Rad, M.; Rezaee, R.; Razavi, B.M.; Hosseinzadeh, H. Pharmacological effects of Safranal: An updated review. Iran. J. Basic Med. Sci. 2023, 26, 1131–1143. [Google Scholar] [PubMed]
- García-Blázquez, A.; Moratalla-López, N.; Lorenzo, C.; Salinas, M.R.; Alonso, G.L. Effect of Crocus sativus L. Stigmas Microwave Dehydration on Picrocrocin, Safranal and Crocetin Esters. Foods 2021, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Cerdá-Bernad, D.; Clemente-Villalba, J.; Valero-Cases, E.; Pastor, J.J.; Frutos, M.J. Novel Insight into the Volatile Profile and Antioxidant Properties of Crocus sativus L. Flowers. Antioxidants 2022, 11, 1650. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, A.M.; Afkhami-Goli, A.; Paul, A.M.; Bhat, R.K.; Acharjee, S.; Ellestad, K.K.; Noorbakhsh, F.; Michalak, M.; Power, C. Neuroinflammation and endoplasmic reticulum stress are coregulated by crocin to prevent demyelination and neurodegeneration. J. Immunol. 2011, 187, 4788–4799. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, M.; Arekhi, S.; Omranzadeh, A.; Sahebkar, A. Saffron in the treatment of depression, anxiety and other mental disorders: Current evidence and potential mechanisms of action. J. Affect. Disord. 2018, 227, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, J.; Liu, C.; Fang, C. Protective effects of crocetin pretreatment on myocardial injury in an ischemia/reperfusion rat model. Eur. J. Pharmacol. 2014, 741, 290–296. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, G.; Nabavi, S.M.; Sancarlo, D.; Greco, A.; Pieretti, S. Crocus sativus L. (Saffron) in Alzheimer’s Disease Treatment: Bioactive Effects on Cognitive Impairment. Curr. Neuropharmacol. 2021, 19, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, 13394. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Shi, X.; Chen, L.; Kou, J.; Meng, J.; Chen, H. Antioxidant Peptides from Goat Milk Fermented by Lactobacillus casei L61: Preparation, Optimization, and Stability Evaluation in Simulated Gastrointestinal Fluid. Nutrients 2018, 10, 797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, G.X.; Zhao, Y.Q.; Qiu, Y.T.; Chi, C.F.; Wang, B. Identification and Active Evaluation of Antioxidant Peptides from Protein Hydrolysates of Skipjack Tuna (Katsuwonus pelamis) Head. Antioxidants 2019, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; John, A. Structure identification of walnut peptides and evaluation of cellular antioxidant activity. Food Chem. 2022, 388, 132943. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Khalil, A.A. Nutritional and health beneficial properties of saffron (Crocus sativus L): A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2683–2706. [Google Scholar] [CrossRef]
- Zhang, A.; Shen, Y.; Cen, M.; Hong, X.; Shao, Q.; Chen, Y.; Zheng, B. Polysaccharide and crocin contents, and antioxidant activity of saffron from different origins. Ind. Crops Prod. 2019, 133, 111–117. [Google Scholar] [CrossRef]
- Ouahhoud, S.; Khoulati, A.; Kadda, S.; Bencheikh, N.; Mamri, S.; Ziani, A.; Baddaoui, S.; Eddabbeh, F.E.; Lahmass, I.; Benabbes, R. Antioxidant Activity, Metal Chelating Ability and DNA Protective Effect of the Hydroethanolic Extracts of Crocus sativus Stigmas, Tepals and Leaves. Antioxidants 2022, 11, 932. [Google Scholar] [CrossRef]
- Paredi, G.; Raboni, S.; Marchesani, F.; Ordoudi, S.A.; Tsimidou, M.Z.; Mozzarelli, A. Insight of Saffron Proteome by Gel-Electrophoresis. Molecules 2016, 21, 167. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, G.; Dong, Y.; Qian, X.; Li, J.; Xu, X.; Huang, H.; Xu, L.; Li, L. Screening of Key Proteins Affecting Floral Initiation of Saffron Under Cold Stress Using iTRAQ-Based Proteomics. Front. Plant Sci. 2021, 12, 644934. [Google Scholar] [CrossRef]
- Habiba, U.; Nisar, J.; Choohan, M.A.; Shah, S.A.; Nisar, Z.; Mustafa, I. Antibacterial Activity of Tris NaCl and PBS Buffer Protein Extract of Cassia fistula, Saccharum officinarum, Albizia lebbeck and Cymbopogon citrates Against Bacterial Strains. Dose Response 2021, 19, 1559325821992239. [Google Scholar] [CrossRef]
- Alashi, A.M.; Blanchard, C.L.; Mailer, R.J.; Agboola, S.O.; Mawson, A.J.; He, R.; Girgih, A.; Aluko, R.E. Antioxidant properties of Australian canola meal protein hydrolysates. Food Chem. 2014, 146, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Durrani, R.; Meiyun, Y.; Yang, B.; Durand, E.; Delavault, A.; Bowen, H.; Weiwei, H.; Yiyang, L.; Lili, S.; Fei, G. Identification of novel bioactive proteins and their produced oligopeptides from Torreya grandis nuts using proteomic based prediction. Food Chem. 2023, 405, 134843. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Gao, T.; Hou, Y.; Li, D.; Fu, L. Identification and characterization of two novel α-glucosidase inhibitory peptides from almond (Armeniaca sibirica) oil manufacture residue. LWT-Food Sci. Technol. 2020, 134, 110215. [Google Scholar] [CrossRef]
- Fan, L.; Mao, X.; Wu, Q. Purification, Identification and Molecular Docking of Novel Antioxidant Peptides from Walnut (Juglans regia L.) Protein Hydrolysates. Molecules 2022, 27, 8423. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.H.; Li, J.; Jiang, G.X.; Li, H.Y.; Zhao, M.M.; Jiang, Y.M. Effects of combined high pressure and enzymatic treatments on physicochemical and antioxidant properties of peanut proteins. Food Sci. Nutr. 2019, 7, 1417–1425. [Google Scholar] [CrossRef]
- Yu, X.; Chen, Y.; Qi, Z.; Chen, Q.; Cao, Y.; Kong, Q. Preparation and identification of a novel peptide with high antioxidant activity from corn gluten meal. Food Chem. 2023, 424, 136389. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Purification and identification of novel antioxidant peptides from watermelon seed protein hydrolysates and their cytoprotective effects on H2O2-induced oxidative stress. Food Chem. 2020, 327, 127059. [Google Scholar] [CrossRef]
- Bollati, C.; Cruz-Chamorro, I.; Aiello, G.; Li, J.; Bartolomei, M.; Santos-Sánchez, G.; Ranaldi, G.; Ferruzza, S.; Sambuy, Y.; Arnoldi, A.; et al. Investigation of the intestinal trans-epithelial transport and antioxidant activity of two hempseed peptides WVSPLAGRT (H2) and IGFLIIWV (H3). Food Res. Int. 2022, 152, 110720. [Google Scholar] [CrossRef]
- Han, R.; Shao, S.; Zhang, H.; Qi, H.; Xiao, F.; Shen, Y.; Fan, L.; Wang, H.; Zhao, D.; Li, G.; et al. Physico-chemical properties, antioxidant activity, and ACE inhibitory activity of protein hydrolysates from wild jujube seed. J. Food Sci. 2022, 87, 2484–2503. [Google Scholar] [CrossRef]
- Gao, J.; Li, T.; Chen, D.; Gu, H.; Mao, X. Identification and molecular docking of antioxidant peptides from hemp seed protein hydrolysates. LWT-Food Sci. Technol. 2021, 147, 111453. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhao, G.X.; Suo, S.K.; Wang, Y.M.; Chi, C.F.; Wang, B. Purification, Identification, Activity Evaluation, and Stability of Antioxidant Peptides from Alcalase Hydrolysate of Antarctic Krill (Euphausia superba) Proteins. Mar. Drugs. 2021, 19, 347. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, S.; Han, R.; Zhang, R.; Ma, X.; Wang, M.; Wan, Z.; Zhao, D.; Yan, M. Structural, physicochemical and functional properties of Semen Ziziphi Spinosae protein. RSC Adv. 2020, 10, 29555–29566. [Google Scholar] [CrossRef]
- Wu, R.; Huang, J.; Huan, R.; Chen, L.; Yi, C.; Liu, D.; Wang, M.; Liu, C.; He, H. New insights into the structure-activity relationships of antioxidative peptide PMRGGGGYHY. Food Chem. 2021, 337, 127678. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT-Food Sci. Technol. 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Liu, W.Y.; Fang, L.; Feng, X.W.; Li, G.M.; Gu, R.Z. In vitro antioxidant and angiotensin I-converting enzyme inhibitory properties of peptides derived from corn gluten meal. Eur. Food Res. Technol. 2020, 246, 2017–2027. [Google Scholar] [CrossRef]
- Yokomizo, A.; Takenaka, Y.; Takenaka, T. Antioxidative activity of peptides prepared from okara protein. Food Sci. Technol. Res. 2002, 8, 357–359. [Google Scholar] [CrossRef]
- Amigo, L.; Martínez-Maqueda, D.; Hernández-Ledesma, B. In Silico and In Vitro Analysis of Multifunctionality of Animal Food-Derived Peptides. Foods 2020, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Z.R.; Chi, C.F.; Zhang, Q.H.; Luo, H.Y. Preparation and evaluation of antioxidant peptides from ethanol-soluble proteins hydrolysate of Sphyrna lewini muscle. Peptides 2012, 36, 240–250. [Google Scholar] [CrossRef]
- Peña-Ramos, E.A.; Xong, Y.L.; Arteaga, G.E. Fractionation and characterisation for antioxidant activity of hydrolysed whey protein. J. Sci. Food Agric. 2004, 84, 1908–1918. [Google Scholar] [CrossRef]
- Clausen, M.R.; Skibsted, L.H.; Stagsted, J. Characterization of major radical scavenger species in bovine milk through size exclusion chromatography and functional assays. J. Agric. Food Chem. 2009, 57, 2912–2919. [Google Scholar] [CrossRef]
- Chai, T.T.; Xiao, J.; Dass, S.M.; Teoh, J.Y.; Ee, K.Y.; Ng, W.J.; Wong, F.C. Identification of antioxidant peptides derived from tropical jackfruit seed and investigation of the stability profiles. Food Chem. 2021, 340, 127876. [Google Scholar] [CrossRef]
- Yu, Y.P.; Lai, S.J.; Chang, C.R.; Chen, W.C.; Wu, S.H.; Lu, C.P. Peptidomic analysis of low molecular weight antioxidative peptides prepared by lotus (Nelumbo nucifera Gaertn.) seed protein hydrolysates. LWT-Food Sci. Technol. 2021, 144, 111138. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Sun, Q.; Song, G.; Huang, J. Extraction, identification and structure-activity relationship of antioxidant peptides from sesame (Sesamum indicum L.) protein hydrolysate. Food Res. Int. 2019, 116, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Yingchutrakul, Y.; Krobthong, S.; Choowongkomon, K.; Papan, P.; Samutrtai, P.; Mahatnirunkul, T.; Aonbangkhen, C. Discovery of a Multifunctional Octapeptide from Lingzhi with Antioxidant and Tyrosinase Inhibitory Activity. Pharmaceuticals 2022, 15, 684. [Google Scholar] [CrossRef]
- Cai, X.; Chen, S.; Liang, J.; Tang, M.; Wang, S. Protective effects of crimson snapper scales peptides against oxidative stress on Drosophila melanogaster and the action mechanism. Food Chem. Toxicol. 2021, 148, 111965. [Google Scholar] [CrossRef]
- Byeon, J.C.; Lee, S.E.; Kim, T.H.; Ahn, J.B.; Kim, D.H.; Choi, J.S.; Park, J.S. Design of novel proliposome formulation for antioxidant peptide, glutathione with enhanced oral bioavailability and stability. Drug Deliv. 2019, 26, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, B.; Luo, X.; Zhao, M.; Zheng, F.; Sun, J.; Li, H.; Sun, X.; Huang, M. Cytoprotective effects of a tripeptide from Chinese Baijiu against AAPH-induced oxidative stress in HepG2 cells via Nrf2 signaling. RSC Adv. 2018, 8, 10898–10906. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Wang, Y.; Zhang, Y.; Xu, N. Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from angelica sinensis. Int. J. Biol. Macromol. 2018, 115, 281–286. [Google Scholar] [CrossRef]
- Wong, F.C.; Xiao, J.; Wang, S.; Ee, K.Y.; Chai, T.T. Advances on the antioxidant peptides from edible plant sources. Trends in Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Liu, K.; Du, R.; Chen, F. Stability of the antioxidant peptide SeMet-Pro-Ser identified from selenized brown rice protein hydrolysates. Food Chem. 2020, 319, 126540. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.R.; Qiu, Y.T.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Purification and Characterization of Antioxidant Peptides Derived from Protein Hydrolysate of the Marine Bivalve Mollusk Tergillarca granosa. Mar. Drugs. 2019, 17, 251. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Z.; Zhang, W.G.; Kang, Z.L.; Zhou, G.H.; Xu, X.L. Stability of an antioxidant peptide extracted from Jinhua ham. Meat Sci. 2014, 96, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Lisboa, C.R.; Santos, T.D.; Costa, J. Bioactive stability of microalgal protein hydrolysates under food processing and storage conditions. J. Food Sci. Technol. 2019, 56, 4543–4551. [Google Scholar] [CrossRef] [PubMed]



| Accession | Amino Acids | Molecular Weight (kDa) | Abundance | Annotation |
|---|---|---|---|---|
| D2T0A5 | 91 | 8.8 | 2173002816 | Non-specific lipid-transfer protein (fragment) |
| A0A5J6ANU7 | 497 | 53.4 | 1602808441 | Aldehyde dehydrogenase |
| A0A2U8ZTY0 | 537 | 58.3 | 1467403203 | Aldehyde dehydrogenase 2B4 |
| A0A6H0C818 | 462 | 51.2 | 1347643392 | Glycosyltransferase |
| A0A075M6P3 | 477 | 53.2 | 818608184.3 | Glycosyltransferase |
| A0A1S5T4X6 | 240 | 26.7 | 485796513.8 | SOUL heme-binding protein |
| A0A3G1GZP7 | 537 | 58.4 | 360497340 | Aldehyde dehydrogenase |
| A0A5J6ANM0 | 504 | 55.1 | 347338259 | Aldehyde dehydrogenase |
| A0A1S5VK40 | 507 | 57.3 | 335751151 | Beta-glucosidase 12 |
| A0A075M6K1 | 434 | 48 | 262639198 | Glycosyltransferase |
| Peptide | Temperature (°C) | pH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 37 | 60 | 80 | 100 | 2 | 4 | 6 | 8 | 10 | |
| DGGSDYLGK | 0.08612 | 0.07396 | 0.07549 | 0.07357 | 0.074 | 0.09748 | 0.13064 | 0.12197 | 0.13675 | 0.1374 |
| VDPYFNK | 0.13439 | 0.13967 | 0.11136 | 0.18938 | 0.01905 | 0.09583 | 0.09639 | 0.09556 | 0.09276 | 0.09625 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Y.; Tao, H.; Wang, S.; Xing, B.; Wang, Z.; Liu, K.; Shao, Q.; Gao, F. Identification and Functional Validation of Two Novel Antioxidant Peptides in Saffron. Antioxidants 2024, 13, 378. https://doi.org/10.3390/antiox13030378
Long Y, Tao H, Wang S, Xing B, Wang Z, Liu K, Shao Q, Gao F. Identification and Functional Validation of Two Novel Antioxidant Peptides in Saffron. Antioxidants. 2024; 13(3):378. https://doi.org/10.3390/antiox13030378
Chicago/Turabian StyleLong, Yiyang, Han Tao, Shiyu Wang, Bingcong Xing, Zhineng Wang, Kexin Liu, Qingsong Shao, and Fei Gao. 2024. "Identification and Functional Validation of Two Novel Antioxidant Peptides in Saffron" Antioxidants 13, no. 3: 378. https://doi.org/10.3390/antiox13030378







